-
Neuroblastoma (NB) is the most common extracranial solid tumor of childhood. It accounts for 8%–10% of all malignant tumors in children[1] and results in a heavy burden to the life and health of children. NB is a complex disease, and its occurrence is related to exposure to environmental risk factors. Children of parents who are occupationally exposed to environmental endocrine disruptors (EEDs) such as paint have a significantly higher risk of NB[2]. Di-2-ethylhcxylphthalate (DEHP) is a widespread EED that can interfere with a variety of neurotransmitters, affect the differentiation, proliferation and migration of neurons, and cause damage to the nervous system, thereby increasing the risk of neurological tumors such as NB in children[3]. DEHP enters the human body, where it is metabolized into mono-2-ethylhexyl phthalate (MEHP). MEHP has been detected in human blood, breast milk, and urine. In a previous study, we found that MEHP promoted the proliferation of SH-SY5Y cells and upregulated matrix metalloproteinase-2 (MMP-2) and MMP-9 to promote the migration of SH-SY5Y cells[3]. However, the mechanism by which MEHP promotes the migration of SH-SY5Y cells remains unclear.
The epithelial-mesenchymal transition (EMT) is a fundamental process induced by growth factors and cytokines, which involves a shift from an epithelial-like phenotype to a mesenchymal phenotype, thereby promoting metastasis. E-cadherin, N-cadherin, vimentin, and Snail are the key molecules involved in the EMT, which can affect tumor cell migration and invasion. Tumor tissues secrete various chemokines, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-β1 (TGF-β1). These chemokines play an important role in tumor angiogenesis, which provides oxygen and energy to tumor tissues, thus, promoting the proliferation, migration, and invasion of tumor cells. In malignant melanoma cells, E-cadherin, N-cadherin, hypoxia-inducible factor 1 (HIF-1α), and VEGF play roles in cell proliferation and migration, because downregulation of the HIF-1α/VEGF signaling pathway and modulation of the expression of E-cadherin and N-cadherin inhibit the EMT[4]. However, whether MEHP promotes the migration of SH-SY5Y cells by affecting EMT remains to be determined. Here, we expanded the findings of our previous study and determined the effect of MEHP exposure on SH-SY5Y cell migration, as well as the mechanism underlying its effects on the EMT.
Human NB cells (SH-SY5Y) (KeyGEN, Nanjing, China) were cultured in high glucose Dulbecco’s Modified Eagle’s Medium (BI Kibbutz, Beit-Haemek, Israel) with 10% fetal bovine serum (BI Kibbutz). When the cells reached 70%–80% confluence, different concentrations of MEHP (0, 10, 50, and 250 µmol/L) (Sigma-Aldrich, St. Louis, MO, USA) were added for 12 and 24 h, and 0.1% dimethyl sulfoxide was used as the solvent control. A scratch test was performed to examine the distance of cell migration. A cell invasion assay was then performed to detect the effects of MEHP on SH-SY5Y cell invasion; the numbers of invaded cells were observed under the microscope in randomly selected fields. The levels of secreted VEGF, PDGF, and TGF-β1 were detected using ELISA kits (Yeasen Biotechnology, Shanghai, China). The expression of migration-related proteins was determined using western blotting.
Data are presented as the mean ± SD, and each experiment was performed independently at least three times. Differences between two groups were tested using one-way analysis of variance. A value of P < 0.05 was considered statistically significant.
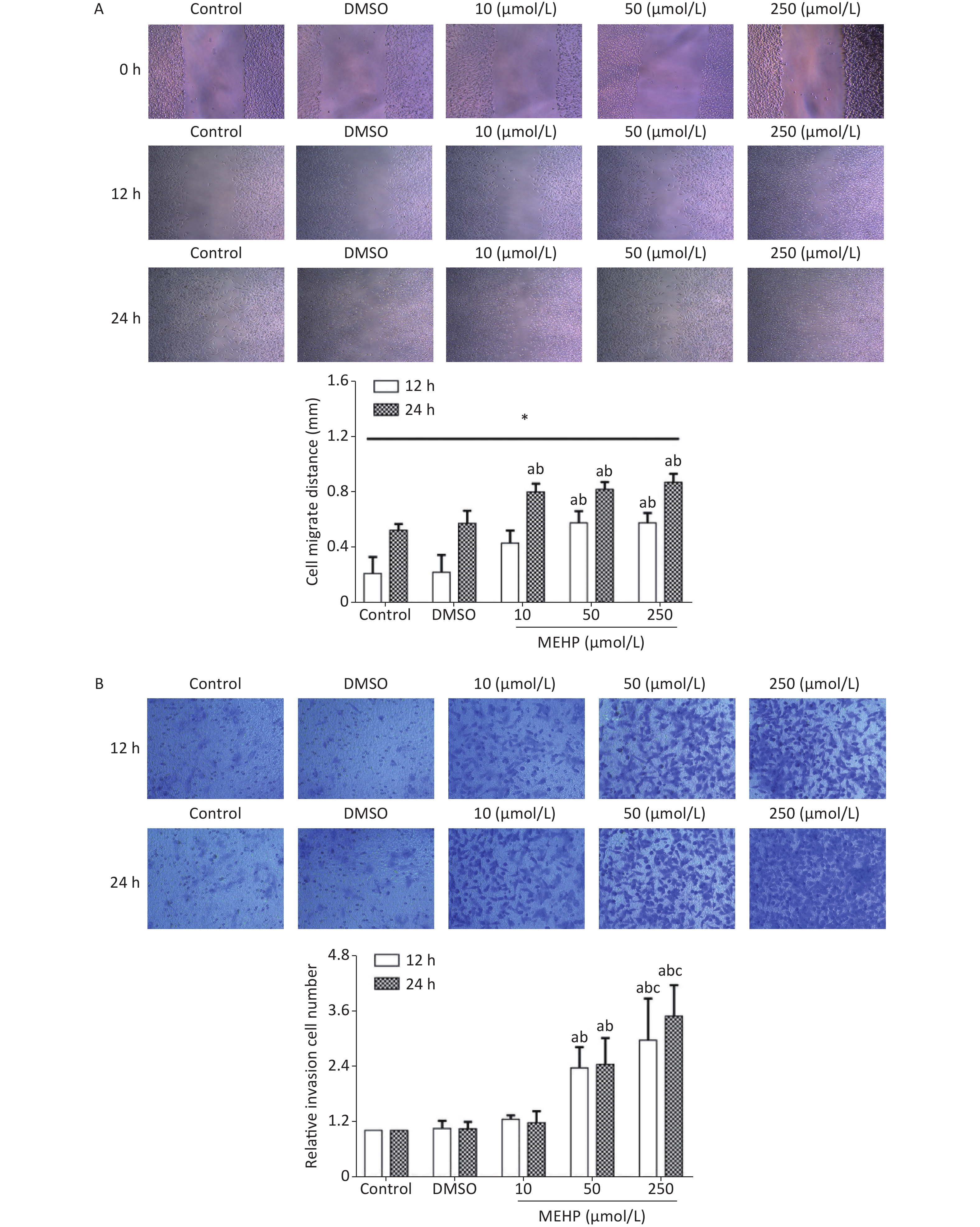
Analysis of migration and invasion showed that exposure to MEHP for 12 and 24 h caused a concentration-dependent increase in the migration distance and the invasion level of SH-SY5Y cells. The cell migration distances and the invasion levels were significantly higher in the 50 and 250 µmol/L MEHP groups than in the control and DMSO groups (P < 0.05). The cell migration distance of the MEHP-treated group was significantly higher at 24 h than at 12 h (P < 0.05), and cell invasion levels were higher in the 250 µmol/L MEHP group than in the 10 µmol/L MEHP group at 12 and 24 h (P < 0.05) (Figure 1). Together, these results indicated that MEHP, an environmental endocrine disruptor, induced tumor cell migration and invasion.
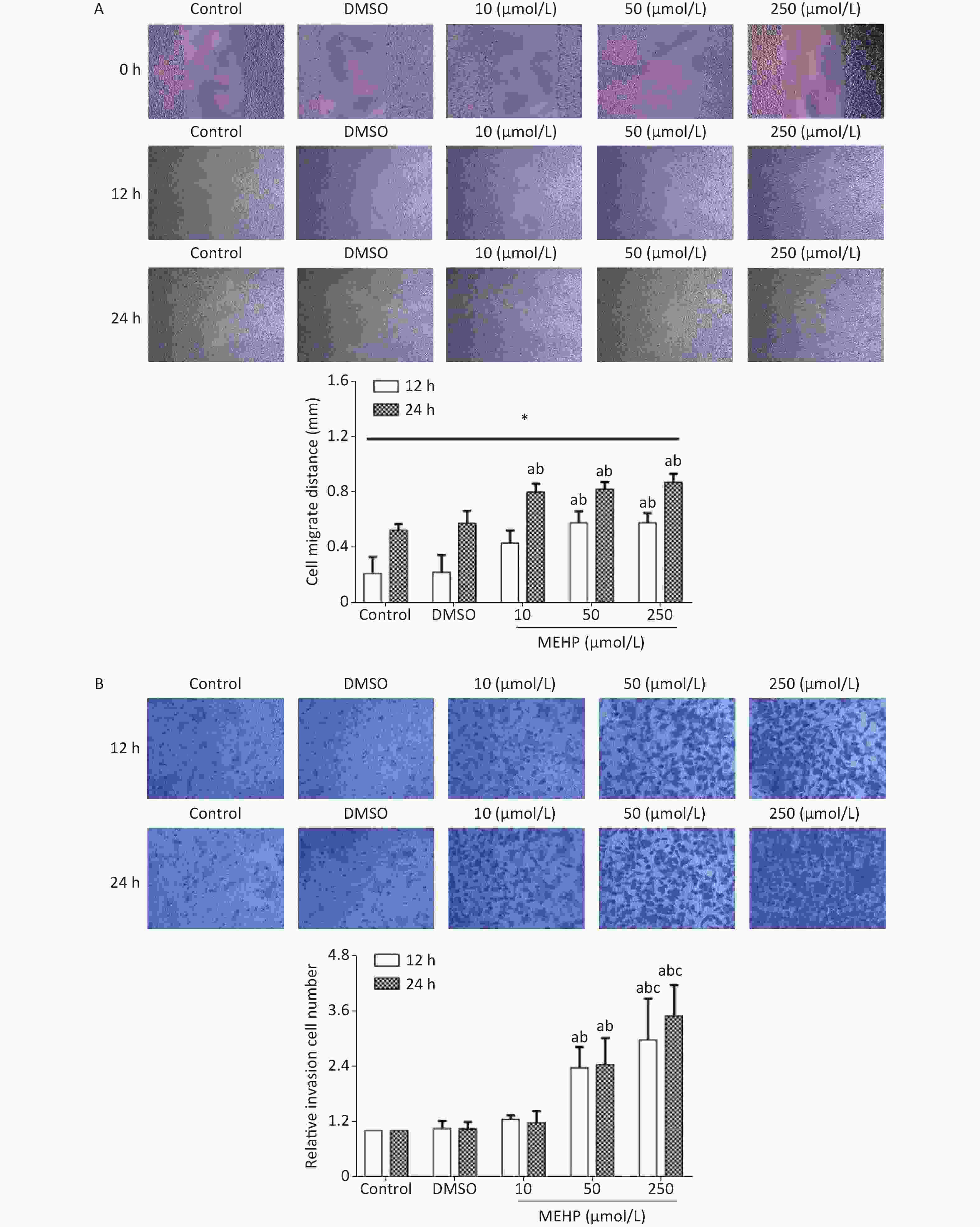
Figure 1. The effects of MEHP on the migration and invasion of SH-SY5Y cells. (A) The effects of MEHP on the migration of SH-SY5Y cells; *comparison with the 12 h group; (P < 0.05). (B) the effects of MEHP on the invasion of SH-SY5Y cells; acomparison with the control group (P < 0.05); bcomparison with the dimethyl sulfoxide group (P < 0.05); ccomparison with the 10 µmol/L MEHP group (P < 0.05). Values are presented as the mean ± SD (n ≥ 3).
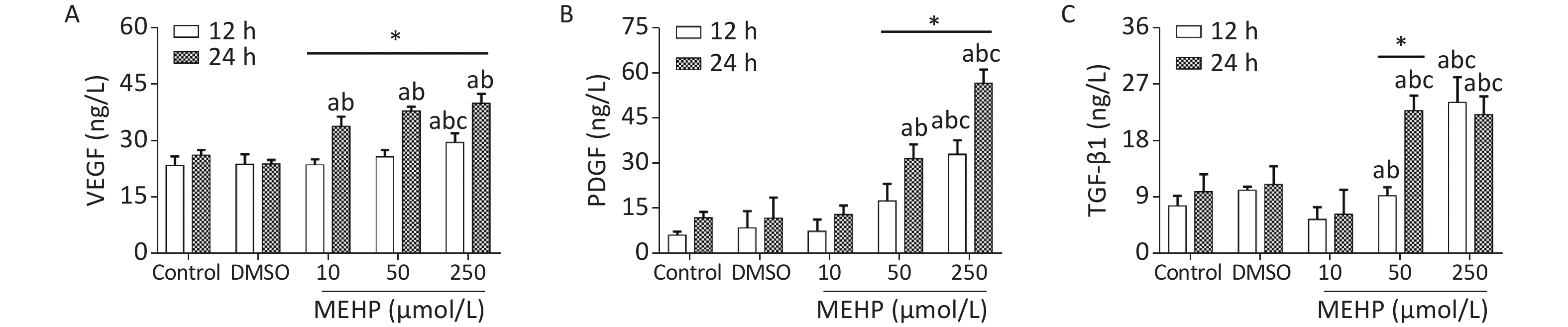
The effect of MEHP on the secretion of migration-related cytokines in SH-SY5Y cells is shown in Figure 2. Treatment with MEHP increased the levels of VEGF, PDGF, and TGF-β1. The levels of VEGF, PDGF, and TGF-β1 were significantly higher in the 250 µmol/L MEHP group than in the control, DMSO, and 10 µmol/L MEHP groups at 12 h (P < 0.05). At 24 h of treatment, the levels of VEGF, PDGF, and TGF-β1 were higher in the 50 and 250 µmol/L MEHP groups than in the control and DMSO groups (P < 0.05). The levels of VEGF in the MEHP group, PDGF in the 50 and 250 µmol/L MEHP groups, and TGF-β1 in the 50 µmol/L MEHP group were higher at 24 h than at 12 h (P < 0.05). The results indicated that MEHP regulated the secretion of tumor angiogenesis-related cytokines, including VEGF, PDGF, and TGF-β1.
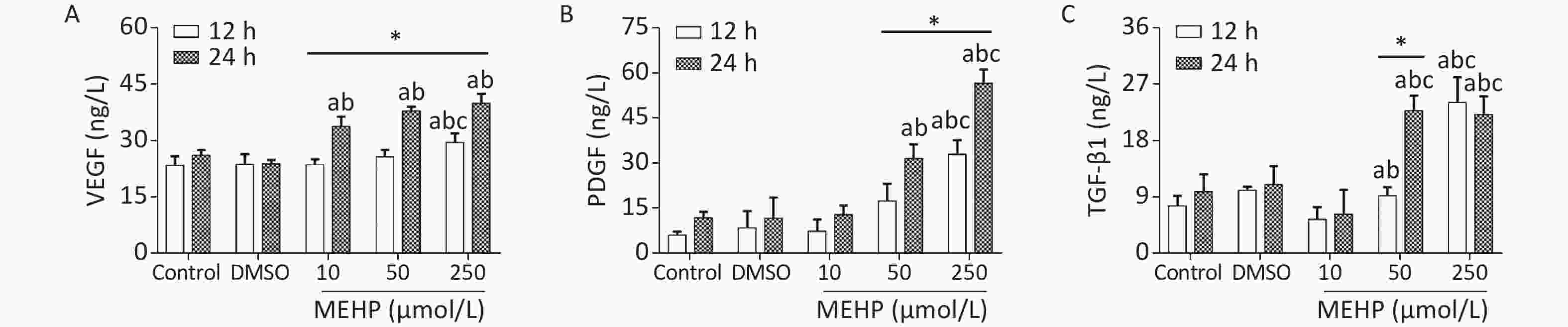
Figure 2. Effects of MEHP on cytokine secretion of SH-SY5Y cells. (A) VEGF secretion; (B) PDGF secretion; (C) TGF-β1 secretion; acomparison with the control group (P < 0.05); bcomparison with the dimethyl sulfoxide group (P < 0.05); ccomparison with 10 µmol/L MEHP group (P < 0.05); *comparison with the 12 h group (P < 0.05). Values are presented as the mean ± SD (n ≥ 6).
VEGF is expressed at high levels in the tumor microenvironment and promotes tumor angiogenesis. New blood vessels can provide oxygen and energy to tumor tissues, which is important for promoting tumor proliferation and migration[4]. PDGF can stimulate the proliferation and chemotaxis of NB cells, promote mitosis, and participate in tumor malignant transformation. PDGF plays an important role in the migration of SH-SY5Y cells. TGF-β1 is involved in cell growth, immune regulation, angiogenesis, and tumor development. The secretion of TGF-β1 by tumor cells can promote tumor tissue angiogenesis, maintain the stability of tumor cells, provide a tumor microenvironment favoring tumor growth and metastasis, and increase tumor migration. In this study, we showed that exposure to MEHP significantly increased the levels of VEGF, PDGF and TGF-β1, indicating that MEHP may affect the migration of SH-SY5Y cells by regulating the secretion levels of VEGF, PDGF, and TGF-β1.
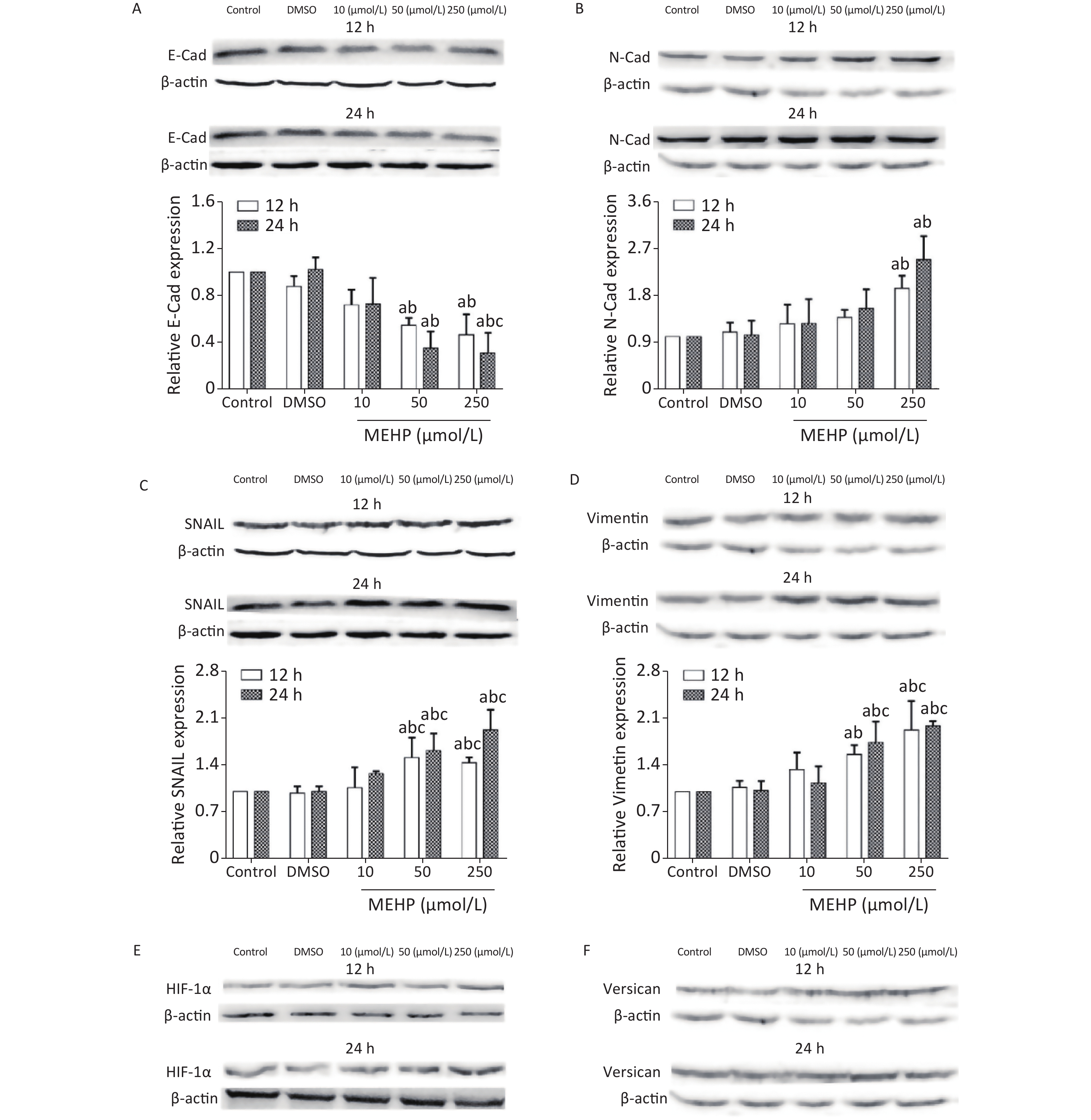
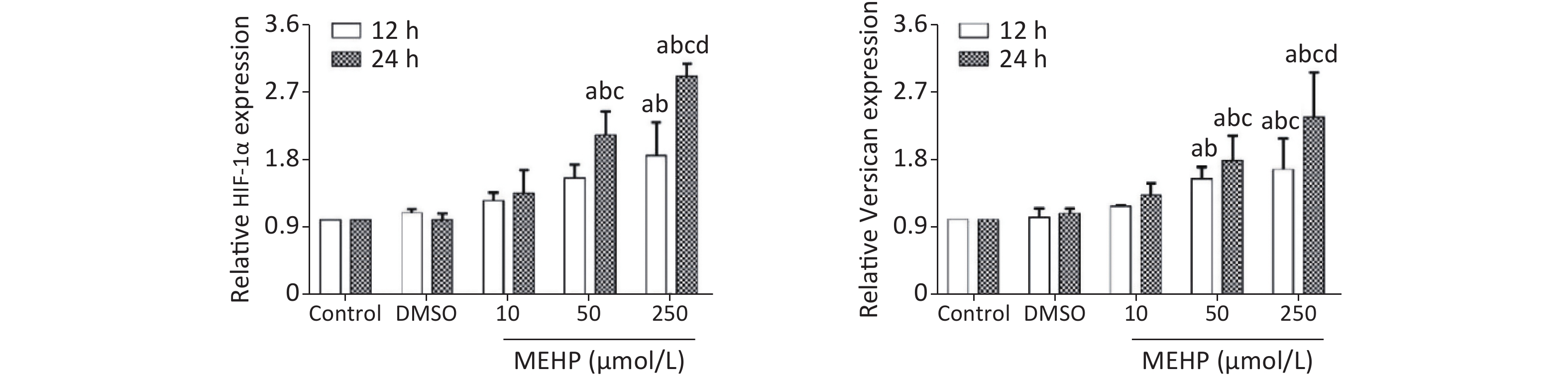
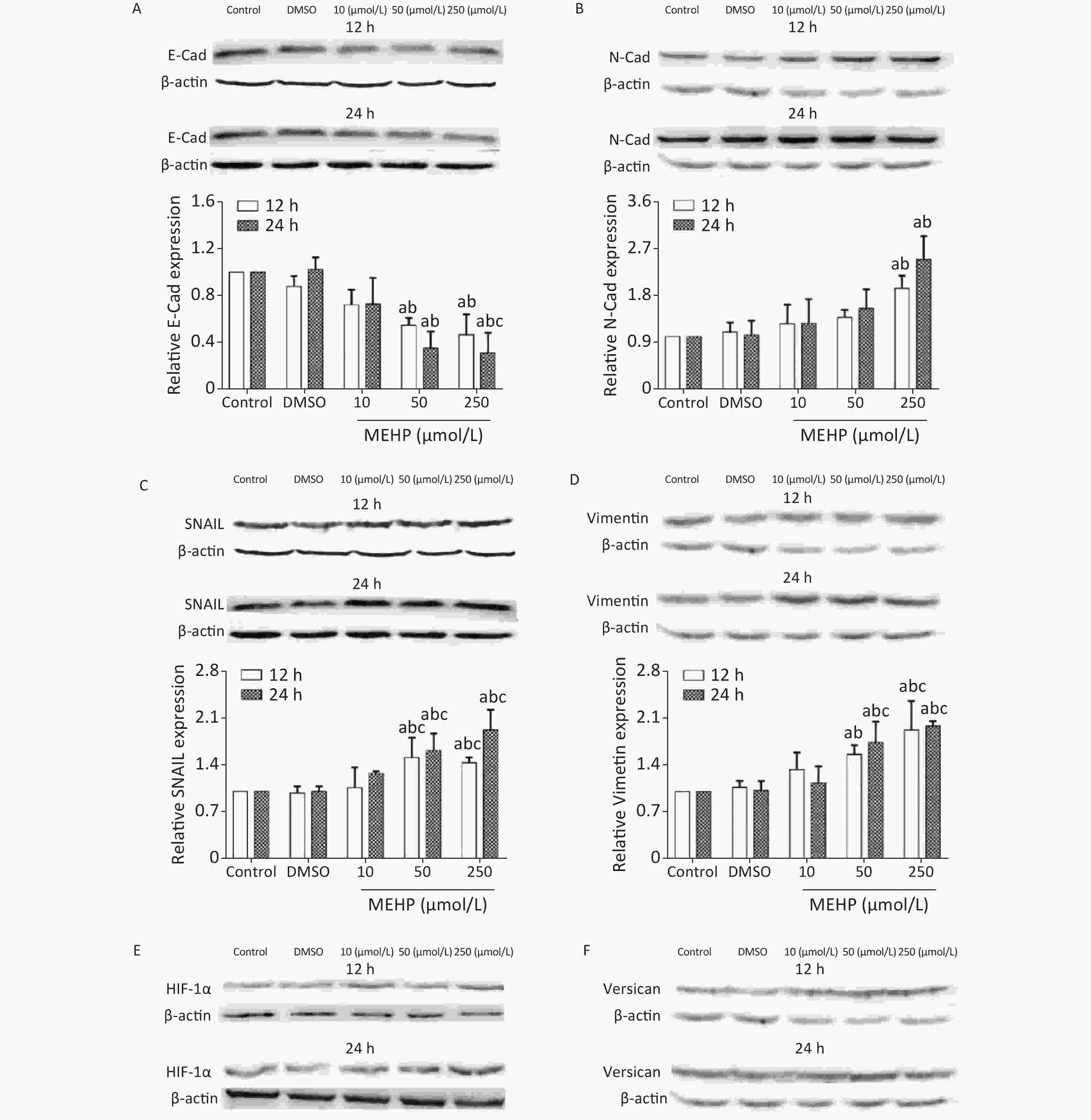
The effect of MEHP on the expression of migration-related proteins in SH-SY5Y cells is shown in Figure 3. MEHP exposure decreased the levels of E-cadherin at 12 and 24 h, and E-cadherin was lower in the 50 and 250 µmol/L MEHP groups than in the control and DMSO groups (P < 0.05). MEHP upregulated the expression of N-cadherin in a dose-dependent manner. The expression of SNAIL, vimentin, HIF-1α, and versican was significantly higher in the 50 and 250 µmol/L MEHP groups than in the control, DMSO, and 10 µmol/L MEHP groups at 12 and 24 h, and the expressions of HIF-1α and versican were significantly higher in the 250 µmol/L MEHP group than in the other groups at 24 h (P < 0.05).

Figure 3. Effects of MEHP on migration-related proteins of SH-SY5Y cells. (A) E-Cad protein expression; (B) N-Cad protein expression; (C) SNAIL protein expression; (D) vimentin protein expression; (E) HIF-1α protein expression; (F) versican protein expression; acomparison with the control group (P < 0.05); bcomparison with the dimethyl sulfoxide group (P < 0.05); ccomparison with the 10 µmol/L MEHP group (P < 0.05). Values are presented as the mean ± SD (n ≥ 5).
The present results showed that MEHP regulated the expression levels of E-cadherin, N-cadherin, vimentin, and SNAIL, which are key proteins affecting tumor cell migration because of their involvement in the EMT, which is a process induced by growth factors and cytokines by which epithelial cells lose polarity and intercellular adhesion, thus, acquiring the migration and invasion characteristics of mesenchymal cells. NB cells migrate after undergoing the EMT, and the EMT is essential to initiate NB cell migration.
Studies of the association between N-cadherin expression and the clinical characteristics of NB showed that N-cadherin mRNA levels are low in metastatic tumors, suggesting that N-cadherin signaling plays an important role in the metastasis of NBs[5]. Three types of NBs were described in 17 neuroblastoma cell lines, and analysis of differentially-expressed genes showed that vimentin was widely expressed in basal adhesion NBs with migration characteristics[6]. The transcription factor, SNAIL, may activate the EMT by regulating the expression of EMT marker proteins to promote tumor cell migration[7]. The present results showed that exposure to MEHP significantly downregulated the expression of E-cadherin and upregulated N-cadherin in SH-SY5Y cells. In addition, MEHP promoted the expressions of vimentin and SNAIL, and enhanced the migration ability of SH-SY5Y cells. The results suggested that MEHP promoted the migration of SH-SY5Y cells by modulating the expressions of key EMT proteins. This is consistent with previous studies showing that DEHP exposure affected the expression of E-cadherin and promoted the migration of A549 cells[7]. These studies indicated that MEHP may induce tumor metastasis by regulating the expression of EMT-related proteins, a key pathway of cell migration.
The tumor cell microenvironment plays an important role in tumor occurrence and progression, as well as in tumor metastasis. The abnormal tumor microenvironment can cause the destruction of the tumor basement membrane and promote the spread of tumor cells from the original location to surrounding or distant tissues and organs, leading to metastasis and infiltration of the tumor. Versican is a key protein in the tumor microenvironment. It is involved in regulating cell growth, migration, and adhesion. Versican is highly expressed in tumor tissues and adjacent tissues in brain tumors, breast cancer, and other tumors[8]. The hypoxic environment of the tumor tissue increases the secretion of HIF-1 and VEGF. HIF-1 and VEGF can induce the occurrence of EMT, thereby promoting tumor migration. HIF-1α can promote tumor cell migration by affecting EMT-related molecules such as SNAIL, activate EMT-related signal pathways, and regulate the release of cytokines. VEGF can promote tumor tissue angiogenesis. The NB phenotype is related to the expression of VEGF, and tumors with high VEGF expression are more malignant[9]. PDGF is a growth factor that can stimulate cell proliferation and chemotaxis in NBs, and is involved in tumor malignant transformation[10]. In this study, exposure to MEHP upregulated the expressions of HIF-1α and versican, and increased the migration of SH-SY5Y cells. In addition, MEHP upregulated the expressions of VEGF, PDGF, E-cadherin, and N-cadherin. These results indicated that MEHP increased the expressions of HIF-1α and versican, promoted the secretion of VEGF and other cytokines, and activated the EMT pathway, thereby promoting SH-SY5Y cell migration.
In conclusion, MEHP exposure promoted the migration of SH-SY5Y cells, possibly by regulating the expressions of the EMT-related proteins, E-cadherin, N-cadherin, SNAIL, and vimentin, as well as the tumor microenvironment-related proteins HIF-1α and versican, and the secretion of VEGF, PDGF, and TGF-β1.
HTML
Reference







 Quick Links
Quick Links
 DownLoad:
DownLoad: