-
The cesarean section (CS) rate has risen rapidly worldwide. In high-income countries, national studies have revealed that more than a quarter of births are performed by CS, while in China the rate increased from 28.8% in 2008 to 34.9% in 2014[1]—far above the World Health Organization (WHO)-recommended rate of 10%–15%. Due to the high global cesarean rates, the promotion of trial of labor after cesarean (TOLAC) may be an option that can be used to reduce the overall number of CSs. In 1982, the American College of Obstetrics and Gynecology (ACOG) recommended TOLAC due to its safety and general acceptability[2]. A large number of studies have shown that with strict indications, the success rate for TOLAC can reach 60%–80%[3]. In 2010, ACOG published a new clinical practice guideline on TOLAC, recommending that obstetricians offer a trial of labor to women with two previous low-transverse CSs[2].
Some investigators have addressed the risks and benefits of the trial of labor after more than one CS, but they have not reached a consistent conclusion[4]. In addition, there have been no randomized trials designed to compare adverse maternal or neonatal outcomes between women who had a trial of labor after two cesarean sections (TOLAC-2) with those who underwent a third CS. As China is in its early stages of adopting TOLAC—and although a trial of labor after a cesarean section (TOLAC-1) is encouraged and acceptable—the rate of TOLAC remains low [8.5% (1,013/11,958) in our previous study] compared with other countries such as Israel [75.6% (7,473/9,888)][5] and France [70% (367/523)][6]. TOLAC-2 is still controversial and rarely accepted, even by obstetricians and midwives. In the present study, we collected data on pregnancies with a prior 1 or 2 CSs from 5 public tertiary hospitals. The purpose of this study, then, was to compare maternal and neonatal outcomes in women with TOLAC-1 vs. TOLAC-2 and TOLAC-2 vs. repeated CS (RCS).
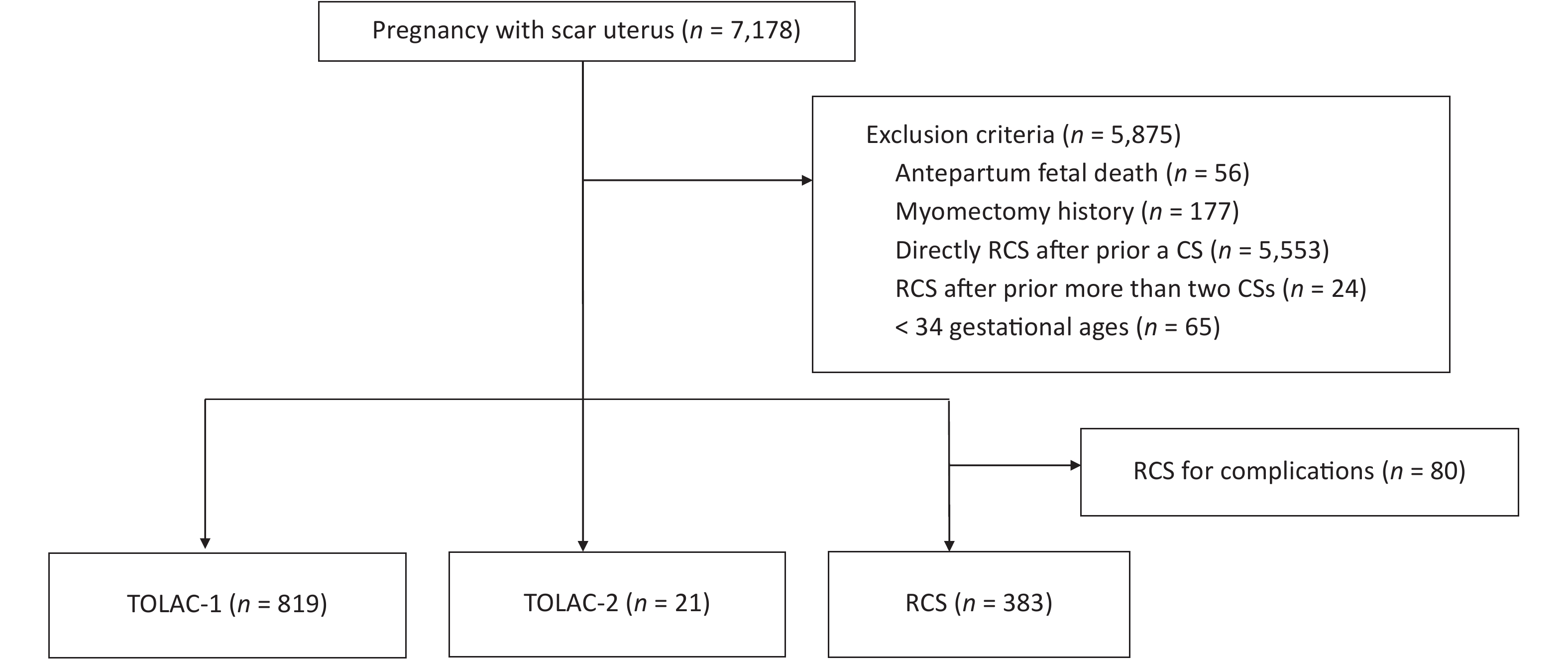
This was a multicenter, retrospective, cross-sectional study conducted in five public tertiary hospitals. The study comprised 7,178 women with singleton pregnancies and a scar uterus who delivered again between January and December of 2017. We excluded cases of antepartum fetal death, a gestational age < 34 weeks, a scarred uterus caused by myomectomy, CS directly after a prior CS, or more than two prior CSs. Considering that a majority of women with TOLAC were without severe obstetric complications, we excluded women who chose RCS due to complications such as placenta previa, severe pre-eclampsia, heart disease, threatened uterine rupture, or fetal distress. Supplementary Figure S1 (available in www.besjournal.com) shows a flow diagram of the patient enrollment process. The indication for TOLAC was according to the Chinese expert consensus of 2016[7]. It is necessary for pregnant women to demonstrate a willingness to attempt vaginal delivery, and medical institutions possess the facilities to deal with potential complications of vaginal birth after cesarean (VBAC) and the appropriate corresponding emergency plans. Women with only one previous low-transverse incision CS without uterine incision laceration, postpartum hemorrhage, or infection were included, and the current pregnancy manifested a cephalic presentation of the fetus without indications for CS. The interpregnancy interval was over 18 months, and ultrasonographic examination indicated that the muscle layer of the lower uterine segment was continuous and that fetal weight was under 4,000 g. TOLAC-2 is not recommended in China, and labor must be closely monitored by senior obstetricians for women who show a strong willingness to undergo TOLAC-2 after being informed of the risk of adverse maternal and neonatal outcomes.
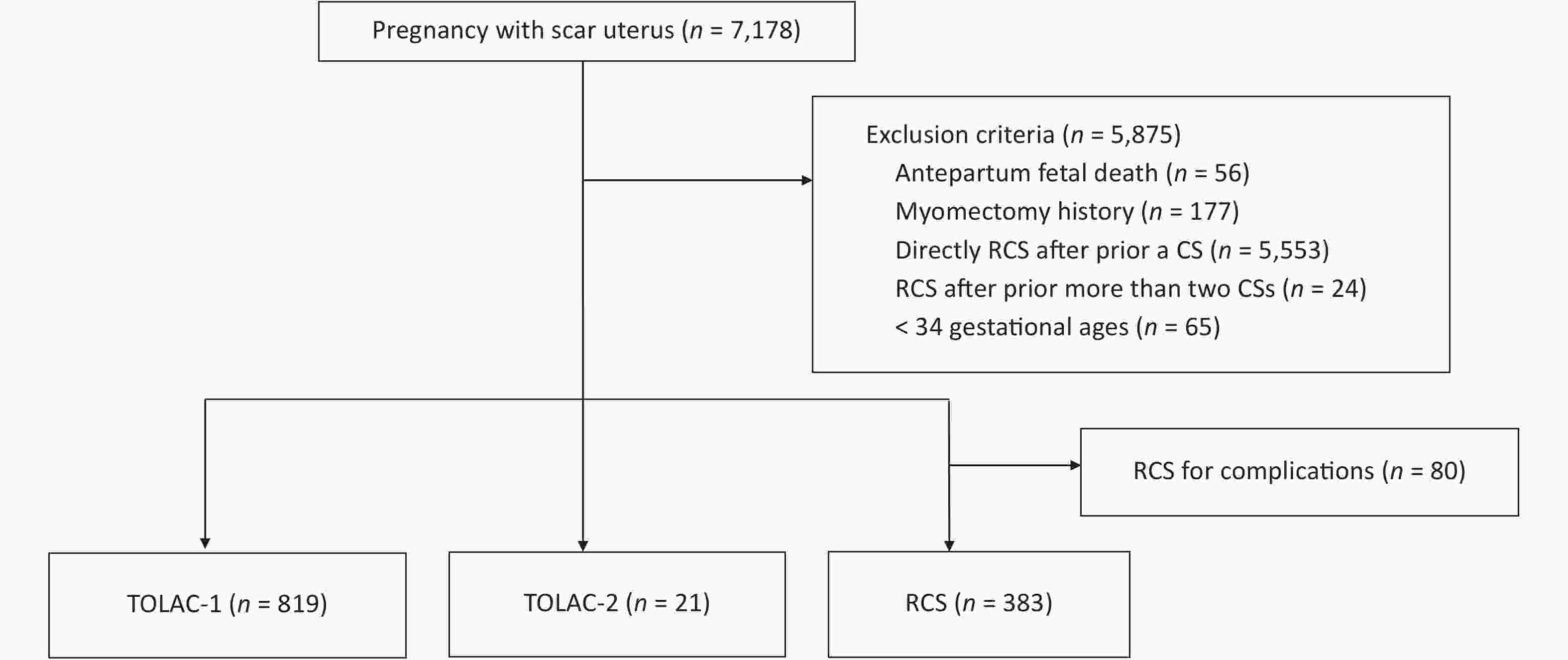
Figure S1. A flow diagram of the patients’ enrollment process. RCS, repeat cesarean section; TOLAC-1, trial of labor after one prior cesarean section; TOLAC-2, trial of labor after two prior cesarean sections.
This retrospective study was approved by the Medical Ethics Committee of Guangzhou Medical University with Medical Research No. 2016 (0406), with approval on April 6, 2016.
Statistical analysis was performed using IBM SPSS v24.0. Continuous variables were presented with mean ± SD. We performed Student’s t-test or 1-way ANOVA with parametric data, or Mann-Whitney U or Kruskal–Wallis test for data following non-normal distributions to compare continuous variables between two groups or multiple groups, respectively. Categorical variables were reported as frequency (percentage), and the differences between the groups were compared using the χ2 test or Fisher exact test in cases of small numbers, as appropriate. Differences with P-values of < 0.05 were considered to be statistically significant.
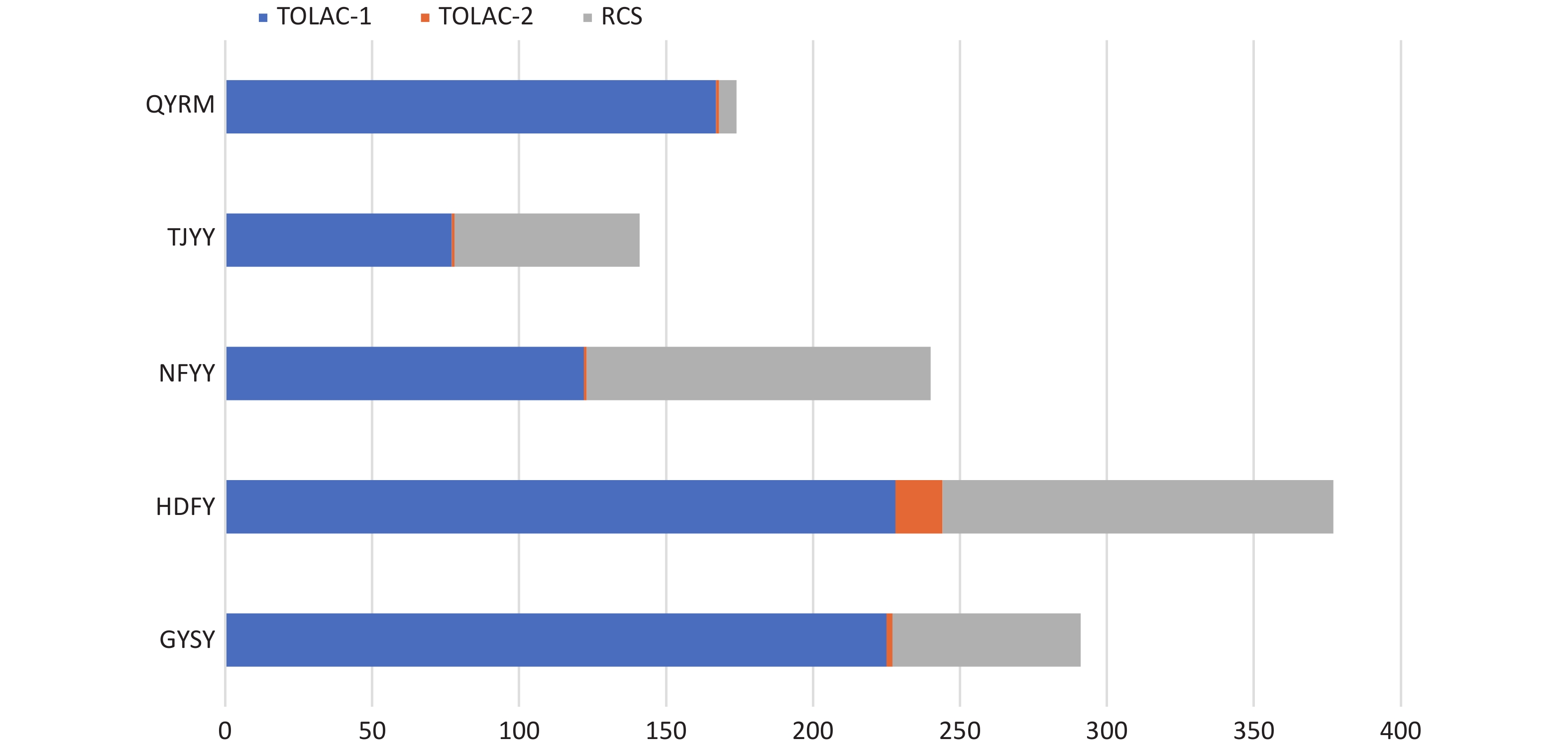
Of the 7,178 women with singleton pregnancies and a scarred uterus, we excluded 5,875 women because they did not meet the inclusion criteria. After exclusion, 819 women were in the TOLAC-1 group, 21 women were in TOLAC-2, and 383 women chose repeat cesarean section (RCS) (Supplementary Figure S1). The distribution of the three types of patients in 5 hospitals was shown in Supplementary Figure S2, available in www.besjournal.com. The hospital with the largest number of deliveries also had the most women in TOLAC-1 and TOLAC-2 groups; these higher numbers might be associated with the hospital’s success rate and experience with trials of labor after CS.
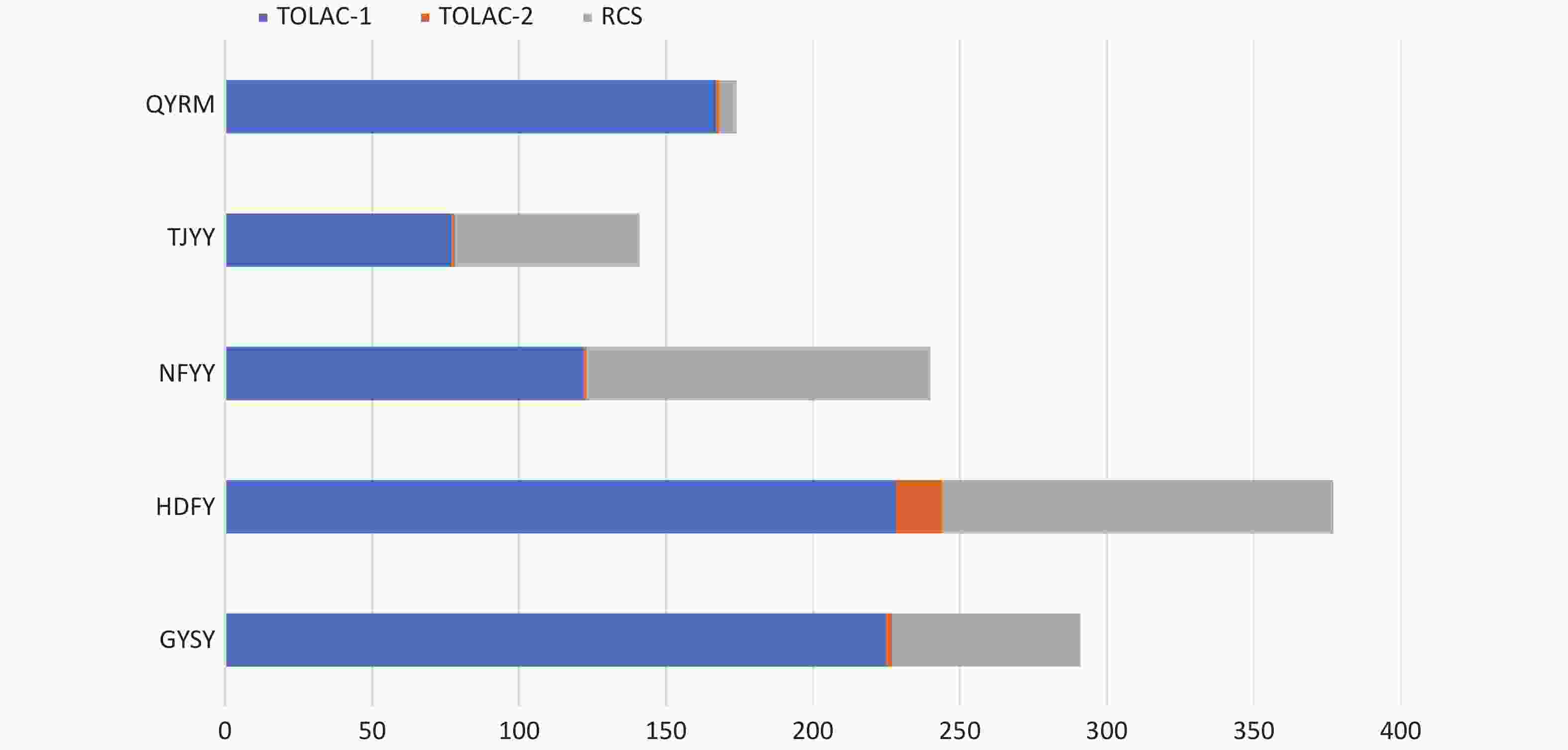
Figure S2. The distribution of women with TOLAC-1, TOLAC-2, or RCS in five hospitals. RCS, repeat cesarean section; TOLAC-1, trial of labor after one prior cesarean section; TOLAC-2, trial of labor after two prior cesarean sections.
The general characteristics of the women in our study are listed in Table 1. The age of the women who chose RCS was higher than for the other two groups (32.84 ± 4.69 vs. 31.51 ± 4.60 vs. 31.81 ± 2.80, respectively; P < 0.05). The proportion of women whose BMI was ≥ 30 kg/m2 before pregnancy was higher, and their weight gained (kg) during pregnancy was higher in the TOLAC-2 group relative to the other two groups (4.8% vs. 1.8% vs. 3.9%, 13.34 ± 4.02 vs. 12.97 ± 3.75 vs. 12.23 ± 3.91, respectively; P < 0.05). Of the three groups, the number of gestational weeks was the lowest (37.86 ± 1.14 vs. 38.51 ± 1.49 vs. 38.29 ± 1.42, P < 0.05) and the number of women showing complications of diabetes mellitus was the highest (20.4% vs. 14.7% vs. 9.5%, respectively; P < 0.05) in the RCS group. The number of women with complications of hypertensive disorders was the least in the TOLAC-1 group relative to the other two groups (1.7% vs. 4.8% vs. 4.7%, respectively; P < 0.05).
Variables TOLAC-1 (n = 819) TOLAC-2 (n = 21) RCS (n = 383) P value Age (year) 31.51 ± 4.60 31.81 ± 2.80 32.84 ± 4.69 < 0.05 Weight gain (kg) 12.97 ± 3.75 13.34 ± 4.02 12.23 ± 3.91 < 0.05 BMI (kg/m2), n (%) < 0.05 < 18.5 144 (17.6) 2 (9.5) 27 (7.0) 18.5–24.9 576 (70.3) 14 (66.7) 259 (67.6) 25.0–30.0 84 (10.3) 4 (19.0) 82 (21.4) > 30.0 15 (1.8) 1 (4.8) 15 (3.9) Nationality, n (%) Han 813 (99.3) 21 (100) 380 (99.2) 0.92 Others 6 (0.7) 0 3 (0.8) Gravity, n (%) 2 395 (48.2) 0 0 < 0.05 3 250 (30.5) 11 (52.4) 157 (41.0) 4 124 (15.1) 6 (28.6) 109 (28.5) ≥ 5 50 (6.1) 4 (19) 117 (30.5) Parity, n (%) < 0.05 1 709 (86.6) 0 0 2 98 (12.0) 20 (95.2) 373 (97.4) 3 10 (1.2) 0 9 (2.3) ≥ 4 2 (0.2) 1 (4.8) 1 (0.3) Gestational week 38.51 ± 1.49 38.29 ± 1.42 37.86 ± 1.14 < 0.05 Source, n (%) 0.55 Hospital 736 (89.9) 18 (85.7) 350 (91.4) Referral 83 (10.1) 3 (14.3) 33 (8.6) ART 16 (2.0) 0 12 (3.1) 0.35 DM 120 (14.7) 2 (9.5) 78 (20.4) < 0.05 Hypertension 14 (1.7) 1 (4.8) 18 (4.7) < 0.05 Gender, n (%) < 0.05 Male 412 (50.3) 11 (52.4) 223 (58.2) Female 407 (49.7) 10 (47.6) 160 (41.8) Note. TOLAC-1, trial of labor after a prior cesarean; TOLAC-2, trial of labor after two prior cesareans; RCS, repeat cesarean section; BMI, body mass index; ART, assisted reproduction technology; DM, diabetes mellitus. Table 1. Maternal general characteristics
The success rate of TOLAC-2 was 81.0%, which was comparable to that for TOLAC-1 (86.7%) (P = 0.511). One systematic review and meta-analysis reported that the success rates for TOLAC-2 and TOLAC-1 were 71.1% and 76.5%, respectively (P < 0.001)[8]. The high success rates for TOLAC in China might be due to stricter indications than those presented in the guidelines of the ACOG[2], although the stricter indication for TOLAC in China might also lead to a lower rate of TOLAC compared to other countries.
The adverse pregnancy outcomes in the TOLAC-2 group were similar to those of the TOLAC-1 group. Compared with the TOLAC-2 group, women who chose RCS exhibited greater blood loss (494.22 ± 335.55 mL vs. 325.24 ± 182.52 mL, respectively; P < 0.05) and more hospitalization days (7.6 ± 3.26 vs. 6.24 ± 4.34, P < 0.05), while other adverse outcomes were similar (Table 2). There were no significant differences between TOLAC-1 and TOLAC-2, or between TOLAC-2 and RCS, in neonatal outcomes—except that the 1-min Apgar score was higher in the TOLAC-1 group compared with the TOLAC-2 group (Table 3). Investigators who generated a systematic review reported uterine rupture rates in TOLAC-1 and TOLAC-2 groups of 1.59% and 0.72%, respectively (P < 0.001), and hysterectomy rates of 0.56% and 0.19% (P = 0.001), respectively. There were no differences in hysterectomy rates (0.40% vs. 0.63%), transfusion rates (1.68% vs. 1.67%), or febrile morbidity (6.03% vs. 6.39%) (P = 0.27) between the TOLAC-2 and RCS groups, respectively[7]. While Davidson et al. reported that composite maternal morbidity was similar between the two groups (18.3% vs. 23.3%), they observed no differences in neonatal outcomes[9]. However, in the study by Dombrowski et al., TOLAC-2 was associated with a higher risk for composite morbidity that included severe newborn complications (OR, 1.78; 95% CI, 1.04–3.04)[10], and liability and safety concerns still limited the availability of TOLAC-2.
Variables TOLAC-1 (n = 819) TOLAC-2 (n = 21) RCS (n = 383) Vaginal delivery, n (%) 710 (86.7)a 17 (81.0) 0 Instrument delivery, n (%) 25 (3.1) 0 0 Cesarean, n (%) 109 (13.3)a 4 (19.0) 383 (100)b* Infection, n (%) 2 (0.3)a 0 8 (2.5)b Uterine rupture, n (%) 6 (0.7)a 0 5 (1.3)b Bladder injury, n (%) 9 (1.1)a 0 22 (5.7)b Blood loss (mL) 367.74 ± 183.57a 325.24 ± 182.52 494.22 ± 335.55b* PPH, n (%) 77 (9.7)a 2 (9.5) 35 (9.1)b Transfusion, n (%) 31 (3.8)a 1 (4.8) 32 (8.4)b Days in hospital (d) 4.95 ± 2.99a 6.24 ± 4.34 7.6 ± 3.26b* Hysterectomy, n (%) 0 0 0 Maternal mortality, n (%) 0 0 0 Note. TOLAC-1, trial of labor after a prior cesarean; TOLAC-2, trial of labor after two prior cesareans; RCS, repeat cesarean section; PPH: postpartum hemorrhage. aTOLAC-1 vs. TOLAC-2; bTOLAC-2 vs. RCS; *P < 0.05. Table 2. Maternal adverse outcomes
Variables TOLAC-1 (n = 819) TOLAC-2 (n = 21) RCS (n = 383) Fetal weight (g) 3110.67 ± 416.76a 3186.67 ± 322.87 3177.45 ± 430.17b 1 min 9.34 ± 0.83a* 8.95 ± 0.97 9.05 ± 0.83b 5 min 9.85 ± 0.53a 9.86 ± 0.48 9.74 ± 0.71b 10 min 9.86 ± 0.57a 9.86 ± 0.48 9.77 ± 0.67b Mild (%) 16 (2.0)a 0 12 (3.1)b Severe, n (%) 2 (0.2)a 0 2 (0.5)b NICU, n (%) 67 (8.2)a 2 (9.5) 31 (8.1)b Neonatal complication, n (%) 20 (2.4)a 1 (4.8) 9 (2.3)b Neonatal mortality, n (%) 1 (0.1)a 0 1 (0.3)b Note. TOLAC-1, trial of labor after a prior cesarean; TOLAC-2, trial of labor after two prior cesareans; RCS, repeat cesarean section; NICU, neonatal intensive care unit. aTOLAC-1 vs. TOLAC-2; bTOLAC-2 vs. RCS; *P < 0.05. Table 3. Neonatal adverse outcomes
In the survey by Doret et al., 23.8% vs. 100% of obstetricians would offer TOLAC-2 vs. TOLAC-1, respectively, principally because TOLAC-2 increased maternal or fetal mortality and morbidity—particularly uterine rupture with severe neonatal outcomes. As randomized trials on the safety of TOLAC-2 are lacking, all present recommendations are based on retrospective observational studies. The question of whether TOLAC-2 increases adverse maternal outcomes has not yet achieved a consistent answer, and there are few studies that focus on the effects of TOLAC-2 on adverse neonatal outcomes. In addition, a repeated (third) CS can cause surgical complications and provoke pregnancy risks in future pregnancies. Overestimation of immediate maternal and fetal risks and a lack of consideration of long-term outcomes might thus explain current obstetric-practice patterns.
In China, a trial of labor is not recommended for women with two prior CSs—which is likely due to the lack of evidence-based medical practice. Obstetricians are reluctant to propose a trial of labor to women with two prior CSs due to their fear of complications and medico-legal issues, and because fewer patients are aware of the availability of TOLAC-2.
In our study, we first discussed the feasibility of using TOLAC-2 in China and found that a trial of labor might be an option for women with two prior CSs and without other contraindications to vaginal birth. This study was limited by the small size of the TOLAC-2 group and the study’s overall retrospective nature, which impacted precise risk estimates and conclusions; however, the size of the entire study was quite large. A benefit of our study is that it provides a reference point that shows that if patients are carefully selected, TOLAC-2 could be an option that reduces the complications associated with RCS. More research is needed, however, to further assess the feasibility of TOLAC-2.
In conclusion, in a small sample of women in public tertiary hospitals in China where we compared TOLAC-1 and TOLAC-2, our results showed that TOLAC-2 did not significantly increase adverse maternal and neonatal outcomes. Our study also provided some references for obstetricians with respect to the management and counseling of women with two prior CSs. TOLAC-2, without any contraindications, constitutes an option for pregnant women, but both TOLAC-2 and RCS are high-risk procedures and should be performed only if highly trained personnel and appropriate resources are available.
Conflicts of Interest The authors declare no competing financial interest.
Author Contributions BI Shi Lei and ZHANG Li Zi: design, planning, data analysis, writing original draft, review and editing; LIANG Xin Yue, HUANG Li Jun, ZENG Shan Shan, LIANG Ying Yu, LI Yu Lian, and HUANG Min Shan: investigation, data collection; JIA Jin Ping, WEN Sui Wen, and FENG Ling: investigation and resources; DU Li Li, WANG Zhi Jian, and CHEN Dun Jin: supervision, project administration, and funding acquisition.
HTML
Reference
 21092Supplementary Materials.pdf
21092Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: