-
Silicosis is a common occupational disease caused by long-term inhalation of free silica (SiO2)-containing dusts, with progressive pulmonary fibrosis as the main lesion, leading eventually to respiratory failure. The formation of fibrosis in the lungs is thought to be preceded by the activation of alveolar macrophages (AM) by SiO2 through molecular signaling pathways, leading to a series of inflammatory reactions, apoptosis of AM, and the stimulation of pulmonary fibrosis[1]. Nevertheless, the exact molecular mechanisms for the initiation of AM damage remains to be identified. Stromal interaction molecule 1 (STIM1) is an important component of store-operated calcium entry (SOCE) (a Ca2+ channel in response to various physiological/pathological factors), which contributes to SOCE activation as a Ca2+ sensor in the endoplasmic reticulum[2]. STIM1 has been reported to participate in various lung diseases, such as lung cancers, pulmonary inflammation, lung intoxication and injury, and pulmonary fibrosis. However, whether and how STIM1 is involved in the toxic effects of silica on AM, which is the initial damage during the pathogenesis of silicosis, has not been reported.
The PI3K (phosphatidylinositol-3 kinase)/Akt (serine/threonine kinases Akt, also known as protein kinase B) signaling pathway participates in cell survival/proliferation, inflammatory activation, and tumorigenesis, and the phosphorylation of Akt is considered to be an indicator of the activation of the PI3K/Akt pathway, because PI3K phosphorylates Akt, thus, leading to changes in the downstream signaling molecules of Akt. In particular, the activation of Akt plays a vital role in the pathogenesis of silicosis, based on experimental results in both human pulmonary fibroblastic cells (in vitro) and an intact mouse (in vivo) model, where modulators of Akt were used[3,4]. Moreover, there has been evidence suggesting that the PI3K/Akt pathway is involved in STIM1-associated pathological processes such as inflammation, cell proliferation, and apoptosis[5]. However, whether the PI3K/Akt pathway participates in the regulation of STIM1, whose activation may initiate silicosis-associated changes, is also unclear and needs to be determined. Early growth response 1 (Egr-1) is a protein serving as a prototypic Cys2-His2 type zinc finger transcription factor. Egr-1 has been reported to regulate the expression of more than 30 downstream target genes, and it is closely related to cell proliferation, differentiation, apoptosis, and the inflammatory response. In a recent study, Egr-1 expression was significantly enhanced in cultured lung epithelial cells and mouse macrophage (RAW264.7) cells by silica dust, suggesting that it might be one of the critical factors in the formation/progression of silicosis[6]. Previous studies have also shown that promoters of transforming growth factor beta1 (TGF-β1) and the tumor necrosis factor-alpha (TNF-α) (both being inflammatory mediators) may serve as Egr-1 binding sites. Both TGF-β1 and TNF-α are related to the progression of pulmonary fibrosis in silicosis, and RAW264.7 cells are stimulated by silica to release both cytokines. We hypothesized that SiO2 might enhance the release of TGF-β1 and TNF-α via activating an Egr-1-related signaling pathway, and proteins such as STIM1, Akt, and Egr-1 may participate in the effect of SiO2 in macrophages. The aim of this study was therefore to identify the role of STIM1 in SiO2-stimulated release of inflammatory cytokines from macrophages, and to determine the involvement of associated proteins, Akt and Egr-1, using the RAW264.7 cell line.
Silica (SiO2, 0.5–10 µm) powder, 2-aminoethoxydiphenyl borate (2-APB), and Deguelin were purchased from Sigma-Aldrich (St. Louis, MO, USA). SiO2 particles at a concentration of 100 mg/mL were stored in DMEM containing 0.25% fetal bovine serum (FBS). The 2-APB and Deguelin were both dissolved in dimethyl sulfoxide prior to exposure to cells. RAW264.7 cells were cultured in DMEM containing 10% FBS at 37 °C in a humidified atmosphere with 5% CO2. For the transfection of STIM1 plasmids, RAW264.7 cells were seeded in a 60 mm culture dish or 35 mm confocal dish (with a cover glass on the bottom). After 24 h, STIM1 plasmids (STIM1 OE) and control plasmids [pcDNA3.1(+)], obtained from Genepharma (Shanghai, China), were transfected into the cells by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. The transfected cells were further cultured for 24 h, then were used in subsequent SiO2-stimulating experiments. For RNA interference of STIM1, RAW264.7 cells were seeded as described above. After 24 h, siRNAs specific for STIM1 were transfected into RAW264.7 cells to achieve transient knockdown of STIM1 using Lipofectamine 2000 according to the manufacturer’s instructions. The siRNAs for STIM1 (STIM1 siRNA) and a non-silencing control RNA (control siRNA) were designed and provided by Shanghai GenePharma (Shanghai, China). Their sequences are listed in Supplementary Table S1, available in www.besjournal.com. RAW264.7 cells were exposed to SiO2 at the density of 7 μg/cm2 for 1 h, followed by collection of the culture medium, which was centrifuged at 3,000 rpm for 15 min. The supernatant was retained and kept at –80 °C for further analysis. The secretion of TGF-β1 or TNF-α from cells to the medium was determined using ELISA kits for TGF-β1 and TNF-α (CUSABIO, Wuhan, China). All measurements were conducted strictly according to the manufacturer’s instructions. For the Western blotting assays of STIM1, Akt, p-Akt, and Egr-1, the following primary antibodies were used: rabbit polyclonal antibodies against mouse phospho-Akt (at Ser473), Akt, Egr-1, STIM1, β-actin, and GAPDH (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA); secondary antibodies: goat-anti-rabbit IgG-conjugated horseradish peroxidase and fluorescein isothiocyanate-labeled goat anti-rabbit IgG (ZSGB Biotech, Beijing, China). The distribution and contents of Akt and p-Akt in cells were analyzed by immunofluorescence analysis of RAW264.7 cells treated with or without SiO2.
siRNA Sense (5’–3’) Antisense (5’–3’) STIM1 siRNA-1 GAC UUC UGA AGA GUC UAC CTT GGU AGA CUC UUC AGA AGU CGC STIM1 siRNA-2 UGC UGG UUU GCC UAU AUC CTT GGA UAU AGG CAA ACC AGC AGC STIM1 siRNA-3 GAG AUG AGA UCA ACC UUG CTT GCA AGG UUG AUC UCA UCU CGC control siRNA UUC UCC GAA CGU GUC ACG UTT ACG UGA CAC GUU CGG AGA ATT Note. The siRNA sequences were designed and synthesized by Shanghai GenePharma Co., Ltd (Shanghai, China). Table S1. The sequences of siRNAs for STIM1 (STIM1 siRNA) and a non-silencing control RNA (control siRNA)
Due to the similarity between RAW264.7 (a mouse peritoneal macrophage line) and pulmonary macrophages, the experimental model used in this study may mimic the initial pulmonary damage induced by SiO2 inhalation, which may progress to pulmonary fibrosis (a central pathological process in silicosis). Our results showed the release of inflammatory mediators from RAW264.7 cells after SiO2 treatment, and the influence of various modulators of Akt, STIM1, and Egr-1. Because activation of Akt by SiO2 is involved in the release of TGF-β1 and TNF-α[7], both being typical inflammatory mediators, and two other proteins, STIM1 and Egr-1, have been observed to be functionally associated with Akt, we determined the effects of various modulators of each protein on SiO2-induced release of TGF-β1 and TNF-α from RAW264.7 cells.
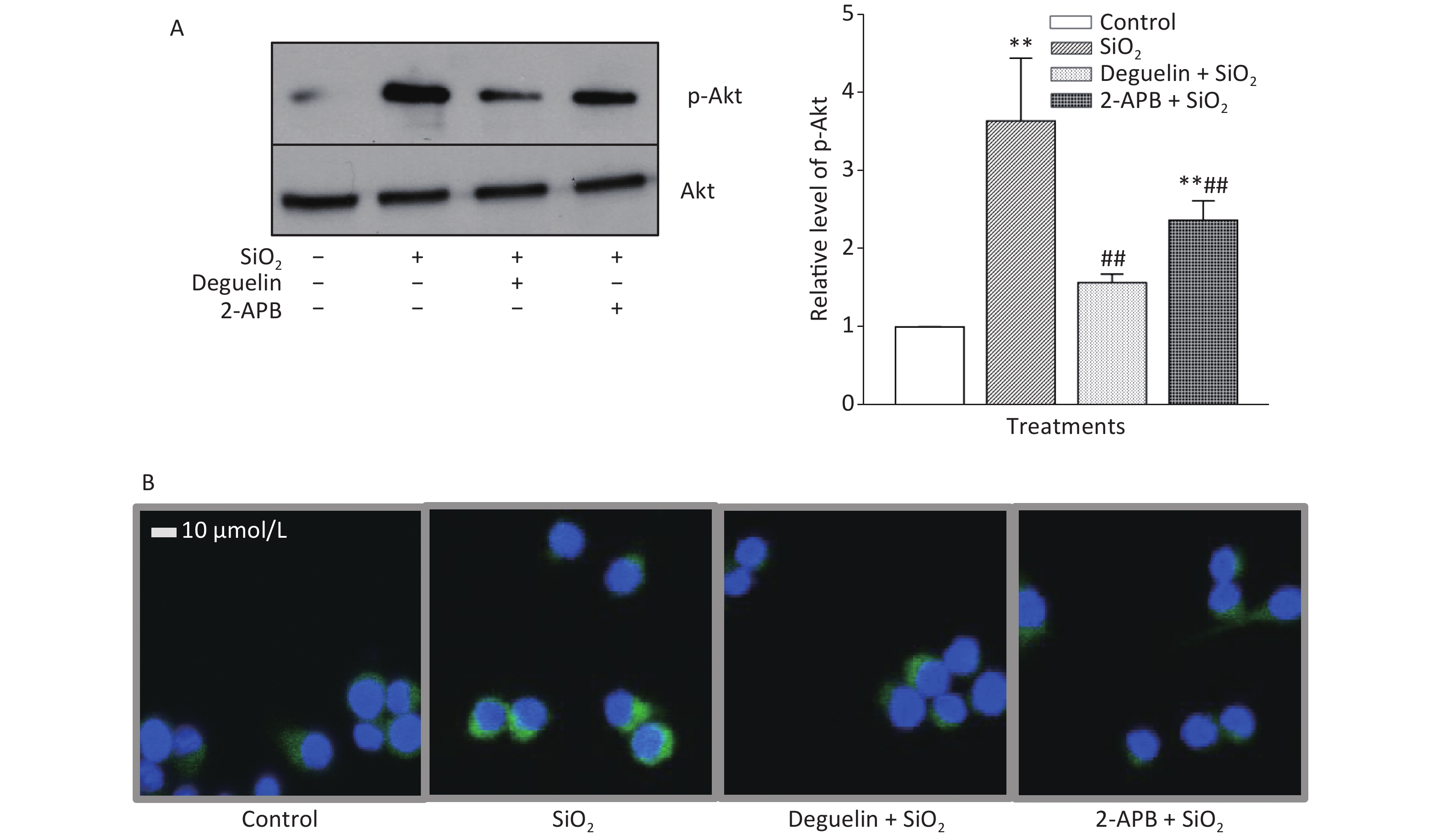
Table 1 shows that SiO2 (7 μg/cm2, exposing for 60 min, a duration with maximal effects as previously determined)[7] significantly increased the release of TGF-β1 and TNF-α from RAW264.7 cells, and this effect was blocked or reduced by Deguelin (100 nmol/L, a specific inhibitor of Akt), 2-APB (100 μmol/L, a specific inhibitor of STIM1), siRNAs of STIM1, and an antibody specific for Egr-1 (20 and 50 μg/mL); while the effect of SiO2 was further enhanced by overexpression of STIM1 (STIM1 OE). We then determined the effect of SiO2 on Akt activation (phosphorylation) and the influence of Deguelin and 2-APB. Previously, we showed that SiO2 (7 µg/cm2) enhanced Akt phosphorylation in a time-dependent manner, which peaked at 60 min of exposure[7]. In the present study, we used the same concentration of SiO2 and exposure duration (60 min), based on the influence of Deguelin, a natural inhibitor of Akt, and 2-APB as an inhibitor of STIM1, on the effect of SiO2 in RAW264.7 cells, using Western blotting and immunofluorescent assays. Supplementary Figure S1A (available in www.besjournal.com) shows that SiO2 significantly induced the activation (phosphorylation) of Akt, and that this effect was suppressed by Deguelin (100 nmol/L) and 2-APB (100 µmol/L). Likewise, using immunofluorescence experiments (Supplementary Figure S1B), SiO2 was found to increase the signal of phosphorylated Akt (p-Akt, as reflected by the green fluorescence in the cytoplasm); however, in the presence of Deguelin and 2-APB, the cytoplasmic p-Akt signal was reduced. These results suggested that STIM1 was involved in the activation of Akt by SiO2 in macrophages.
Target proteins Specific modulators SiO2, µg/cm2 Release of inflammatory mediators TGF-β1, ng/mL TNF-α, pg/mL − None 0 0.75 ± 0.15 234 ± 41.9 − None 7 1.60 ± 0.17** 485 ± 8.09** Akt Deguelin (100 nmol/L) 7 1.06 ± 0.11** 347 ± 5.48** Egr-1 Egr-1 antibody (20 μg/mL) 7 1.07 ± 0.11**†† 442 ± 12.5**†† Egr-1 antibody (50 μg/mL) 7 0.79 ± 0.02*†† 282 ± 27.2*†† STIM1 2-APB (100 μmol/L) 7 0.94 ± 0.08**†† 271 ± 11.8*†† Control siRNA 0 0.39 ± 0.07 362 ± 8.18 Control siRNA 7 0.95 ± 0.05## 441 ± 12.0## STIM1 siRNA-1 7 0.65 ± 0.03##‡‡ 365 ± 11.1##‡‡ STIM1 siRNA-2 7 0.66 ± 0.04##‡‡ 391 ± 12.0##‡‡ pcDNA3.1(+) 0 0.49 ± 0.04 370 ± 7.87 pcDNA3.1(+) 7 0.87 ± 0.19¦¦ 398 ± 7.49¦¦ STIM1 OE 7 1.24 ± 0.12¦¦§§ 443 ± 8.43¦¦§§ Note. Cells were exposed to SiO2 at the density of 7 μg/cm2 for 60 min. Interference RNAs 1 and 2 demonstrated preferred extent of STIM1 knockdown among the three siRNAs applied (see Figure 2A in the following Section 3.3), they were therefore chosen in relevant experiments. STIM1 OE stands for a mammalian expression vector encoding STIM1 for over-expression of STIM1, and pcDNA3.1(+) was the blank vector. Data are means ± SD (n = 3); *P < 0.05, **P < 0.01, compared with the control (untreated cells); ††P < 0.01, compared with the treatment of SiO2 (7 μg/cm2) alone; ##P < 0.01, compared with control siRNA-treatment; ‡‡P < 0.01, compared with treatments of both control siRNA and SiO2; ¦¦P < 0.01, compared with the treatment of pcDNA3.1(+) alone; §§P < 0.01, compared with the treatments of both pcDNA3.1(+) and SiO2. Table 1. Effect of SiO2 on the expression of TGF-β1 and TNF-α in RAW264.7 cells and the influence of modulators of several cell signal proteins
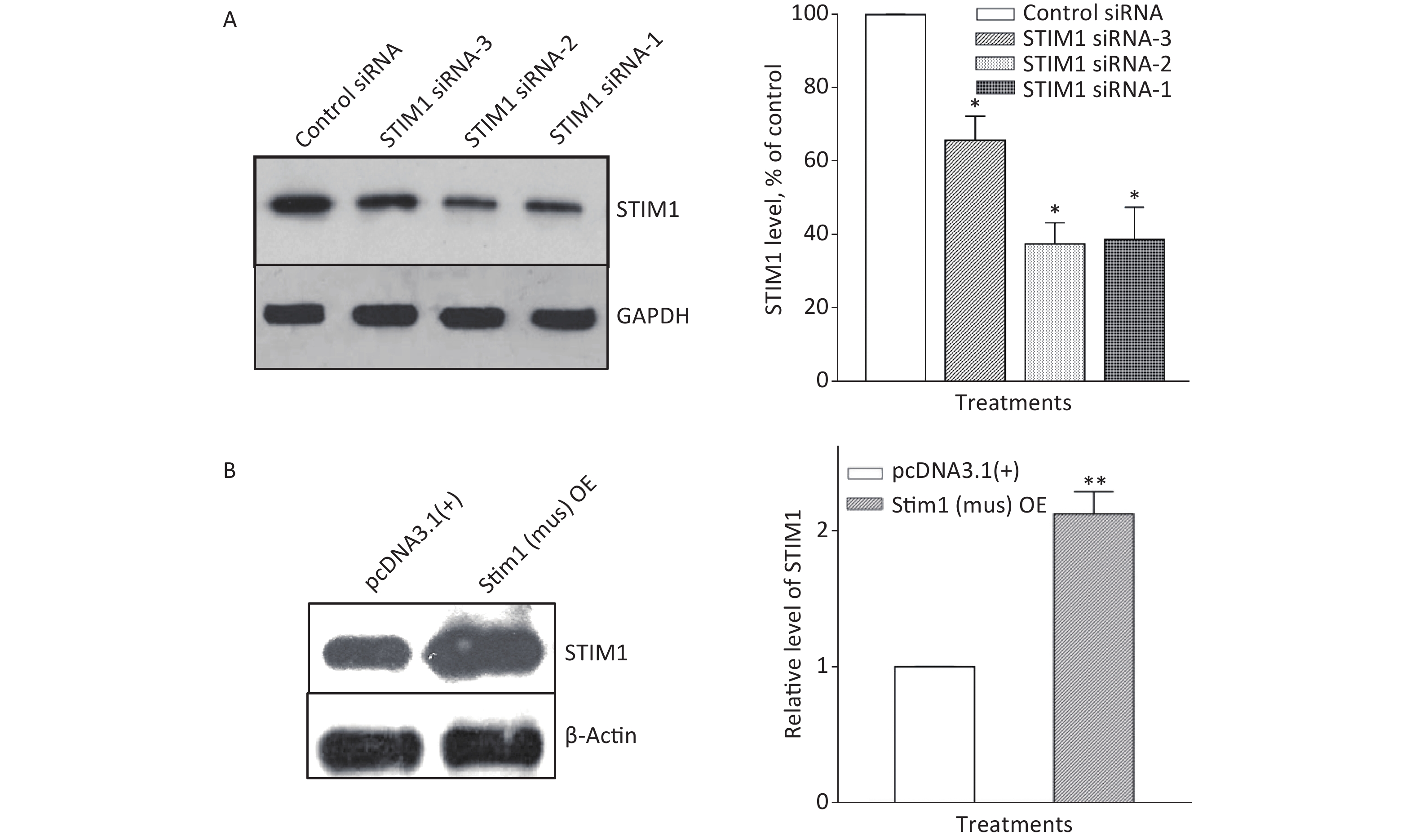
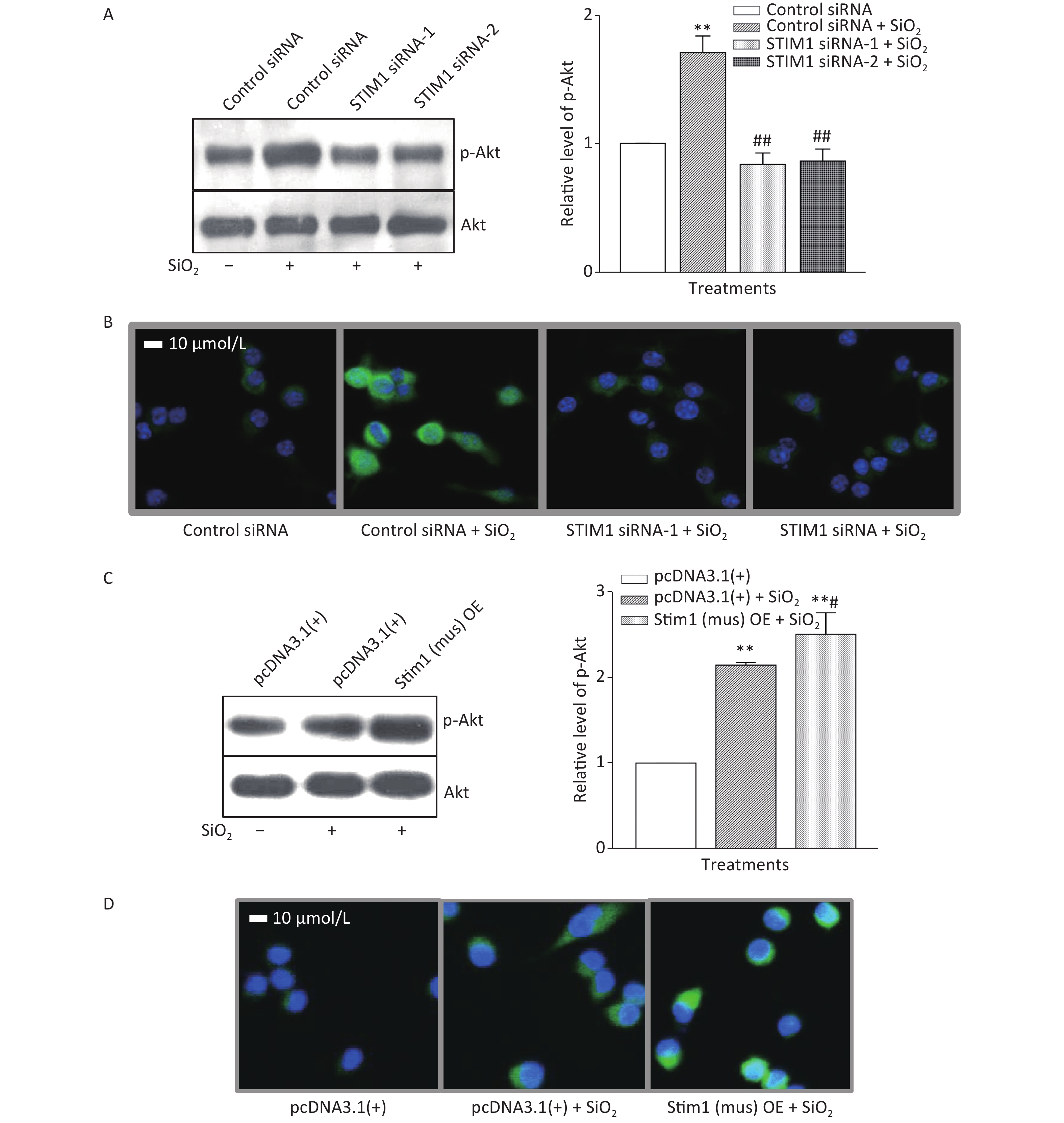
To further confirm the role of STIM1 in SiO2-stimulated activation of the PI3K/Akt pathway, three independent sets of specific siRNA sequences were used to selectively inhibit the intracellular expression of STIM1. Supplementary Figure S2A (available in www.besjournal.com) shows that the level of STIM1 protein in RAW264.7 cells was significantly reduced by all three gene silencing sequences (STIM1 siRNA-1, STIM1 siRNA-2, and STIM1 siRNA-3, the first two being more effective than siRNA-3). Figure 1A shows that STIM1 siRNA-1 and -2 completely blocked the activation of Akt by SiO2. Similarly, an immunofluorescent assay showed that siRNA-1 and -2 blocked the increases of p-Akt in the cytoplasm of RAW264.7 cells (Figure 1B). These results confirmed that STIM1 was upstream of Akt in the SiO2-induced phosphorylation of Akt. To clarify whether STIM1 was upstream or downstream of Akt in SiO2-induced PI3K/Akt pathway activation, RAW264.7 cells were transfected with STIM1 plasmids [Stim1 (mus) OE] or control plasmids [pcDNA3.1(+)], and the cells were then treated with SiO2 for 60 min. Supplementary Figure S2B shows that the level of STIM1 protein in Stim1 (mus) OE-transfected cells was 2-fold higher than when transfected with the control pcDNA3.1(+) sequence; moreover, STIM1 overexpression in RAW264.7 cells further enhanced the activation of Akt by SiO2 (Figure 1C). An immunofluorescent assay showed the effect of STIM1 overexpression, which augmented the p-Akt signal in the cytoplasm, when compared with treatment with SiO2 alone (Figure 1D). These results confirmed that STIM1 was upstream of Akt in the SiO2-stimulated PI3K/Akt pathway.
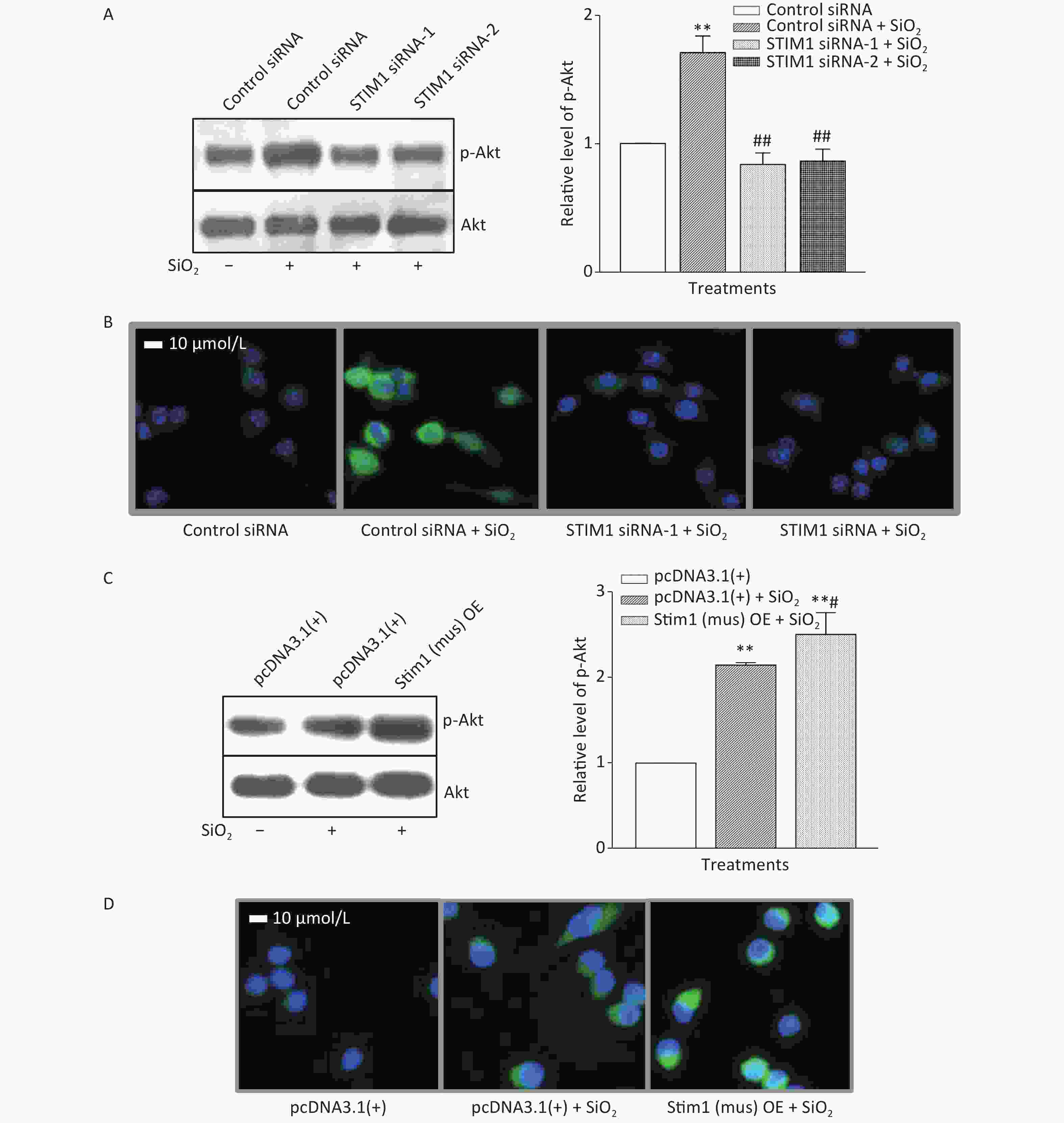
Figure 1. Knockdown and overexpression of stromal interaction molecule 1 (STIM1) in RAW264.7 cells, and the effects on SiO2-stimulated activation of Akt as determined by Western blotting (A & C) and immunofluorescent assays (B & D). For knockdown experiments, STIM1 siRNA-1 and -2 were used to transfect RAW264.7 cells, which were then stimulated with SiO2 (7 µg/cm2) for 60 min, with serum-free DMEM serving as the control. The p-Akt was determined using Western blotting (A) and an immunofluorescent assay (B). For the overexpression experiments, RAW264.7 cells were transfected with STIM1 plasmids [Stim1 (mus) OE] or control plasmids [pcDNA3.1(+)], then the cells were stimulated with SiO2 (7 µg/cm2) for 60 min, with serum-free DMEM serving as the control. The p-Akt was determined using Western blotting (C) and an immunofluorescent assay (D). Data of the right parts of panel A and C are the means ± SD (n = 3); **P < 0.01, compared with the control siRNA group (A) or control pcDNA3.1(+) group (C); ##P < 0.01, compared with treatment with both control siRNA and SiO2 (A); #P < 0.05, compared with treatment with both pcDNA3.1(+) and SiO2 (C).
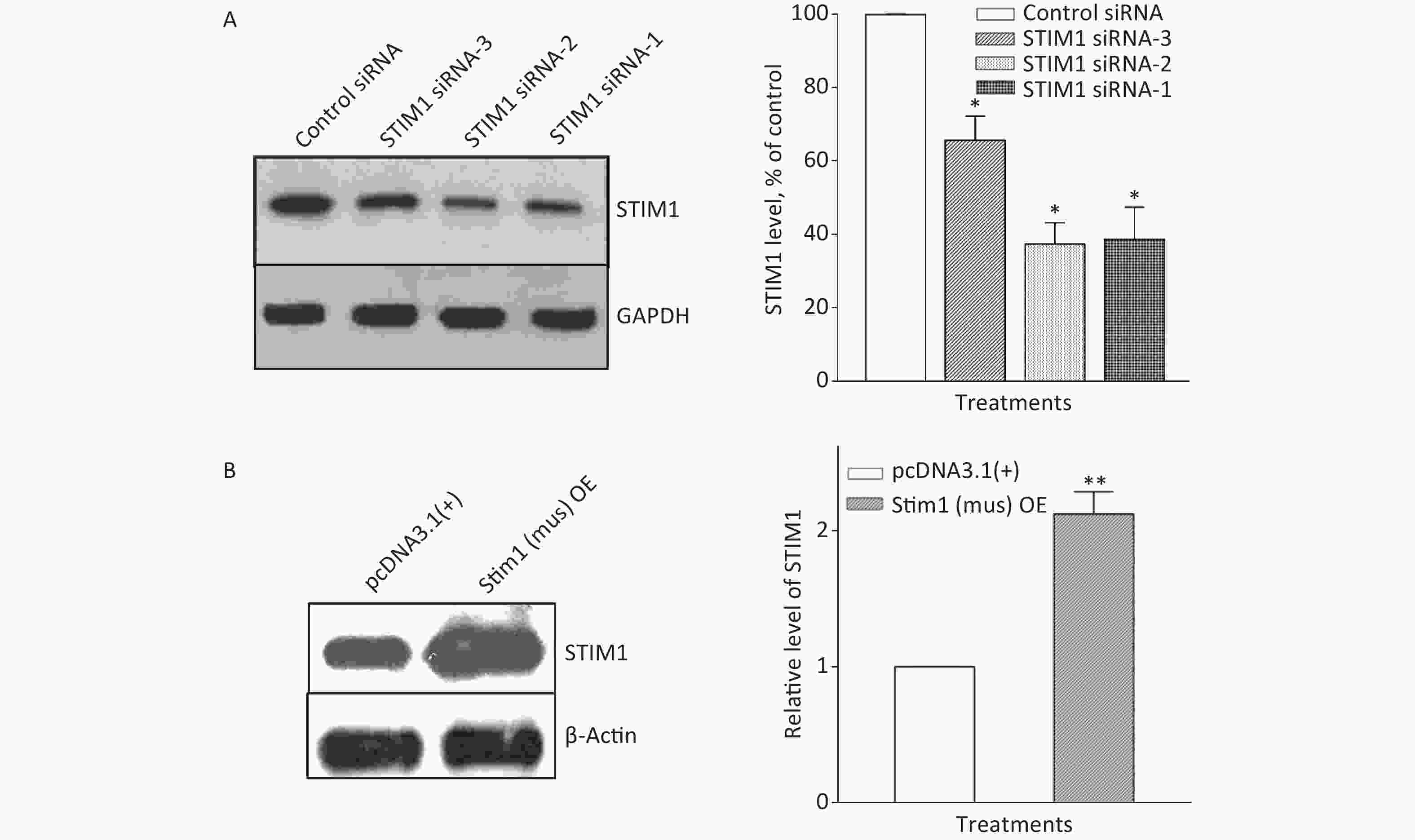
Figure S2. Knockdown and overexpression of STIM1 in RAW264.7 cells. RAW264.7 cells were interfered with STIM1 siRNA-1, -2, and -3 (a negative siRNA used as the control) (A), or transfected with STIM1 plasmids [Stim1 (mus) OE] or control plasmids [pcDNA3.1(+)] (B), and the level of STIM1 were determined by using Western blot assay (A & B). Data of the right parts of panel A & B are means ± SD (n = 3); *P < 0.05, **P < 0.01, compared with the control siRNA group or control plasmids group (A & B).
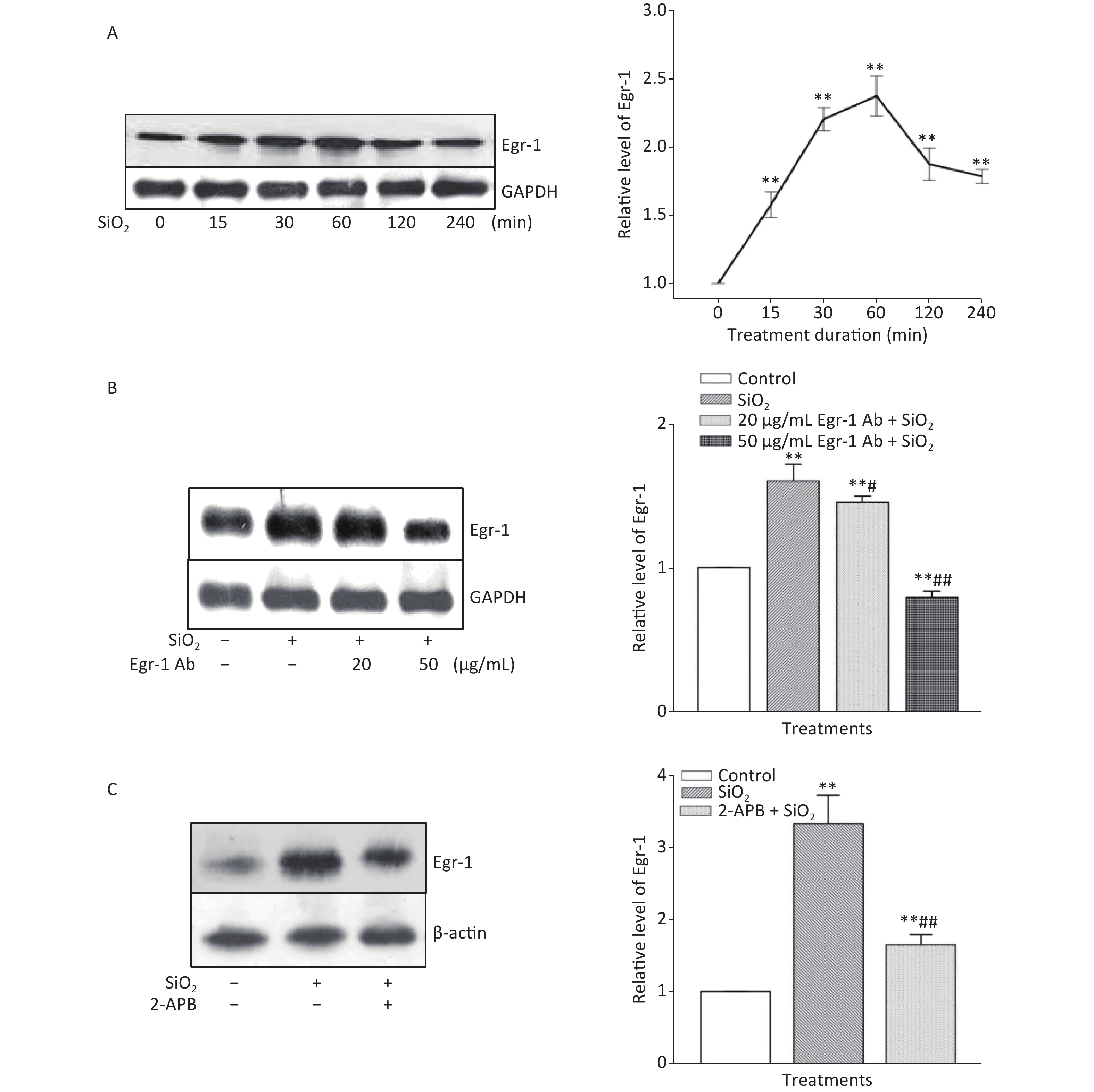
The time course of the effect of SiO2 on the expression of Egr-1 in RAW264.7 cells is shown in Supplementary Figure S3A, available in www.besjournal.com. The time-dependent elevation of Egr-1 expression peaked at 60 min. Pretreatment of RAW264.7 cells with an antibody specific for Egr-1 at a concentration of 50 μg/mL blocked the elevation of (unbound) Egr-1 in cells exposed to SiO2 for 60 min; while Egr-1 at a lower concentration (20 μg/mL) showed only mild inhibition of the effect of SiO2 (Supplementary Figure S3B). Moreover, inhibition of Akt by Deguelin (100 nmol/L) led to significant reduction of the levels of Egr-1 protein in cells exposed to SiO2 (Figure 2A). In contrast, suppression of Egr-1 using a specific antibody (50 μg/mL) showed no effect on SiO2-stimulated Akt activation (Figure 2B). These results showed that although both Akt and Egr-1 were involved in SiO2-induced release of inflammatory mediators, they were at different levels of regulation, with Akt being upstream of Egr-1.
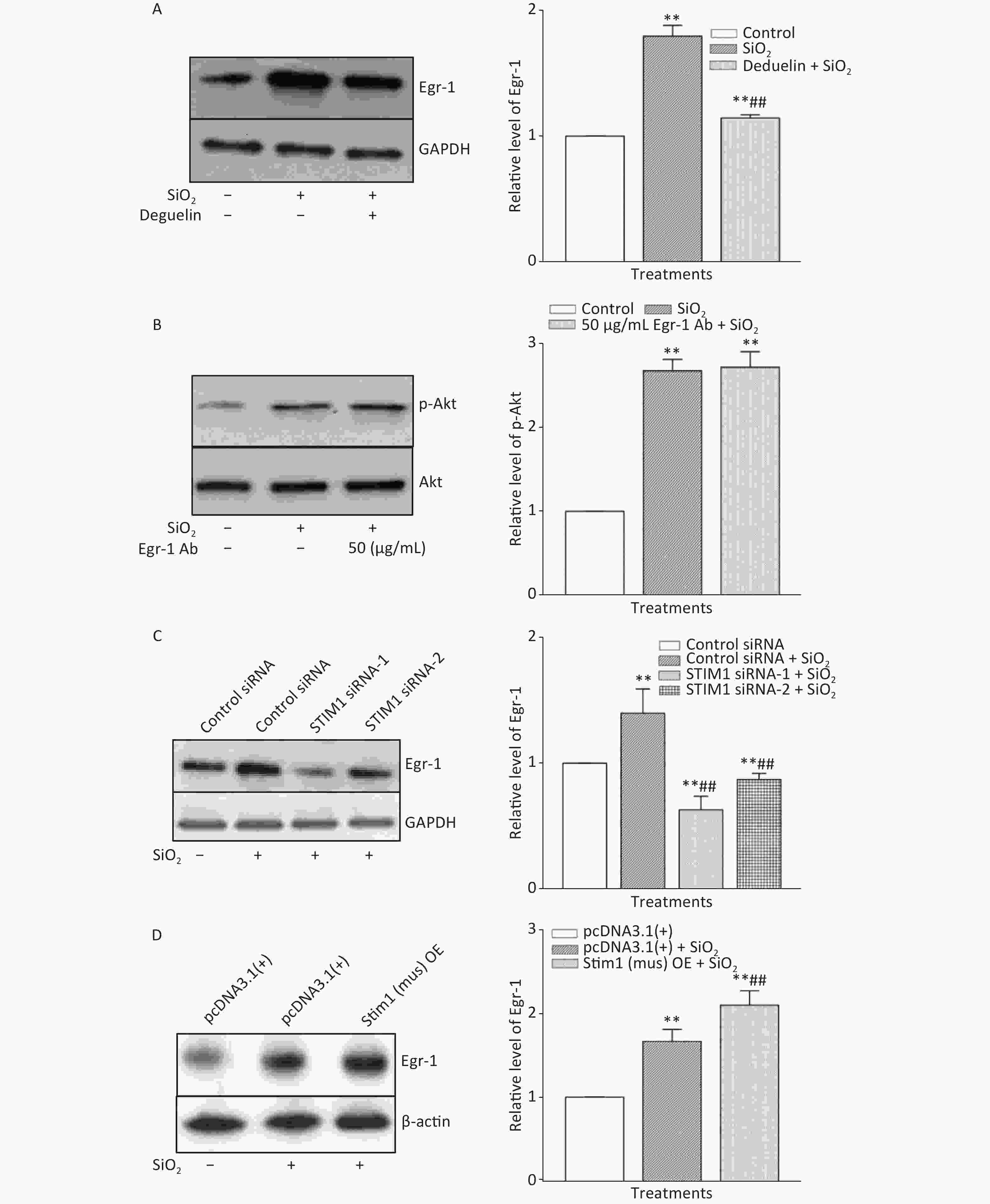
Figure 2. The influence of Deguelin (Akt inhibitor) (A), stromal interaction molecule 1 (STIM1) siRNAs (C), and STIM1 overexpression (D) on the expression of Egr-1 and that of Egr-1 antibody on phosphorylation of Akt (B) in SiO2-stimulated RAW264.7 cells. All proteins were determined using Western blotting; data on the right part of each panel are the means ± SD (n = 3); **P < 0.01, compared with the control group (A & B), control siRNA group (C), and control pcDNA3.1(+) group (D); ##P < 0.01, compared with the treatment with SiO2 alone (A, C, & D).
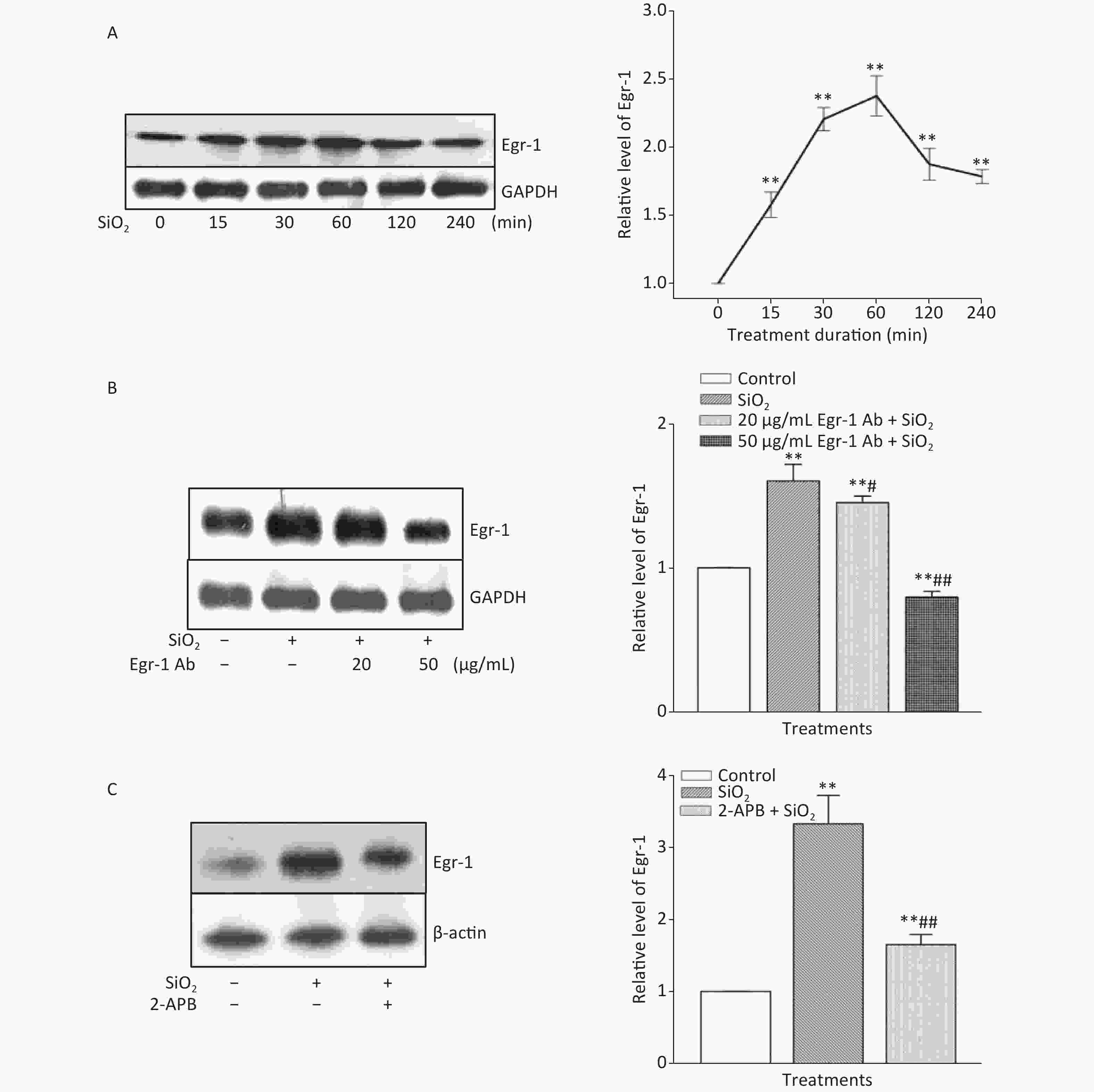
Figure S3. Effect of SiO2 on the expression of Egr-1 following various exposure durations and the impact of Egr-1 antibody and 2-APB. The time-course of Egr-1 elevation after stimulation of RAW264.7 cells by SiO2 (7 µg/cm2) was determined by Western blot assay after an exposure period of 30, 60, 120, 240, and 360 min (A). The influence of pretreatment of RAW264.7 cells with Egr-1 antibody at the concentration of 20 and 50 μg/mL for 60 min on the level of Egr-1 (B). The effect of Deguelin (100 nmol/L) on SiO2-stimulated expression of Egr-1 protein (C). Data on the right part of each panel are means ± SD (n = 3); *P < 0.05, **P < 0.01, compared with the control group (A, B, & C); #P < 0.05, ##P < 0.01, compared with the treatment with SiO2 alone.
To ascertain whether STIM1 was upstream of Egr-1 in the SiO2-induced release of inflammatory mediators, varying modulators of STIM1 were used in a model of SiO2-augmented Egr-1 expression in RAW264.7 cells. Supplementary Figure S3C shows that the increase of Egr-1 expression by SiO2 was significantly decreased by 2-APB (100 μmol/L) and completely blocked by STIM1 siRNA-1 and -2 (Figure 2C). In contrast, overexpression of STIM1 in RAW264.7 cells further enhanced SiO2-stimulated expression of Egr-1 (Figure 2D), showing that STIM1 was upstream of Egr-1 in their interactive regulation in macrophages.
In the present study, the effects of SiO2 on the levels of STIM1, Akt, and Egr-1 in RAW264.7 cells and the impact of various modulators of these genes/proteins suggest a sequence of signal transductions involving STIM1→Akt→Egr-1. Egr-1. A protein downstream of Akt in mediating SiO2-stimulated release of inflammatory mediators from macrophages as observed in this study, has been reported to regulate the expression of TGF-β1 [in human lung cancer (A549) cells][8] and TNF-α (in human keratinocytes)[9]. Studies based on other models, such as the chronic ethanol-induced liver injury[10], consistently suggest that Egr-1 is an immediate-early gene transcription factor involved in various stimulator-induced releases of inflammatory mediators. Taken together, our study indicates that STIM1 may participate in controlling SiO2-induced TGF-β1 and TNF-α expressions via the Akt/Egr-1 cascade pathway; however, it cannot be excluded that some other proteins are also involved in this cascade, either upstream, between these proteins, or downstream. For example, mitogen-activated protein kinase (MAPK) pathways have been reported to be involved in SiO2-induced Egr-1 activation[6], so the possibility for their interaction with Akt and STIM1 may also be worthy of further studies. It is likely that all these signaling pathways work together to form a complex network regulating the release of inflammatory mediators from macrophages. Studies in intact animals should therefore be conducted to confirm the findings of this study.
Acknowledgments We are thankful to MS Fanglan Liu, Director of the Guangdong Center for Disease Control, for proofing our manuscript.
Conflicts of Interest The authors declare that they have no conflicts of interest to report regarding the present study.
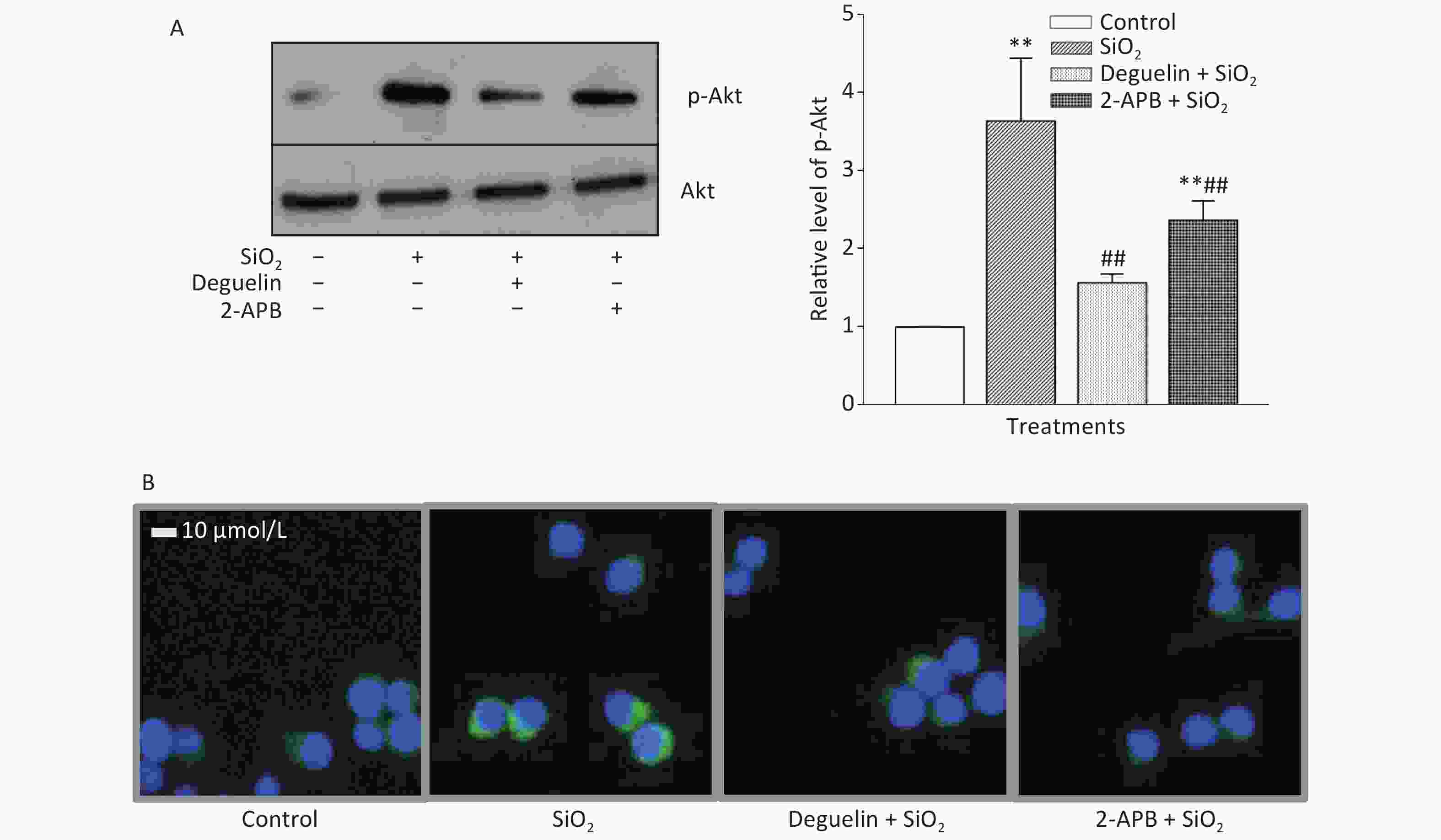
Figure S1. Effects of Deguelin and 2-APB on SiO2-activated phosphorylation of Akt in RAW264.7 cells, by Western blot (A) and immunofluorescent assay (B). RAW264.7 cells were pretreated with Deguelin (100 nmol/L), 2-APB (100 µmol/L) or DMSO (vehicle, 0.1%. v:v) for 60 min, followed by incubation with SiO2 at the density of 7 µg/cm2 for 60 min, serum-free DMEM serving as the control. Triplicate experiments were set up for each treatment. Cell lysate of each culture was prepared, and analyzed by SDS-PAGE, followed by Western blot assay using antibodies raised in rabbit against phosphorylated mouse Akt at Ser473 (p-Akt) and the total Akt. Data in the right part of panel A are means ± SD (n = 3); **P < 0.01, compared with the control; ##P < 0.01, compared with the treatment with SiO2 alone.
HTML
 21423Supplementary Materials.pdf
21423Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: