-
Experimental evidence has demonstrated that environmental hazards can induce neurodegeneration such as Alzheimer's disease (AD) [1]. The pathogenesis of AD, characterized by a gradual cognitive decline, overproduction of β-amyloid (Aβ), and hyperphosphorylated tau in specific brain areas, is considered to involve the interaction of several internal (individual) and external environmental factors.
External environmental factors affecting the risk of AD include chronic exposure to physical, chemical, and psychosocial hazards, as well as lifestyle factors. Internal factors contributing to AD include genetics, aging, and other factors that are largely inherited and cannot be changed [2].
Therefore, we hypothesized that gene-environment interactions between internal ApoE4 overexpression and external environmental noise hazards might accelerate hippocampus-dependent cognitive decline, and promote the occurrence and development of AD-like pathological changes.
This study aimed to test the synergistic effects of environmental noise exposure and ApoE4. We established an ApoE4 transfected male rat model, in which we explored the combined effects of noise and ApoE4 gene interactions on AD-like neuropathy.
Our study used a 2 × 2 factorial design with four randomly allocated groups: a control group, noise group, ApoE4 group, and noise+ApoE4 (N+E4) group. We used a sound generator (BECAUse 3560C, B&K Instruments, Denmark) to deliver sound through a loudspeaker. Animals were kept on a standard 12/12 h light/dark schedule (lights on at 8 AM), and all testing was performed during the light phase. The animals were allowed to acclimate to the laboratory environment for 5 days before the start of the experiment.
All data were analysed in SPSS 19.0 software (SPSS, Inc., USA) and GraphPad Prism 7 (GraphPad software). Data were considered statistically significant when P < 0.05 (α = 0.05). Data are expressed as the mean ± standard error of the mean.
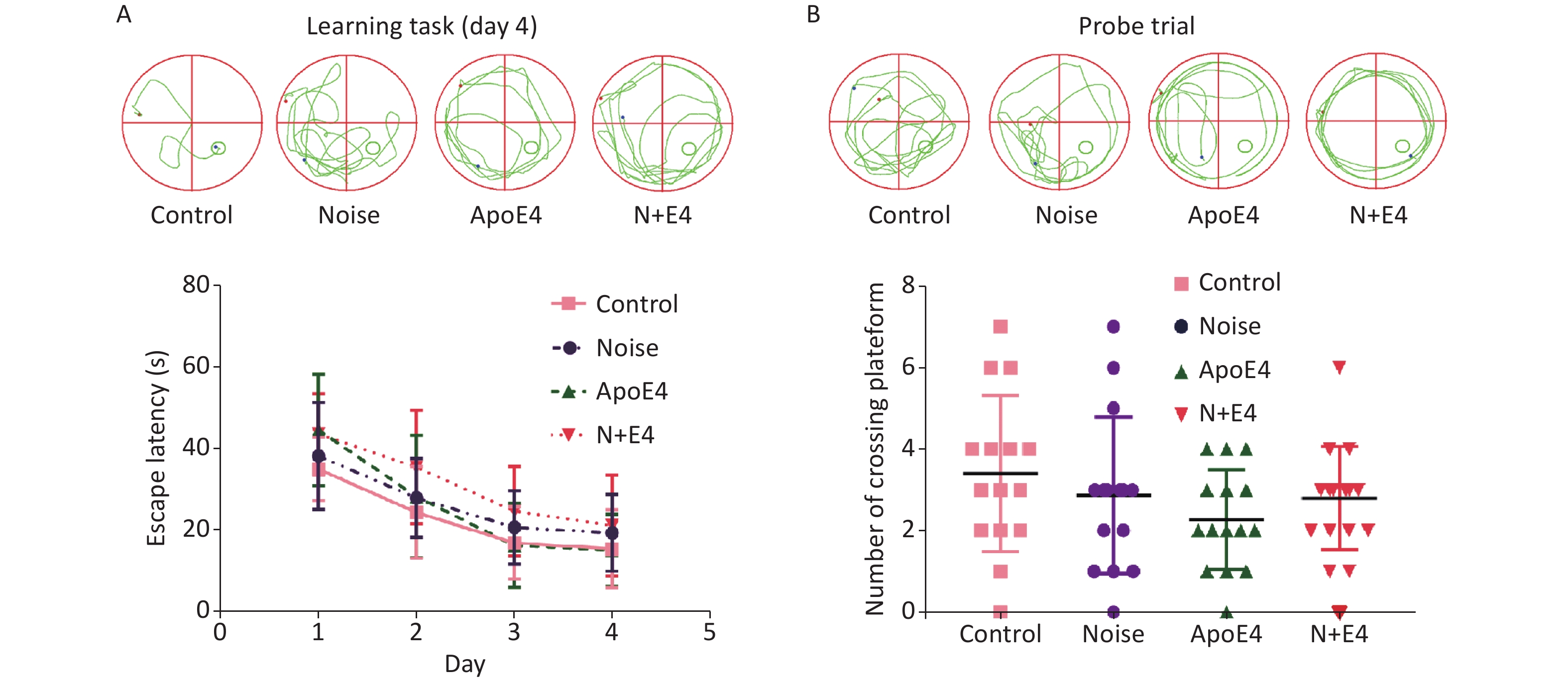
During the training phase of the morris water maze (MWM) task, all rat groups exhibited improvements in performance, as indicated by decreases in the escape latency over four successive days of training. The escape latency showed an increasing trend in the noise, ApoE4, and N+E4 groups; the value in the N+E4 group was highest with respect to the latencies observed in the controls (Supplementary Figure S1A, available in www.besjournal.com). During probe trial testing, the number of platform crossings showed a decreasing trend, although statistically significant changes were not found (Supplementary Figure S1B). These results indicated that exposure to chronic noise and ApoE4 had no significant effects on cognitive impairment (F = 0.000, P > 0.05, df = 1).
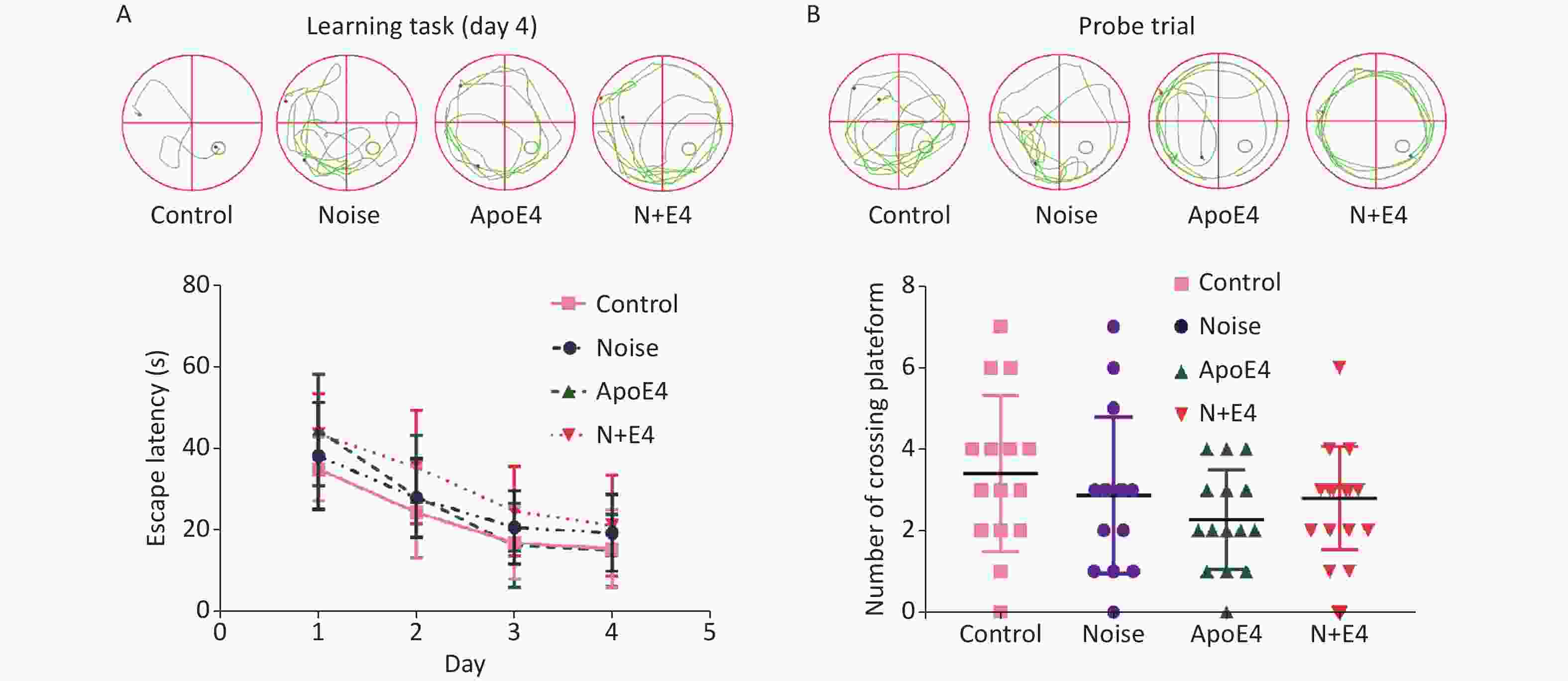
Figure S1. Effects of chronic noise and ApoE4 exposure on cognitive performance in the Morris water maze task. (A) Representative traces and effects of noise and ApoE4 exposure on escape latency in the training phase in rats. (B) Representative traces and effects of noise and ApoE4 exposure on performance in the probe trial. Data are shown as the mean ± standard deviation (n = 15 per condition). Two-way ANOVA with Fisher’s LSD post-test shows no significant effects on cognitive performance.
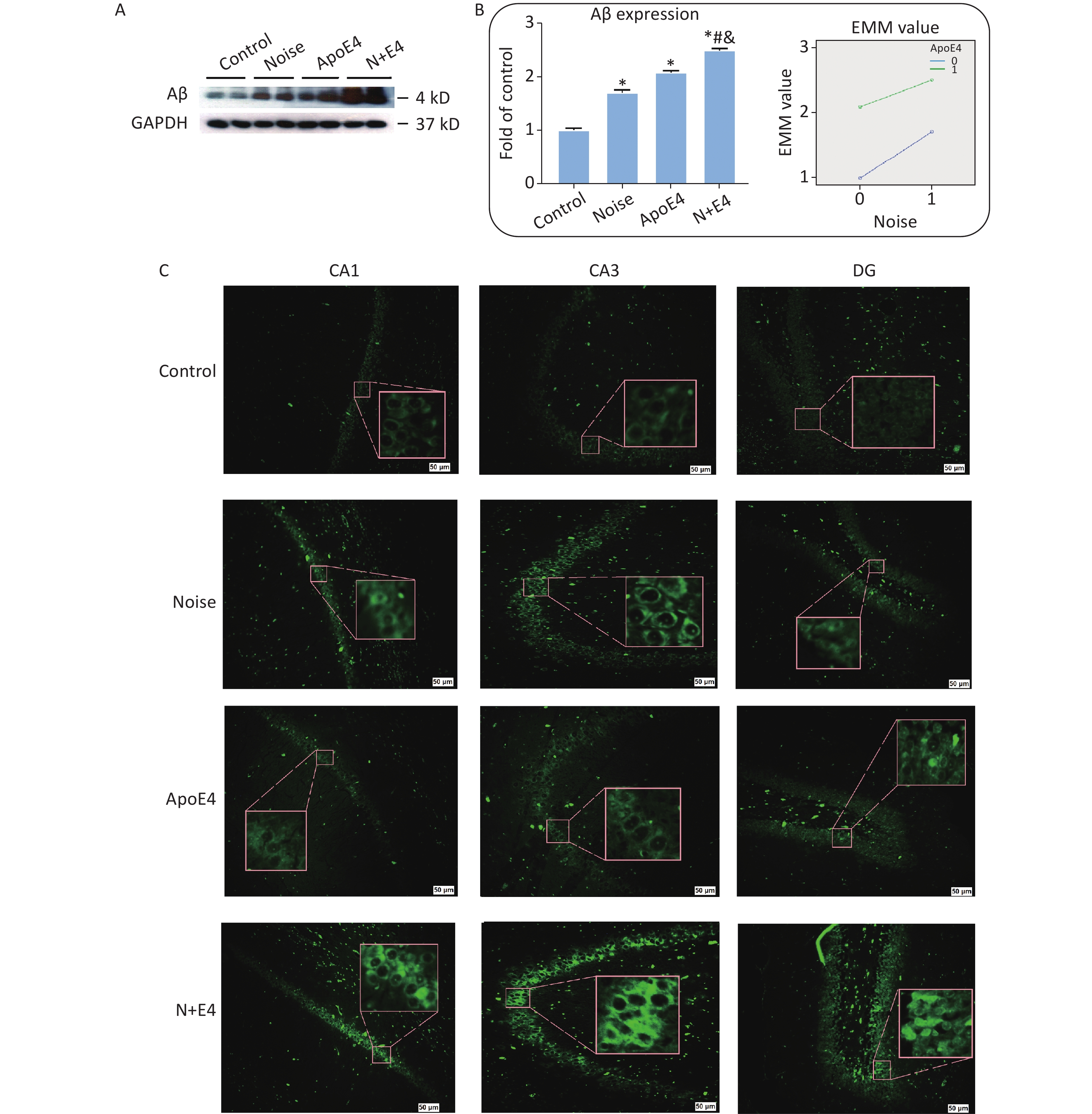
Levels of Aβ expression were analysed via quantitative immunoblot analysis of hippocampus tissue (Figure 1A). Aβ expression significantly increased after exposure to 98 dB white noise for 21 days (Figure 1B). Factorial ANOVA indicated that chronic noise (F = 1285.387, P < 0.001, df = 1) and ApoE4 transfection (F = 3619.645, P < 0.001, df = 1) significantly affected the expression of Aβ, and revealed a significant interaction effect of chronic noise and ApoE4 (F = 88.839, P < 0.001, df = 1) (Figure 1B). These results indicated that chronic noise exposure and ApoE4 interact in accelerating senescence-related AD-like pathological alterations.
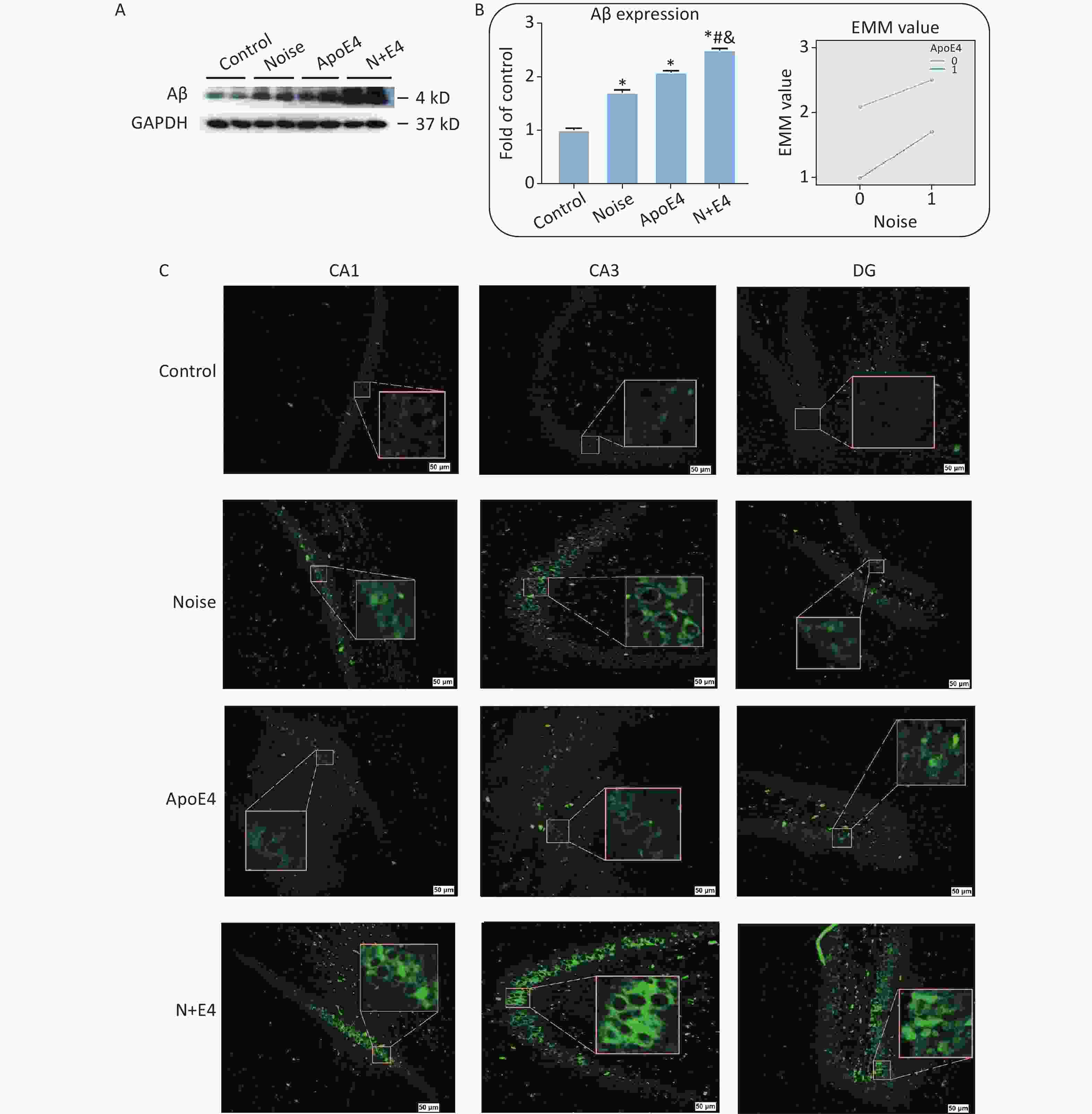
Figure 1. Chronic noise and ApoE4 exposure induce changes in Aβ accumulation in rats. (A) Western blot analysis of Aβ expression in the hippocampus. GAPDH was used as a loading control. (B) Changes in Aβ immunoreactivity, represented as percentage change relative to the control. *P < 0.01 versus the control group, #P < 0.01 versus the noise group, and &P < 0.01 versus the ApoE4 group. Data are presented as the mean ± standard deviation (n = 8 per condition). Two-way analysis of variance (ANOVA) with Fisher’s LSD post-test was used to analyse the statistical changes. (C) Representative images of thioflavin staining in hippocampal CA1, CA3, and DG immediately after cessation of chronic noise and ApoE4 exposure. Scale bar = 50 μm.
We analysed brain paraffin sections via thioflavin T staining to determine the patterns of Aβ distribution in different regions of the hippocampus. Aβ expression was observed in the CA1, CA3, and dentate gyrus (DG) regions, but was present at low levels in the hippocampus in control rats (Figure 1C).
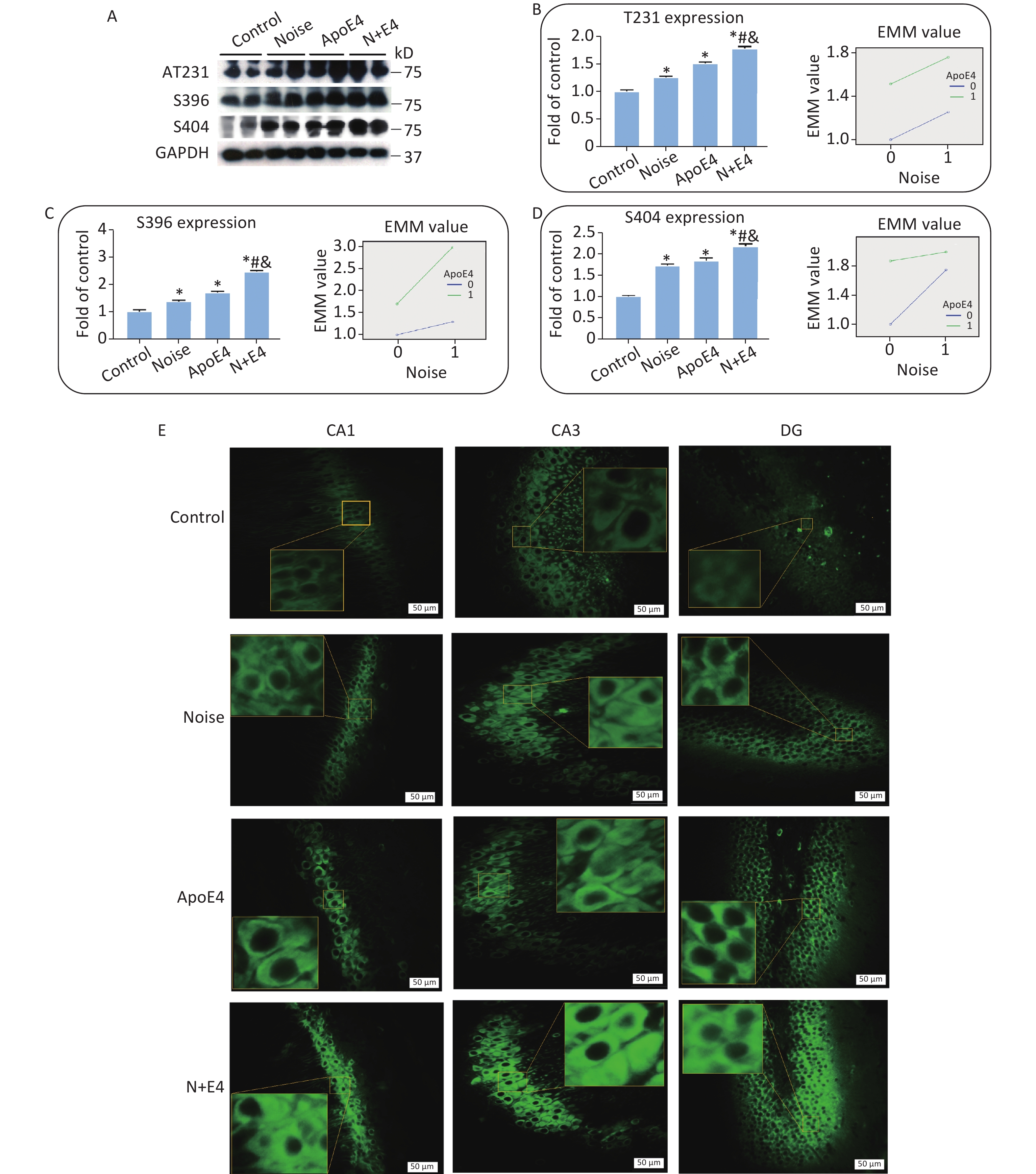
To assess the synergistic effects of chronic noise exposure and ApoE4 transfection on the phosphorylation of tau, we used western blot analysis to detect tau phosphorylated at the Thr231, Ser396, and Ser404 sites in hippocampal extracts. The hippocampal levels of tau phosphorylated at the Thr231, Ser396, and Ser404 sites significantly increased, and the highest levels of p-tau were observed in the N+E4 group, followed by the ApoE4 and noise groups (Figure 2A–D). Factorial ANOVA indicated that only at Ser404 sites chronic noise (F = 734.074, P < 0.001, df = 1) and ApoE4 transfection (F = 1200.737, P < 0.001, df = 1) significantly affected the expression of p-tau, and revealed a significant interaction effect of chronic noise and ApoE4 (F = 378.126, P < 0.001, df = 1) (Figure 2D).
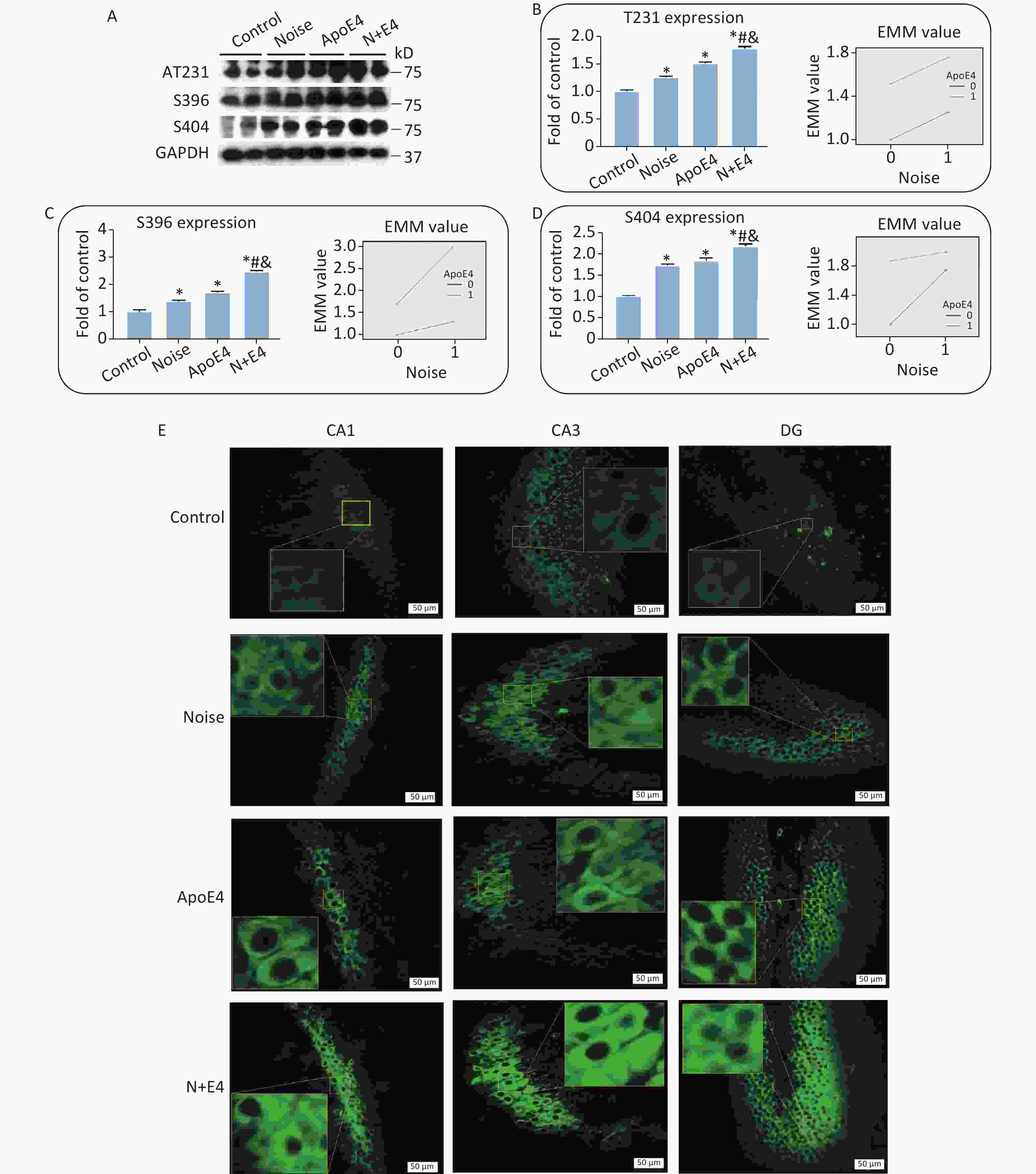
Figure 2. Chronic noise and ApoE4 exposure increase tau hyperphosphorylation in the hippocampus. (A) Immunoblot panels of RIPA-soluble hyperphosphorylated tau (P-tau) were probed with phosphorylation-dependent anti-tau antibodies as indicated. (B–D) The density of the immunoreactive bands was quantified and is presented as the percentage change relative to control samples. GAPDH was used as a loading control. Data are represented as the mean ± standard deviation (n = 8 per condition). *P < 0.01 versus the control group, #P < 0.01 versus the noise group, and &P < 0.01 versus the ApoE4 group. (E) Immunofluorescence staining for tau (S396) expression in the CA1, CA3, and DG regions of the hippocampus. Scale bar = 50 μm.
We analysed brain paraffin sections by immunofluorescence to further determine the distribution patterns of p-tau in the hippocampus. P-tau (Ser396) immunoreactivity was observed at high levels in the CA1, CA3, and DG regions in the noise exposure group, ApoE4 group, and N+E4 group, but at low levels in the control group (Figure 2E).
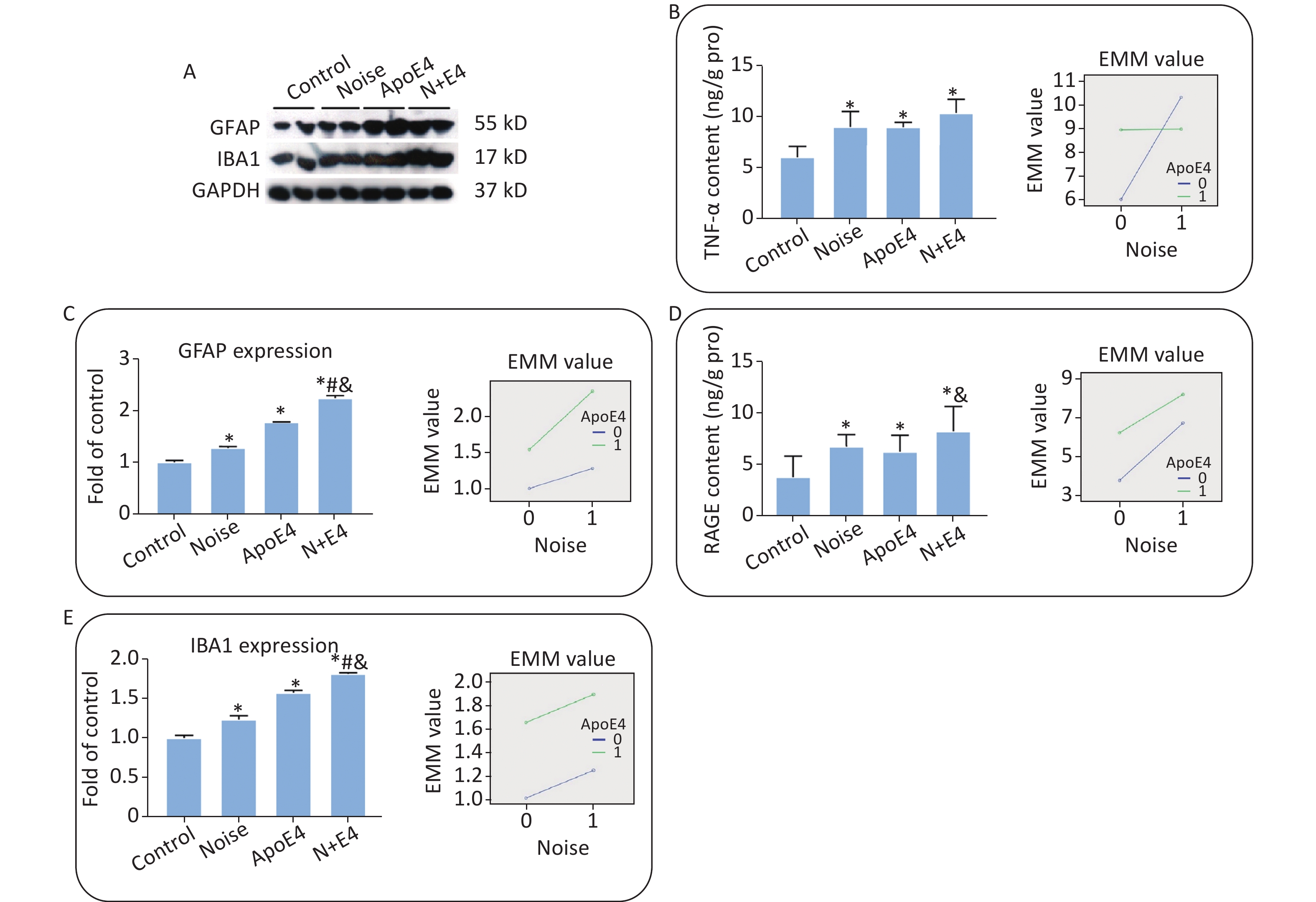
The expression of GFAP and Iba1 (Supplementary Figure S2A–C, available in www.besjournal.com), and the content of TNF-α and RAGE (Supplementary Figure S2D, E) in the hippocampus significantly increased after exposure. Factorial ANOVA indicated that chronic noise (F = 1454.501, P < 0.001, df = 1) and ApoE4 transfection (F = 3183.522, P < 0.001, df = 1) significantly affected the expression of GFAP, and similar effects of chronic noise (F = 309.328, P < 0.001, df = 1) and ApoE4 (F = 2285.265, P < 0.001, df = 1) were observed on Iba1 expression. Moreover, a significant interaction effect was observed between chronic noise and ApoE4 transfection (F = 348.118, P < 0.001, df = 1) on GFAP expression only (Supplementary Figure S2B). However, no such interaction effects were observed for Iba1, TNF-α, or RAGE expression (Supplementary Figure S2C–E).
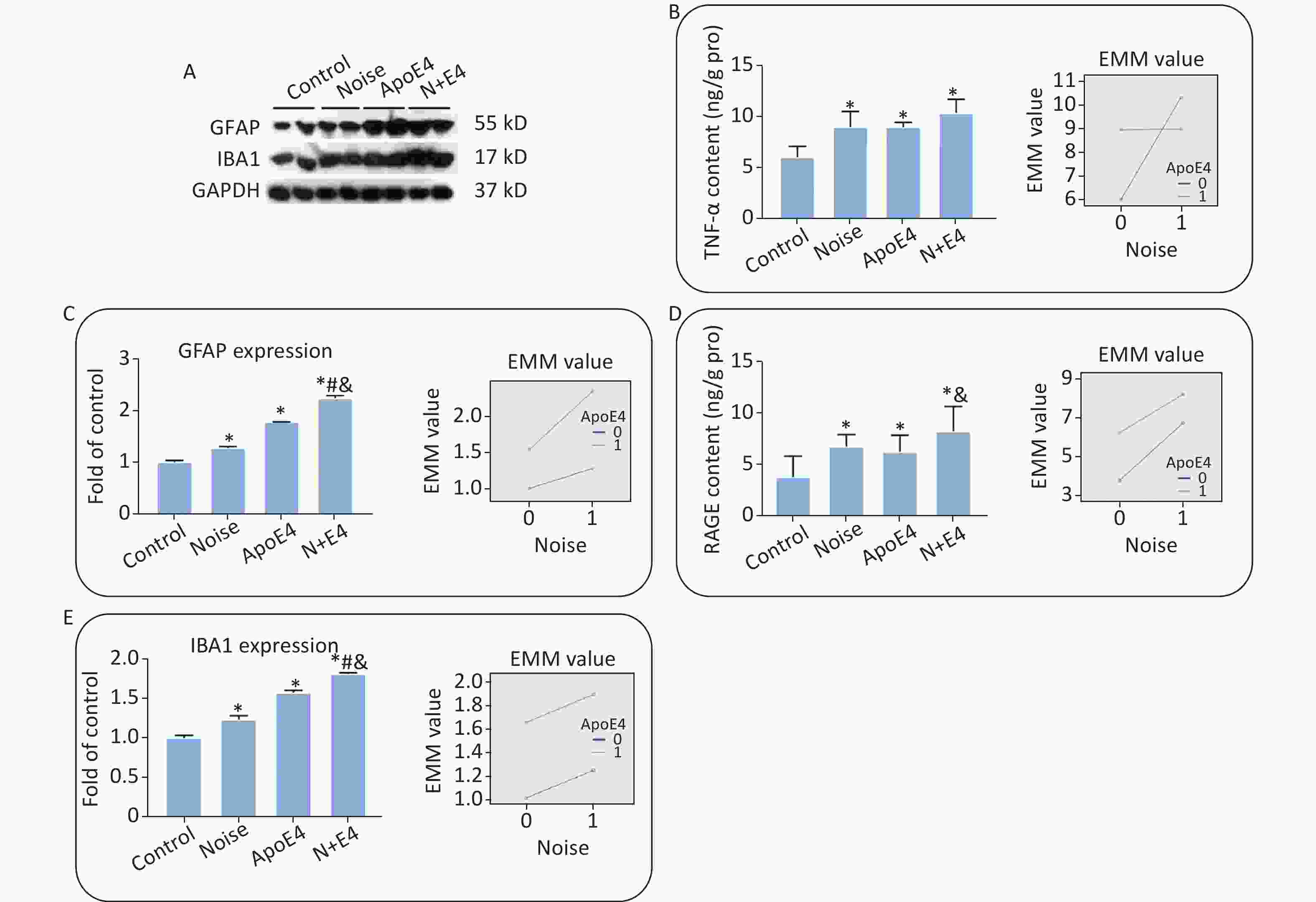
Figure S2. Chronic noise and ApoE4 exposure induce neuroinflammation in the hippocampus. (A) Western blot analysis of GFAP and IBA1 expression in the hippocampus. GAPDH was used as a loading control. (B, C) The density of the immunoreactive bands was quantified and is presented as the percentage change relative to the control. (D, E) TNF-α and RAGE levels in the hippocampus after exposure, as determined by ELISA. *P < 0.01 versus the control group, #P < 0.01 versus the noise group, and &P < 0.01 versus the ApoE4 group. Data are presented as the mean ± standard deviation (n = 8 per condition).
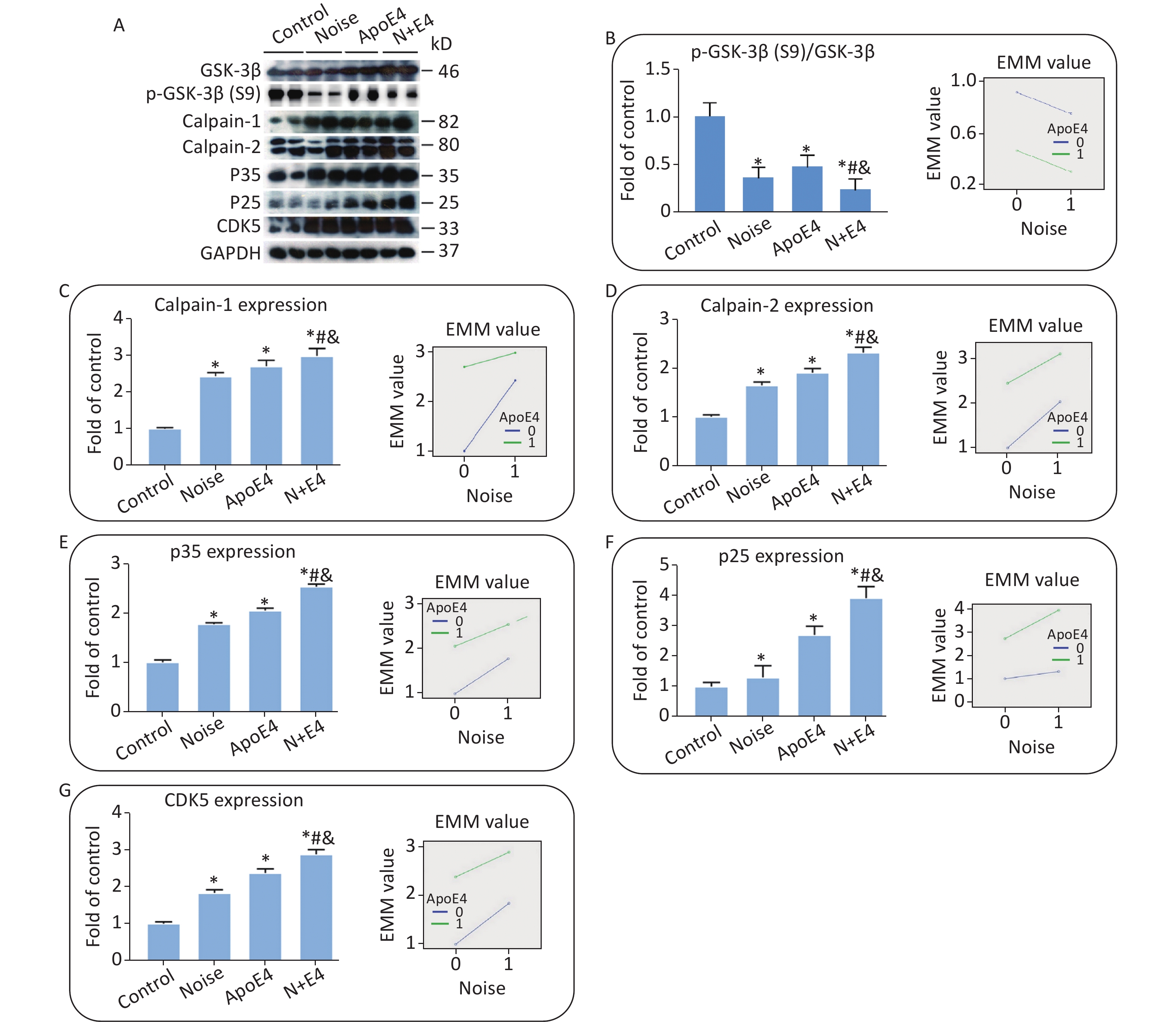
The same set of tissue homogenates in the analyses described above was analysed with available antibodies specific to components of the AKT-GSK-3β and calpain-CDK5 signalling pathways to clarify the underlying mechanisms. The levels of GSK3β, calpain-1, calpain-2, P35, P25, and CDK5 in the hippocampus markedly increased in both the noise-exposed and ApoE4 transfected groups. The level of p-GSK3β at site S9 showed an opposite trend (Figure 3), and the activity of GSK-3β increased significantly in the noise-exposed and ApoE4 transfected groups. Factorial ANOVA indicated that chronic noise (F = 804.896, P < 0.001, df = 1) and ApoE4 transfection (F = 2542.078, P < 0.001, df = 1) significantly influenced the expression of proteins in the GSK-3β and calpain-CDK5 signalling pathways, and revealed a significant interaction effect of chronic noise and ApoE4 (F = 40.381, P < 0.001, df = 1).
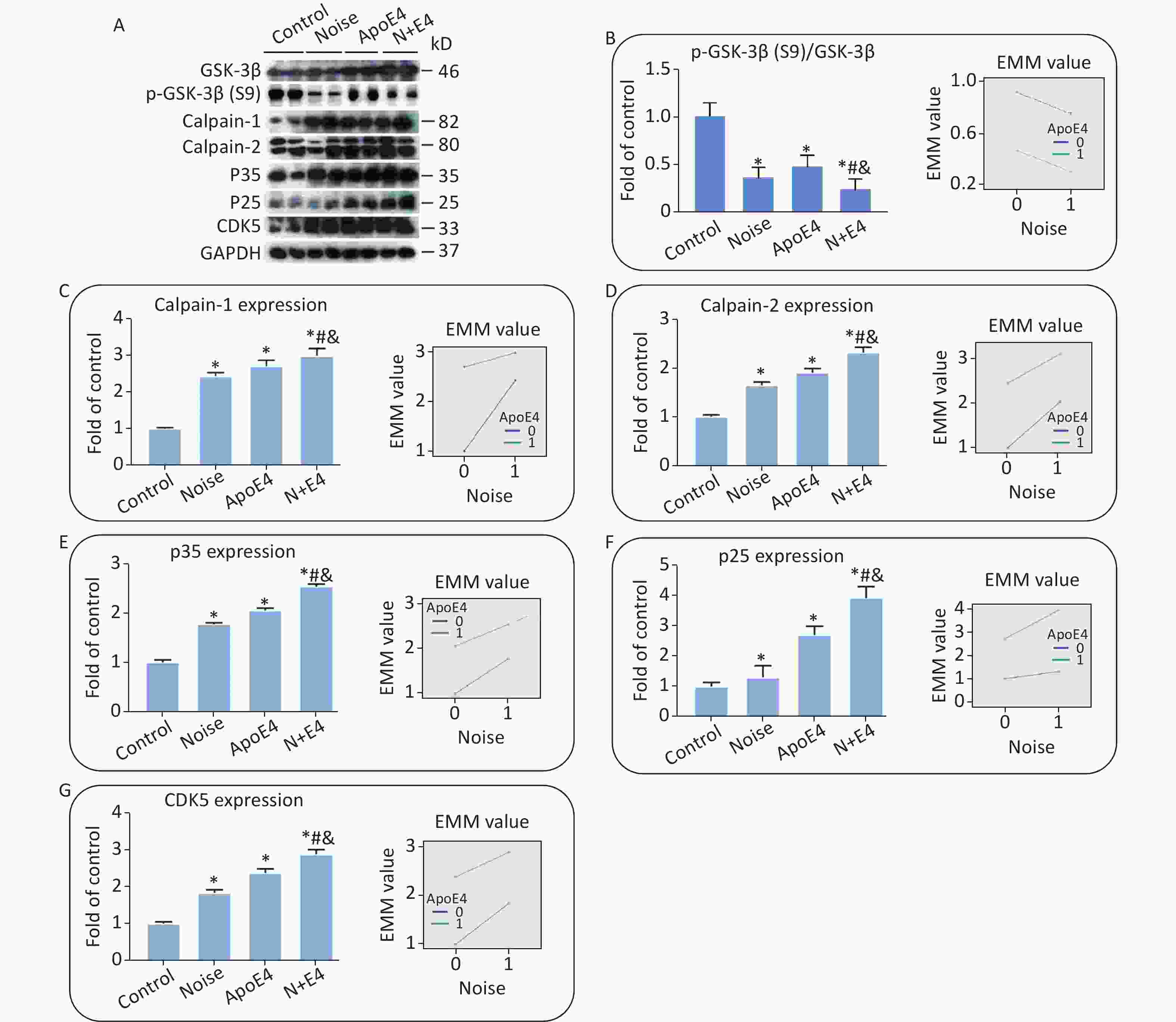
Figure 3. Combined effects of chronic noise and ApoE4 activation on the AKT-GSK-3β and calpain-CDK5 signalling pathways. (A) Western blot analysis of AKT-GSK-3β and calpain-CDK5 signalling pathways in the hippocampus. GAPDH was used as a loading control. (B–H) The density of the immunoreactive bands was quantified and is presented as the percentage change relative to the control. GAPDH was used as a loading control. *P < 0.01 versus the control group, #P < 0.01 versus the noise group, and &P < 0.01 versus the ApoE4 group. Data are represented as the mean ± standard deviation (n = 8 per condition).
In the present study, we investigated whether combined exposure to chronic noise and the ApoE4 gene might synergistically produce an AD-like neuropathology. Dual exposure to chronic noise and ApoE4 exacerbated Aβ accumulation and tau phosphorylation. Strikingly, tau phosphorylation was accompanied by enhancements in AKT-GSK-3β and calpain-CDK5 signalling after combined exposure. Our results indicated that chronic noise exposure and ApoE4 activation may be a gene-environment interaction that accelerates the development of AD.
Previous studies have revealed that noise exposure causes impairments in cognitive function[3]. However, ApoE4 expression accelerates hippocampus-dependent cognitive deficits[4]. In the present study, the exacerbation of cognitive decline after combined exposure suggested that chronic noise exposure and ApoE4 activation synergistically increase the risk of cognitive dysfunction.
We observed a marked increase in the levels of Aβ expression and tau phosphorylation in the rat hippocampus after exposure to chronic noise and ApoE4 transfection, thus supporting the cumulative effects of these factors on the onset and progression of AD-like neuropathological damage. Further findings showed that the synergistic interaction of these genetic and environmental factors underlies the progression of AD-related pathological changes.
The AKT-GSK-3β and calpain-CDK5 signalling pathways play important roles in the development of AD[5]. In the present study, synergetic increases in the CDK5/GSK-3β signalling pathway paralleled increases in tau phosphorylation and Aβ accumulation, thus, suggesting that CDK5/GSK-3β may play a causal role in the induction and development of AD-like neuropathology. (The levels of GSK3β at site S9 increased in the AKT-GSK-3β signalling pathways. The levels of calpain-1, calpain-2, P35, P25, and CDK5 increased in the calpain-CDK5 signalling pathways.)
Our study is the first to demonstrate that chronic noise exposure and ApoE4 activation synergistically affect tau phosphorylation and Aβ accumulation in the hippocampus, thus, potentially contributing to the cognitive deficits characteristic of neurodegenerative diseases such as AD. Additionally, our findings indicate that this synergism may be associated with the CDK5/GSK-3β signalling pathways. Further studies are required to further elucidate the mechanisms underlying this synergism.
Our results may enable the prevention and delay of the course of AD. In daily life, AD onset may be delayed in people with AD prone genes, and reducing noise exposure may delay disease progression in patients with AD or prevent noise from causing AD in people without AD. Simultaneously, the disease course in patients with AD may be delayed, or the onset of in normal people may be prevented, by controlling environmental factors and genetic factors.
Conflicts of Interest None of the authors have any financial relationships with the organizations that sponsored the research. The authors declare that they have no competing interests.
Author Contributions QZ and YL designed the research; WL and DS performed the exposure and behaviour experiments; DS, WL, and HC performed the molecular biology experiments; WL, DS, and FW analysed data; YL and WL wrote the paper. All authors read and approved the final manuscript.
HTML
 21387Supplementary Materials.pdf
21387Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: