-
Human glioblastoma (GBM) is a grade-IV diffuse glioma and represents the most frequent malignant primary brain tumor with an extremely poor prognosis[1]. Although the current therapies, including a maximum surgical resection followed by temozolomide (TMZ) in combination with ionizing radiation (IR), show an effective response, tumor recurrence invariably occurs for most patients with a median overall survival of 15 months and a 5-year survival rate of less than 5%[2]. Given the complex development of therapeutic resistance in GBM and controversial therapeutic efficacy[3], a comprehensive understanding of the resistance mechanism implicated in the cellular response to TMZ and/or IR is necessary to exploit new therapies to improve the outcome of the current GBM therapy.
Primary cilia are non-motile, antenna-like microtubule-based cellular sensing organelles that protrude from the apical surface of virtually every type of mammalian cell[4]. Cilia maintain normal physiological homeostasis but are now postulated to be involved in the pathogenesis of various cancers[5]. Several studies have shown the loss of primary cilia in some cancer types, including breast, skin, and thyroid cancers and GBM cells[6-9]; however, other cancer types, such as cervical carcinoma HeLa cells, osteosarcoma MG63 cells, and gastrointestinal stromal tumor cells, harbor primary cilia[10,11]. Nevertheless, whether human GBM cells possess primary cilia and knowledge of the effect of primary cilia on the cellular resistance to TMZ/IR remain elusive.
Notably, suppressing cilium formation in GBM cells by disrupting critical ciliogenesis genes, such as IFT88, slows tumor progression[12]. Additionally, primary cilia show significant increases in incidence and length in drug-resistant tumor cell lines, and the inhibition of ciliogenesis can reverse their drug resistance[13]. The signals received and transduced by cilia include mechanical cues and a suite of soluble ligands via sonic hedgehog, WNT, transforming growth factor-β, and NOTCH, which are closely correlated to the proliferation, malignant development, and therapeutic resistance of tumor cells[14]. Notwithstanding the potential functions for cilia-related genes and signaling pathways, it warrants in-depth analyses of the regulation mechanisms by which primary cilia affect GBM resistance to TMZ/IR.
TMZ/IR-induced DNA strand breaks lead to the activation of DNA damage response (DDR), including the separation of centrioles, autophagy, and cell cycle arrest. The emerging interplay between cilia and autophagy represents additional evidence that strongly links cilia and cancer[15]. NudC-like protein 2, an autophagy receptor, mediates the selective autophagic degradation of CP110 at mother centrioles to promote ciliogenesis[16]. Specifically, primary cilia in glioma cells can significantly improve survival rates in chemotherapeutic treatment by regulating the autophagy induced by mitochondrial stress[17]. The current evidence for the promotion of autophagy as a fundamental response to cancer therapy suggests the complexity of its functional contributions to cell survival and disease outcomes[18].
IR-induced DNA double-strand break (DSB) induces the activation of ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3-related protein (ATR), and DNA-dependent protein kinase (DNA-PK), three members of the phosphatidylino-sitol-3-kinase-related kinase (PIKK) family with specialized and overlapping functions. A recent study suggested that the activated DNA-PK localized to the base of the primary cilium contributed to ciliogenesis and inhibition of ciliogenesis through the depletion of IFT88, which attenuated the genotoxic stress-induced DDR[19]. Controversially, DNA-PK can bind to histone deacetylase 6 (HDAC6), a negative regulator of ciliogenesis, by deacetylating acetylated α-tubulin (Ac-α-tub) [5] and facilitate its acetylase activity to promote primary cilia disassembly[20]. Meanwhile, the inhibition of HDAC6 activity enhances the effects of therapeutic resistance in GBM cells[21]. Our recent work has proven that GBM cell lines with distinct DNA-PK genotypes, such as M059K and M059J cells, showed significant differences in the cellular radiosensitivity associated with primary cilia[22]. However, the relationship between primary cilia, cellular resistance to TMZ/IR, and DNA-PK has not been fully understood. This study aimed to uncover the role of primary cilia involved in TMZ/IR resistance in GBM cells, which can be considered a promising target for innovative “smart” therapies in GBM.
-
The human GBM cell lines M059K and M059J were purchased from the American Type Culture Collection. The tumor tissue-derived primary cell line GS1910 cells were derived from a GBM patient (67-year-old male, WHO IV), and the GBM surgical specimens were obtained from the Neurosurgery Department of Gansu Provincial Hospital following local ethical board approval (ID# 2020-031). The tissues were dissociated into single cells by placing them in phosphate-buffered saline (PBS) with 0.25% trypsin and 2,000 U/mL collagenase for 30 min at 37 °C and plated into 60 mm dishes. Single colonies were formed about a week later and subsequently expanded for experiments. All cells were cultured in Dulbecco’s Modified Eagle Medium/F-12 medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (BI, Beit Haemek, Israel) and 1% penicillin and streptomycin in a humidified atmosphere at 37 °C under 5% CO2. All cell lines were negative for mycoplasma, as tested by a Mycoplasma Detection Kit (TaKaRa, Kusatsu, Shiga, Japan). The human GBM specimens were provided by the Department of Pathology of 940th Hospital of Joint Logistics Support Force of CPLA and the Neurosurgery Department of Gansu Provincial Hospital in accordance with the local ethical board approval (ID# 2020-031).
-
The TMZ molecules were purchased from MedChemExpress (HY-17364, MCE, NJ, USA), diluted in dimethylsulfoxide (DMSO, Sigma-Aldrich, Darmstadt, Hesse, Germany) for later use, and stored at −80 °C. The cells were treated with different doses from 1 µmol/L to 1,000 µmol/L for 1–3 days in a single experiment.
For IR exposure, X-rays were produced by an X-RAD225 cabinet X-ray irradiator (PXI, North Branford, CT, USA) at a dose rate of 2 Gy/min (225 kV, 13.3 mA), and carbon ion beams were generated by the Heavy Ion Research Facility at Lanzhou (HIRFL, Institute of Modern Physics, Chinese Academy of Sciences, Lanzhou, China) at a dose rate of 2 Gy/min (80 MeV/u, 35 keV/µm).
-
Cells were fixed with 4% paraformaldehyde for 10 min following the pre-chilling with methanol for 10 min. The fixed cells were permeabilized in 0.5% Triton X-100 for 10 min and blocked with 5% goat serum for 2 h at room temperature. For primary cilia examination, the primary antibody rabbit anti-Arl13b antibody (17711-1-AP, Proteintech, Rosemont, IL, USA) was used to detect primary cilia, and mouse-anti-γ-tubulin (γ-tub, 66320-1-Ig, Proteintech) antibody was employed to detect the basal body. The tumor tissue samples fixed with 4% formalin immediately after the surgery were embedded in paraffin and cut to sections of 4 µm widths, followed by immunostaining with Arl13b antibody to detect the primary cilia. For DSB detection, the primary antibody mouse-anti-γH2AX (phospho Ser139, ab26350, Abcam, Cambridge, UK) antibody or rabbit anti-phosphorylated DNA-PK (pDNA-PK) (phospho S2056, ab18192, Abcam) antibody were used. The primary antibodies were incubated for 2 h at room temperature. The bound antibodies were visualized using goat anti-rabbit IgG secondary antibody (A32731, Invitrogen, Carlsbad, CA, USA) and goat anti-mouse IgG secondary antibody (A11005, Invitrogen). The nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI, molecular probes, Eugene, OR, USA). The images were captured under an RVL-100-G (ECHO, San Diego, CA, USA) fluorescence microscope. The number of ciliated cells, total cells, and foci were counted in ten randomly selected fields from at least three independent experiments. The length of the primary cilium was measured using ImageJ software (version 1.52, National Institutes of Health, Bethesda, MA, USA).
-
The cells were lysed with radioimmunoprecipitation assay buffer (Beyotime, Shanghai, China). The total cellular proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a methanol-activated polyvinylidene difluoride membrane (Merck Millipore, Darmstadt, Hesse, Germany). The membrane was blocked with 5% skim milk containing 0.1% Tween-20 in PBS for 2 h and probed with the primary antibodies, including mouse-anti-α-tub (acetyl K40, Ac-α-tub, ab24610, Abcam), rabbit anti-HDAC6 (12834-1-AP, Proteintech), rabbit anti-IFT88 (13967-1-AP, Proteintech), rabbit-anti-LC-3B (14600-1-AP, Proteintech), mouse-anti-BCL2 (YM3041, Immunoway, Plano, TX, USA), mouse-anti-DNA-PK (ab18321, Abcam), rabbit anti-phosphorylated DNA-PK (pDNA-PK, phospho S2056, ab18192, Abcam), rabbit anti-ATM (ab32420, Abcam), rabbit anti-phosphorylated ATM (pATM, phospho S1981, ab36810, Abcam), mouse-anti-glyceraldehyde-3-phosphate dehydrogenase (MA5-15738, Invitrogen), and mouse-anti-β-actin (MA5-15739, Invitrogen) for 2 h at room temperature. After incubation with goat-anti-rabbit (SA00001-2, Proteintech) or goat-anti-mouse (SA00001-1, Proteintech) horseradish peroxidase-conjugated secondary antibody, the protein bands were detected with luminal reagents (Merck Millipore), and the images were captured using an Alliance LD4 gel imaging system (UVItec, Cambridge, UK). The density of bands was measured using ImageJ software.
-
To remove the primary cilia, we treated the cells by depleting the IFT88 expression or using chloral hydrate (CH, 47335-U, 2 mmol/L, Sigma-Aldrich). Briefly, a stealth siRNA targeting IFT88 (si-IFT88, HSS111980, Thermo Fisher Scientific, Waltham, MA, USA) and a negative control siRNA (NC, 12935200, Thermo Fisher Scientific) were introduced to the cells at a final concentration of 50 nmol/L using Lipofectamine 2000 (Thermo Fisher Scientific) following the manufacturer’s instructions. The sequence of si-IFT88 is 5′-GAAGAAAGCUGUAUUGCCAAUAGUU-3′.
-
siRNAs against DNA-PK (si-DNA-PK, Bioneer, Shanghai, China) and NC (Thermo Fisher Scientific) were introduced into cells to knock down the target gene expression at a final concentration of 50 nmol/L using Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific), in accordance with the manufacturer’s instruction. The medium was replaced with fresh culture medium 5 h after transfection. The depletion efficiency of siRNA was evaluated by Western blotting analysis. The sequence of si-DNA-PK is 5′-GAUUUCGGUUUGCUUGUAU-3′.
-
Cells (1 × 105) were plated into 12 well plates and incubated for 24 h before they were treated with lithium chloride (LiCl, 5 mmol/L) or left untreated. The cell number was counted on days 0–6 using a Z2 Coulter Counter system (Beckman, Brea, CA, USA).
-
Cells transfected with si-IFT88 or NC were exposed to 2 Gy X-rays or carbon ion beams, and an identical number of cells were plated onto 60 mm dishes to produce approximately 50–150 colonies. After culturing for 12 days, the cells were fixed with 75% ethanol and stained with 0.5% crystal violet. Colonies containing more than 50 cells were counted as survivors, and the plating efficiency (PE) of the cell line was calculated as the colony number/plated cell number ×100. The value of survival fraction at 2 Gy (SF2), which equals the colonies counted at 2 Gy/[cells seeded × (PE/100)], was used to evaluate the cellular radiosensitivity. At least three independent experiments were performed.
-
Cells (4 × 103) were seeded in 96-well plates and incubated for 24 h. Cells were exposed to TMZ with 0–1,000 µmol/L or autophagy inhibitor for 48 h, and the medium was refreshed. Then, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, M1020, Solarbio, Beijing, China) solutions were added at a final concentration of 0.5 mg/mL, and the cells were incubated for 4 h. Afterward, 150 µL DMSO was added, and the cells were vibrated by a table concentrator for 10 min. The absorbance values at 570 nm (OD570) were detected by an Infinite M200 pro microplate reader (TECAN, Mannedorf, Switzerland), and the cell viability was calculated and normalized by the control group.
-
Cellular autophagy was evaluated by measuring autophagolysosome levels in cells stained with AO. The cells were plated on a slide in a 35 mm dish for 24 h and then transfected with si-IFT88 (50 nmol/L) or treated with CH following the exposure to 10 Gy X-ray or TMZ (500 µmol/L). Subsequently, the cells were labeled with AO solution buffer (0.1 mg/mL, Solarbio) in PBS for 15 min at 48 h post-exposure. The green and red images at the same position were collected under a fluorescence microscope. The merged pictures and semi-quantitative analysis of the red fluorescence were performed with ImageJ software.
-
3-Methyladenine (3-MA, HY-19312, MCE) and brefeldin A (BFA, HY-16592, MCE) were introduced to inhibit cellular autophagy. Cells cultured for 24 h were exposed to TMZ (500 µmol/L), followed by the administration of 3-MA (10 mmol/L) or BFA (0.5 µg/mL) for 48 h, and the MTT assay was performed at a wavelength of 570 nm.
-
All the experiments were repeated at least three times, and the data are shown as mean ± standard deviation (SD). The P value was determined using Student’s t-tests, and *P
< 0.05 was considered a statistically significant difference. -
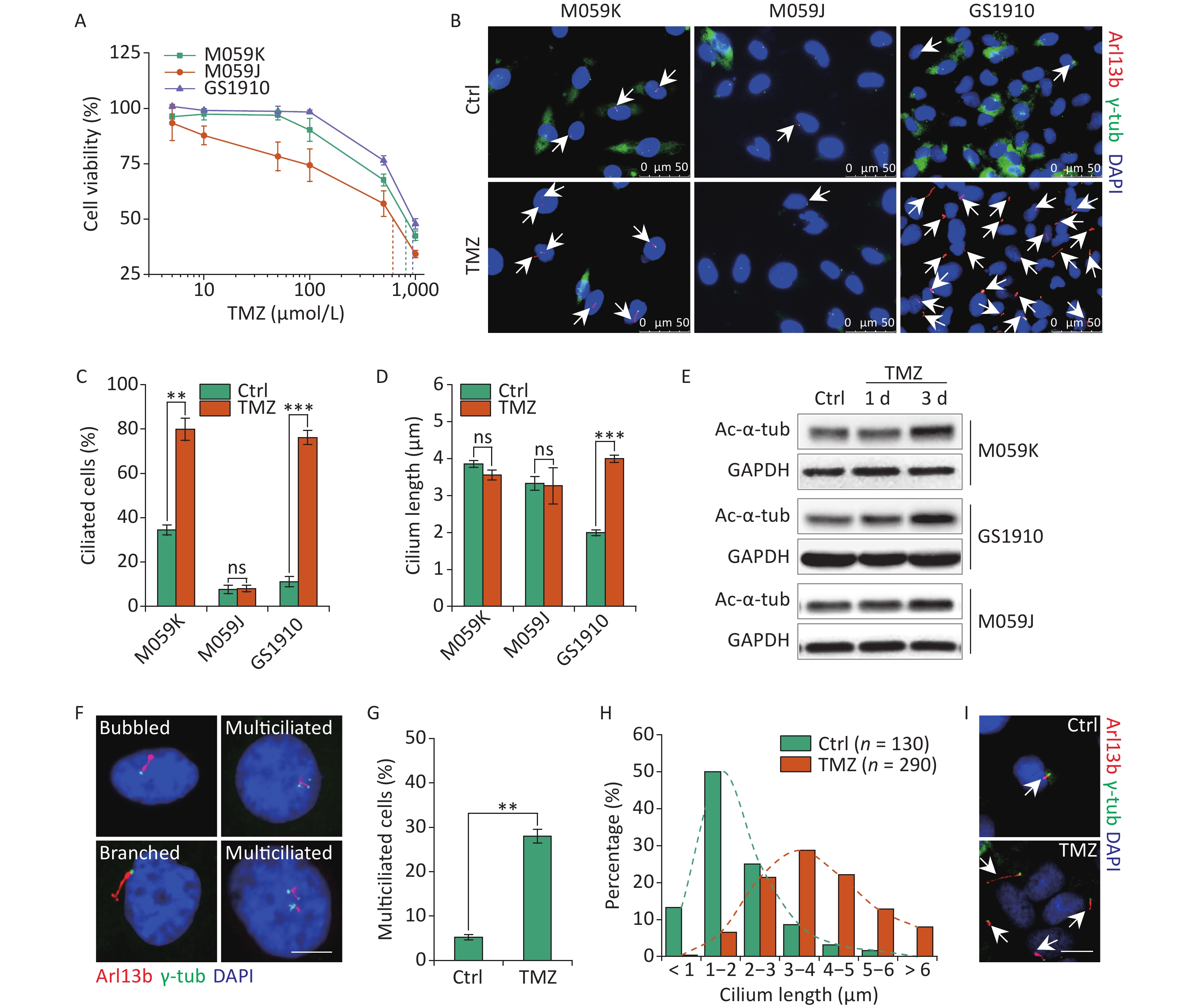
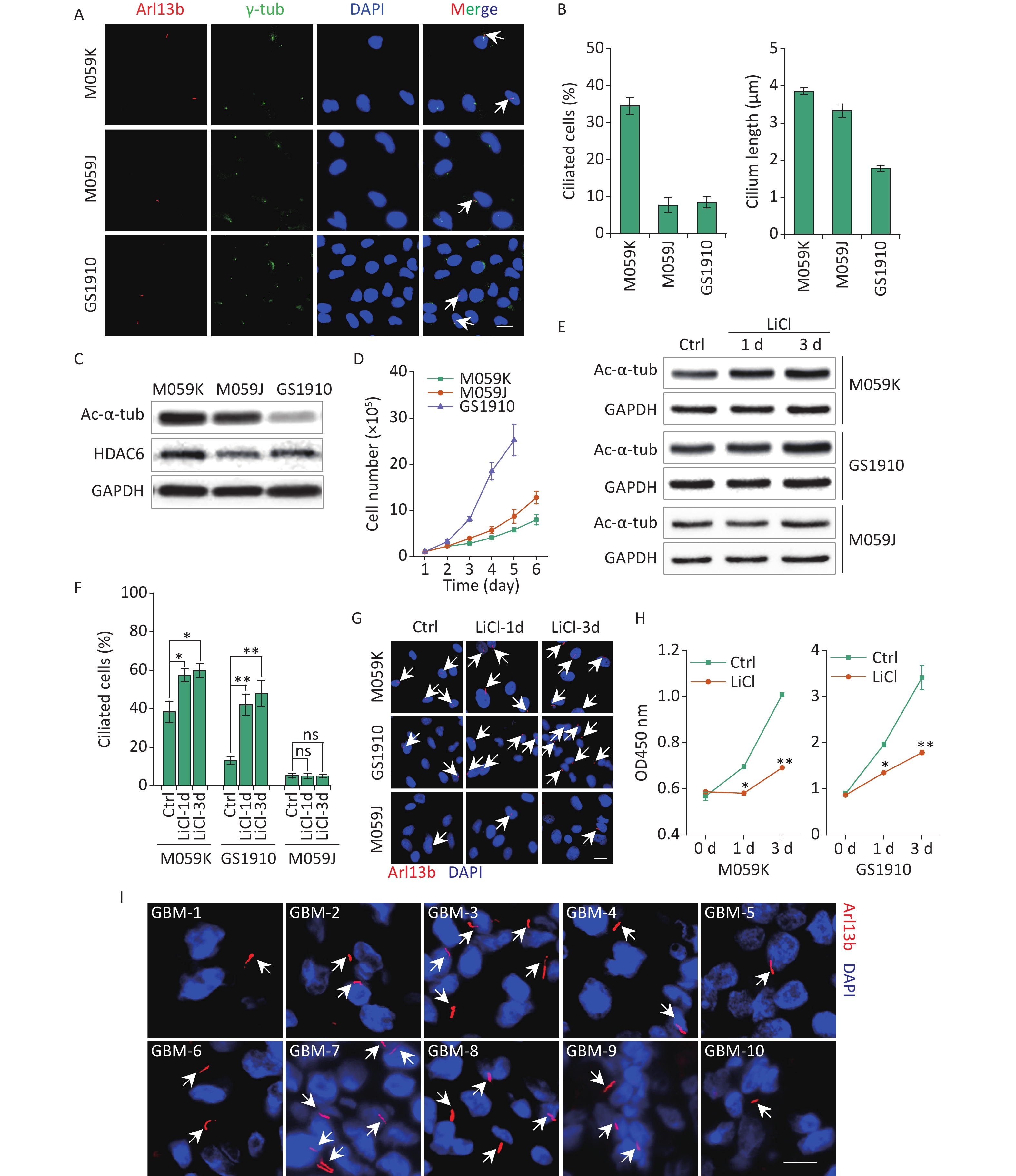
To investigate the prevalence of primary cilia in GBM cells, we examined the expression of two ciliary markers, including a marker specific for ciliary membrane Arl13b and a basal body marker γ-tub, in human GBM cell lines M059K, M059J, and GBM patient-derived primary cell line GS1910 cells. Immunostaining assay showed that cells stained for colocalization of Arl13b with γ-tub harbored an intact ciliary structure (Figure 1A). The M059K cells displayed the highest cilia frequency (% of ciliated cells in a population, 34.45% ± 2.26%) compared with M059J and GS1910 cells, whose values were both less than 10% (Figure 1B). The ciliary length was also measured. The average length of primary cilia in M059K and M059J cells reached 3.0–4.0 µm, and it was less than 2.0 µm in GS1910 cells. Moreover, the expression levels of Ac-α-tub protein, the major component of the cilia axoneme, exhibited similar patterns, but HDAC6 exhibited the opposite expression (Figure 1C). Thus, these findings indicate the preservation of primary cilia in GBM cells.
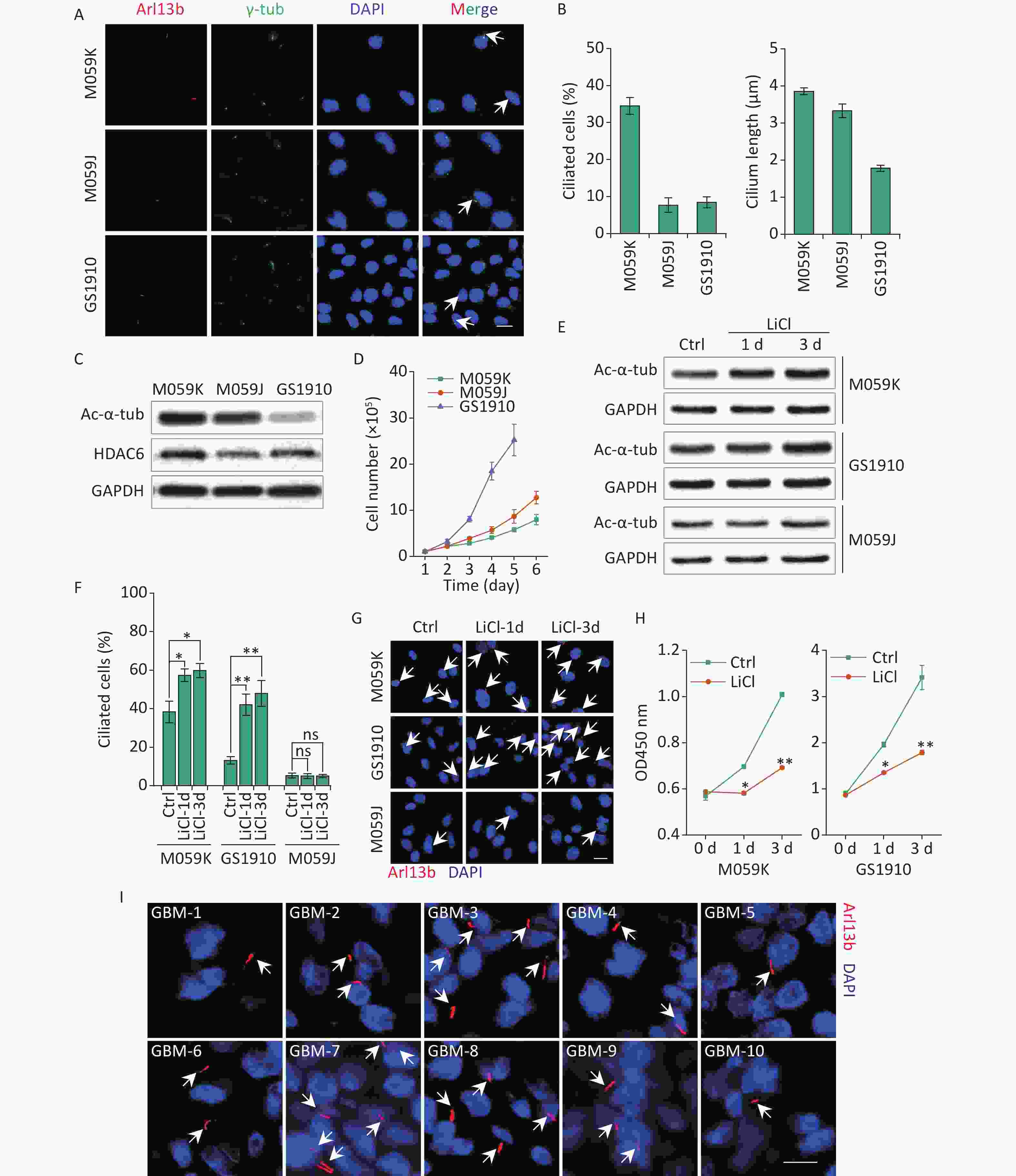
Figure 1. GBM cells express primary cilia. (A) Representative images of the primary cilia in M059K, M059J, and GS1910 cells. Cilium was stained for ciliary membrane marker Arl13b (red) and basal body marker γ-tub (green). DNA was stained with DAPI (blue). Scale bar, 20 µm. (B) Quantification of ciliated cell and the ciliary length counted in ten randomly selected fields from at least three independent experiments. (C) Expression levels of Ac-α-tub and HDAC6 proteins detected by Western blot in M059K, M059J, and GS1910 cells. (D) Cell growth curves. (E) Expression levels of Ac-α-tub protein measured by Western blot before and after treatment with LiCl (5 mmol/L) at 1 and 3 days. (F) Quantification of ciliated cells in three GBM cell lines treated with LiCl at 1 and 3 days. (G) Representative images of primary cilia induced by LiCl in three GBM cell lines at 1 and 3 days and stained for Arl13b (red). DNA was stained with DAPI (blue). Scale bar, 20 µm. (H) Cell proliferation assay of M059K (left) and GS1910 (right) cells in (F). (I) Representative images of primary cilia in GBM biopsies collected from 10 patients and stained for Arl13b (red). DNA was stained with DAPI (blue). Scale bar, 10 µm. Ctrl, control; white arrows indicate primary cilia; ns, not significant; *P < 0.05 and **P < 0.01. GBM: glioblastoma.
Notably, the cellular high proliferative capacity was negatively correlated with the ciliary frequency and length of GBM cells (Figure 1D), suggesting that the induction of primary cilia may be a potential therapeutic strategy for GBM treatment. To confirm this conjecture, we restored the ciliary incidence by LiCl, a common stimulus for ciliogenesis[23]. The cellular ciliogenesis was enhanced in M059K and GS1910 cells but not in M059J cells with the addition of LiCl for 3 days, as ascertained by the increased Ac-α-tub levels (Figures 1E, 1F, and 1G). However, the restoration of primary cilia in M059K and GS1910 cells markedly decreased the cell growth speed (Figure 1H). Ultimately, ten individual clinical GBM tissues were collected, and the expression of Arl13b was examined by immunostaining assay. The positive staining of primary cilia was observed in all ten samples (Figure 1I), and the number of ciliated cells in them ranged from approximately 5% to 35% of the population of tumor cells, indicating the complicated roles of primary cilia in the pathogenesis of GBM.
-
The abrogation of ciliogenesis by depletion of kinesin family member 3A or pericentriolar material 1, a component of centriolar satellites surrounding centrosomes, increased the cellular sensitivity to TMZ in GBM cells[24]. A recent study determined that resistant cells showed increases in cilium numbers and length, which can facilitate drug resistance[13], suggesting the positive relation of primary cilia with cellular TMZ resistance. Thus, we examined the cellular resistant ability to TMZ in M059K, M059J, and GS1910 cells by cell viability assay. GS1910 cells with the lowest ciliary frequency and length displayed the poorest TMZ sensitivity (Figure 2A), indicating ciliogenesis induction responding to TMZ stimulation. Next, we investigated the effects of TMZ on primary cilium biogenesis. The proportion of ciliated cells sharply increased to approximately 80% in M059K and GS1910 cells during TMZ treatment but barely changed in M059J cells (Figures 2B and 2C). Moreover, TMZ exposure evidently elongated the cilia in GS1910 cells, resulting in a longer average cilium length compared with those in M059K and M059J cells, which had modest changes in response to TMZ stimulation (Figure 2D). Furthermore, the significantly elevated expression levels of Ac-α-tub protein after TMZ treatment confirmed the induction effect of TMZ on ciliogenesis in M059K and GS1910 cells (Figure 2E). Abnormal morphology phenotypes, including bubbled and branched cilia, were frequently observed in TMZ-treated M059K cells, and especially, the population of multiciliated (no less than 2 cilia per cell) cells climbed from near 5% to 30% of the total ciliated cells in response to TMZ stimulation (Figures 2F and 2G). Although the increases in ciliated cells with abnormal morphology and multiciliation were not detected in GS1910 cells, the overall ciliary length was intensively increased by TMZ exposure, and more than half of the measured lengths ranged between 3–5 µm in cells treated with TMZ compared with the 1–2 µm observed in the control cells (Figures 2H and 2I). Overall, these results indicate that TMZ-induced ciliogenesis, including multiciliation and elongation of primary cilia, may contribute to the TMZ resistance of GBM cells.
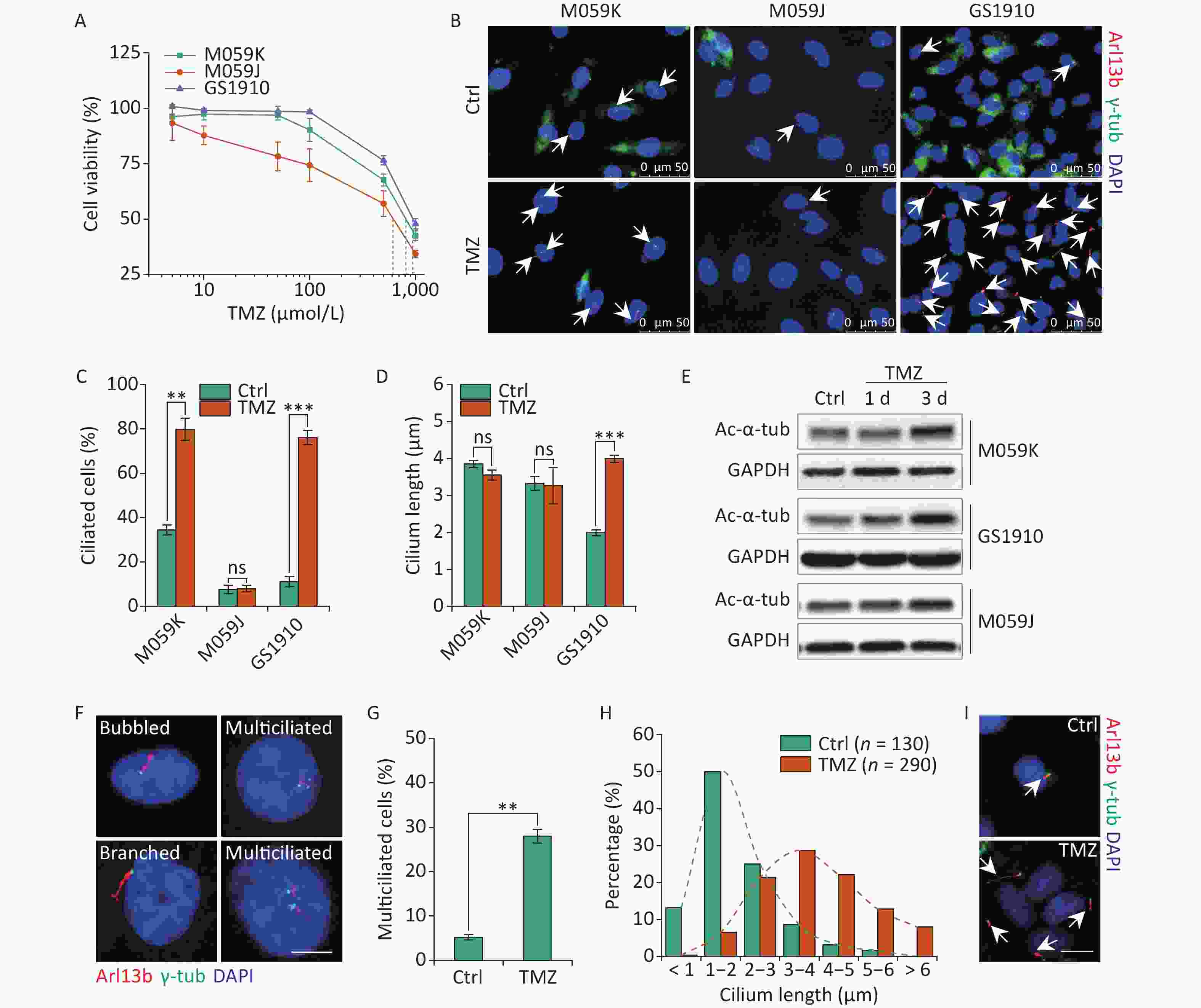
Figure 2. TMZ induces ciliogenesis in GBM cells. (A) Cell viability assay of three GBM cell lines treated with different concentrations of TMZ for 3 days. (B) Representative images of TMZ-induced primary cilium formation in M059K, M059J, and GS1910 cells. Double staining with antibodies against Arl13b (red) and γ-tub (green). DNA was stained with DAPI (blue). Scale bar, 20 µm. Quantitative results of the frequency of ciliated cells (C) and ciliary length (D) in three GBM lines before and after treatment with 500 µmol/L TMZ for 3 days. The data are presented as the mean ± SD of three independent experiments, and more than 100 cilia were counted and measured in each individual group. (E) Western blot of Ac-α-tub expression in TMZ-induced ciliogenesis of three GBM cell lines for 1 and 3 days. (F) Representative images of TMZ-induced abnormal cilia in M059K cells treated with 500 µmol/L TMZ for 3 days and stained for Arl13b (red) and γ-tub (green). DNA was stained with DAPI (blue). Scale bar, 10 µm. (G) Quantitative results of the multiciliated cells in M059K cells prior to and posttreatment with 500 µmol/L TMZ for 3 days. (H) Quantitative results of the length of cilium in GS1910 cells after the treatment with 500 µmol/L TMZ for 3 days. (I) Representative images of elongated cilia in GS1910 cells treated with TMZ and stained for Arl13b (red) and γ-tub (green). DNA was stained with DAPI (blue). Scale bar, 10 µm. Ctrl, control; white arrows indicate primary cilia; ns, not significant; **P < 0.01 and ***P < 0.001. TMZ: temozolomide; GBM: glioblastoma.
-
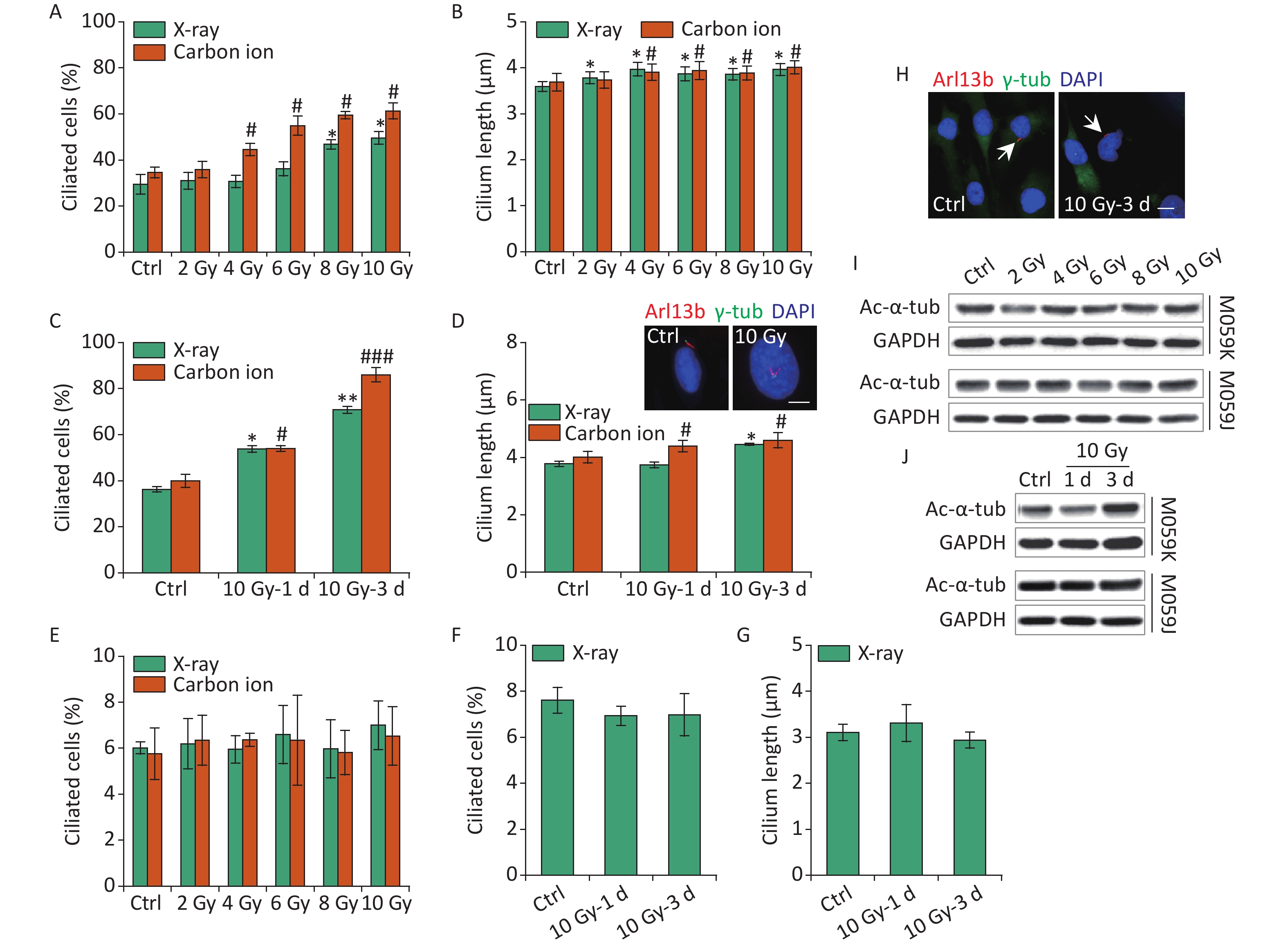
Primary cilia in osteoblasts and skin cells sense electromagnetic fields or ultraviolet (UV) radiation affecting cell function and fate[25, 26]. IR leads to DNA damage, cell cycle block, and various types of cellular responses, and the harmful effects are used to kill the tumor cells in tumor therapy. To date, radiotherapy remains an integral treatment modality included in the standard therapy for GBM[1]. Previous data have identified TMZ-induced ciliogenesis in GBM cells. Thus, the effects of IR on ciliogenesis have been determined. First, M059K cells were exposed to 2–10 Gy X-rays or carbon ions, and the ciliary frequency and length were examined at 1 day post-irradiation. The results showed that X-rays and carbon ions markedly promoted the population of ciliated cells, and the cilium length elongated slightly in M059K cells along with the increase in the dose of IR (Figures 3A and 3B, respectively). Notably, the ciliary induction capacity of carbon ions was more efficient than X-rays at the same radiation dose (Figure 3A). Next, M059K cells were exposed to 10 Gy IR, and the cilium expression was examined at 1 and 3 days post-irradiation. The proportion of ciliated cells increased from less than 40% of non-irradiated control cells to approximately 60% after 1 day of irradiation and rose to more than 80% at 3 days post-irradiation with carbon ions (Figure 3C). The cilium length was elongated following the IR treatment, and multiciliated cells were frequently identified (Figure 3D), displaying time dependence. Although the percentage of multiciliated cells increased, the average cilium length did not increase because the ciliary length in multiciliated cells did not change or was shortened (Figure 3D). Nevertheless, the primary cilia displayed almost no response to IR in M059J cells (Figures 3E, 3F, 3G, and 3H). Moreover, IR triggered the expression of Ac-α-tub protein in M059K cells but not in M059J cells (Figures 3I and 3J, respectively). Collectively, these data indicate the dose and time dependence of ciliogenesis in response to IR of GBM cells.
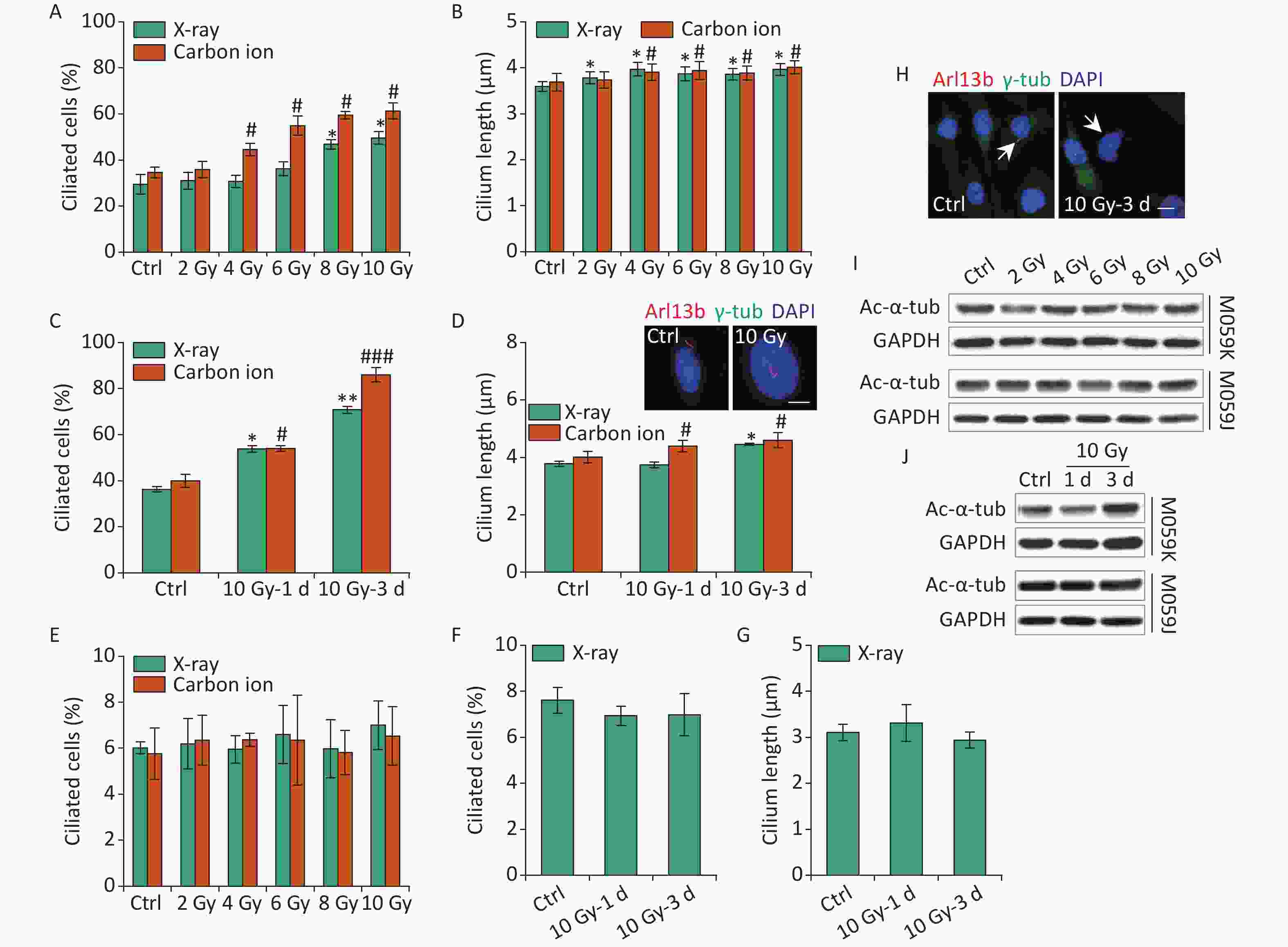
Figure 3. IR triggers primary cilium formation and elongation in GBM cells. (A) Fraction of ciliated cells in M059K cells exposed to 0–10 Gy X-rays or carbon ions on day 1. (B) Average cilium length of cells in (A). (C) Frequency of ciliated cells in M059K cells treated with 10 Gy X-rays or carbon ions at 1 and 3 days after irradiation. (D) Average cilium length of cells in (C) and representative images of the elongated cilium. Double staining with antibodies against Arl13b (red) and γ-tub (green). DNA was stained with DAPI (blue). Scale bar, 10 µm. (E) Frequency of ciliated cells in M059J cells after 0–10 Gy X-rays or carbon ion exposure at 1 day. (F) Frequency of ciliated cells in M059J cells treated with 10 Gy X-rays at 1 and 3 days after irradiation. (G) Cilium length of cells in (F). (H) Representative images of primary cilia in M059J cells during 10 Gy X-ray treatment for 3 days and double staining with antibodies against Arl13b (red) and γ-tub (green). DNA was stained with DAPI (blue). Scale bar, 10 µm. (I) Western blot of Ac-α-tub expression in M059K and M059J cells after X-ray exposure for 1 day. (J) Western blot of Ac-α-tub expression in M059K and M059J cells after 10 Gy X-ray exposure at 1 and 3 days. Ctrl, control; white arrows indicate primary cilia; *P < 0.05 and **P < 0.01 vs. Ctrl (X-ray); #P < 0.05 and ###P < 0.001 vs. Ctrl (Carbon ion). IR: ionizing radiation; GBM: glioblastoma.
-
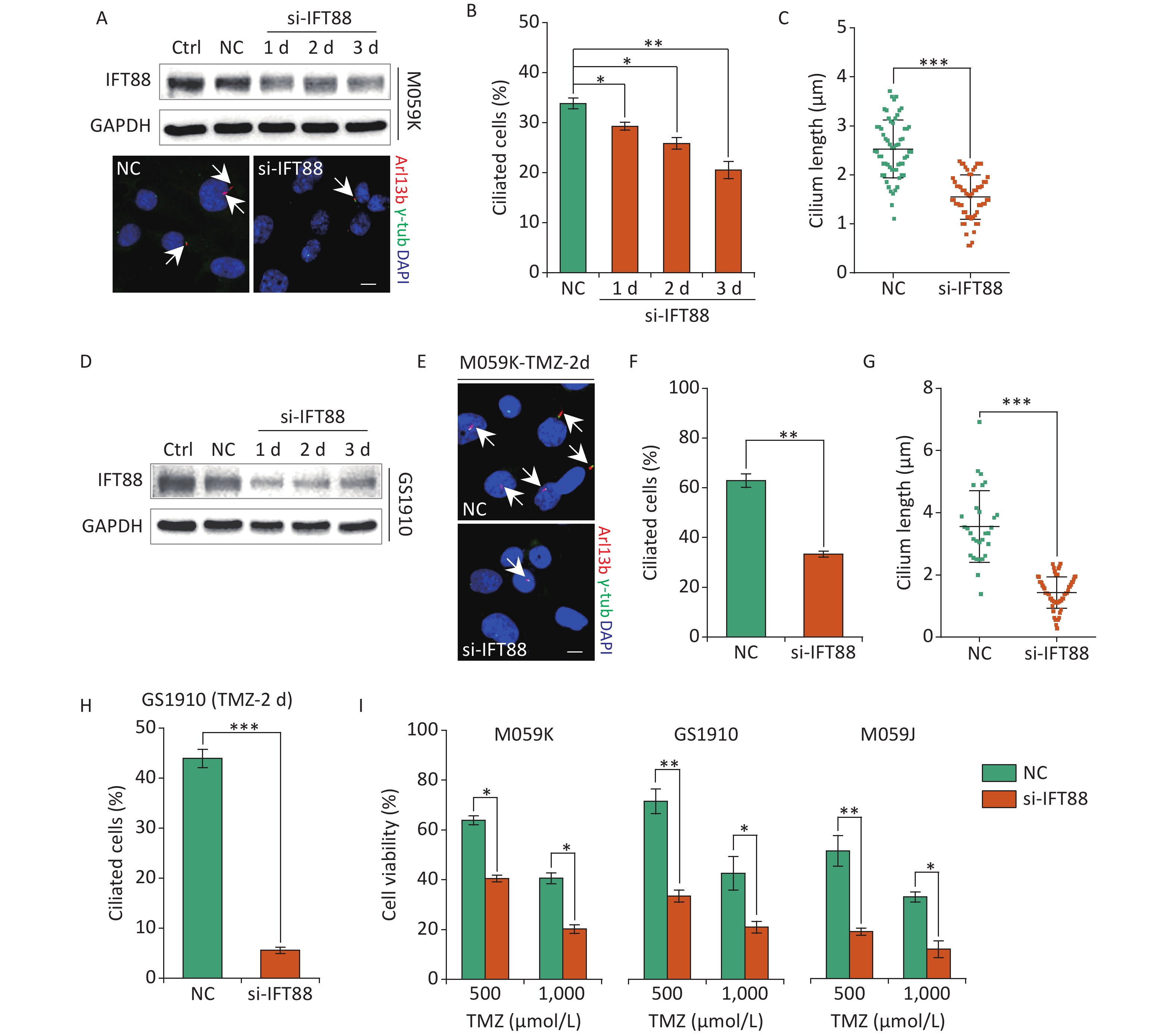
Recent accumulating evidence has suggested the emerging contribution of primary cilia to the survival of cells administrated with chemotherapy drugs[19]. To investigate the effect of primary cilia on the cellular resistance to TMZ in GBM cells, we introduced siRNAs against IFT88 (si-IFT88), an intraflagellar transporter protein required for ciliogenesis, to ablate ciliogenesis by knockdown of IFT88 gene expression[27]. The ectopic expression of si-IFT88 in M059K cells cultured in normal conditions persistently reduced IFT88 protein levels along with the significantly reduced cilium formation and ciliary length (Figures 4A, 4B, and 4C). The deleterious effect of si-IFT88 on IFT88 protein expression was verified in GS1910 cells (Figure 4D), implying a strong inhibitory efficacy to primary cilia biogenesis in GBM cells. Moreover, si-IFT88 induction abrogated ciliogenesis in M059K (Figures 4E, 4F, and 4G) and GS1010 cells (Figure 4H) treated with TMZ for 2 days, inducing a significant increase in the cellular sensitivity to TMZ compared with NC (Figure 4I), which did not rely on the administration dosage of TMZ and the cell type of GBM. These rustles were consistent with those of a previous report[24], suggesting that the loss of primary cilia is associated with increased cellular sensitivity to TMZ in GBM cells.

Figure 4. Interference of ciliogenesis by IFT88 siRNA decreases the cellular resistance to TMZ. (A) Top, Western blot of IFT88 expression in M059K cells transfected with IFT88 siRNA (si-IFT88) or an NC siRNA at a final concentration of 50 nmol/L; bottom, representative images of primary cilia in M059K cells depleting the IFT88 expression and double staining with antibodies against Arl13b (red) and γ-tub (green); DNA was stained with DAPI (blue). Scale bar, 10 µm. (B) Quantitative results of the frequency of ciliated cells in M059K cells after the depletion of IFT88 during different periods. (C) Quantitative results of the cilium length in M059K cells treated with si-IFT88 for 3 days. (D) Western blot of IFT88 expression in GS1910 cells transfected with si-IFT88 or NC at a final concentration of 50 nmol/L. (E) Representative images of primary cilia in IFT88-deficient M059K cells treated with 500 µmol/L TMZ on day 2. Scale bar, 10 µm. Ciliary frequency (F) and length (G) of cells in (E). (H) Quantitative results of the frequency of ciliated cells in IFT88-deficient GS1910 cells treated with 500 µmol/L TMZ at 2 days. (I) Cell viability assay of three GBM cells with the depletion of IFT88 the following treatment with 500 or 1,000 µmol/L TMZ at 2 days. Ctrl, control; white arrows indicate primary cilia; *P < 0.05, **P < 0.01, and ***P < 0.001. TMZ: temozolomide; GBM: glioblastoma.
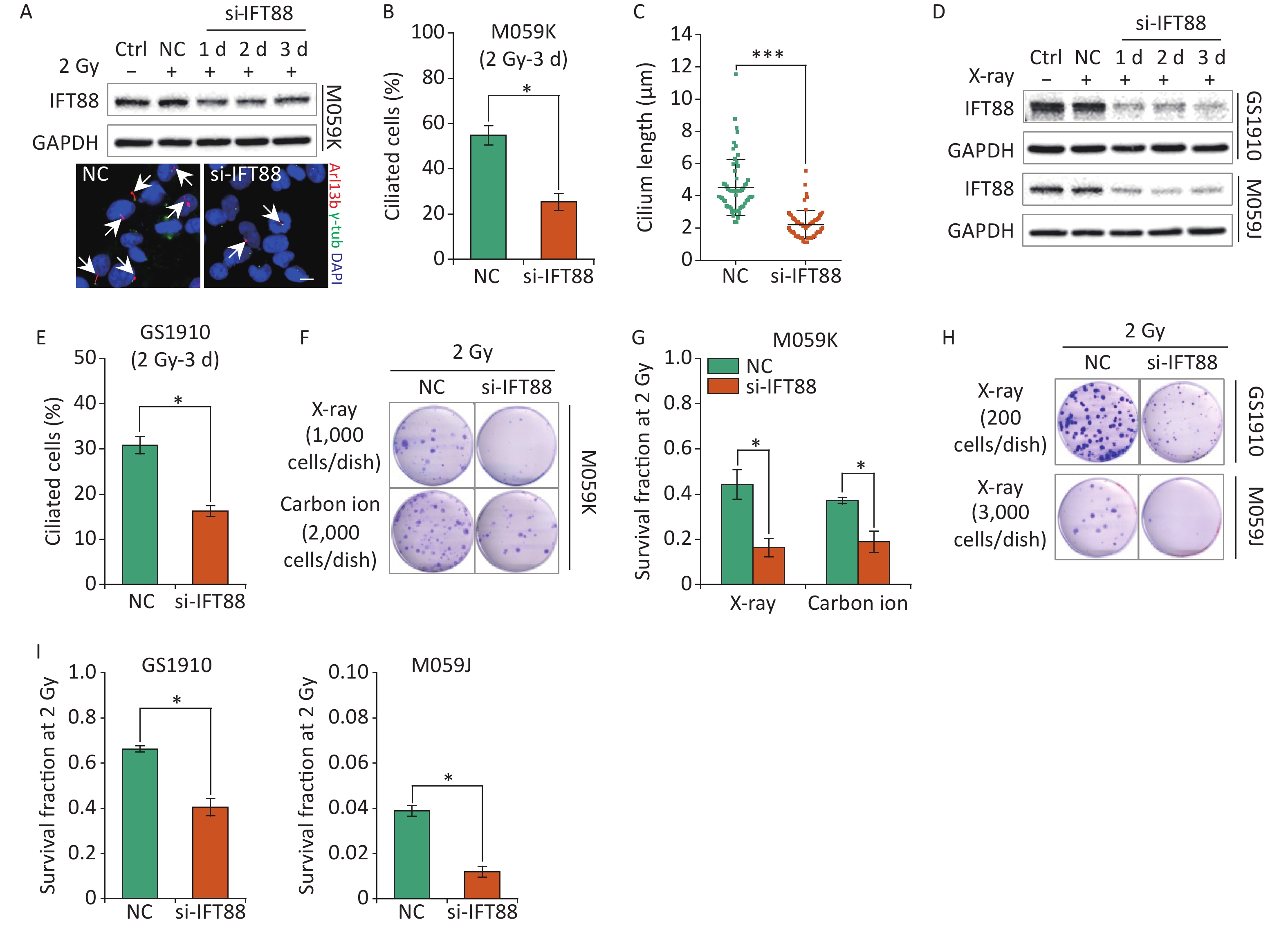
In addition to participation in cellular resistance to chemotherapeutic drugs, primary cilia have been linked to the regulation of radiosensitivity for the interplay with the cellular DDR process[28]. Thus, we further assessed the influence of primary cilia on the cellular sensitivity to IR in GBM cells. We first verified the detrimental effects of si-IFT88 on IFT88 protein expression and ciliogenesis in M059K cells during 2 Gy X-ray exposure for 3 days (Figures 5A, 5B, and 5C). Likewise, the depletion of IFT88 by si-IFT88 continuously abolished the IR-induced upregulation of IFT88 protein expression in GS1910 cells and M059J cells (Figure 5D) and the ciliogenesis in GS1910 cells (Figure 5E). Next, a colony formation assay was performed, and the SF2 was measured to estimate the cellular radiosensitivity. The results showed that the abrogation of ciliogenesis profoundly reduced the cell survival ability upon IR treatment (Figure 5F), and the SF2 value declined by more than 50% in M059K cells treated with X-ray or carbon ions (Figure 5G). Similar decreases in cell survival and SF2 value were observed in GS1910 cells (approximately 30%) exposed to X-ray (Figures 5H and 5I, respectively). An intense lethal effect occurred in M059J cells (Figure 5H), and the SF2 value declined by more than 70% during si-IFT88 treatment (Figure 5I), similar to the cell viability assay results in TMZ treatment (Figure 4I), indicating the unexpected determinant functions of primary cilia in GBM cell resistance to IR and TMZ. Collectively, the interference of primary cilia by si-IFT88 increased the GBM cellular sensitivity to IR, suggesting that targeting primary cilia significantly improves the cellular radiosensitivity of GBM cells.
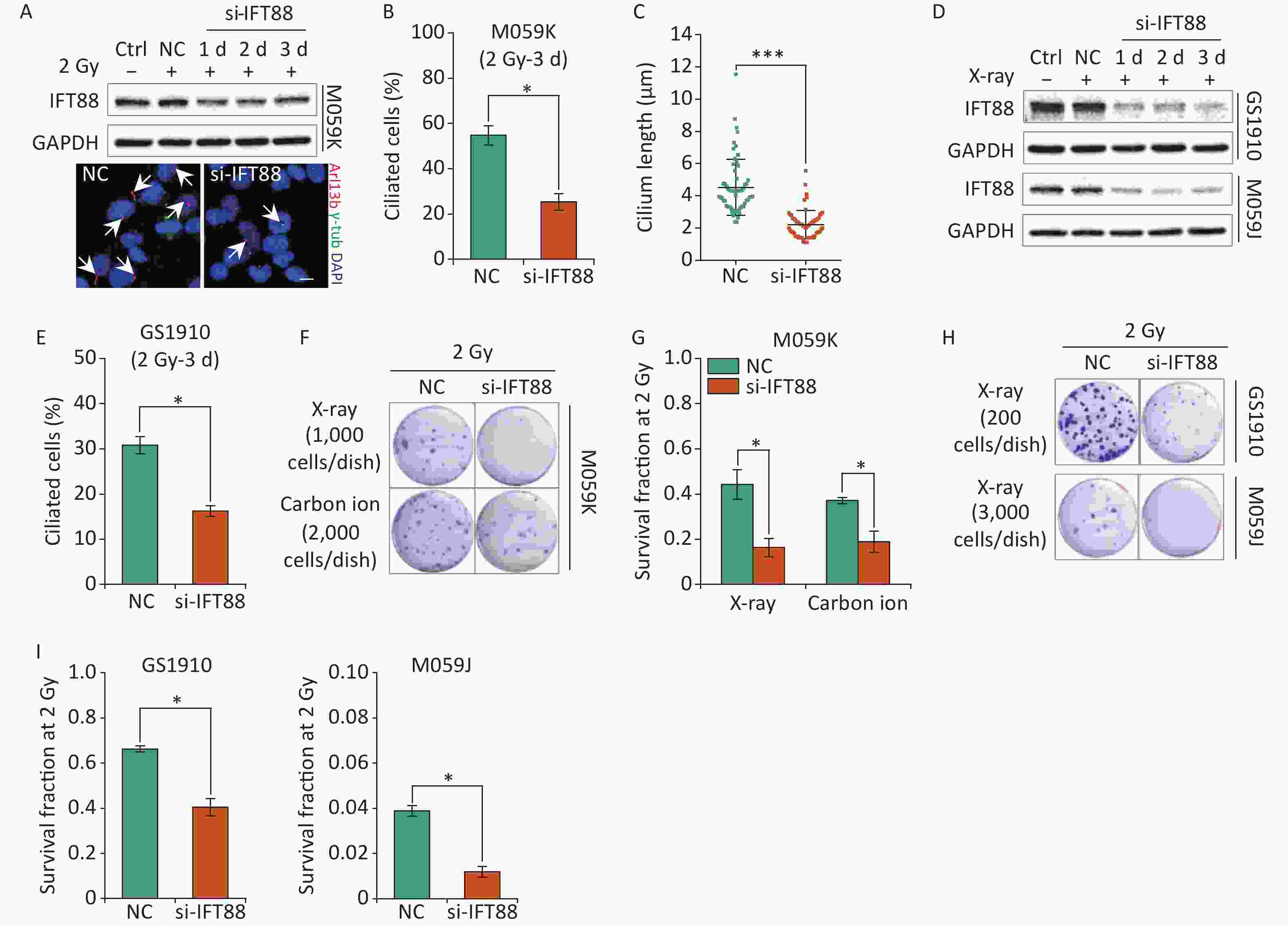
Figure 5. Interference of primary cilia by si-IFT88 decreases the cellular resistance to IR. (A) Top, Western blot of IFT88 expression in M059K cells transfected with si-IFT88 or NC at a final concentration of 50 nmol/L followed by 2 Gy X-ray exposure; bottom, representative images of primary cilia in M059K cells depleting IFT88 expression and double staining with antibodies against Arl13b (red) and γ-tub (green); DNA stained with DAPI (blue). Scale bar, 10 µm. (B) Quantitative results on the frequency of ciliated cells in M059K cells after depletion of IFT88 followed by 2 Gy X-ray exposure on day 3. (C) Cilium length of cells in (B). (D) Western blot of IFT88 expressions in GS1910 and M059J cells transfected with si-IFT88 or NC followed by 2 Gy X-rays at different times. (E) Quantitative results on the frequency of ciliated cells in GS1910 cells after IFT88 depletion followed by 2 Gy X-ray exposure on day 3. (F) Representative images of colonies of M059K cells transfected with si-IFT88 or NC following exposure to 2 Gy X-rays or carbon ions; stained with 0.5% crystal violet. At least three independent experiments were performed. (G) SF2 values of IFT88-deficient M059K cells by X-ray or carbon ion exposure. (H) Representative image of colonies of M059J and GS1910 cells transfected with si-IFT88 or NC following exposure to 2 Gy X-rays. (I) SF2 values of IFT88-deficient GS1910 cells (left), IFT88-deficient M059J cells (right), and NC cells exposed to 2 Gy X-rays. Ctrl, control; SF2, survival fraction at 2 Gy; white arrows indicate primary cilia; *P < 0.05 and ***P < 0.001.
-
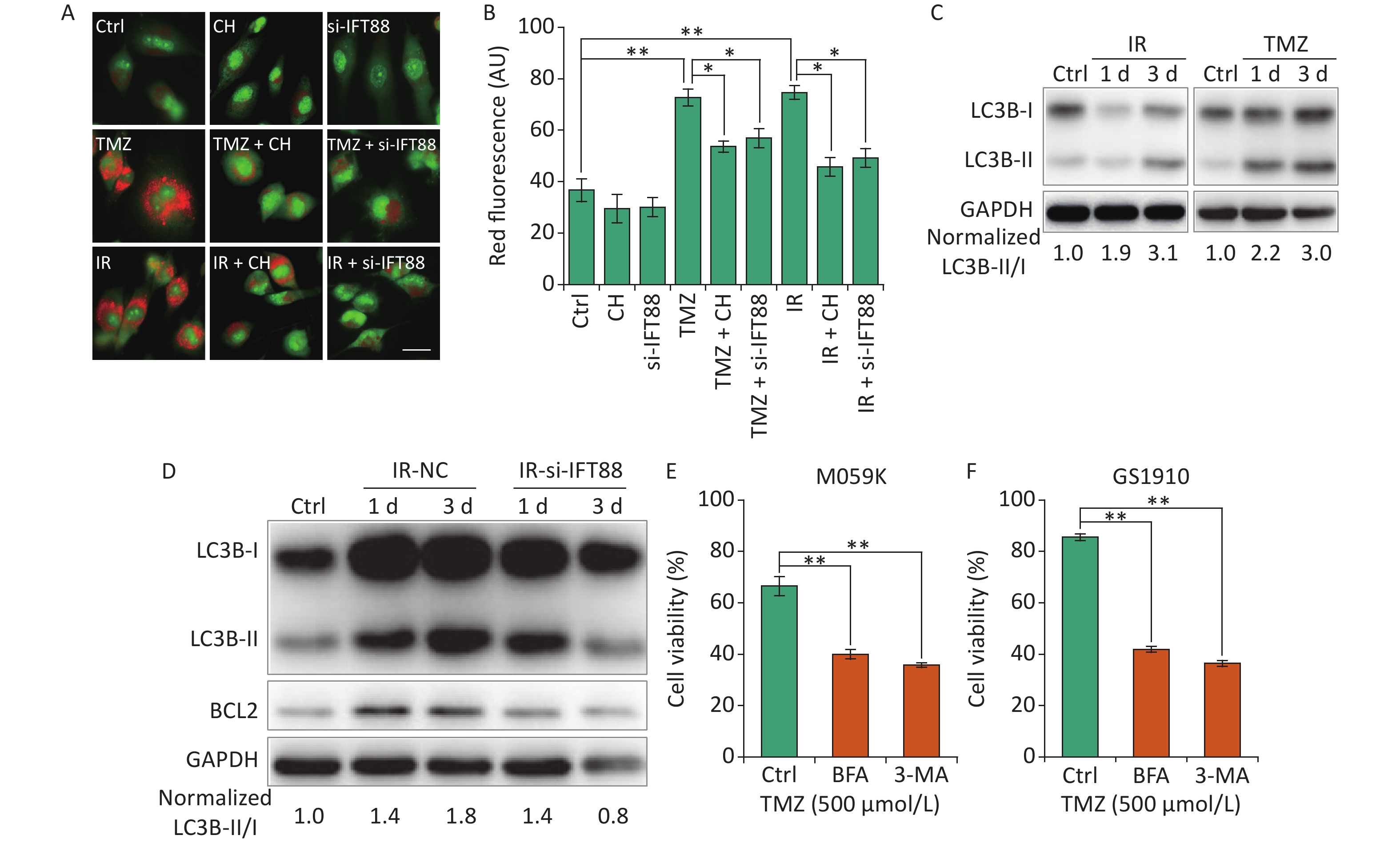
Autophagy is a lysosome-related program involved in response to multiple types of stresses, including chemotherapeutic modalities and IR, and plays an important role in radio- and chemoresistance [18]. Consistent with previous reports in GBM cells[29, 30], we observed that the autophagy levels showed by autophagolysosomes labeled with AO staining were strongly enhanced by IR/TMZ administration (Figures 6A and 6B) and the activation of LC3B (ratio of LC3B-II/I, Figure 6C). For the intricate relationship between primary cilia and autophagy[15], we speculated that the inhibition of ciliogenesis may abolish the autophagy induced by TMZ or IR. Ciliogenesis interference by si-IFT88 or CH evidently decreased the AO staining levels in M059K cells following the exposure to 10 Gy X-rays or TMZ (500 μmol/L) (Figures 6A and 6B). Meanwhile, the IR-induced accumulation of LC3B-II was diminished by the introduction of si-IFT88 (Figure 6D), implying that the interference of cilia inhibited IR-induced autophagy, as indicated by the conversion of LC3B-II to LC3B-I. Of note, the expression of BCL2, a key regulator of the anti-apoptosis signaling pathway preserving survival by binding and inhibiting pro-apoptotic proteins [31], was elevated by IR in parallel with autophagy induction in the NC group, indicating the non-activation of apoptosis (Figure 6D). When primary cilia were inhibited by si-IFT88, the expression of BCL2 was also reduced, suggesting the activation of apoptosis. Ultimately, the role of autophagy in cellular resistance was examined in M059K and GS1910 cells treated with autophagy inhibitor BFA or 3-MA in combination with TMZ. The results showed that the inhibition of autophagy substantially impaired the cell survival ability of M059K and GS1910 cells (Figures 6E and 6F, respectively). Altogether, these data suggest the involvement of autophagy in primary cilia-mediated radio- and chemoresistance in GBM cells.
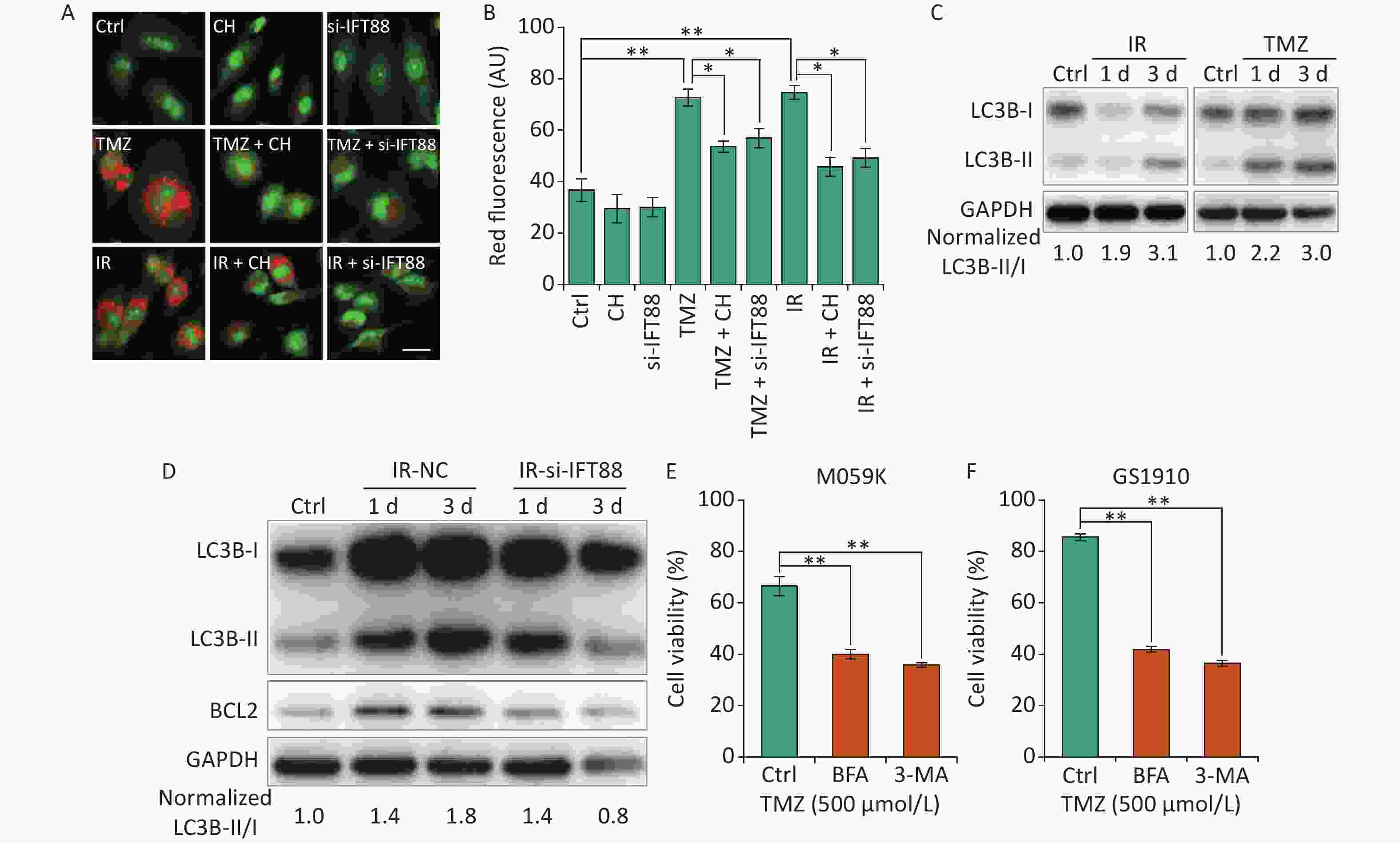
Figure 6. Primary cilia regulate autophagy to affect the cellular sensitivity to TMZ and IR. (A) AO staining assay for the detection of autophagolysosomes levels in M059K cells transfected with si-IFT88 (50 nmol/L) or treated with CH (2 mmol/L) following the exposure to IR (10 Gy X-rays) or TMZ (500 µmol/L). Scale bar, 50 µm. (B) Bar diagram quantifies the red fluorescence signal intensity of cells in (A). At least 100 cells from each group were tested in three independent experiments. (C) Western blot of LC3B expression in M059K cells after the exposure to IR or TMZ at 1 and 3 days. (D) Western blot of LC3B and BCL2 expressions in M059K cells transfected with si-IFT88 or NC following the exposure to X-rays at 1 and 3 days. Cell viability assay of M059K (E) and GS1910 (F) cells exposed to TMZ (500 µmol/L), followed by the administration of 3-MA (10 mmol/L) or BFA (0.5 µg/mL) at 2 days. Ctrl, control; *P < 0.05 and **P < 0.01. TMZ: temozolomide; IR: ionizing radiation.
-
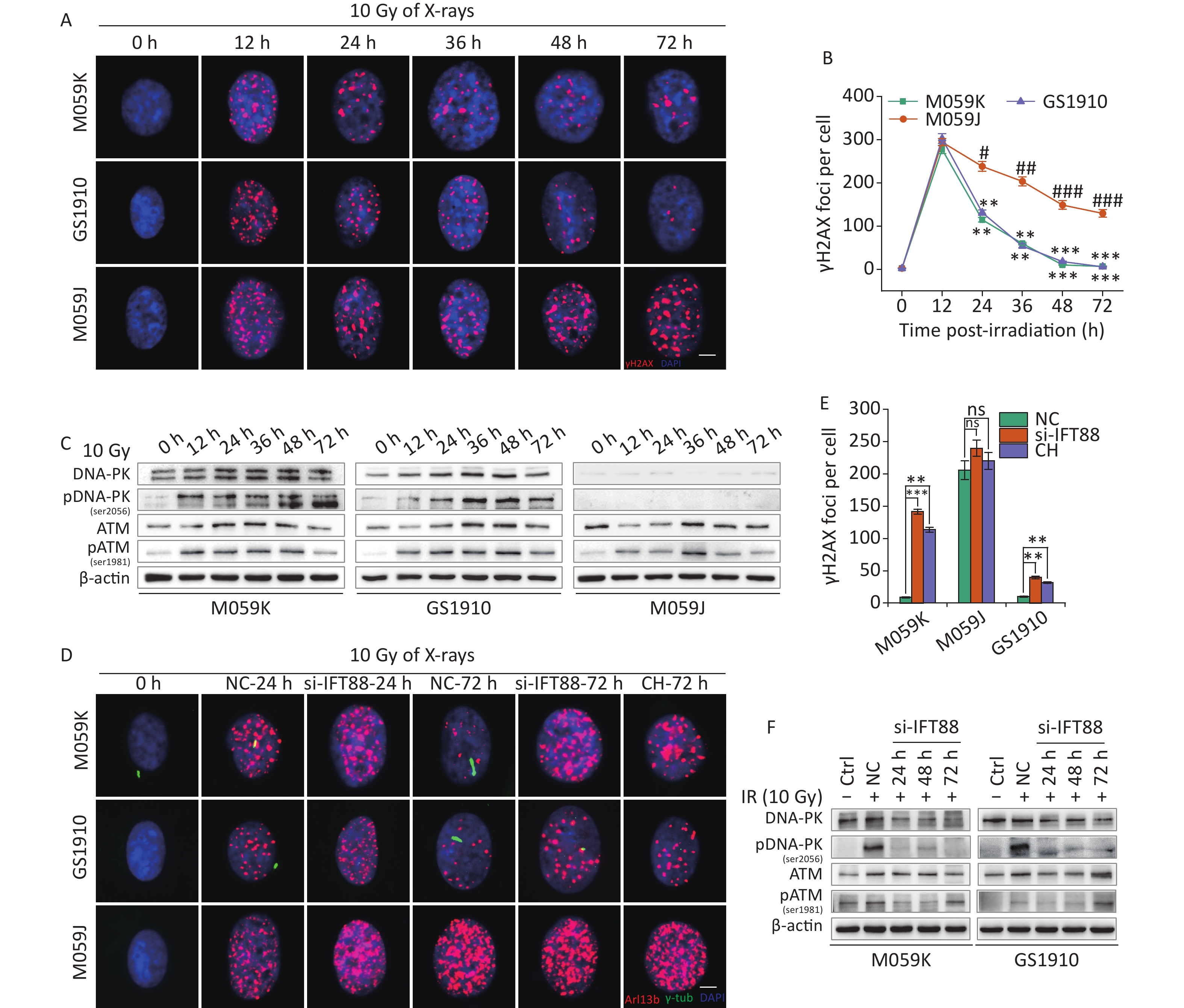
IR kills tumor cells by inducing DNA damage. However, in eukaryotic cells, an intracellular DDR network has been evolved and orchestrated to maintain genome stability, in which the PIKK family plays a principal role to be responsible for the initiation of signaling in response to DNA damage[32]. Increasing evidence has shown the function of primary cilia in cellular DDR[28], which was investigated here in GBM cells. First, the kinetic curves of γH2AX foci were measured in M059K, GS1910, and M059J cells treated with 10 Gy X-rays. M059J cells were more sensitive to IR than the other two cell lines (Figures 7A and 7B), and this finding may be correlated with their defect in DNA-PK activation (Figure 7C), as has been reported previously[33]. ATM was activated in all three cell lines but at a slower response compared with DNA-PK, and the activation decreased gradually over time (Figure 7C). When IR-induced ciliogenesis was abrogated by IFT88 depletion or CH administration (Figure 7D), the cellular DNA damage repair progress was blocked consequently in M059K and GS1910 cells (Figure 7D and 7E, respectively) but barely in M059J cells. Importantly, the IR-induced activation of DNA-PK and ATM was prevented by IFT88 depletion (Figure 7F). Thus, these findings suggest the potential role of the PIKK family, especially DNA-PK, in linking primary cilia and DDR.

Figure 7. Primary cilia mediate cellular DNA damage repair progression. (A) Representative images of γH2AX foci formed in DNA DSB regions by immunostaining with a specific antibody against γH2AX (red) in three GBM cell lines during 10 Gy X-ray exposure at different time periods. DNA was stained with DAPI (blue). Scale bar, 10 µm. (B) Kinetic curves of γH2AX foci in three GBM cell lines. (C) Western blot of DNA-PK, pDNA-PK (Ser2056), ATM, and pATM (Ser1981) expressions in (A). (D) Representative images of γH2AX foci (red) in three GBM cell lines transfected with si-IFT88 or exposed to 2 mmol/L CH following 10 Gy X-ray exposure at 24 and 72 h and double staining with antibodies against Arl13b (green) and γH2AX (red). DNA was stained with DAPI (blue). Scale bar, 10 µm. (E) Quantity of γH2AX foci per cell in (D) at 72 h. (F) Western blot of DNA-PK, pDNA-PK, ATM, and pATM expressions in M059K and GS1910 cells transfected with si-IFT88 or NC following 10 Gy X-ray exposure at different time periods. **P < 0.01 and ***P < 0.001 vs. M059J at the same time point; Ctrl, control; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. M059J at 12 h.
-
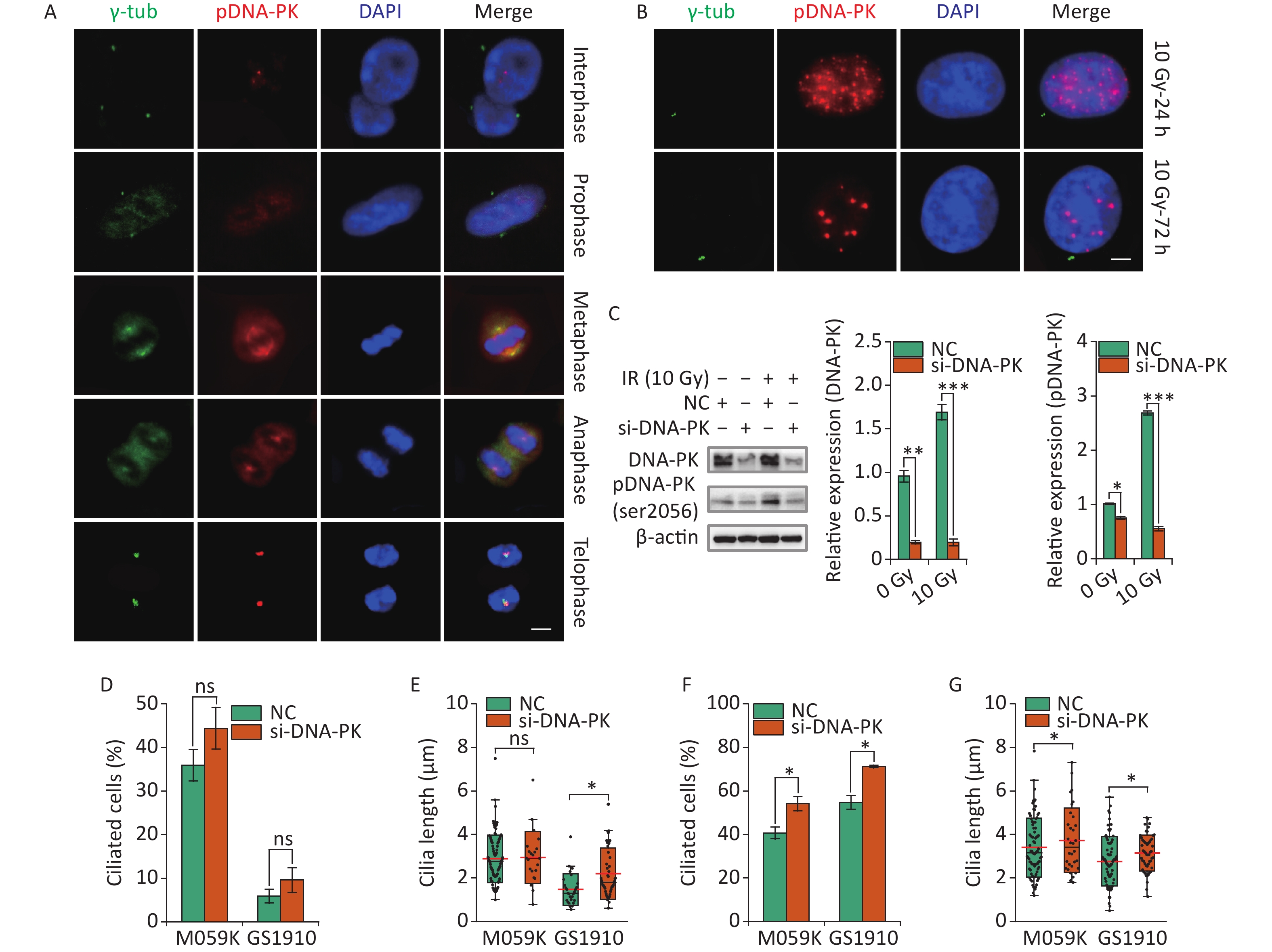
Recent work has highlighted the links between DDR and primary cilia. Etoposide-activated DNA-PK led to centrosome amplification and primary cilium formation[34]. Aberrant cilia may affect mitogenic signaling and responses to genotoxic stress differently. However, whether DNA-PK plays a role in IR-induced ciliogenesis remains vague. Here we first examined the subcellular localization of activated DNA-PK under normal conditions. The results showed that phosphorylated DNA-PK were hardly detected in the nucleus, but the activated DNA-PK signal increased dramatically when cells entered the M phase (Figure 8A). Surprisingly, the activated DNA-PK was colocalized with the centriole and the emitted microtubules, suggesting the important roles of DNA-PK in cell cycle progression and cilia reabsorption. Next, we observed that DNA-PK was highly activated in the nucleus but not in the cytoplasm after 10 Gy X-ray treatment at 24 and 72 h (Figure 8B). Then, si-DNA-PK was transfected to M059K and GS1910 cells, resulting in marked decreased expression levels of DNA-PK and pDNA-PK compared with NC, especially during the 10 Gy X-ray treatment (Figure 8C). Notably, the inhibition of DNA-PK promoted cellular ciliogenesis in the frequency of ciliated cells and cilium length in M059K and GS1910 cells in the presence or absence of 10 Gy X-ray treatment (Figures 8D, 8E, 8F, and 8G). Additionally, the abnormal nuclei that frequently occurred during IR treatment were reduced when treated with si-DNA-PK (data not shown), suggesting the increased cell death. Meanwhile, the surviving cells had a regular shape with large nuclei and increased cilia incidence compared with the control group. Therefore, the data above all indicate that the activated DNA-PK negatively regulates ciliogenesis in GBM cells.
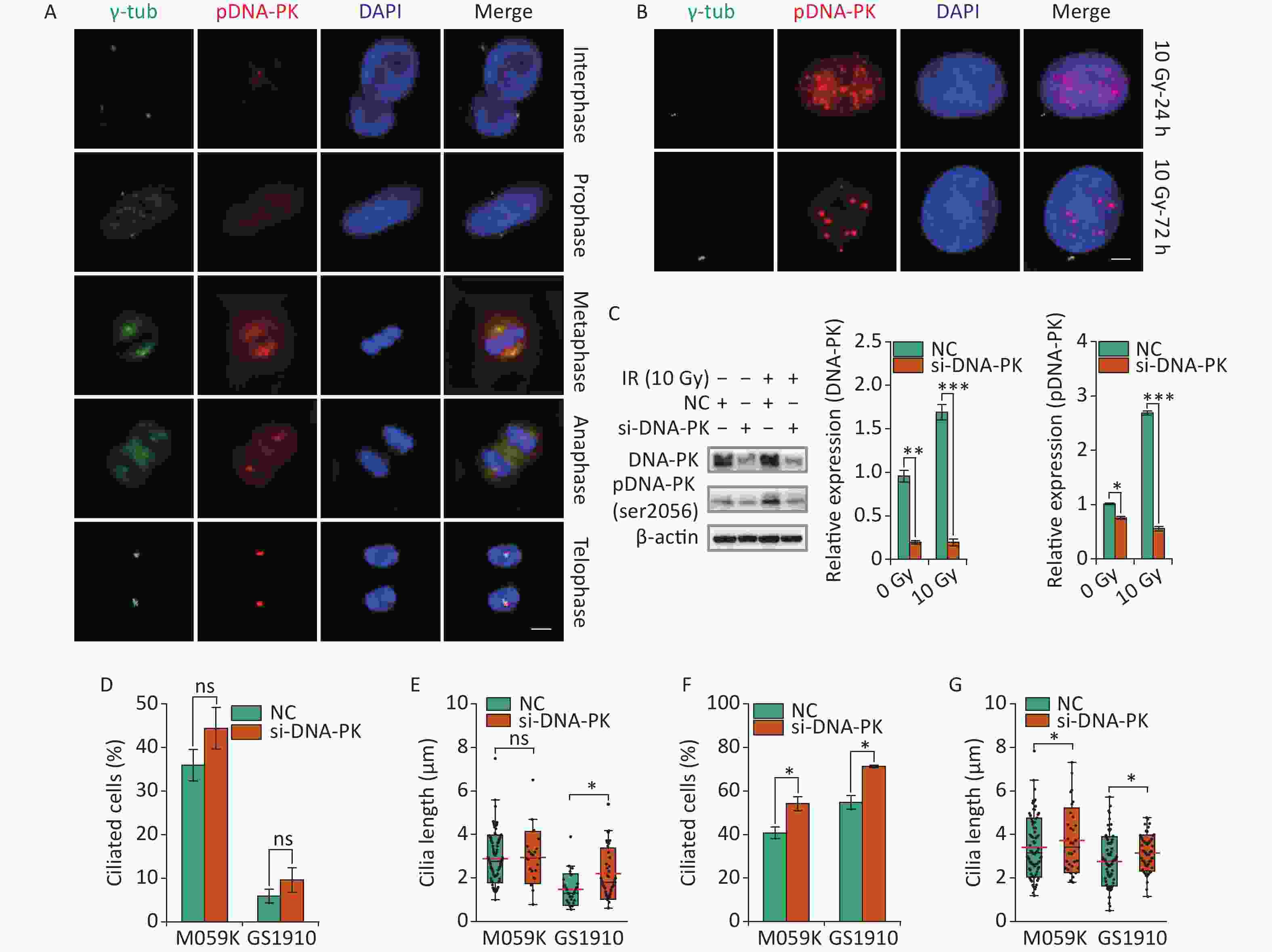
Figure 8. DNA-PK contributes to ciliogenesis. (A) Representative images of subcellular localization of pDNA-PK at different cell division phases in M059K cells. Double staining with antibodies against γ-tub (green) and pDNA-PK (red). DNA was stained with DAPI (blue). Scale bar, 10 µm. (B) Representative images of subcellular localization of pDNA-PK in M059K cells during 10 Gy X-ray exposure at 24 and 72 h. Scale bar, 10 µm. (C) Western blot of DNA-PK and pDNA-PK expressions in M059K cells transfected with si-DNA-PK followed by treatment or nontreatment with 10 Gy X-rays and the quantitative results. Quantitative results of the ciliary frequency (D) and length (E) in M059K and GS1910 cells transfected with si-DNA-PK or NC. The red line indicates the mean cilium length. Quantitative results of the ciliary frequency (F) and length (G) in M059K and GS1910 cells transfected with si-DNA-PK followed by 10 Gy X-ray exposure. The red line indicates the mean cilium length. ns, not significant; *P < 0.05.
-
In this study, we reported for the first time that TMZ and IR facilitates ciliogenesis in GBM cells. Moreover, we demonstrated that the inhibition of ciliogenesis in GBM cells enhanced the cellular sensitivity to TMZ and IR by affecting autophagy and DDR progression. Importantly, we observed that primary cilia were involved in the regulation of DNA damage-induced DNA-PK activation, and DNA-PK activity played a critical role in mediating ciliogenesis, suggesting the intense interplay between primary cilia and TMZ/IR-induced DDR.
Primary cilia have been reported to be commonly lacking or extremely restricted in various types of human tumors, including GBM. On the contrary, Sarkisian and colleagues reported that GBM biopsies displayed frequent primary cilia expressions with ciliated cells ranging from 1% to 25%[35]. Consistently, we detected 5% to 35% ciliated cells in GBM tissue samples and 10% to 40% in GBM cell lines. Although the ciliary incidence in GBM cells is controversial, which may be related to the detection methods, cell types, and individual patient differences, all the studies indicated a profound role of primary cilia in the pathophysiology of GBM. In addition, primary cilia function as sensors of extracellular signals in response to a wide range of mechanical and chemical stimuli. Emerging evidence has shown that genotoxic stresses elicited from chemo drugs or UV/IR exposure trigger primary cilium biogenesis in human fibroblasts and epithelial cells[36]. Our data further revealed the induction of ciliogenesis by DNA damage resulting from TMZ and IR in GBM cells (Figures 2 and 3, respectively). In addition, the dose and time dependency of ciliogenesis and multiciliation processes were observed in M059K and GS1910 cells treated with TMZ or IR. This phenomenon has been recently reported in human myoblasts[37], retina pigmented epithelial cells[38], adrenocortical tumor cells[34], and other cell types[19] and has been linked to the centriole splitting caused by DNA damage[39]; however, the related molecular mechanism remains largely unknown.
M059K and M059J cell lines, which are derived from single tumor tissue with differences in the cellular sensitivity to IR and chemo agents[33], are well-established models for the cellular resistance study in GBM. M059J cells are more sensitive to IR than M059K cells in regard to the defects of DNA-PK[40]. In this study, our results confirmed that M059J cells were approximately 12-fold more sensitive to X-rays than M059K cells (the SF2 values were 0.038 and 0.45, respectively). Meanwhile, our results showed that TMZ/IR treatment induced cilium formation and elongation in M059K cells but not in M059J cells, and the depletion of primary cilia enhanced the cellular sensitivity to IR in both cells, indicating correlations between primary cilia and cellular radiosensitivity. We observed that X-ray and carbon ion led to ciliogenesis in a dose- or time-dependent manner, especially the higher ciliogenesis induction capacity of carbon ion than X-ray at the same radiation dose (Figure 3), suggesting that IR-induced ciliogenesis is a general effect of radiotherapy. Combining with the data from GS1910 cells, we proposed that the ciliary response to TMZ or IR may be a useful marker for evaluating the cellular resistant ability or the treatment outcome in GBM therapy. Although the restoration of primary cilia has been demonstrated to inhibit cell proliferation in GBM cells (Figure 1H), we discovered robust improvements in the cellular adaption to IR or TMZ treatment in the context of ciliogenesis, which highlights the limitations in the development of therapeutic strategy via triggering primary cilia biogenesis in tumor therapy.
Primary cilia have been linked to therapeutic resistance in multiple types of tumor cells. The ciliary incidence and length exhibit evident increases in drug-resistant cell lines[13, 41]; enhanced ciliogenesis can facilitate resistance, whereas ablation of primary cilia overcomes cellular resistance through promoting cell death[8]. Notably, the inhibition of ciliogenesis in GBM cells recovers the sensitivity to TMZ[24]. Furthermore, our recent study suggested that primary cilia are involved in the cellular radiosensitivity in M059K and M059J cells[22]. In concordance with previous observations, here, we revealed that the interference of ciliogenesis was sufficient to sensitize GBM cells to TMZ and IR, at least partly by affecting autophagy and DNA damage repair processes. Although TMZ- and IR-induced ciliogenesis blocked the cell cycle progression, it was accompanied by an increase in cellular resistance. Thus, the pharmacological inhibition of ciliogenesis with specific chemical compounds, including cytoskeletal drugs, kinase inhibitors, signaling pathway inhibitors, and so on[42], in combination with chemoradiotherapy, may be a valuable strategy for GBM treatment. On the other hand, primary cilium loss is sufficient to promote and maintain highly proliferative phenotypes in GBM cells[43] and primary cilia in kidney tubular epithelial cells, and bronchoalveolar epithelial cells participate in the regulation of cisplatin-induced acute kidney injury and lung damage[44, 45], implying potent limitations and side effects of ciliogenesis inhibitors for GBM treatment.
Therapy-induced resistance concerns a series of cell processes, including cell survival, DNA damage repair, reactive oxygen species (ROS) clearance, epithelial-to-mesenchymal transition induction, cancer stem cell-like phenotype enrichment, and tumor microenvironment modification[46], which are coordinated by precise molecular signaling networks. Accumulating evidence indicates crosstalk between autophagy and ciliogenesis. Classically, autophagy exhibits a pro-survival function in cells exposed to chemotherapy or radiation through DNA damage repair, removal of misfolded proteins and damaged organelles, scavenging of ROS, energetic regulation, and the activation of cytoprotective signals that block apoptosis[47]. In addition, autophagy is involved in the regulation of ciliogenesis by degrading ciliary proteins or by removing them from centriolar satellites[48]. Importantly, we demonstrated for the first time that primary cilia regulate IR/TMZ-induced autophagy in GBM cells. The positive regulation of autophagy by primary cilia is consistent with the results of a previous study on renal carcinoma cells[49], and the knockdown of IFT88 to perturb cilium formation can depress autophagy in proximal tubular cells in the human kidney through the upregulation of mammalian target of rapamycin signaling pathway[50]. However, when cilium formation was inhibited in thyroid tumor cells, the autophagy activity was extremely high, and such finding was associated with the poor prognosis of patients[51]. Meanwhile, the inhibition of autophagy prevented GBM cell resistance to TMZ, indicating that cilia regulated the cellular sensitivity by affecting autophagy, which is consistent with the result of a previous study[52].
DNA-PK is a member of the PIKK family, and it plays a vital role in cellular DDR progression[53]. The centrosome, which transforms into the basal body in the ciliogenesis process, serves as a target and a regulator of DDR, suggesting a potential inter-regulatory relationship between primary cilia and the PIKK family. The deficiency in ATR, a PIKK family member determining replication for stability and DDR, causes primary cilium abnormality and profoundly confers ciliary dysfunction[54]. More recent studies have tied DNA-PK to ciliogenesis. Chen et al. observed that genotoxic stress activated ciliogenesis in parallel with DNA-PK, which localizes to the basal body of primary cilia[19]. Meanwhile, the depletion of DNA-PK decreased ciliogenesis and vice versa, and autophagy and senescence were involved. In this work, activated DNA-PK localized to the centrosomes during the mitosis phase in GBM cells (Figure 8A). The knockdown of DNA-PK expression and activation slightly promoted ciliogenesis (Figures 8D and 8E, respectively). These observations are consistent with Chen’s report and indicate a detrimental role of DNA-PK in ciliogenesis under normal conditions. Upon IR treatment, we did not observe the colocalization of pDNA-PK and basal body (Figure 8B), and the suppression of DNA-PK, instead of reducing, intensively facilitated primary cilium formation and elongation (Figures 8F and 8G, respectively), which are contrary to the findings in Chen’s report. We speculated that the inhibition of DNA-PK may promote ciliogenesis via phosphorylating distinct signaling cascades. The binding of activated DNA-PK in the cytoplasm to the centriole facilitates mitosis and prevents primary cilium formation, whereas under DNA damage stress, activated DNA-PK enters the nucleus without the inhibition of ciliogenesis. This hypothesis still needs to be further deciphered in the future. Given that serum starvation induces primary cilium formation but without DNA damage[55], whether the depletion of DNA-PK has an effect on starvation-induced ciliogenesis needs to be investigated to further confirm the relationship between the activation of DNA-PK and ciliogenesis. Considering the complicated roles of DNA-PK in mediating HDAC6, suppressing its expression but promoting its deacetylase activity[21], further understanding is needed about its role in ciliogenesis during genotoxic stress to clarify the underlying mechanism resulting in inconsistent observations.
In summary, certain limitations still need to be targeted during the induction or inhibition of cilia. Targeted cilia treatment may trigger abnormalities in all cilia-related signaling pathways, which can result in ultimate effects beyond our expectations. Some scholars commonly use small-molecule chemicals to interfere with ciliogenesis along with the effects on other cellular processes. For example, as the only currently used ciliary inhibitory molecule, CH has an effect on M phase cell division[56]. The interference of the IFT family by siRNA to disturb ciliogenesis has off-target and limited inhibition efficiency and affects intracellular material transport and secretion[57]. Therefore, more specific and efficient cilia interference methods should be deeply explored.
-
We thank the support of the Heavy Ion Research Facility in Lanzhou (HIRFL) at the Institute of Modern Physics, Chinese Academy of Science (Lanzhou, China) for carbon ion radiation. We thank the Large Instrument Sharing Management Platform of Lanzhou University for its assistance in this study. We thank Mr. Puzhong Ji and Ms. Liangliang Jin (Department of Pathology, 940th Hospital of Joint Logistics Support Force of CPLA, Lanzhou, China) for technical assistance in clinical sample immunostaining experiments.
-
Conceptualization, GAO L and HE JP; investigation, WEI L and MA W; methodology and software, WEI L, MA W, PENG SP, and TIAN HB; formal analysis and data curation, WEI L, MA W, and HE JP; supervision and validation, CAI H, WANG JF, and GAO L; writing-original draft preparation, WEI L and MA W; writing-review and editing, GAO L and HE JP; funding acquisition, GAO L, WANG JF, and HE JP. All authors have read and agreed to the published version of the manuscript.
-
The authors declare no competing financial interests.
Inhibition of Ciliogenesis Enhances the Cellular Sensitivity to Temozolomide and Ionizing Radiation in Human Glioblastoma Cells
doi: 10.3967/bes2022.058
- Received Date: 2021-12-31
- Accepted Date: 2022-03-29
-
Key words:
- Primary cilia /
- Glioblastoma /
- Cellular sensitivity /
- Temozolomide /
- Ionizing radiation /
- Autophagy /
- DNA damage response /
- DNA-PK
Abstract:
| Citation: | WEI Li, MA Wei, CAI Hui, PENG Shao Peng, TIAN Huan Bing, WANG Ju Fang, GAO Lan, HE Jin Peng. Inhibition of Ciliogenesis Enhances the Cellular Sensitivity to Temozolomide and Ionizing Radiation in Human Glioblastoma Cells[J]. Biomedical and Environmental Sciences, 2022, 35(5): 419-436. doi: 10.3967/bes2022.058 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: