-
Measles is an acute, highly contagious, and severe respiratory disease caused by the measles virus. Infected patients can develop fever, maculopapular rashes, pneumonia, encephalitis, and other complications, including death[1]. A measles epidemic has persisted in China for years. Cross-contamination can easily occur in hospitals, and medical staff is at high risk for cross-infection. Therefore, it is important to understand the measles immunization status of this special population to prevent potential nosocomial infections.
According to a national measles surveillance program issued by the Chinese Center for Disease Control and Prevention, the surveillance of the measles immunity of medical staff is performed by collecting and detecting serum IgG antibodies. However, many medical staff members refuse blood draw since it is invasive, traumatic, and increases the risk for infection. Oral fluids (OFs) are a mixture of saliva and gingival crevicular fluids, which contain IgA, IgM, and IgG antibodies [2]. The detection of measles antibodies in OFs is a non-invasive, self-sampling, alternative method. In 2008, WHO recommended the OF assay for measles diagnosis and surveillance to all countries [3].
To explore and discuss the significance of this new, non-invasive OF sampling method for the prevention and control of measles in Beijing, China, the measles antibodies in medical staff were investigated for the first time using the OF assay.
By random sampling, a cross-sectional study was performed, and the medical staff of the Beijing Haidian Hospital and three affiliated community hospitals (Towns of Wenquan, Haidian, and Shuangyushu) were tested from March to December 2018. The inclusion criteria were: (1) medical staff from intensive care units, clinical laboratories, and emergency, pediatrics, infection, respiratory, dermatology, obstetrics and gynecology, internal medicine, surgery, anesthesiology, pharmacy, or medical support departments; and (2) participants who provided informed consent and can cooperate in the completion of specimen collection and clinical follow-up. The exclusion criteria were: (1) medical staff with severe diseases, acute or chronic infections, active fever, skin rash, or oral lesions; (2) medical staff with immunocompromised status, such as human immunodeficiency virus infection; (3) medical staff with egg allergy history; and (4) pregnant women. This study was approved by the hospital ethics committee.
Baseline demographic information, history of measles infection, vaccination history, and recent contact history with a patient with measles were obtained using a standardized questionnaire.
Serum and OF samples were collected from each study participant at the same time. Blood samples were collected by venipuncture and centrifuged to obtain the serum. OFs were self-collected using Oracol swabs (Malvern Medical Developments, UK), following the manufacturer’s instructions, and the collection time was maintained at 90 s. The OF samples were eluted in 1 mL of phosphate-buffered saline containing 10% fetal bovine serum and 1% gentamicin [4]. All samples were stored at –20 ℃ before laboratory testing.
Serum measles IgG antibodies were detected by indirect enzyme-linked immunosorbent assay (EIA; EuroImmun Medizinische Labordiagnostika AG, Germany). The detected optical density values, standard curves, and IgG antibody concentrations were calculated according to the manufacturer’s instructions. Measles IgG concentrations of < 200 IU/L, ≥ 200 and < 275 IU/L, and ≥ 275 IU/L were considered negative, equivocal, and positive, respectively. The equivocal results were counted as negative when calculating the positive rate [5]. A measles IgG concentration of ≥ 800 IU/L was considered as a protection level [6]. Study participants with serum measles IgG antibody levels < 800 IU/L were voluntarily vaccinated with a monovalent measles vaccine (Shanghai Institute of Biological Products Co., Ltd.).
Measles IgG antibodies in OFs were detected by anti-measles IgG capture EIA (Clin-Tech Ltd., Guildford, UK). The assay was performed, following the instructions from the manufacturer. The mean values of three OD450−620 nm (
${\overline{NC}} $ ) measurements were calculated. A value of ≥${\overline{NC}} $ *1.25 was positive, ≤${\overline{NC}} $ *1.1 was negative, and >${\overline{NC}} $ *1.1 and <${\overline{NC}} $ *1.25 was equivocal. When a positive rate was calculated, ≥${\overline{NC}} $ *1.1 (cut-off value) was counted as positive. The S/CO of each sample (S represents the OD450−620 nm of the OF samples, while CO represents the cut-off value) was calculated as the relative level of the measles OF IgG.Data analysis was performed in SPSS (version 20.0, IBM, New York, USA) and GraphPad (version 6.0, GraphPad, California, USA). The diagnostic performance of OFs was evaluated based on the serum measles antibody measurements. A receiver operating characteristic (ROC) curve was created to evaluate the performance of the measles IgG antibody test on OF samples. The area under the corresponding ROC curve (AUC) was used to calculate its diagnostic accuracy. Kappa (κ) analysis was used to evaluate the consistency of the two methods. A chi-squared test was used to compare the antibody-positive rates between the different groups. P < 0.05 was considered statistically significant.
A total of 495 medical staff were enrolled in this study. There were 103 males (20.8%) and 392 females (79.2%), with a median age of 33 years old. Among these participants, 54 received the measles vaccine. Finally, 499 pairs of serum and oral fluid samples were investigated, including the initial 495 pairs and 54 pairs of pre- and post-vaccination samples.
In total, 549 pairs of serum and OF samples were tested for measles IgG antibodies. The result of the ROC curve analysis showed an AUC value of 0.84, which was statistically significant (P < 0.01).
Measles-specific IgG antibodies in paired OF and serum samples are presented in Table 1. The sensitivity, specificity, positive predictive value, negative predictive value, and Youden index were 90.2%, 67.9%, 94.2%, 54.5%, and 58.1%, respectively. Previous studies reported that the sensitivity and specificity of the OF assay were 97.4%–98% and 87%–90.0%, respectively [7, 8]. These variations in sensitivity and specificity might be due to different reagents used in different studies.
Items Serum Positive Negative Total OF Positive 422 26 448 Negative 46 55 101 Total 468 81 549 Table 1. Comparison of detected measles-specific IgG antibodies in the serum by indirect EIA and in the OF samples by capture EIA
Kappa (κ) analysis was used to evaluate the consistency between the OF and serum assays. The kappa value was 0.53, suggesting a medium and high agreement between the two assays. Among the 468 positive serum samples, 422 (90.2%) OF samples were positive. The consistent rate of positive results was 90.2% (422/468). Of the 81 negative serum samples, 55 were also negative in the OF samples. The consistent rate of negative results was 67.9% (55/81). Among the 72 samples with inconsistent results, 46 serum samples were positive but negative in OF, and 26 serum samples were negative but positive in OF. The results showed a high agreement between the serum and OF antibody assays.
The positive rates in serum or OF measles IgG antibodies were 83.6% (414/495) and 80.2% (397/495), respectively (Table 2). These values are close to those of the general population in 2012 (84.7%) and 2017 (79.8%) in Beijing, China [5, 9]. These data indicate that a significant percentage of the population has not been vaccinated against measles. Appropriately targeted immunization strategies and measures should be considered and carried out, such as measles antibody monitoring and vaccination rate improvement, especially in medical staff.
Characteristics Number Proportion rate (%) Serum IgG positive OF IgG positive Cases Positive rate (%) χ2 P Cases Positive rate (%) χ2 P Gender 2.37 0.12 0.89 0.35 Male 103 20.8 81 78.6 86 83.5 Female 392 79.2 333 85.0 311 79.3 Age (%) 4.31 0.37 12.79 0.01 20–24 36 7.3 32 88.9 29 80.6 25–29 109 22.0 88 80.7 78 71.6 30–34 153 30.9 123 80.4 118 77.1 35–39 103 20.8 88 85.4 88 85.4 40– 94 19.0 83 92.8 84 89.7 Measles contact history 21.33 < 0.01 23.69 < 0.01 Yes 157 31.7 149 94.9 146 93.0 No 338 68.3 265 78.4 251 74.3 History of measles infection 4.72 0.03 5.95 0.02 No 472 68.3 391 82.8 374 79.2 Yes 23 31.7 23 100.0 23 100.0 History of measles vaccination 19.29 < 0.01 2.44 0.12 Yes 292 59.0 262 89.7 241 82.5 No 203 41.0 152 74.9 156 76.9 Table 2. Measles IgG antibody levels in the serum and OF samples from patients grouped according to their characteristics
Antibody analyses were performed by evaluating the study participants in different subgroup populations.
The percentages of positive serum measles IgG antibodies were 78.6% (81/103) for males and 85.0% (333/392) for females, with no statistically significant difference (P > 0.05). The percentages of positive OF measles IgG antibodies were 83.5% (86/103) for males and 79.3% (311/392) for females, with no statistically significant difference (P > 0.05; Table 2).
The participants were assigned into five age groups (20–24, 25–29, 30–34, 35–40, and > 40 years). The percentages of positive serum measles IgG antibodies in different age groups ranged from 80.4% to 92.8%, with no statistically significant difference (P = 0.37; Table 2). The percentages of positive OF measles IgG antibodies in different age groups ranged from 71.6% to 89.7%, with a statistically significant difference (P = 0.01; Table 2). In the OF assay, the percentage of positive antibodies in the 25–29 age group was significantly different from that in the 35–39 age group and the > 40 age group (P = 0.01 and P < 0.01, respectively). The percentage of positive antibodies in the 30–34 age group was significantly different from that in the > 40 age group (P = 0.02) (Table 2).
In total, 157 study participants were reported to be close contacts of patients with measles. The percentages of positive serum measles IgG antibodies in participants with and without a contact history were 94.9% (149/157) and 78.4% (265/338), respectively, with a statistically significant difference (P < 0.01). The percentages of positive OF measles IgG antibodies in participants with and without a contact history were 93.0% (146/157) and 74.3% (251/338), respectively, with a statistically significant difference (P < 0.01; Table 2).
On the other hand, 472 participants reported no history of measles infection, while 23 participants had a history of measles infection. The percentages of positive serum measles IgG antibodies in participants with and without a history of measles infection were 100.0% (23/23) and 82.8% (391/472), respectively, with a statistically significant difference (P < 0.05). The percentages of positive OF measles IgG antibodies in participants with and without a history of measles infection were 100.0% (23/23) and 79.2% (374/472), respectively, with a statistically significant difference (P < 0.05; Table 2).
In total, 292 participants reported a vaccination history for measles, while 203 participants were not vaccinated. The percentages of positive IgG antibodies in the serum of participants with and without a vaccination history for measles were 89.7% (262/292) and 74.9% (152/203), respectively, with a statistically significant difference (P < 0.01). The percentages of positive IgG antibodies in the OFs of participants with and without a vaccination history for measles were 82.5% (241/292) and 76.9% (156/203), respectively, with no statistically significant difference (P = 0.12) (Table 2). The IgG levels in the OF samples were approximately 1/800 of those found in the serum [10]. There were significant differences in the positivity rates of the OF assay between the different age groups (25–29 age group vs. 35–39 age group; 25–29 age group vs. > 40 age group; and 30–34 age group vs. > 40 age group); however, there were no differences in the serological test results of these age groups. There were no significant differences in the OF assay between the vaccination histories, but in the serological tests, there were statistically significant differences. These differences might be caused by the lower concentrations of antibodies in the OF samples than those in the serum. Thus, it is necessary to improve the sensitivity and specificity of the OF assay.
In this study, 54 participants were voluntarily vaccinated with the monovalent measles vaccine. Among these, eight participants were 20–24 years old, 22 participants were 25–29 years old, 17 participants were 30–34 years old, five participants were 35–39 years old, and two participants were 40 years old and above. The percentages of positive serum measles IgG antibodies pre- and post-vaccination were 64.8% (35/54) and 98.1% (53/54), respectively, with a statistically significant difference (P < 0.01). The median antibody levels pre- and post-vaccination were 485.43 IU/L and 1,229.27 IU/L, respectively (Figure 1A). Among the different age groups, there were no significant differences in their serum measles antibodies pre- and post-vaccination (P = 0.95 vs. P = 0.82).
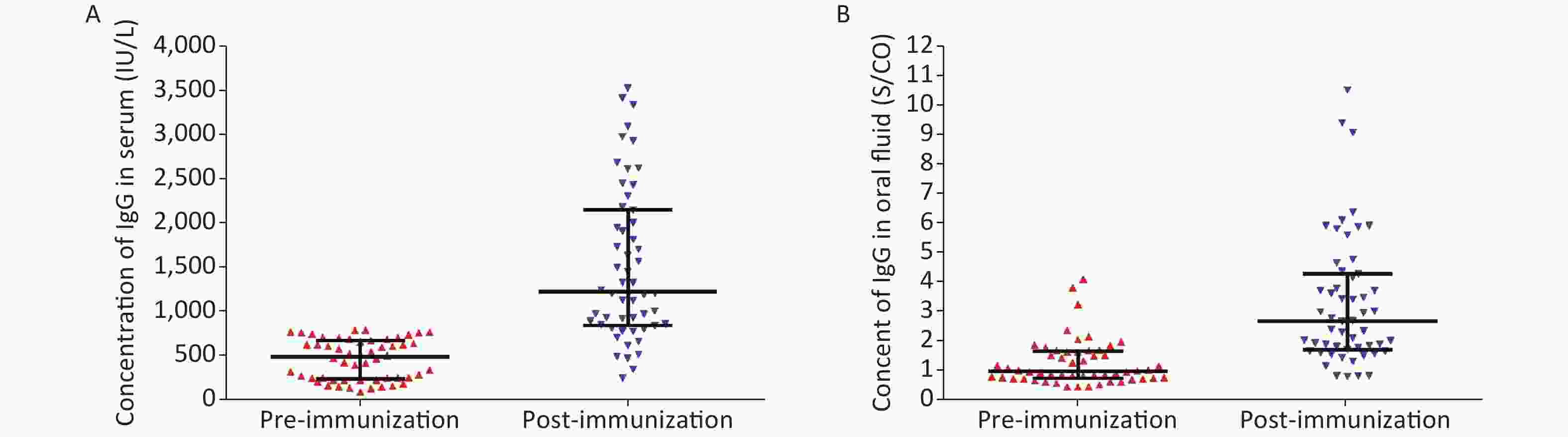
Figure 1. Comparison of measles IgG antibody levels based on the serum and OF assays between pre- and post-vaccination periods. (A) Serum IgG antibody level pre- and post-vaccination. (B) OF IgG antibody level pre- and post-vaccination.
The percentages of positive OF measles IgG antibodies pre- and post-vaccination were 50.0% (27/54) and 94.4% (51/54), respectively, with a statistically significant difference (P < 0.01). The median antibody levels (expressed as S/CO values) pre- and post-vaccination were 1.00 and 2.66, respectively (Figure 1B). Among the different age groups, there was no significant difference in the levels of measles antibodies pre- and post-vaccination (P = 0.05 vs. P = 0.31). After the immunization, the median serum IgG antibody concentration and median OF IgG antibody relative content (expressed as an S/CO value) increased by 2.53 and 2.66 times, respectively. The IgG antibody content in the OF and serum samples also increased. These results suggest that the OF assay could be used to evaluate the immune response of an individual after vaccination.
The OF assay is a suitable, non-invasive, easy-to-use, and low-risk method with wide clinical applications relative to the serum test. China is in a critical stage for measles elimination. The OF assay can greatly improve the efficiency in diagnosing measles while maintaining the safety of sampling.
Conflict of Interest The authors declare that they have no conflict of interest.
Acknowledgments The authors thank all participating medical staff for donating specimens required for this study.
Author Contributions DONG Mei, XIE Hui, and HUANG Fang wrote the paper. DONG Mei, SUN Jing Yi, XIE Hui, DONG Jian Ping, and HUANG Fang conceived and designed the experiments. DONG Mei, LIU Jie, WANG Yi Ting, and FU Yin performed the experiments. All of the authors have read and approved the final manuscript.
Ethics Approval Statement The ethics approvals for the protocol of this study were obtained from the Ethics Committee of Beijing Center for Disease Prevention and Control. Before enrollment, the nature, purpose, procedures, potential health impact, and benefits of this study were explained carefully to each healthcare provider, and written informed consents were obtained.
Application of Oral Fluid in Measles IgG Antibody Detection for Medical Staff
doi: 10.3967/bes2022.008
- Received Date: 2021-04-11
- Accepted Date: 2021-09-03
| Citation: | DONG Mei, SUN Jing Yi, XIE Hui, LIU Jie, WANG Yi Ting, FU Yin, DONG Jian Ping, HUANG Fang. Application of Oral Fluid in Measles IgG Antibody Detection for Medical Staff[J]. Biomedical and Environmental Sciences, 2022, 35(1): 64-68. doi: 10.3967/bes2022.008 |













 Quick Links
Quick Links
 DownLoad:
DownLoad: