-
Nontuberculous mycobacteria (NTM) are opportunistic pathogens of environmental origin that can cause human infections in a wide variety of tissues[1]. Lung involvement is the most common manifestation of NTM infection[1]. The symptoms and radiographic signs are similar to those of patients with pulmonary tuberculosis (TB) who are infected with Mycobacterium tuberculosis complex (MTBC)[1]. Most NTM species are naturally resistant to first-line anti-TB drugs and have different resistance profiles, thus misdiagnosis leads to inappropriate treatment and increases the risk of poor prognosis for patients with an NTM infection, and even wider spread through person-to-person contact[2]. The differential diagnosis of NTM among patients who are suspected to have TB has not received adequate attention and is always limited by the laboratory capacity for NTM detection and species identification in primary healthcare. It has been reported that the global prevalence of NTM infections has increased in recent years and a number of new NTM species have been isolated from human specimens[1]. This situation makes the disease an emerging public health problem and reminds us of the importance of comprehensive detection of NTM species in the clinic for timely and effective treatment.
A series of national TB prevalence surveys in China have suggested a notable increase in the NTM rates among patients suspected to have TB[3], and significant geographic diversity has been observed in the prevalence of NTM infections. With respect to NTM species, limited data has shown longitudinal trends and changes in different NTM species[4]. Shenzhen is a thriving coastal city in southern China that attracts large numbers of internal migrants. Perhaps due to a poor work environment or limited access to medical care, internal migrants account for the majority of TB patients in Shenzhen[5]; however, they might have a lower risk than local residents to be infected with NTM[6]. To comprehensively understand the trends in prevalent NTM species and the associated factors in Shenzhen, the current study summarized the longitudinal database of NTM detection between 2015 and 2020, estimated the prevalence and determined the trends by year of NTM rates among patients who were culture-positive for TB, investigated the epidemiologic changes of different species, and analyzed the risks for NTM infection among different populations and areas.
Patients seeking health care at district-level TB hospitals are routinely asked to provide three sputum specimens for bacteriologic examination if the radiographic signs suggested active pulmonary TB. Positive sputum cultures from Löwenstein-Jensen slants or Mycobacteria Growth Indicator Tubes (Bactec MGIT 960; Becton, Dickinson, Sparks, MD, USA) are sent to the municipal central laboratory for NTM identification. Isolates that are initially identified as NTM strains by the MPB64 immunity colloid gold method and the para-nitrobenzoic acid (PNB) inhibition test are further speciated using the line probe assay (GenoType® Mycobacterium CM/AS; Hain Lifescience GmbH, Nehren, Germany). The results of the identified species are reported clinically and recorded in the electronical database of the municipal laboratory. Patients with positive mycobacterial cultures from the 10 districts of Shenzhen between 2015 and 2020 were enrolled in this study, and those whose strains were identified as species other than mycobacteria were excluded. A total of 12,062 suspected TB patients were culture-positive and their cultures were sent for the identification of mycobacterium species. The isolates from 11,929 patients were successfully tested as mycobacteria and included in our study.
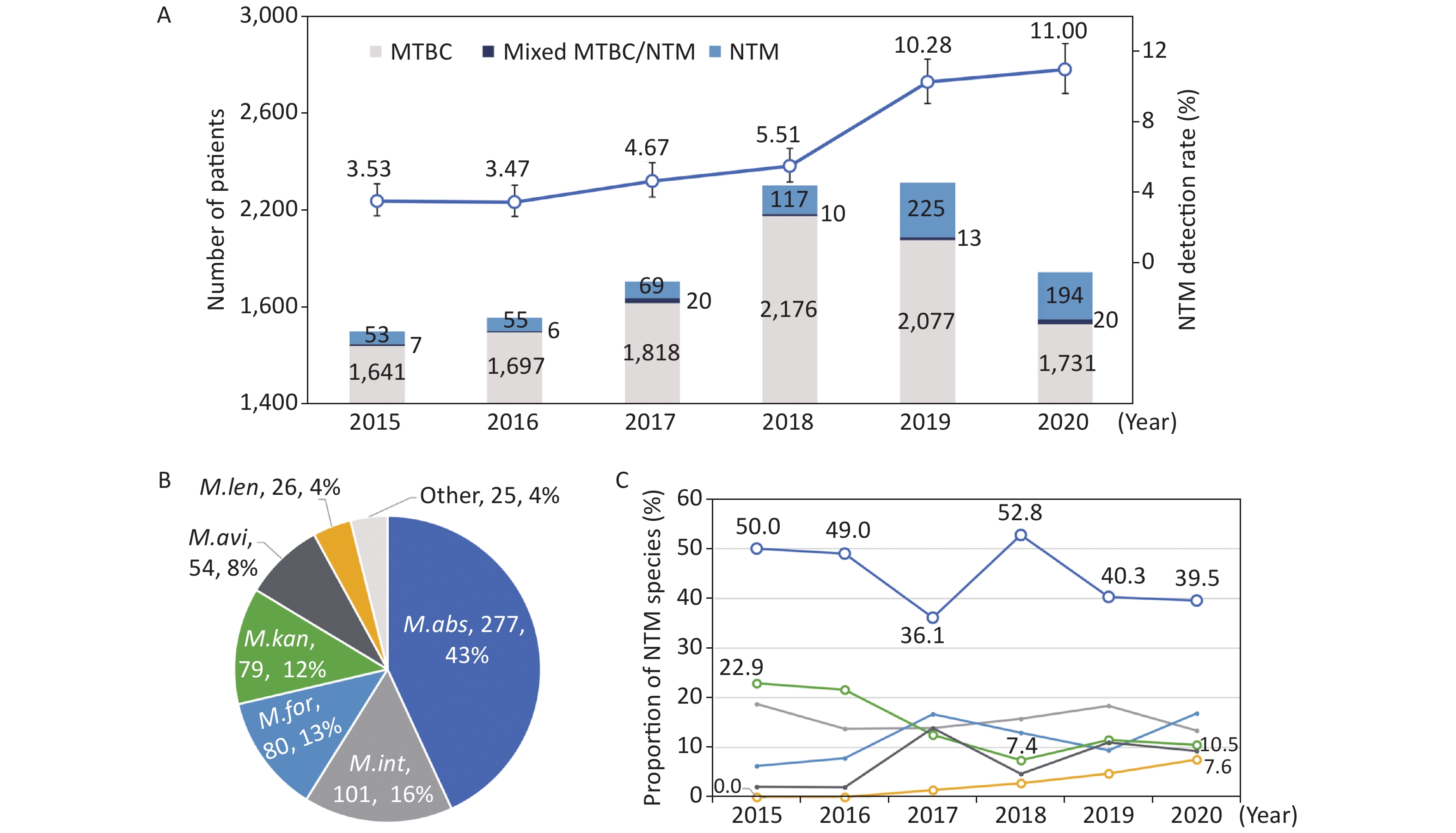
We divided the number of positive NTM reports by the number of tested patients to calculate the isolation rates for NTM. Overall, 6.61% (789/11,929) of culture-positive patients were detected with NTM strains, and the others were shown to be infected with MTBC, including three patients with M. bovis. The isolation rates of NTM among culture-positive patients increased each year, from 3.53% (60/1,701) in 2015 to 11.00% (214/1,945) in 2020 (Figure 1A). The trend was statistically significant (χ2trend = 155.3, P < 0.001); the greatest increase occurred from 2018 (5.51%) to 2019 (10.28%). The number of tested patients dramatically increased in 2018 by 21% (from 1,907 to 2,303 patients) compared to that in the previous year. Fewer patients were tested in 2020 when the NTM rate was the highest.
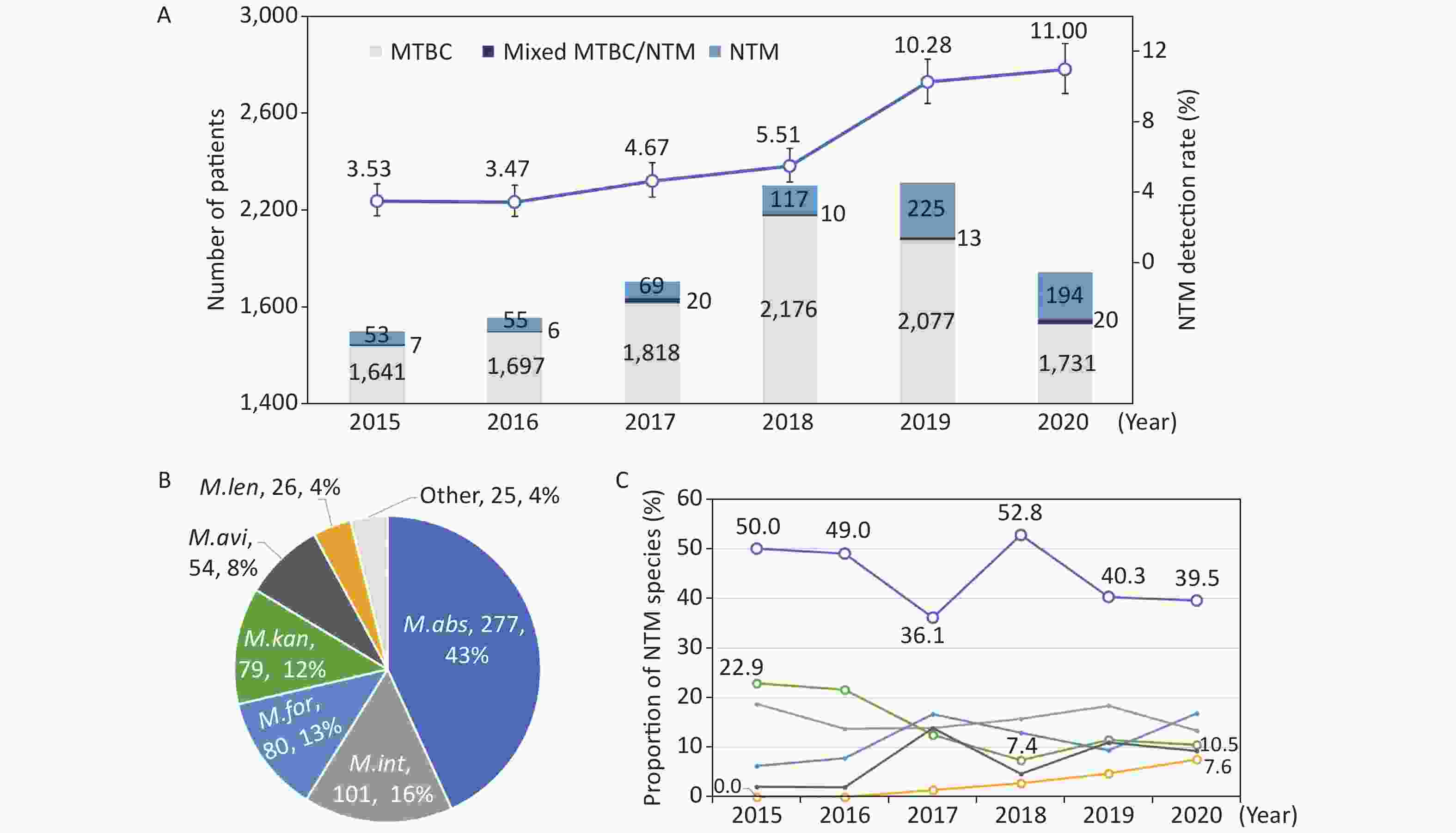
Figure 1. The numbers and isolation rates (A), and the proportions in total (B) and by year (C) of nontuberculous mycobacteria (NTM) species in Shenzhen between 2015 and 2020. The abbreviations in the pie chart were as follows: M. abs - M. abscessus; M. int - M. intracellulare; M. for - M. fortuitum; M. kan - M. kansasii; M. gor - M. gordonae; M. avi - M. avium; and M. len - M. lentiflavum; other - other minority NTM species. The polylines in subfigure C showed the trends of major NTM species in different colors corresponding to subfigure B
During the 6 years of the study, the NTM rates in Shenzhen more than tripled, which indicated a considerable increase in the NTM epidemic. The recent NTM rate in Shenzhen was > 10%, exceeding the average national level of 6.8%, and also higher than the 9.2% in southern China[7]. Although the global data[1] demonstrated an increasing trend in the prevalence of NTM, especially in developing countries, longitudinal data in China have been inconsistent. Specifically, a review[4] of related publications from 2000−2019 revealed no significant trend in the prevalence of NTM over the years, even though the NTM rate was estimated to be 8.15% in the first 5 years and 18.20% in the last 5 years. The discrepancies between studies could result from the different inclusion criteria, culture capacity, and varied methods for NTM detection in different areas or periods.
NTM infection was confirmed if two samples from the patient were detected with the same species[1]; however, among the 789 patients from whom NTM strains were detected in Shenzhen, only 60 (7.73%) provided additional specimens for confirmation of the NTM infection. The resent samples from two patients were contaminated in culture. Paired isolates from seven patients had different species, and the other 51 patients had the same NTM species and were confirmed as NTM infections according to the diagnostic criteria. In fact, over 200 patients were clinically treated for NTM infection during the study period and no deaths were reported. Of note, 76 patients (9.63%) had mixed infections with NTM and MTBC strains, including specimens from 13 patients in which both species were detected simultaneously, and consecutive samples from 63 patients detected of MTBC or NTM.
A total of 17 NTM species were identified from the cultures of 696 patients (88.2%) in Shenzhen’s database. The GenoType® Mycobacterium assay makes rapid and accurate determination of NTM from MTBC with a sensitivity and specificity > 98%[8]; however, approximately 12% of NTM isolates in the site could not be identified, indicating the limitations of the commercial molecular methods of detecting NTM. Identification of NTM species is essential for clinical diagnosis and treatment; however, commonly used commercial methods, such as Hain and the melting curve, could only detect approximately 20 of > 60 NTM species that have been shown to cause human diseases, and would be almost powerless for the newly discovered species[9]. Although multi-loci sequencing of conserved genes, such as 16S rRNA, hsp65, and rpoB, could provide robust results for NTM species, in-house procedures and analysis halted the application in the clinical setting. Whole-genome sequencing could be one of the promising techniques to identify all of the NTM species, as well as to construct the links of possible person-to-person transmission[5].
Based on the positive reports by the GenoType® Mycobacterium assay, M. abscessus (277, 35.1%) was one of the most clinically significant NTM, followed by M. intracellulare (101, 12.8%), M. fortuitum (80, 10.1%), M. kansasii (79, 10.0%), M. gordonae (74, 9.4%), M. avium (54, 6.8%), and M. lentiflavum (26, 3.3%). Excluding isolates that could not be determined of NTM species and M. gordonae isolates, which were regarded as environmental contamination; 625 patients were shown to have common pathogenic NTM species. Seventeen patients were shown to have two NTM species, making a total of 642 detectable NTM strains (Figure 1B).
The contribution of major NTM species among all the NTM strains were stable over the years, except for M. kansasii, which had a decreasing trend, and M. lentiflavum, which had an increasing trend (Figure 1C). M. abscessus constituted 43.1% (277/642) of all detectable NTM strains, and the proportions ranged between 36.1%−52.8% for each year during the study period (χ2trend = 2.207, P = 0.137). M. intracellulare, M. fortuitum, and M. kansasii followed, with proportions of 13.4%−18.8%, 6.3%−16.9%, and 7.4%−22.9%, respectively. M. kansasii accounted for 22.9% among all the detectable NTM strains in 2015, and the proportion decreased to 7.4% in 2018 and stabilized at approximately 10% in the next 2 years (χ2trend = 6.547, P = 0.011). The first M. lentiflavum strain was detected in 2017, and the number of identified strains increased yearly, and contributed to 7.6% of all the detectable NTM strains in 2020 (χ2trend = 10.807, P = 0.001).
M. intracellulare and M. abscessus have been reported to be the two major species of clinical importance in China[4]. These two species accounted for nearly 60% of the NTM strains in Shenzhen during the study period, while the number of M. abscessus was nearly three times that of M. intracellulare. Zhou L, et al.[4] concluded that M. abscessus was replaced by M. intracellulare as the dominant species across the entire country, except for Guangdong Province, which had a similar distribution as Shenzhen. Beyond these common NTM species, several minority species also need attention because the prevalence trend has been on the rise in recent years, such as M. lentiflavum, the representation of which increased from 0% to 7.6% in Shenzhen.
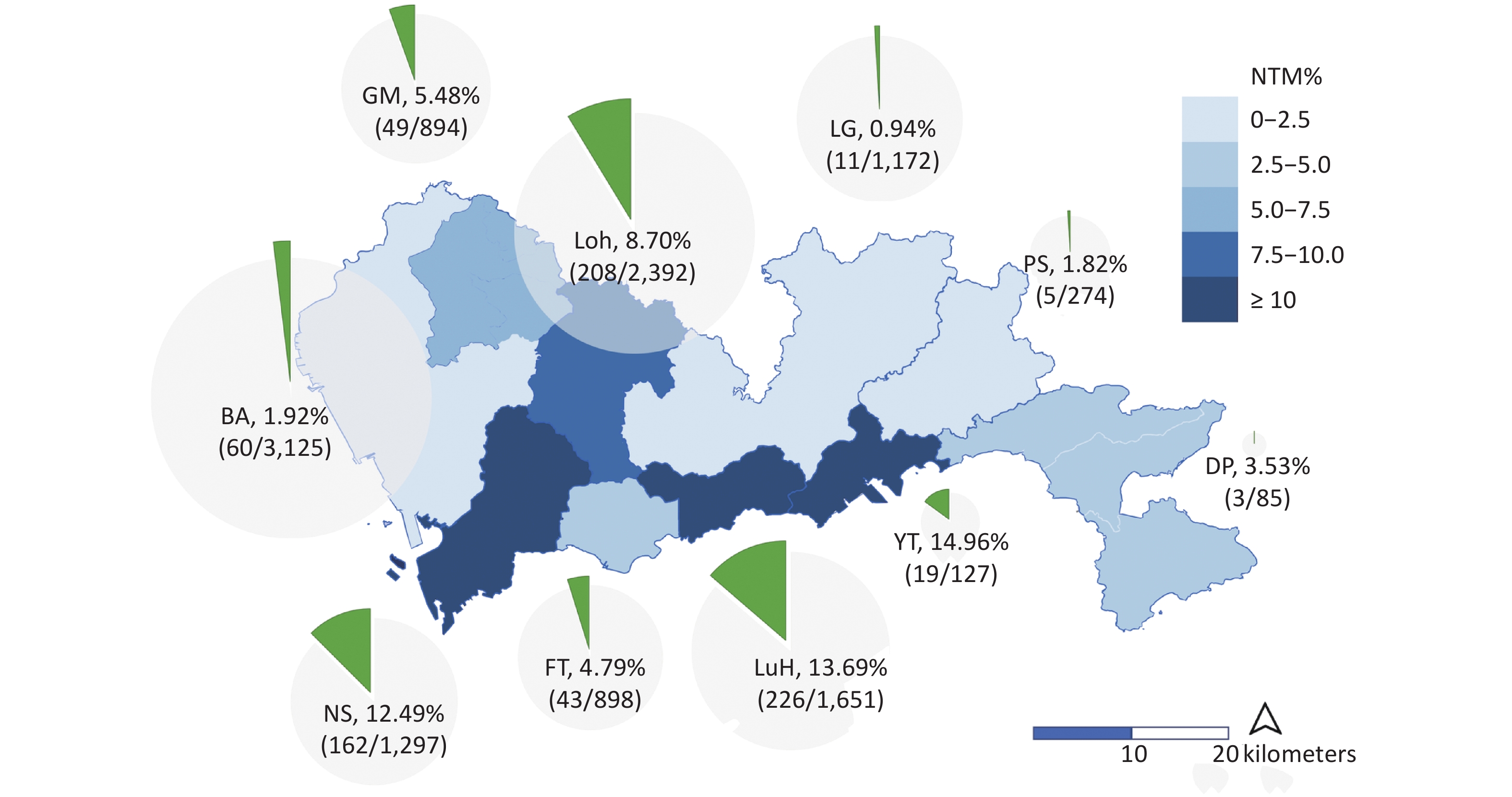
We collected the demographic and clinical characteristics of the enrolled patients to investigate the associated factors for NTM, including sex, age, TB history, migrant status, and the living district. Among the patients from whom NTM was isolated, 39.01% (307/787) were females, the median age was 36 years (IQR: 27−52 years), 13.88% (79/569) were local residents, 57.25% (450/786) lived in the city center, and 22.42% (100/446) had a history of anti-TB treatment (Table 1). Significant differences were shown among NTM rates in the 10 districts of Shenzhen which ranged from 0.94%−14.96% (Figure 2). Three districts in the city center (Nanshan, Luohu, and Yantian) had the highest rates of NTM, with 12.49% (162/1,297), 13.69% (226/1,651), and 14.96% (19/127) among culture-positive patients, respectively.
Characteristics NTM MTBC Univariable regression Multivariable regression N = 789 N = 11,140 OR (95% CI) P value aOR (95% CI) P value Gender: female 307/789 (39.01) 3,645/11,140 (32.75) 1.31 (1.13−1.52) < 0.001 1.21 (0.98−1.48) 0.080 Age, Median (IQR) 36 (27−52) 31 (25−45) − − − − Age increase per 10 years − − 1.24 (1.19−1.30) < 0.001 1.15 (1.08−1.23) < 0.001 Local resident 79/569 (13.88) 704/10,421 (6.76) 2.23 (1.73−2.86) < 0.001 1.86 (1.40−2.46) < 0.001 Sampled in 2019/2020 452/789 (57.29) 3,808/11,140 (34.18) 2.58 (2.23−2.99) < 0.001 1.72 (1.40−2.09) < 0.001 Lived in city center 450/786 (57.25) 3,523/11,130 (31.65) 2.89 (2.50−3.35) < 0.001 2.54 (2.08−3.11) < 0.001 Tuberculosis history 100/446 (22.42) 494/10,130 (4.88) 5.63 (4.43−7.16) < 0.001 5.55 (4.31−7.11) < 0.001 Note. NTM, Nontuberculous mycobacterium; MTBC, Mycobacterium tuberculosis complex; OR, odds ratio; aOR, adjusted OR; IQR, interquartile range. Table 1. Risk factors for NTM infection in univariable and multivariable logistic regression models
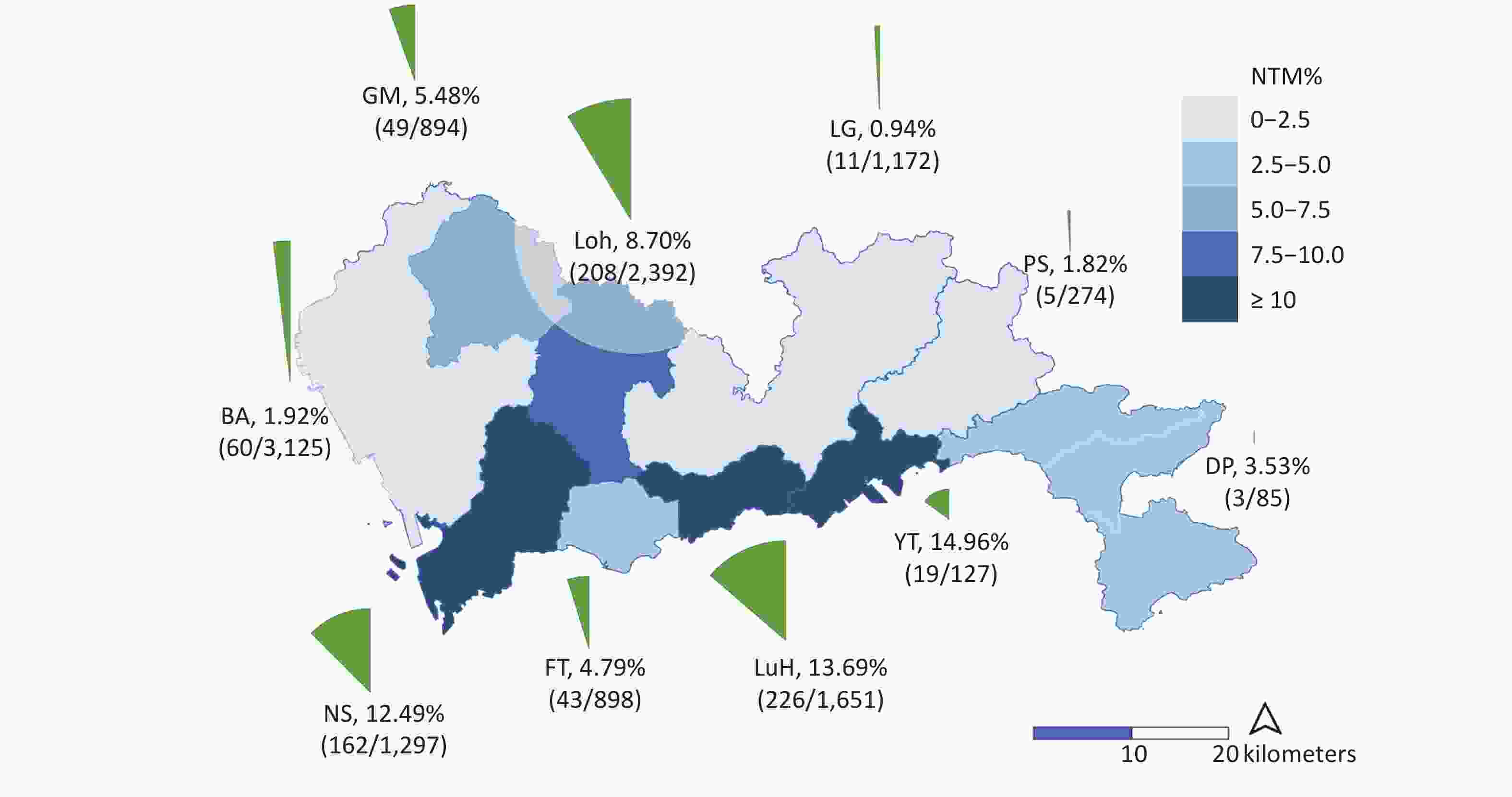
Figure 2. The isolation rates of nontuberculous mycobacteria (NTM) in different districts of Shenzhen. The light blue and dark blue in the map indicate the level of NTM detection rates. The surrounding pie chart provides the specific values of NTM rates in each district, calculated by dividing the total number of patients by the number of NTM isolates.
While most TB patients were migrants, local residents were more likely to be infected by NTM, with rates of NTM being 10.09% (79/783) compared to 4.8% (490/10,207) among migrant patients. More local residents than migrants lived in the city center, which might lead to a geographic difference in the rates of NTM. To assess the independent risk factors for NTM infection, the multivariable logistic regression model included both the resident status and location, as well as the possible confounding factors, including gender, age, sampling time, and TB history (Table 1). The final model indicated that being a local resident was independently associated with NTM detection with a higher risk (1.86; 95% CI, 1.40−2.46) compared to internal migrants. Living in city center had a risk (2.54; 95% CI, 2.08−3.11) of being infected with NTM than people living in the outer districts. Elderly people were independently associated with a higher risk of NTM infection, with an adjusted odds ratio (OR) of 1.15 (95% CI, 1.08−1.23) with every 10-year increase in age. TB history increased the risk of NTM with an adjusted OR of 5.56 (95% CI, 4.31−7.11). Females had a slightly higher risk (1.21; 95% CI, 0.98−1.48) to be infected with NTM than males, although no statistical significance was detected.
Like most studies involving the risk factors for NTM[7], the data from Shenzhen indicated a higher risk among patients with a TB history, the elderly, and possibly females. In addition, it was also shown that local residents and people who lived in the city center had a higher risk for NTM detection. The increased geographic incidence might be affected by a higher population density, which was also reported in Portugal[10]. The decreased risk among migrants has raised concerns about the underdiagnosis of NTM among this population. Migrants are a younger average age than the local residents, more likely to residents in the outer urban districts, and have limited access to medical care, which may lead to a higher risk of delayed diagnosis and incomplete treatment of TB or NTM infection[5]. More attention needs to be paid to the timely and accurate diagnosis of TB and NTM infection among migrants and urban suburbs.
There were some limitations to this study. First, we did not follow the patients detected with NTM, and only a relatively small proportion of patients provided another sample for diagnosis of NTM infection. Therefore, data were limited to analyze of the treatment or the prognosis of these patients. Second, the detected samples were sent from 10 district-level hospitals and only the basic characteristics of the patients were recorded in the municipal central laboratory. More risk factors, such as the radiographic manifestation and underlying diseases, could not be analyzed in this dataset.
In conclusion, this longitudinal surveillance data from Shenzhen demonstrated an increasing trend of NTM rates among culture-positive TB suspects. While M. abscessus was the dominant species over the study period, while the prevalence of other minor species, such as M. lentiflavum, may have a significant increasing trend. Timely identification of NTM species using molecular methods needs further implementation and evaluation in primary medical care to improve accurate diagnosis and effective treatment.
Acknowledgements
We thank the physicians and laboratory technicians of municipal- and district-level Centers for Chronic Disease Control in Shenzhen for their work in the surveillance work.
Conflicts of Interest
The authors have no conflicts of interest to declare. The funders had no role in the study design, data collection, analysis, interpretation, or writing of the paper.
Author Contributions
LCJ and QJ designed and managed the study. CYH and JLL collected, processed, and cleaned the raw data; QJ did the data analysis and interpretation. LCJ and QJ drafted the manuscript. SC, WP, and QJ prepared the final article. All the authors contributed to and reviewed the final version.
Increasing Trends and Species Diversity of Nontuberculous Mycobacteria in A Coastal Migrant City−Shenzhen, China
doi: 10.3967/bes2022.020
- Received Date: 2021-07-10
- Accepted Date: 2021-09-13
| Citation: | JI Le Cai, CHEN Shuai, PIAO Wei, HONG Chuang Yue, LI Jin Li, JIANG Qi. Increasing Trends and Species Diversity of Nontuberculous Mycobacteria in A Coastal Migrant City−Shenzhen, China[J]. Biomedical and Environmental Sciences, 2022, 35(2): 146-150. doi: 10.3967/bes2022.020 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: