-
Skin is the first line of defense against a wide variety of physical and chemical damages. Photodamage of the skin can have an effect on beauty, while it is correlated with various skin diseases. Accordingly, effective prevention of skin photodamage has constantly been a research hotspot in the field of skin.
The precursor protein of amyloid β-protein (APP) refers to a type of transmembrane protein that has been widely found in numerous types of cells. The secretory N-terminal domains of APP (sAPP) synthesized by the hydrolysis of APP are characterized by considerable physiological functions. sAPP is capable of facilitating the proliferation, migration and adhesion of keratinocytes and HaCaT cells, while improving their resistance to ultraviolet (UV) damage, whereas the functional peptide region has not been identified[1]. As indicated by existing studies, sAPP can serve as a novel member of the family of cysteine-rich growth factors, which consists of hepatocyte growth factors secreted by skin fibroblasts that facilitate keratinocyte growth[2]. APP5 peptide is an active part of sAPP, capable of promoting the growth of fibroblasts and having similar antioxidant and anti-apoptotic effects as full-length APP. P165 is a structural optimization product of APP5. As reported by Wang et al.[3, 4], P165 may down-regulate the expression of MMP-1, reduce the degradation of type I collagen in UVB-irradiated fibroblasts via MAPK/AP-1 signaling pathway and play an anti-photoaging role. This study verified whether P165 pretreatment is capable of reducing the production of reactive oxygen species (ROS), up-regulating the expressions of antioxidant enzymes, reducing IL-1β, IL-6 and TNF-α synthesis, and affecting the synthesis of apoptotic proteases (e.g., caspase-9, 3 and 8) in UVB-irradiated HaCaT cells.
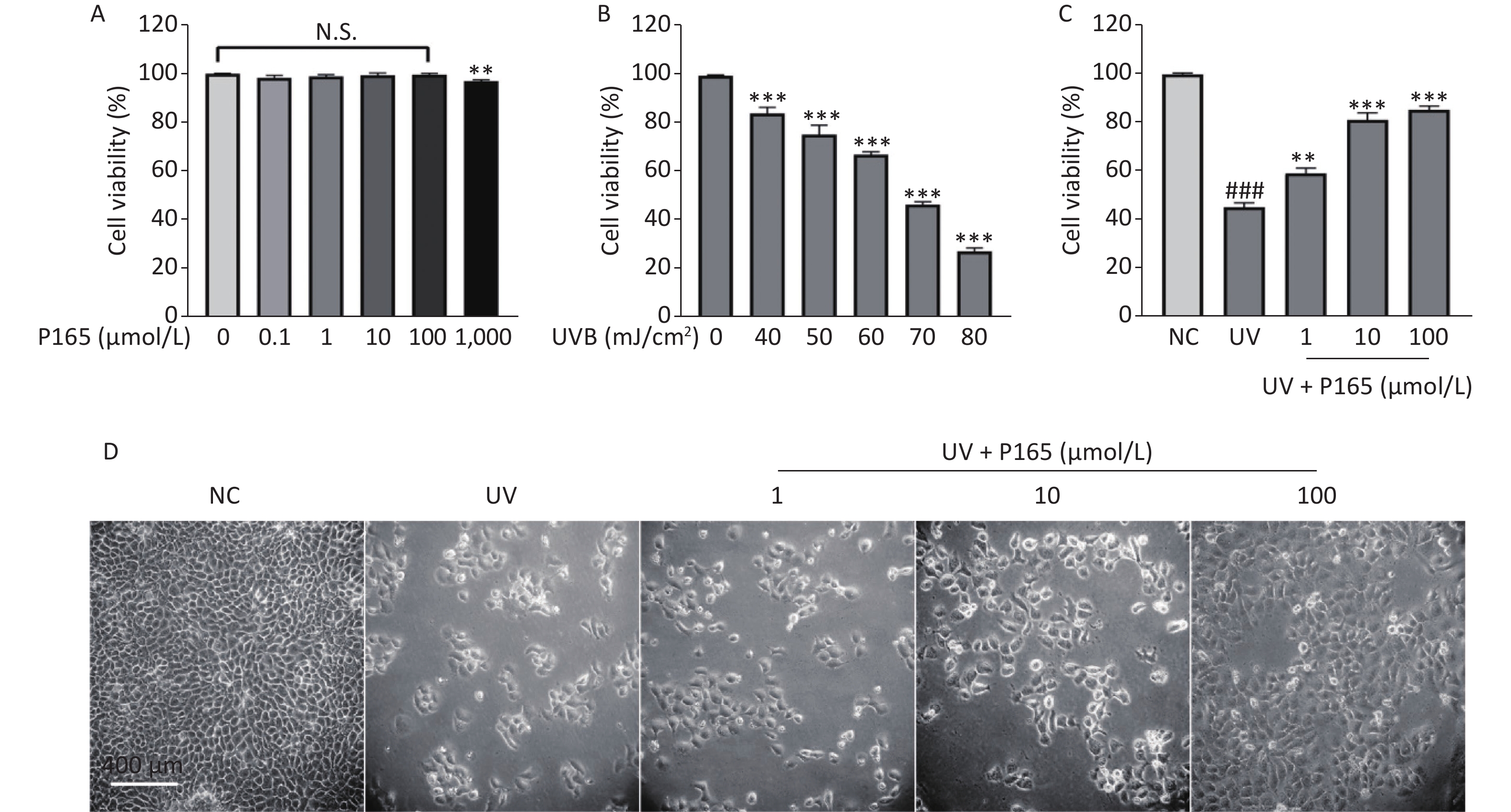
As revealed by the experimental results of this study, 0.1–100.0 µmol/L P165 did not have any cytotoxic effect on HaCaT cells (Supplementary Figure S1A available in www.besjournal.com). Compared with the NC group, the viability of UVB-irradiated HaCaT cells was significantly reduced; with the increase in the UVB irradiation dose, the viability of HaCaT cells tended to decrease; when UVB irradiation dose was 70 mJ/cm2 (under the irradiation time for 50 seconds), the cell viability would be close to 50% (Supplementary Figure S1B). Thus, P165 with the concentrations of 1 μmol/L, 10 μmol/L, 100 μmol/L and the irradiation dose of 70 mJ/cm2 were selected for subsequent experiments. With the addition of P165 at different concentrations, the viability of UVB-irradiated HaCaT cells was significantly improved (P < 0.01) (Supplementary Figure S1C). The number of HaCaT cells decreased after UVB irradiation, the volume increased and rounded, the intracellular particles increased, and some intercellular spaces increased, which indicated that there was obvious damage. In the P165 pretreatment group, the HaCaT cells progressively returned to the normal growth state (Supplementary Figure S1D). As revealed by the above data, P165 pretreatment significantly increased the survival rate of UVB-irradiated HaCaT cells.
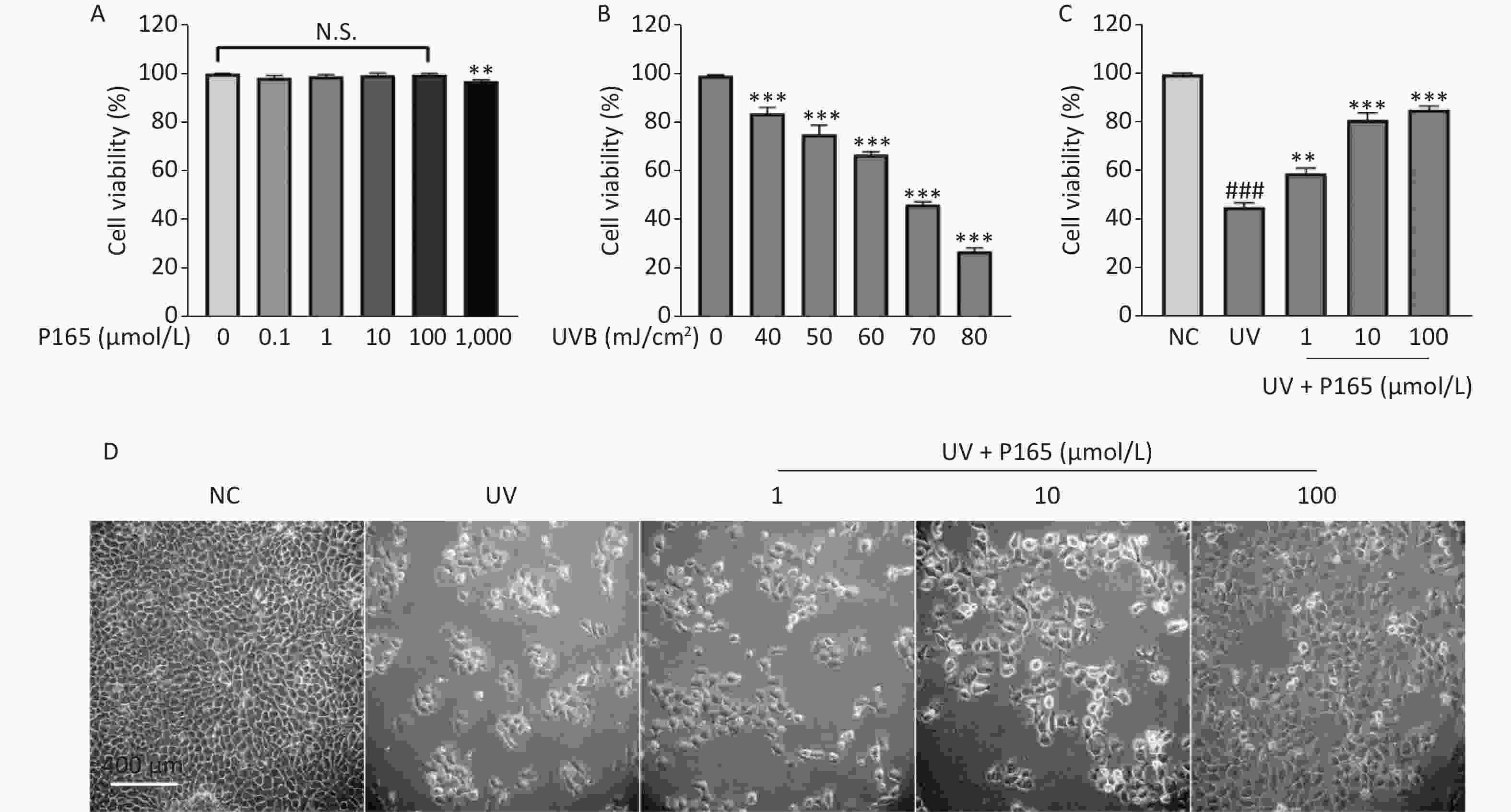
Figure S1. (A) The cells were treated using 0, 0.1, 1, 10, 100, 1,000 µmol/L P165 for 24 h; the cell viability was obtained by CCK8 kit. (B) The cells were irradiated with a wide range of irradiation dose exhibited by UVB, and the cell viability was obtained by CCK8 kit. (C) P165 increased the viability of UVB-irradiated HaCaT cells, and the cell viability was obtained by CCK8 kit. (D) HaCaT cells in P165 treatment group were changed in term of the number, volume and intercellular space. The results are expressed as the mean ± SD. ###P < 0.001 vs. NC group; **P < 0.01, ***P < 0.001 vs. no P165 treatment group (A), no UV irradiation group (B) and UV group (C).
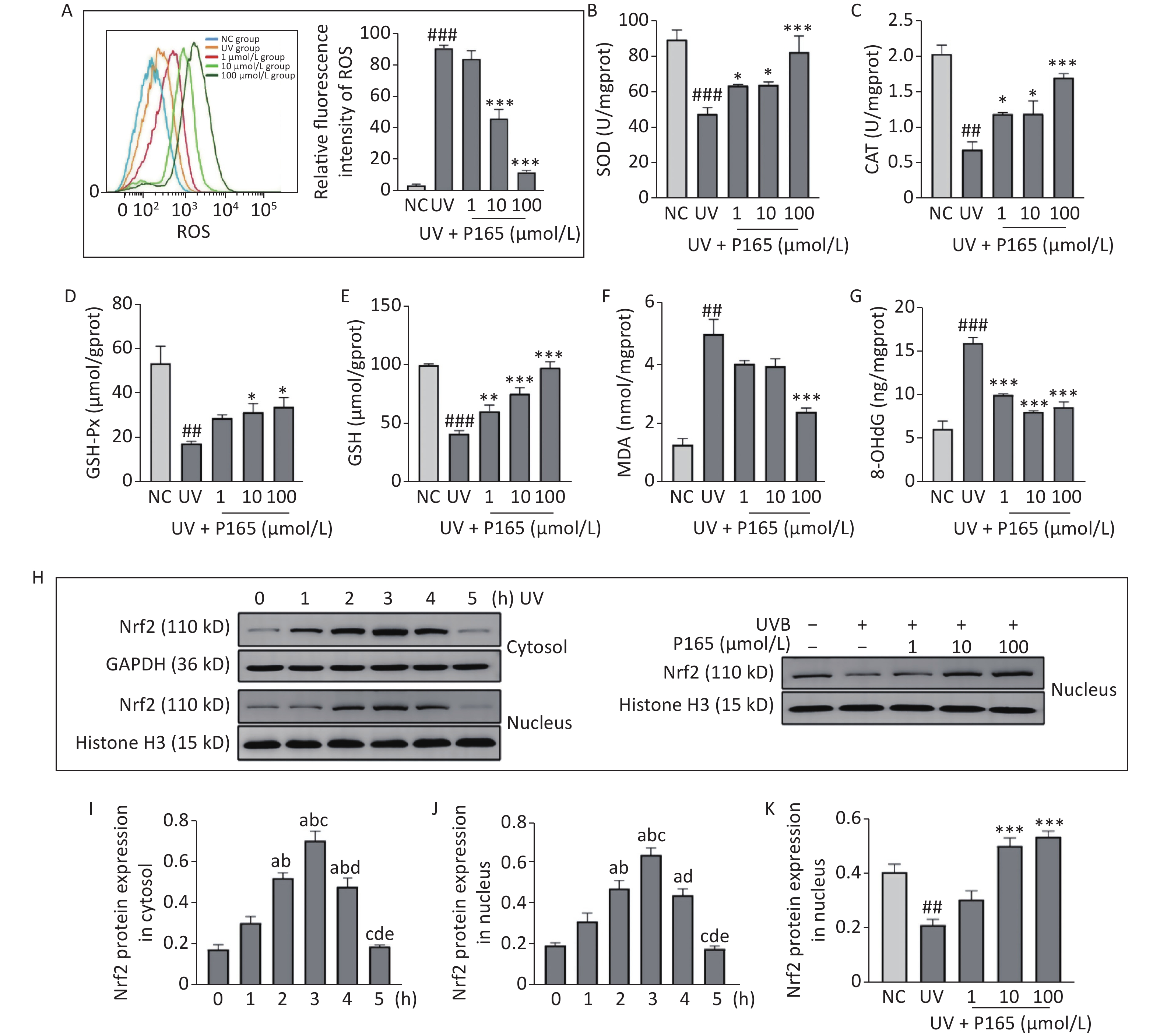
Oxidative stress is considered to be a major cause of UV-induced damage to HaCaT cells. ROS is the core factor of oxidative stress reaction, acts as a commonly indicator indicating oxidative stress, which is generated by oxygen metabolism. ROS, an intracellular signaling molecule, is significantly correlated with cell proliferation and apoptosis, and while playing a role in activating numerous inflammation-related signaling pathways (e.g., the MAPK and NF-κB). Intracellular oxidative stress is unbalanced, and excessive ROS is capable of damaging biological macromolecules (e.g., lipids, proteins and DNA), leading to oxidative damage of cells. ROS can generate lipid peroxidation reaction with enzymes, phospholipids, polyunsaturated fatty acids and nucleic acids related to membrane receptors in the biofilm, and synthesize lipid peroxidation products [e.g., malondialdehyde (MDA)], thereby probably affecting the permeability and fluidity of cell membranes and then changing the structure and function of cells [5]. 8-hydroxy-2 deoxyguanosine (8-OHdG) is a specific product of DNA oxidative damage, and it has been recognized as a biomarker of endogenous and exogenous factors for DNA oxidative damage. In this study, UVB irradiation significantly facilitated the synthesis of ROS in HaCaT cells, while P165 pretreatment significantly hindered the synthesis of ROS on UVB-irradiated HaCaT cells (Figure 1A). 100 μmol/L P165 pretreatment could significantly up-regulate the expressions of antioxidant enzymes, including superoxide dismutase (SOD) (Figure 1B), catalase (CAT) (Figure 1C) and glutathione peroxidase (GSH-Px) (Figure 1D), while promoting the expressions of HaCaT cells antioxidant glutathione (GSH) in a dose-dependent manner (Figure 1E). In addition, MDA content was most significantly lowered in the 100 μmol/L group (Figure 1F), and 8-OHdG in all groups of P165 pretreatment declined significantly (Figure 1G). We observed that with the increase in P165 concentration, ROS, MDA and 8-OHdG tended to decrease, and the expressions of antioxidant (e.g., SOD, CAT, GSH-Px and GSH) all increased. It is well known that the Nrf2/ARE signaling pathway is involved in UV-induced oxidative damage of HaCaT cells[6]. Herein, Nrf2 expression in cytoplasm and nucleus decreased progressively over time and reached the maximum at 3h after irradiation, while pretreatment with 10 μmol/L and 100 μmol/L of P165 could up-regulate Nrf2 expression in nucleus (Figure 1H–K). As indicated by the above data, P165 may improve the antioxidant capacity of UVB-irradiated HaCaT cells by activating Nrf2/ARE signaling pathway.
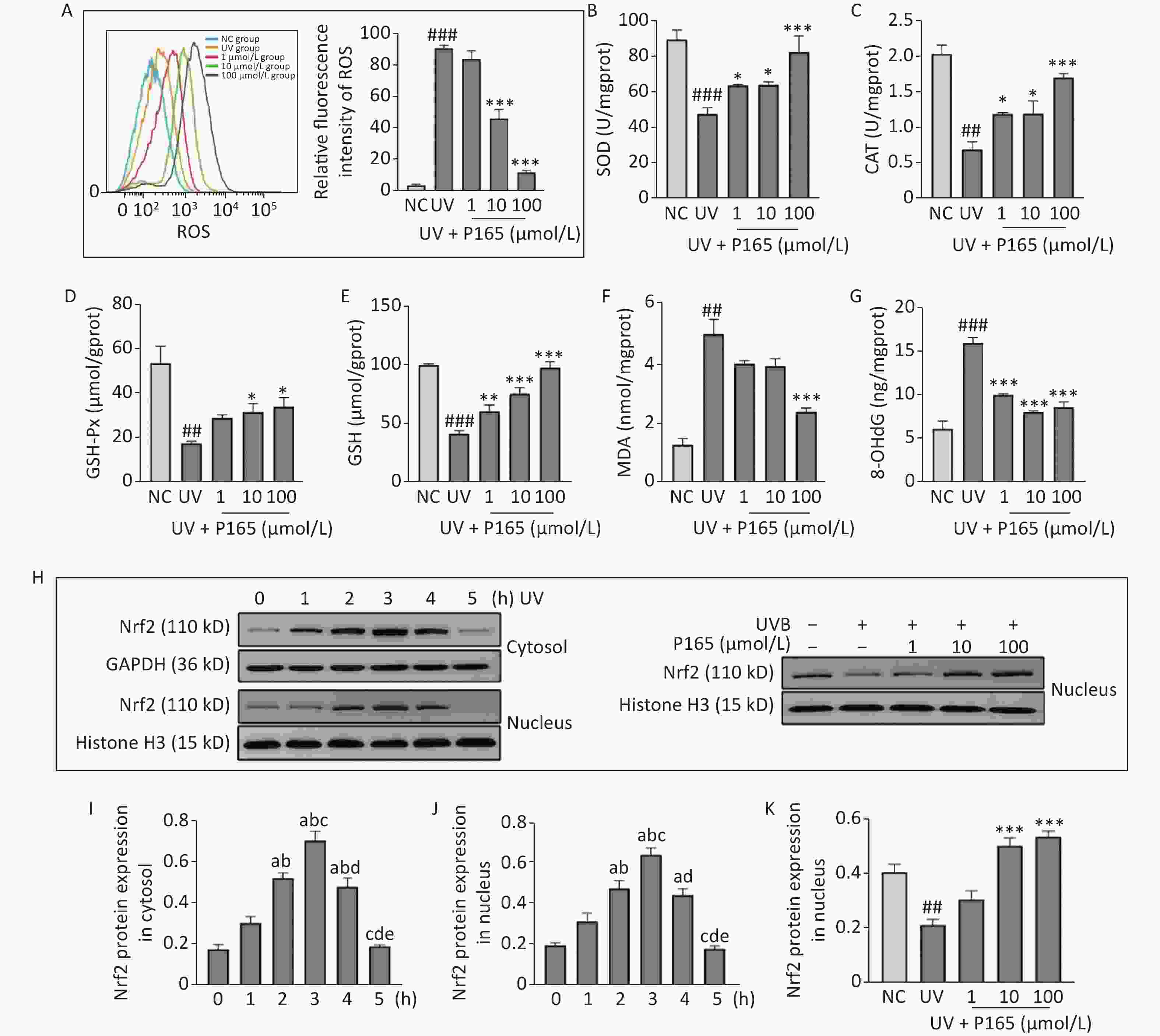
Figure 1. (A) The intracellular ROS within HaCaT cell was evaluated based on flow cytometry. (B–G) The detection was achieved for SOD, CAT, GSH-Px, GSH, MDA, and 8-OHdG based on biochemical methods. The results are expressed as the mean ± SD. ##P < 0.01, ###P < 0.001 vs. NC group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. UV group. (H) Cytoplasm and nucleus Nrf2 protein changed with time in HaCaT cells, and P165 increased nucleus Nrf2 expression in UVB-irradiated HaCaT cells. (I–K) Relative changes within protein intensity received the quantification based on densitometric analysis. The results are expressed as the mean ± SD. aP < 0.05 vs. the 0 h group; bP < 0.05 vs. the 1 h group; cP < 0.05 vs. the 2 h group; dP < 0.05 vs. the 3 h group; eP < 0.05 vs. the 4 h group. ROS, oxygen species; SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase; GSH, glutathione; MDA, malonaldehyde; 8-OHdG, 8-hydroxy-2 deoxyguanosine.
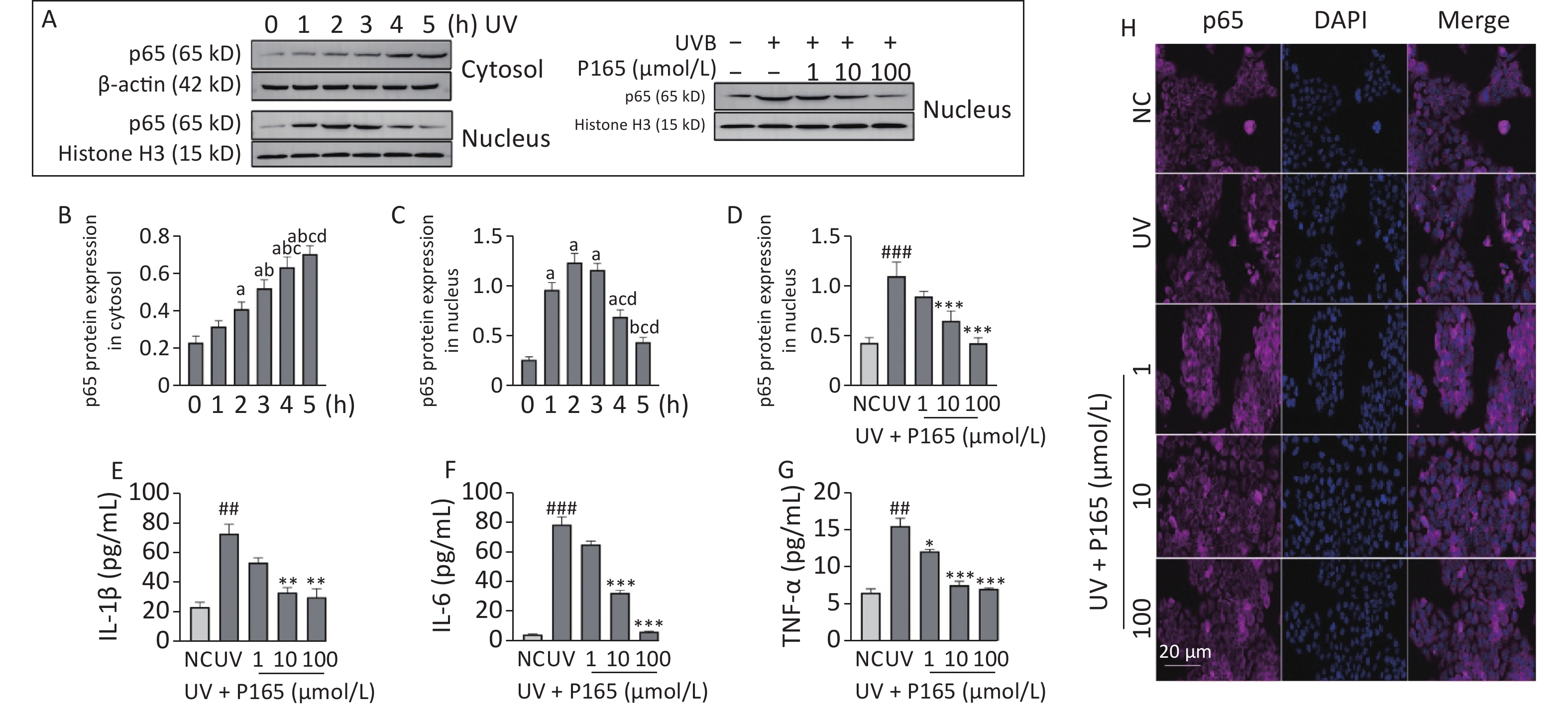
Inflammation refers to another damage effects of UV-induced HaCaT cells. Keratinocytes can be affected by various changes inside and outside the cell (e.g., UV irradiation and endotoxin), which can activate inflammatory signaling pathways and lead to the expressions of apoptosis-related signaling molecules. UV irradiation is capable of causing keratinocytes to release various physiological activators (e.g., IL-1β, IL-6, IL-8, IFNγ, TNF), and above inflammatory factors or chemokines may cause the death of keratinocytes as well[7] . NF-κB is a transcription factor appearing early in the inflammatory injury process, which is capable of entering the nucleus to regulate the gene transcription of various inflammatory cytokines (e.g., TNF-α, IL-8, IL-6 and IL-1β)[8]. In this study, both oxidative stress and inflammation may be involved in the damage of UVB-irradiated HaCaT cells, and NF-κB signaling pathway is well known to be associated with inflammation. NF-κB signaling pathway can be activated by a range of stimulus factors, and ROS acts as a vital factor. On the one hand, the unbalanced redox reaction of HaCaT cells attributed to UV produces excessive ROS, and the resulting ROS activates NF-κB signaling pathway, thereby releasing downstream inflammatory factors. Thus, the cascade of inflammatory factors is initiated, and extensive inflammatory damage will be induced. On the other hand, NF-κB can produce ROS and NO. Besides, the above 2 factors can promote each other. In this study, nuclear p65 expression reached the maximum 2 h after irradiation and then tended to decrease, and UVB irradiation led to the significantly up-regulated expression of nucleus p65. After the pretreatment with P165, nuclear p65 expression was significantly down-regulated with the 10 μmol/L and 100 μmol/L group decreasing the most significantly (Figure 2A–D), and the cytokines IL-1β, IL-6, and TNF-α secreted by UVB-irradiated HaCaT cells declined as well (Figure 2E–G). Moreover, immunofluorescence showed that p65 expression in the nucleus of HaCaT cells decreased with the increase of P165 concentration (Figure 2H). Thus, it was confirmed that P165 can reduce the inflammatory response of UV-irradiated HaCaT cells. Here, P165 may reduce the synthesis of inflammatory factors through down-regulating the NF-κB signaling pathway and attenuated the inflammatory response of UVB-irradiated HaCaT cells.
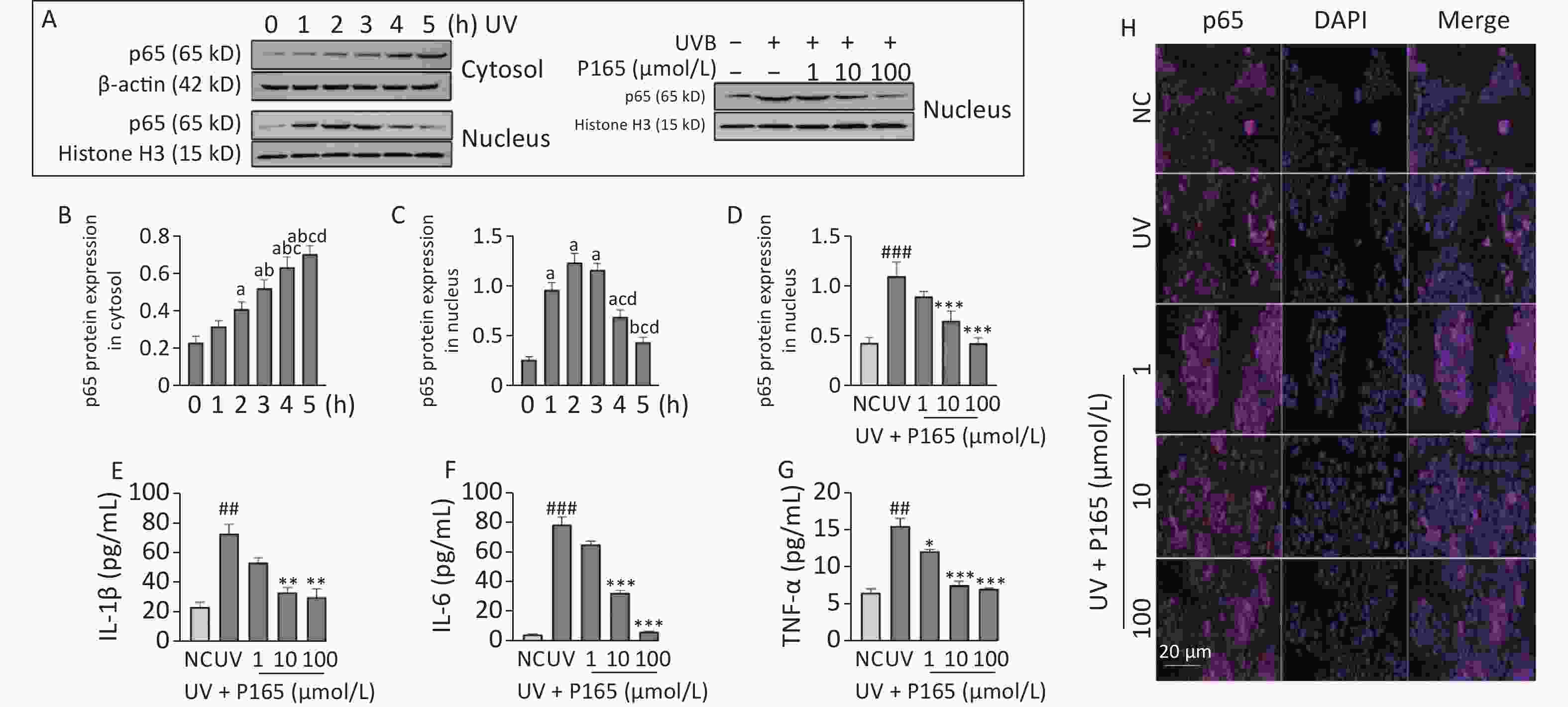
Figure 2. (A) Cytoplasm and nucleus p65 protein changed with time in HaCaT cells, and P165 decreased nucleus p65 expression in UVB-irradiated HaCaT cells. p65 protein expressing states were examined based on western blot assay. (B–D) Relative changes within protein intensity received the quantification based on densitometric analysis. (E–G) P165 decreased the secretion of IL-1β, IL-6, and TNF-α in UVB-irradiated HaCaT cells. (H) Immunofluorescence showed that p165 reduced the nuclear shift of p65 in UVB-irradiated HaCaT cells (The nucleus is blue fluorescence, and the p65 protein is purple fluorescence, both of which are artifacts). The results are expressed as the mean ± SD. ##P < 0.01, ###P < 0.001 vs. NC group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. UV group.
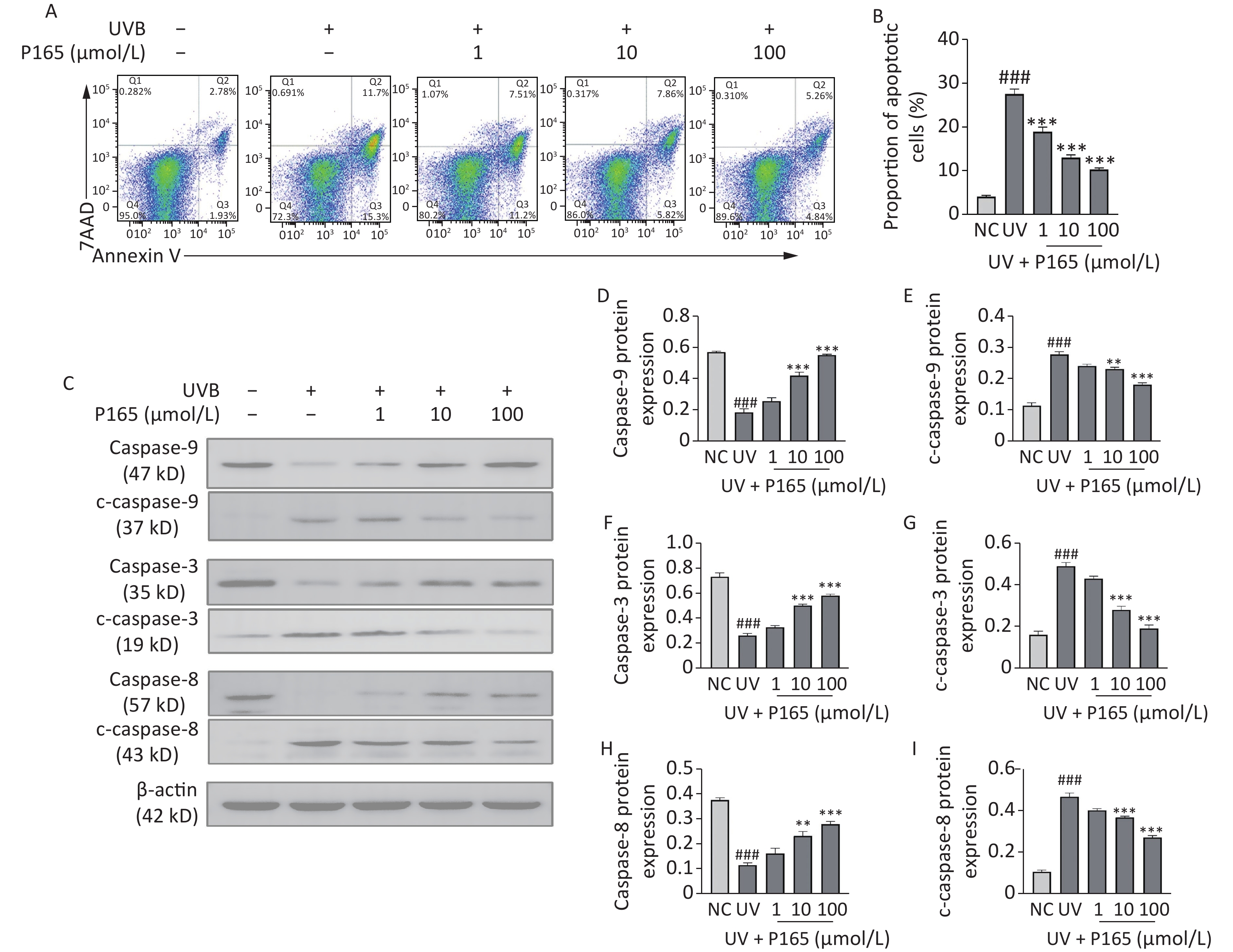
The caspase family pertains to a large category of regulatory genes mediating cell apoptosis. Caspase facilitates apoptosis via mitochondria-dependent and non-mitochondria-dependent pathways. To be specific, caspase-9 is located at the most upstream of caspase cascade, which is capable of self-activating and activating downstream executive caspases with other cytokines involved (e.g., caspase-3). On that basis, it can specifically cleave substrates to cause biochemical and morphological changes in cells and eventually cause cell apoptosis[9]. Caspase-9 and caspase-8 are considered the initiators of the endogenous and exogenous pathways of UV-induced HaCaT cells apoptosis, respectively. During cell apoptosis, caspase is activated in a cascade, and the caspase is cleaved to produce active caspase, thereby participating in cell apoptosis. Cleaved caspase-9, 3, 8 are the active forms of caspase-9, 3, 8 respectively. When HaCaT cells are exposed to UV, their own protective mechanism will be activated to induce appropriate cell apoptosis. When UV reaches a certain dose, HaCaT cells will be damaged. This study verified whether P165 pretreatment could have anti-apoptotic effects on UVB-irradiated HaCaT cells. As revealed by the results, UVB irradiation could promote the apoptosis of HaCaT cells, while P165 pretreatment could dose-dependently reduce HaCaT cells apoptosis (Figure 3A–B). P165 pretreatment could up-regulate caspase-9, 3 and 8, and down-regulate cleaved caspase-9, 3 and 8 in a concentration-dependent manner (Figure 3C–I). Accordingly, P165 may be involved in the apoptosis process of HaCaT cells exposed to UVB irradiation, and may exert an anti-apoptotic effect. Further experimental verification should be performed to clarify the exact process of this mechanism.
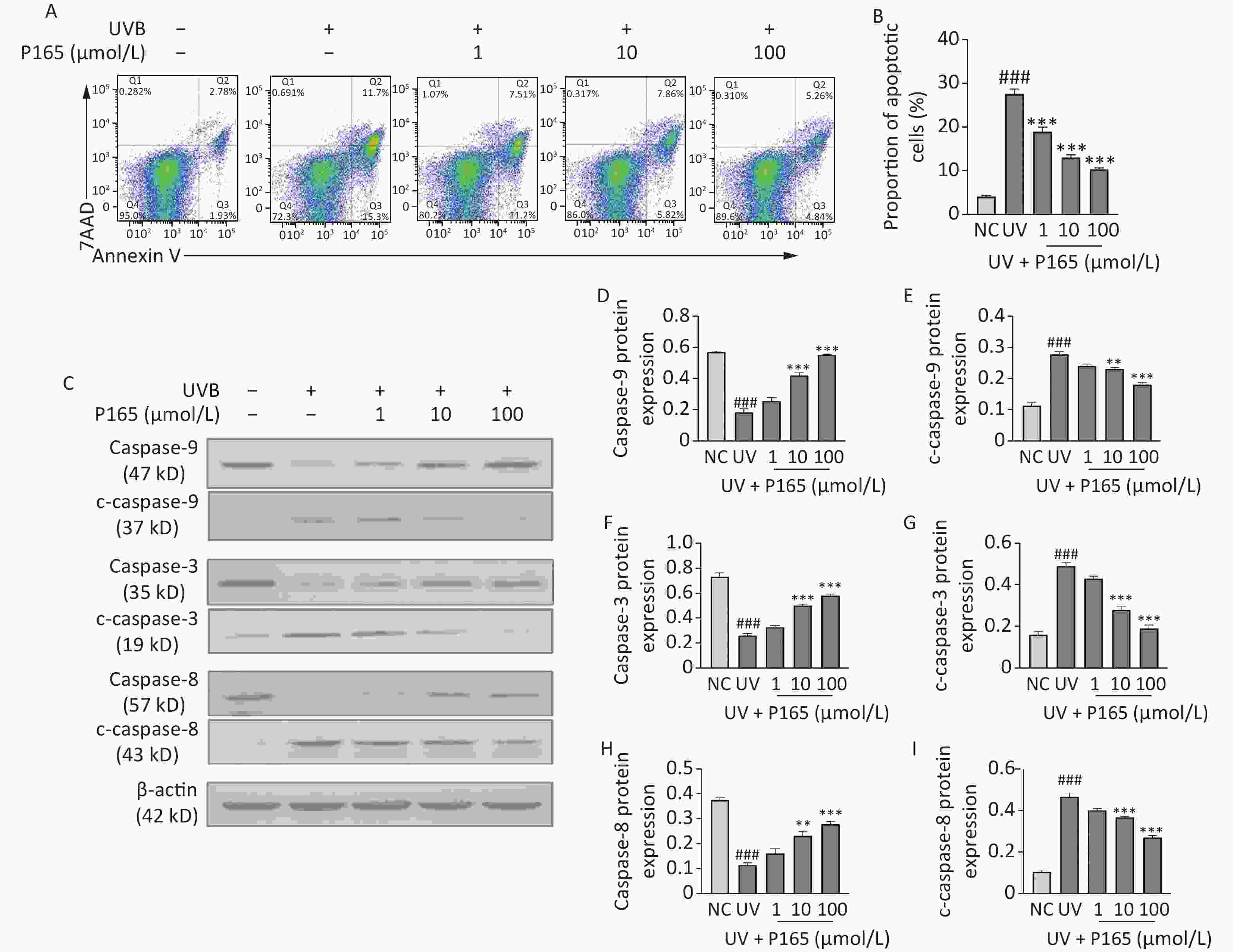
Figure 3. (A) The apoptosis of UVB-irradiated HaCaT cells was evaluated by flow cytometry. (B) Percentage of apoptotic HaCaT cells was illustrated to be bar diagram. (C) The protein expressions of caspase-9, c-caspase-9, caspase-3, c-caspase-3, caspase-8 and c-caspase-8 were measured by western blot assay. (D–I) The relative change of protein intensity was quantified on the basis of densitometric analysis. The results are expressed as the mean ± SD. ##P < 0.01, ###P < 0.001 vs. NC group; *P < 0.05, **P < 0.01 ,***P < 0.001 vs. UV group.
This study is the first to demonstrate the beneficial effects of P165 on UVB-irradiated HaCaT cells. P165 may be involved in antioxidant stress of UVB-irradiated HaCaT cells via Nrf2/ARE signaling pathway, anti-inflammatory response of UVB-irradiated HaCaT cells via NF-κB signaling pathway, and anti-apoptotic effects of UVB-irradiated HaCaT cells. More specific in vitro experiments should be performed to explore the clear mechanism of P165 with antioxidant, anti-inflammatory and anti-apoptotic effects and verify whether P165 can achieve the expected effects in vivo. Accordingly, this study indicated P165 as a probable agent to prevent skin photodamage.
No potential conflicts of interest were disclosed.
HTML
 21386Supplementary Materials.pdf
21386Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: