-
Periodontitis is a chronic infectious disease characterized by plaque biofilm as its main pathogenic factor; its clinical manifestations include gingival inflammation, alveolar bone resorption, attachment loss, and periodontal pocket formation, all of which eventually lead to tooth loss and seriously affect the quality of life of patients[1]. This illness has a close relationship with blood sugar levels in patients with diabetes and is one of the most important complications of diabetes[2]. Macrophages are an important part of host immune responses and exhibit powerful functions of identifying, phagocytizing, and removing bacteria; macrophage polarization is involved in the pathogenesis of periodontitis[3].
Periodontal ligament stem cell (PDLSC)-mediated periodontal tissue regeneration is a potential treatment for periodontitis and has shown favorable results in clinical trials. MicroRNAs play an important role in this process; however, their exact role in macrophage polarization and inflammation development remains poorly characterized. In this study, we examined the effects of high glucose levels on the proliferation and polarization of human myeloid leukemia mononuclear cell (THP-1)-derived macrophages and the modulatory effects of PDLSCs and microRNA on high glucose-induced inflammatory responses.
Human impacted third molars without caries and periodontitis were extracted from 18- to 25-year-old patients under local anesthesia. The periodontal tissues were peeled off gently from the root, and the middle third tissues were used for PDLSC isolation. After repeated cleansing, the periodontal tissues were minced and digested in a solution of 3 mg/mL collagenase type I (Thermo Fisher Scientific, USA) and 4 mg/mL dispase (Boehringer Mannheim, Germany) for 1 hour at 37 °C. The cells were obtained by passing through a 70 μm strainer (Falcon, USA). After being centrifugated at 1,000 rpm for 10 min, the cells were resuspended in 25 cm culture flasks (Corning Costar, USA) with the alpha-modification of Eagle’s medium (α-MEM; GIBCO, USA) (referred to as basal medium) containing 20% fetal bovine serum (FBS; GIBCO), 100 μmol/L ascorbic acid 2-phosphate (WAKO, Tokyo, Japan), 2 mmol/L glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) and then incubated at 37 °C in 5% carbon dioxide. The medium was changed every 3 days, and the PDLSCs in passages 3–5 were used in the following experiments.
The expression profiles of three mesenchymal stem cell (MSC) markers, namely, STRO-1 (R&D systems, Minneapolis, USA), CD146, and CD90 (Abcam, Cambridge, UK) in PDLSCs were tested by flow cytometry (BD Immunocytometry Systems, San Jose, CA, USA). PDLSCs were cultured in osteoinductive (basal medium + 10 mmol/L β-glycerophosphate + 50 mg/L vitamin C + 10 nmol/L dexamethasone; Sigma-Aldrich, St. Louis, MI, USA) and adipogenic (basal medium + 10 μmol/L dexamethasone + 2 mmol/L indomethacin + 10 mg/L insulin + 0.5 mmol/L 3-isobutyl-1-methylxanthine; Sigma-Aldrich) media for 3 weeks. Osteogenic and adipogenic differentiation was checked by Alizarin red staining and oil red O staining, respectively.
In brief, 4.0 × 103 THP-1 cells were seeded in 24-well plates and cultured in RPMI 1640 medium (GIBCO) supplemented with 10% FBS at 37 ℃ in 5% carbon dioxide, followed by the addition of phorbol-12-myristate-13-acetate (5 μg/L, Sigma-Aldrich) for 48 hours to obtain adherent macrophages. The medium was then supplemented with two concentrations of glucose (Sigma-Aldrich), i.e., 5 mmol/L as low glucose and 30 mmol/L as high glucose, for another 48 hours. In some high-glucose wells, PDLSCs (4.0 × 103) were co-cultured with macrophages in Transwell chambers with a 0.4 μm pore size membrane (Costar, USA). The cell suspension was stained with trypan blue, and the number of living cells was counted to determine macrophage proliferation. Three pro-inflammatory cytokines, namely, interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, and two anti-inflammatory cytokines, namely, IL-10 and transforming growth factor (TGF)-β, were analyzed by quantitative real-time polymerase chain reaction (RT-PCR). Additionally, the expression of CD80 and CD206, markers of macrophage M1 and M2 polarization, respectively, was tested by RT-PCR. The primer sequences are shown in Supplementary Table S1 (available in www.besjournal.com).
Gene Sense primers (5’-3’) Antisense primers (3’-5’) IL-1β GGATATGGAGCAACAAGTGGTGT TTTCAACACGCAGGACAGGTA IL-6 AACAACCTGAACCTTCCAAAGA TCAAACTCCAAAAGACCAGTGA TNF-α TGTAGCCCATGTTGTAGCAAAC TTGAAGAGGACCTGGGAGTAGA IL-10 GGGAGAACCTGAAGACCCTC ATAGAGTCGCCACCCTGATG TGF-β CCCACAACGAAATCTATGACAA ACGTGCTGCTCCACTTTTAACT CD80 TGCCTGACCTACTGCTTTGC AGGGCGTACACTTTCCCTTC CD163 CCAACAAGATGCTGGAGTGAC TGACAGCACTTCCACATTCAAG microRNA146a TTACAGGGCTGGGACAGGC GGTCCTCAAGCCCACGATG GADPH AGAACATCATCCCTGCCTCTACT GATGTCATCATATTTGGCAGGTT Table S1. Primers used for quantitative RT-PCR analysis
After the co-culture, the supernatants of different groups were collected and centrifugated at 1,000 g for 20 min to remove cell debris. Enzyme-linked immunosorbent assays (ELISA) were used to quantify the levels of IL-1β, IL-6, TNF-α, IL-10, and TGF-β in the culture supernatants in accordance with the instructions of the manufacturers (ElabScience).
The THP-1 cells were cultured and treated as mentioned above and transfected with microRNA 146a inhibitor or negative control by using Lipofectamine 2000 (Invitrogen) in accordance with the protocols of the manufacturers to further test the role of microRNA 146a. After 48 hours, the cells and supernatants were collected and used for RT-PCR and ELISA, respectively.
All data were expressed as mean ± standard deviation (SD). Statistical significance was assessed by Student’s t-test or analysis of variance, and a P value less than 0.05 was considered statistically significant. For multiple comparisons, the Bonferroni method was used to adjust the probability level.
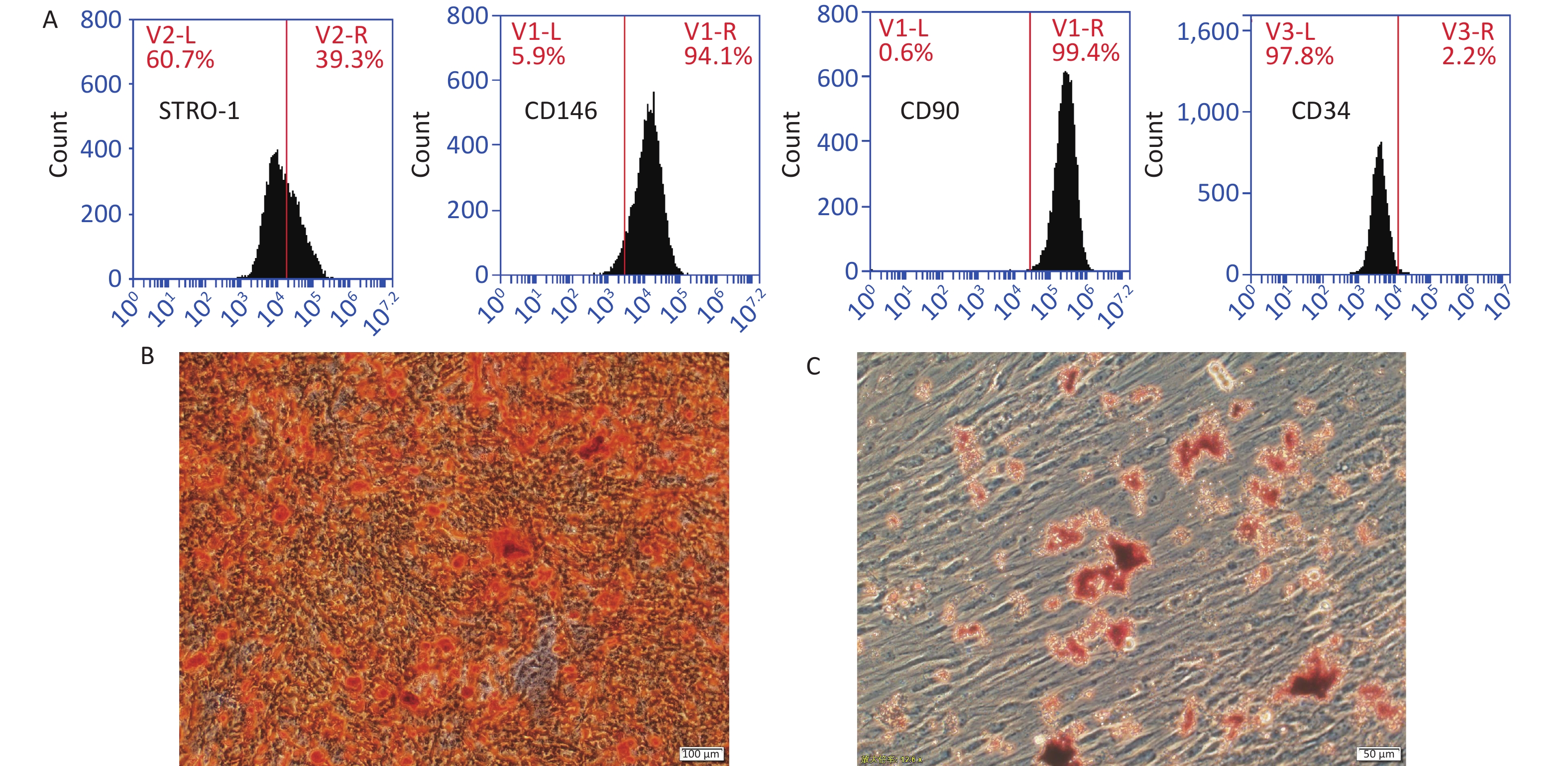
After primary culture for 2–3 days, the PDLSCs were distributed in clusters and formed cell colonies several days later. At post passage, the PDLSCs changed into long spindle appearances, grew in a radial or vortex shape, and gradually fused into pieces. Flow cytometry showed that the PDLSCs were positive for STRO-1, CD146, and CD90, three MSC markers, and were negative for hematopoietic lineage cell marker CD34. Therefore, these cells were identified as MSC. After 3 weeks of culture in an inductive medium, the PDLSCs advanced to osteogenic and adipogenic differentiation as indicated by the positive calcium accumulation dyed orange by Alizarin red staining and the intracellular red positive particles visualized by oil red O staining, respectively (Figure 1).
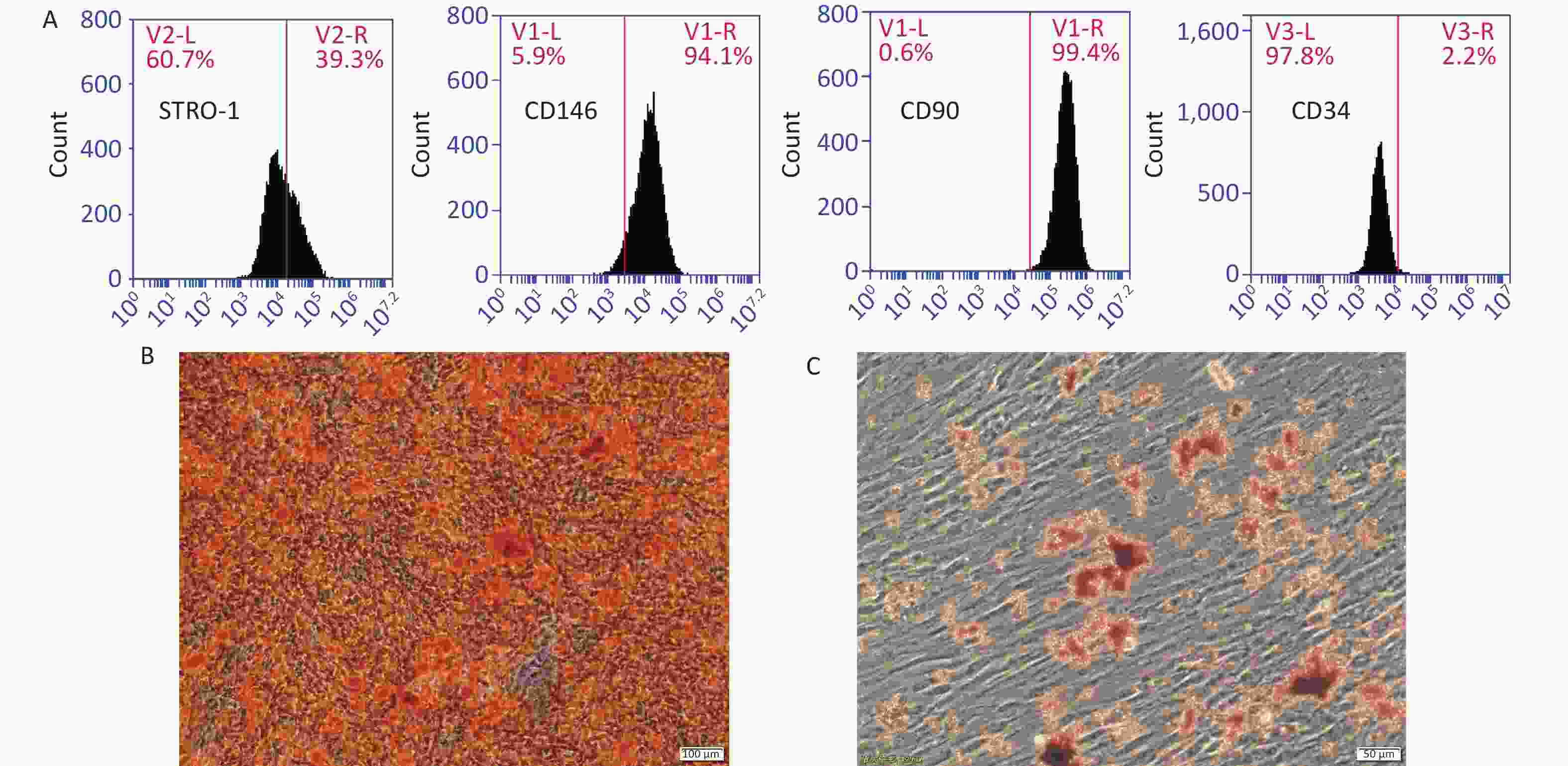
Figure 1. Characterization of PDLSCs. (A) PDLSCs were positive for STRO-1, CD146, and CD90, three MSC markers, but were negative for CD34, a hematopoietic lineage cell marker. PDLSCs could advance into osteogenic (B) and adipogenic (C) differentiation in an inductive medium. Scale bar in (B) and (C): 100 and 50 μm, respectively.
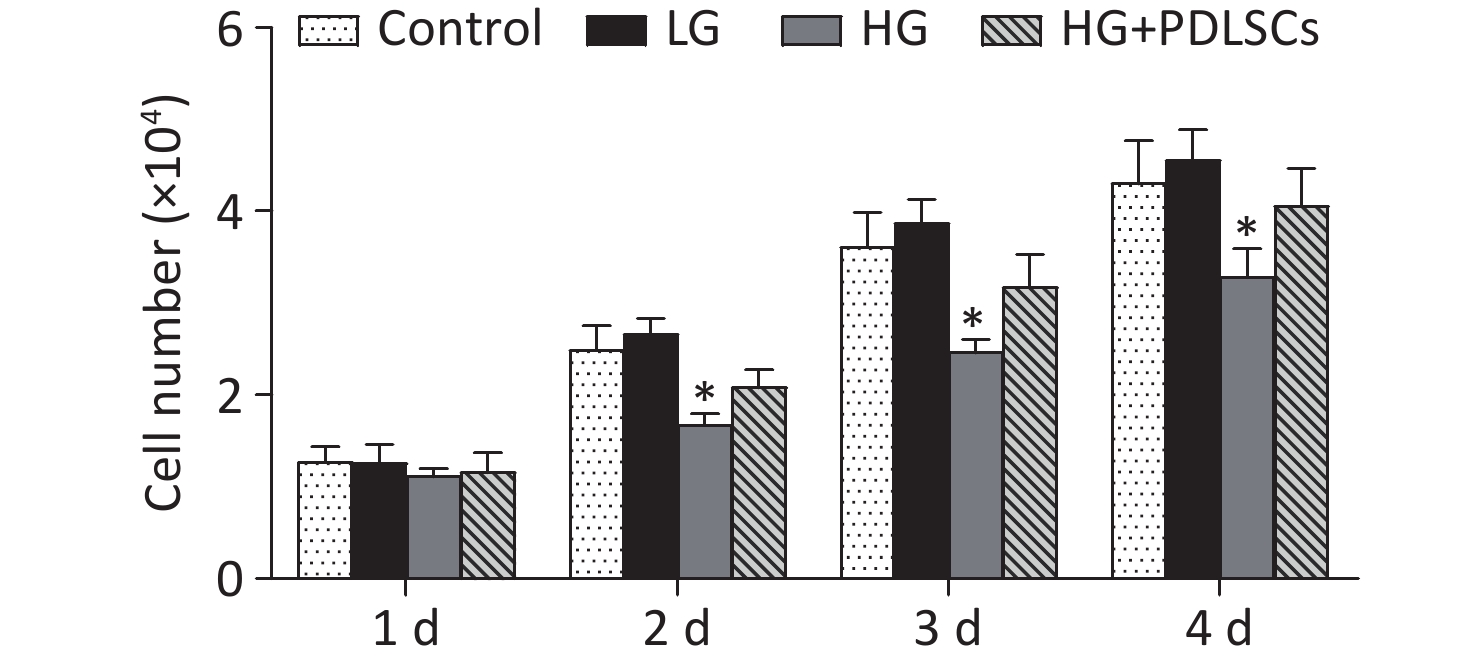
The effects of high glucose levels on the proliferation of THP-1-derived macrophages were evaluated. Macrophages were co-cultured with different glucose concentrations for 4 days. Proliferation tests indicated that the two glucose concentrations did not influence the proliferation of macrophages on the 1st day of co-culture, and the condition was comparable with that during the proliferation of macrophages alone. However, high glucose levels (30 mmol/L) significantly inhibited the proliferation of macrophages on days 2, 3, and 4. The PDLSCs were then added to the high-glucose-treated macrophages to investigate their role in macrophage proliferation. The results showed that the PDLSCs restored the macrophage proliferation inhibited by high-glucose treatment; however, the proliferation rate was still lower than that for macrophages alone or macrophages with low-glucose treatment (Supplementary Figure S1 available in www.besjournal.com).
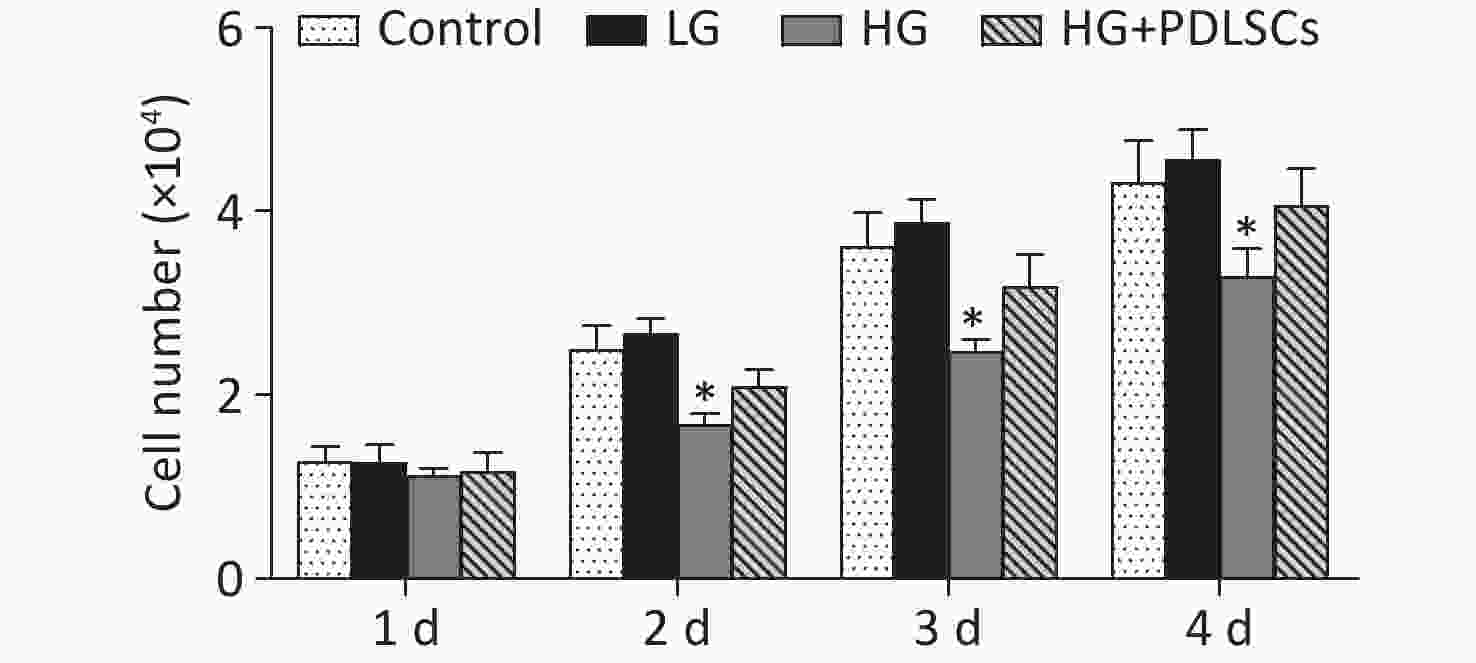
Figure S1. Effect of high glucose on macrophage proliferation. High-glucose treatment (30 mmol/L) did not affect the proliferation of macrophages after 1 day of co-culture but significantly suppressed the proliferation of macrophages at 2–4 days. Meanwhile, low-glucose treatment had no effects on macrophages. PDLSCs restored the macrophage proliferation inhibited by high-glucose treatment. Data represent the mean ± SD. *Means statistically significant compared with other groups of the same day (P < 0.05). The experiments were repeated three times. Control, macrophage culture alone. LG, macrophages + low glucose; HG, macrophages + high glucose; HG+PDLSCs, macrophages + high glucose + PDLSCs.
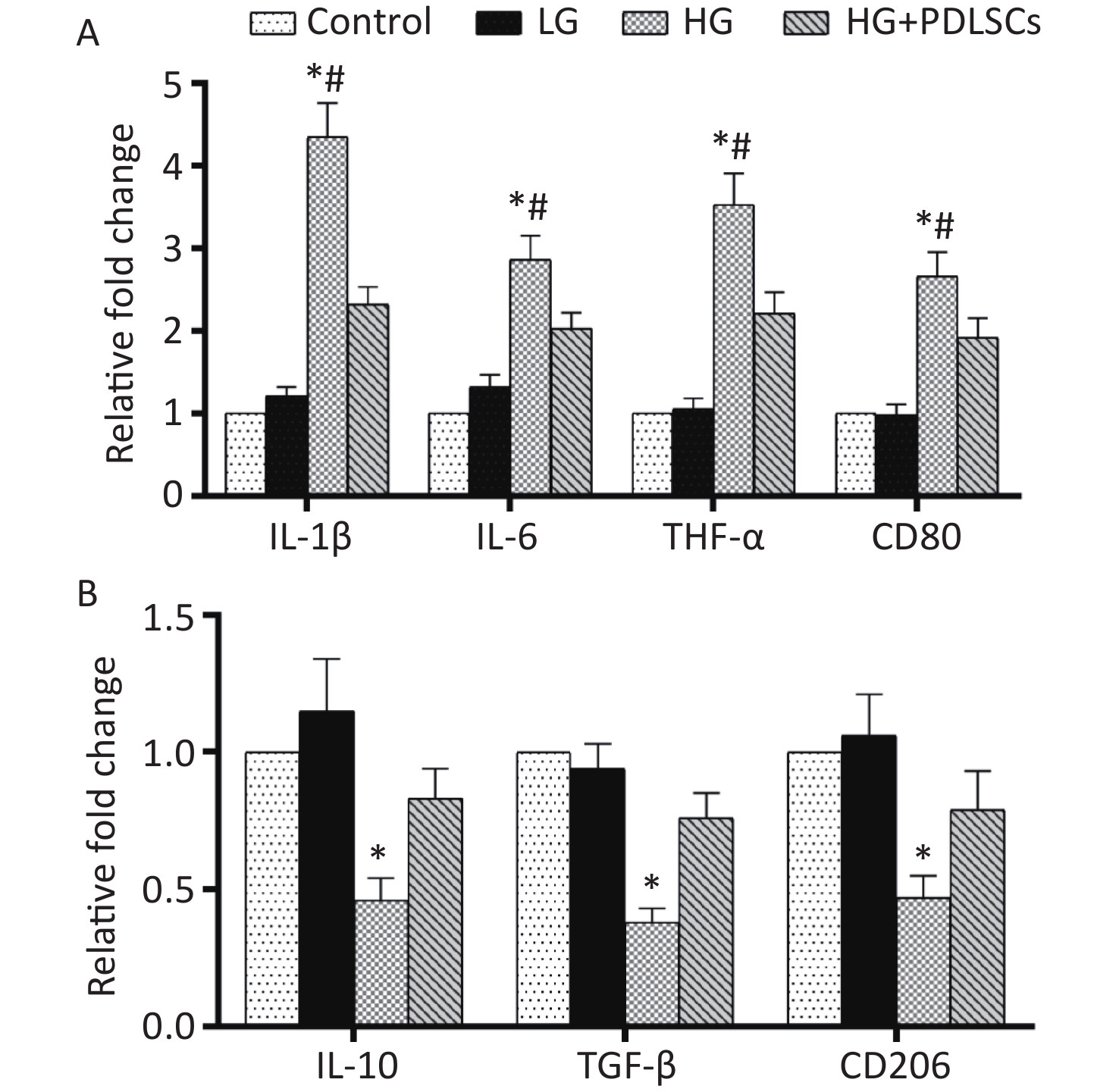
The expression of three pro-inflammatory factors, IL-1β, IL-6, and TNF-α, and two anti-inflammatory cytokines, IL-10 and TGF-β, was quantified at the gene transcription level to test whether high glucose concentration could induce the pro-inflammatory or anti-inflammatory state of macrophages. The results showed that after high-glucose treatment, the expression of IL-1β, IL-6, and TNF-α was up-regulated significantly (Figure 2A), but that of IL-10 and TGF-β was down-regulated (Figure 2B). Consistent with the RT-PCR results, ELISA showed that the macrophages cultured with high glucose concentration dramatically produced a large amount of pro-inflammatory factors, including IL-1β, IL-6, and TNF-α, and secreted less IL-10 and TGF-β (Supplementary Table S2 available in www.besjournal.com). However, low-glucose treatment did not affect the above-mentioned cytokines at the gene transcription (Figure 2A, B) and protein translation levels (Supplementary Table S2). Furthermore, the expression of polarization markers was detected to investigate the effect of high glucose levels on macrophage polarization. The results showed that expression levels of CD80 and CD206 in high-glucose-incubated macrophages increased and decreased, respectively, compared with those in the low-glucose group (Figure 2A, B). When the high-glucose group was directly co-cultured with equal amounts of PDLSCs in Transwell chambers, the up-regulation of IL-1β, IL-6, and TNF-α and the down-regulation of IL-10 and TGF-β were reversed at the gene transcription (Figure 2A, B) and protein translation levels (Supplementary Table S2). Furthermore, the PDLSCs shifted the M1 polarization of high-glucose-treated macrophages into M2 polarization, as manifested by the decline in CD80 (Figure 2A) and the improvement of CD206 (Figure 2B).
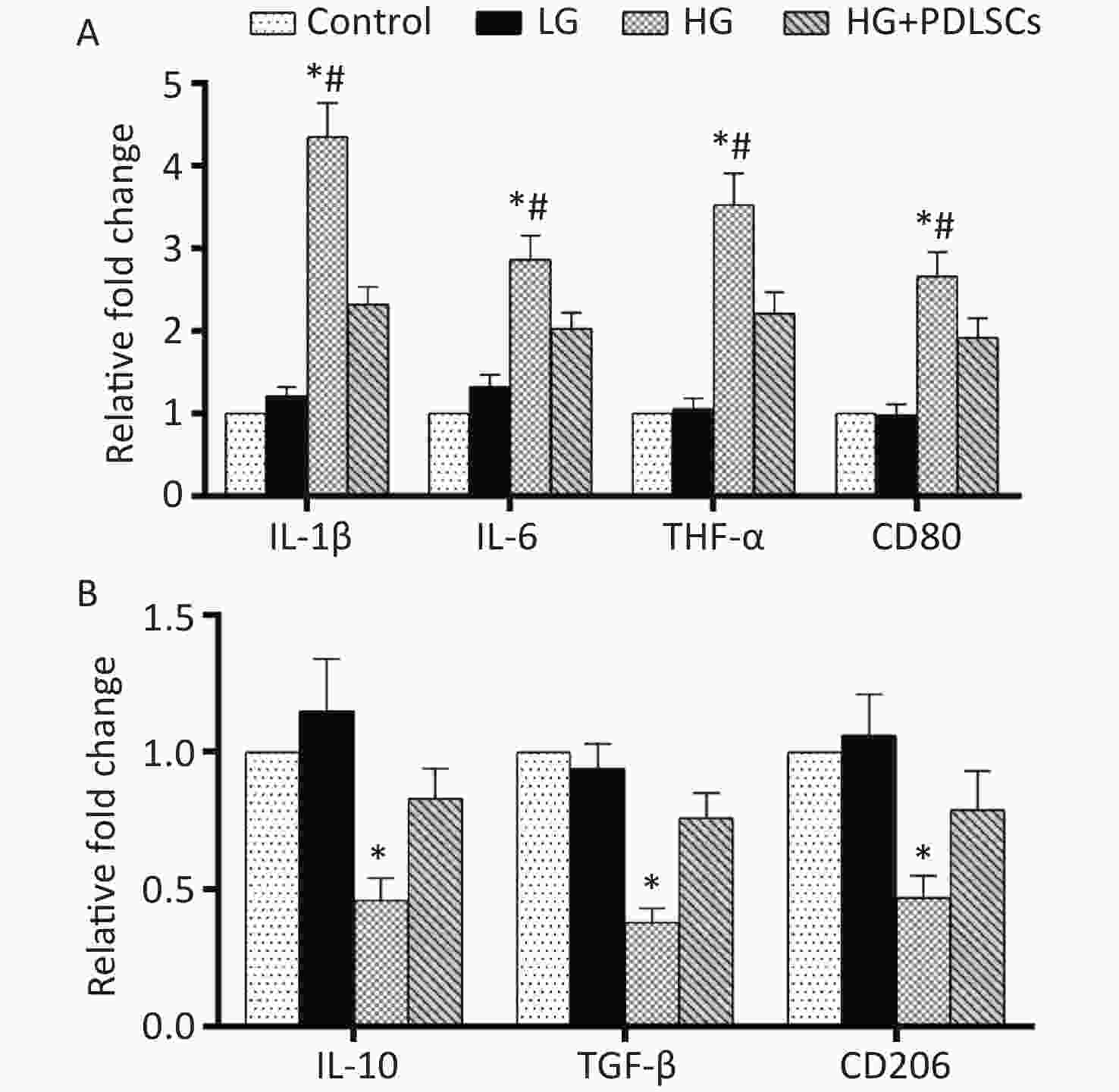
Figure 2. Cytokine identification and macrophage polarization test after high-glucose treatment. (A) RT-PCR analysis showed that high-glucose treatment remarkably up-regulated the expression of IL-1β, IL-6, TNF-α, and CD80 in macrophages. Nevertheless, PDLSCs could reverse this course. *Means statistically significant compared with the control group and LG group (P < 0.01), #means statistically significant compared with HG+PDLSCs group (P < 0.05). (B) RT-PCR analysis showed that high-glucose treatment significantly down-regulated the expression of IL-10, TGF-β, and CD206 in macrophages. However, PDLSCs enhanced the decreased expression of IL-10, TGF-β, and CD206. *Means statistically significant compared with other groups of the same item tested (P < 0.05). The experiments were repeated three times. Control, macrophage culture alone. LG, macrophages + low glucose; HG, macrophages + high glucose; HG+PDLSCs, macrophages + high glucose + PDLSCs.
Groups IL-1β IL-6 TNF-α IL-10 TGF-β Control group 748 ± 69 191 ± 23 103 ± 15 336 ± 47 533 ± 62 Low glucose 907 ± 103 228 ± 31 112 ± 21 364 ± 43 502 ± 49 High glucose 3258 ± 243* 547 ± 62* 366 ± 38* 153 ± 21* 203 ± 33* High glucose+PDLSCs 1367 ± 106 322 ± 41 228 ± 23 275 ± 31 419 ± 49 Note. *Means statistically significant compared with other groups of the same item tested (P < 0.05). Table S2. The concentrations of pro-inflammatory and anti-inflammatory cytokines after high glucose co-culture (pg/mL, mean ± standard deviation)
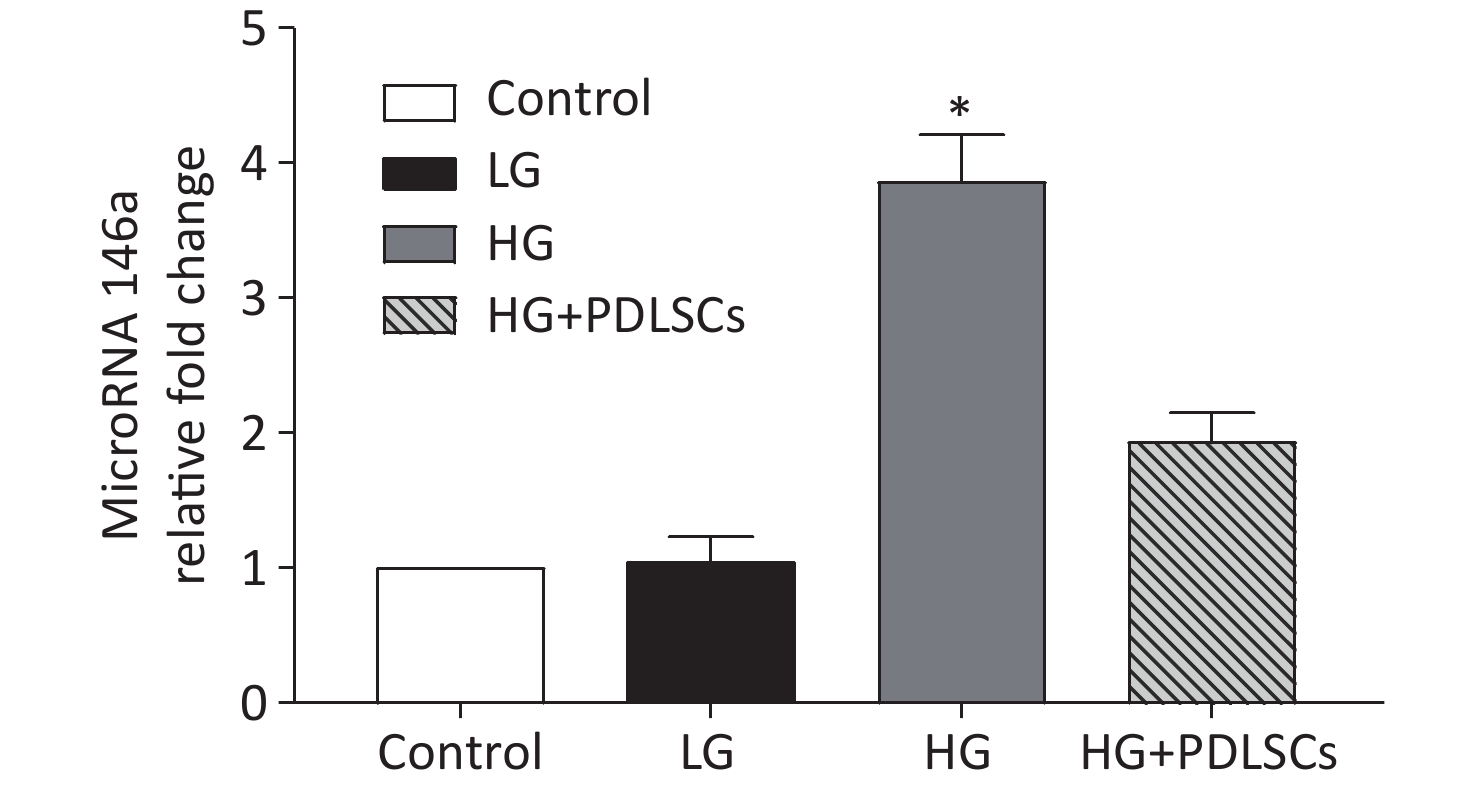
MicroRNA 146a was quantitatively analyzed by RT-PCR to verify its possible role in this course. The results showed that the expression level of microRNA 146a in the high-glucose group was up-regulated statistically compared with that in the low-glucose group and was comparable with that in the macrophages alone. The PDLSCs reduced the expression of microRNA 146a, which was enhanced by incubation with high glucose concentration; however, the rate was still higher than that in the low-glucose group (Supplementary Figure S2 available in www.besjournal.com).
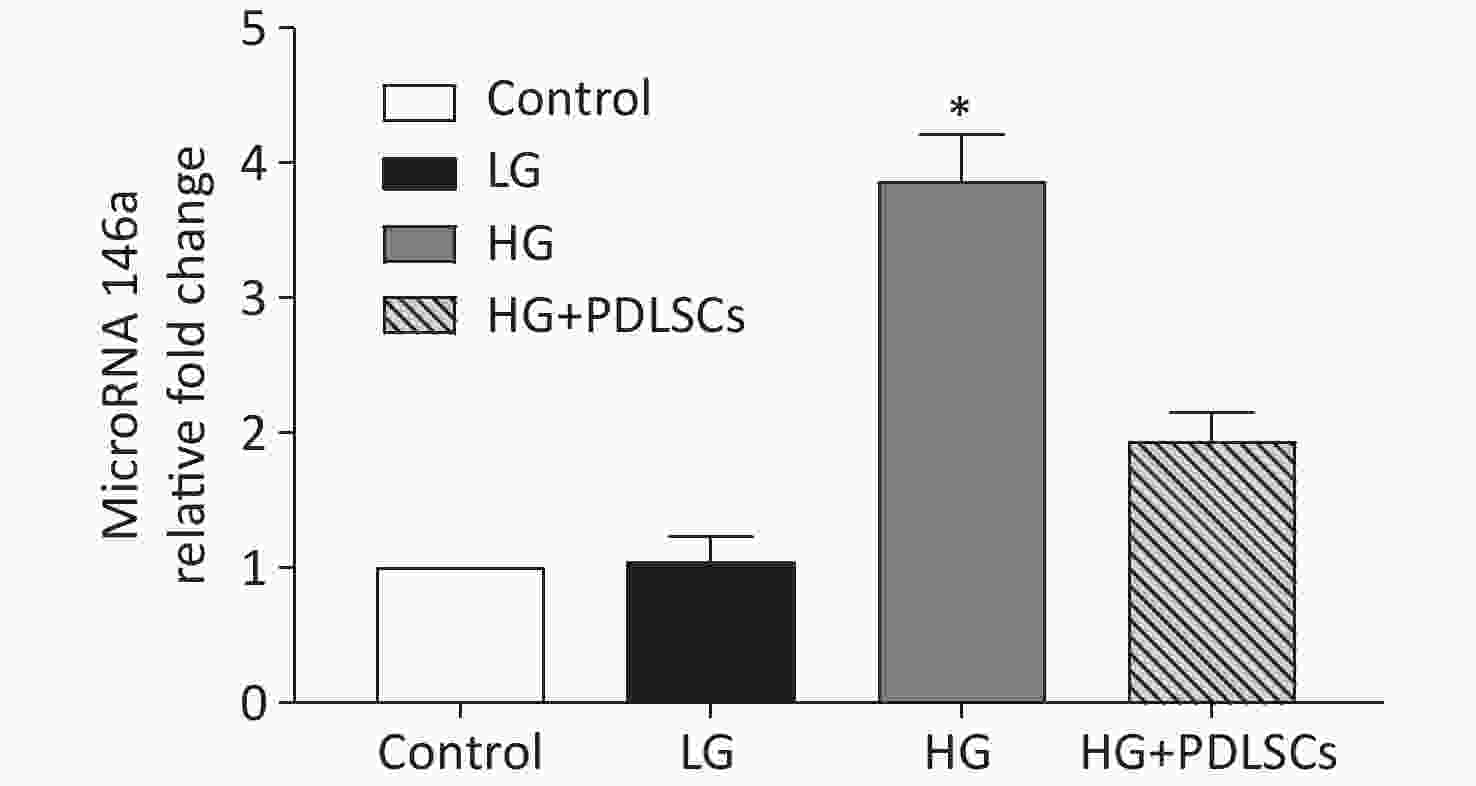
Figure S2. MicroRNA 146a was quantitatively analyzed by RT-PCR, and the data showed that high-glucose treatment up-regulated the level of microRNA 146a. PDLSCs reduced the high-glucose-induced expression of microRNA 146a. *Means statistically significant compared with other groups (P < 0.05). The experiments were repeated three times. Control, macrophage culture alone. LG, macrophages + low glucose; HG, macrophages + high glucose; HG+PDLSCs, macrophages + high glucose + PDLSCs.
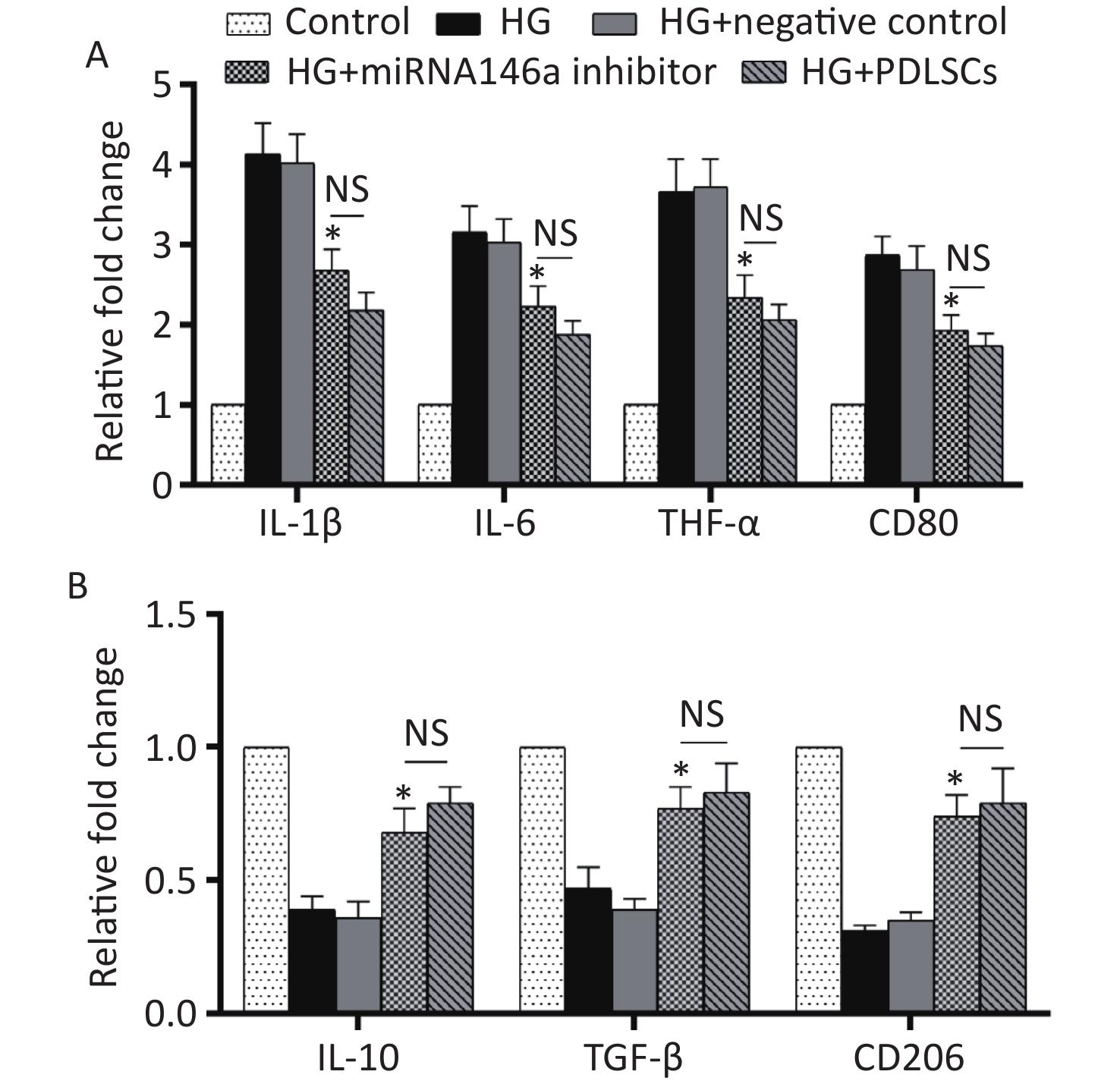
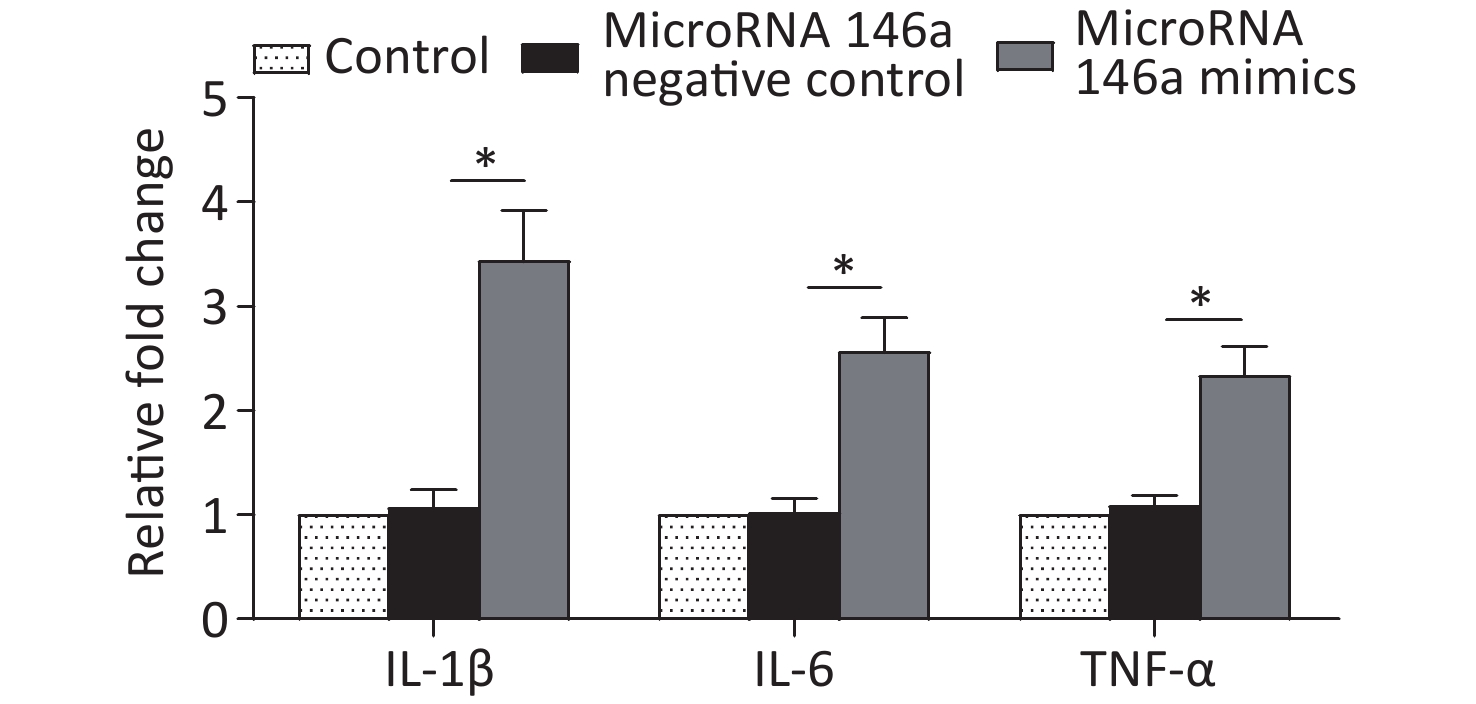
THP-1-derived macrophages were transfected with microRNA 146a inhibitor or negative control for 48 hours to further confirm microRNA 146a’s potential functions in high-glucose-mediated macrophage M1 polarization. The macrophages were co-cultured with different glucose concentrations for another 48 hours. After the co-culture, the cells were harvested and used for RT-PCR analysis. The results showed that microRNA 146a inhibitor suppressed the expression levels of IL-1β, IL-6, TNF-α, and CD80 (Figure 3A), which were up-regulated by high-glucose treatment. Furthermore, the expression levels of IL-10, TGF-β, and CD206 (Figure 3B), which were down-regulated by high-glucose treatment, were increased to the same extent as PDLSCs’ influence (Figure 3A, B). Moreover, the transfection of microRNA 146a mimics promoted the expression of IL-1β, IL-6, and TNF-α in macrophages (Supplementary Figure S3, available in www.besjournal.com). ELISA indicated that microRNA 146a inhibitor reduced the secretion of IL-1β, IL-6, and TNF-α and increased the secretion of IL-10 and TGF-β, and these actions were comparable with the effects of PDLSCs (Supplementary Table S3 available in www.besjournal.com).
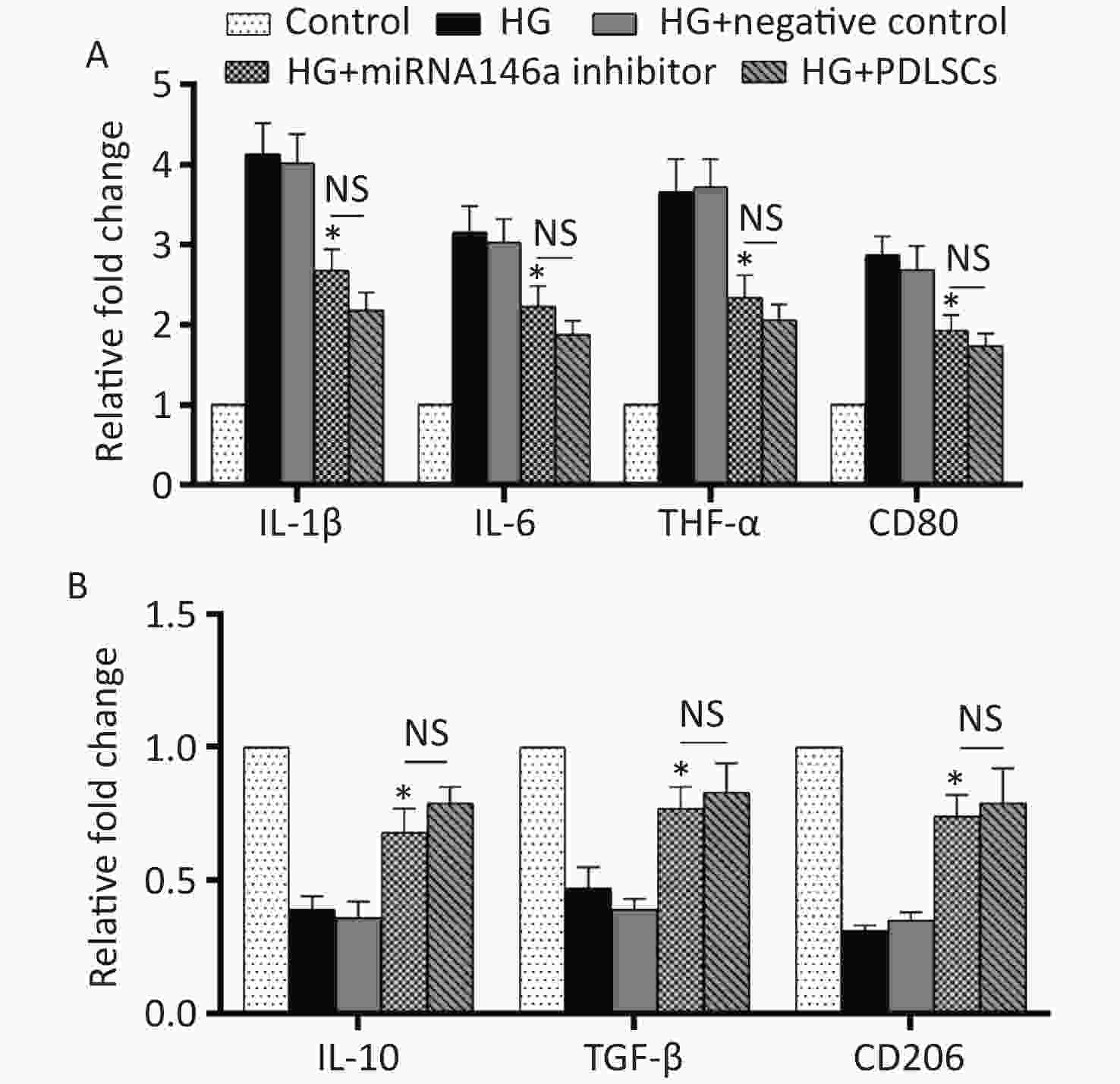
Figure 3. Cytokine identification and macrophage polarization test after the transfection of microRNA 146a inhibitor. MicroRNA 146a inhibitor transfection suppressed the expression levels of IL-1β, IL-6, TNF-α, and CD80 (A), which were up-regulated by high-glucose treatment, and increased the expression levels of IL-10, TGF-β, and CD206 (B), which were down-regulated by high-glucose treatment, to the same extent as PDLSCs’ influence. *Means statistically significant compared with the HG group (P < 0.05). NS, no significant. The experiments were repeated three times. Control, macrophage culture alone. HG, macrophages + high glucose; HG+negative control, macrophages + high glucose + transfection of negative control; HG+microRNA 146a inhibitor, macrophages + high glucose + transfection of microRNA 146a inhibitor; HG+PDLSCs, macrophages + high glucose + PDLSCs.
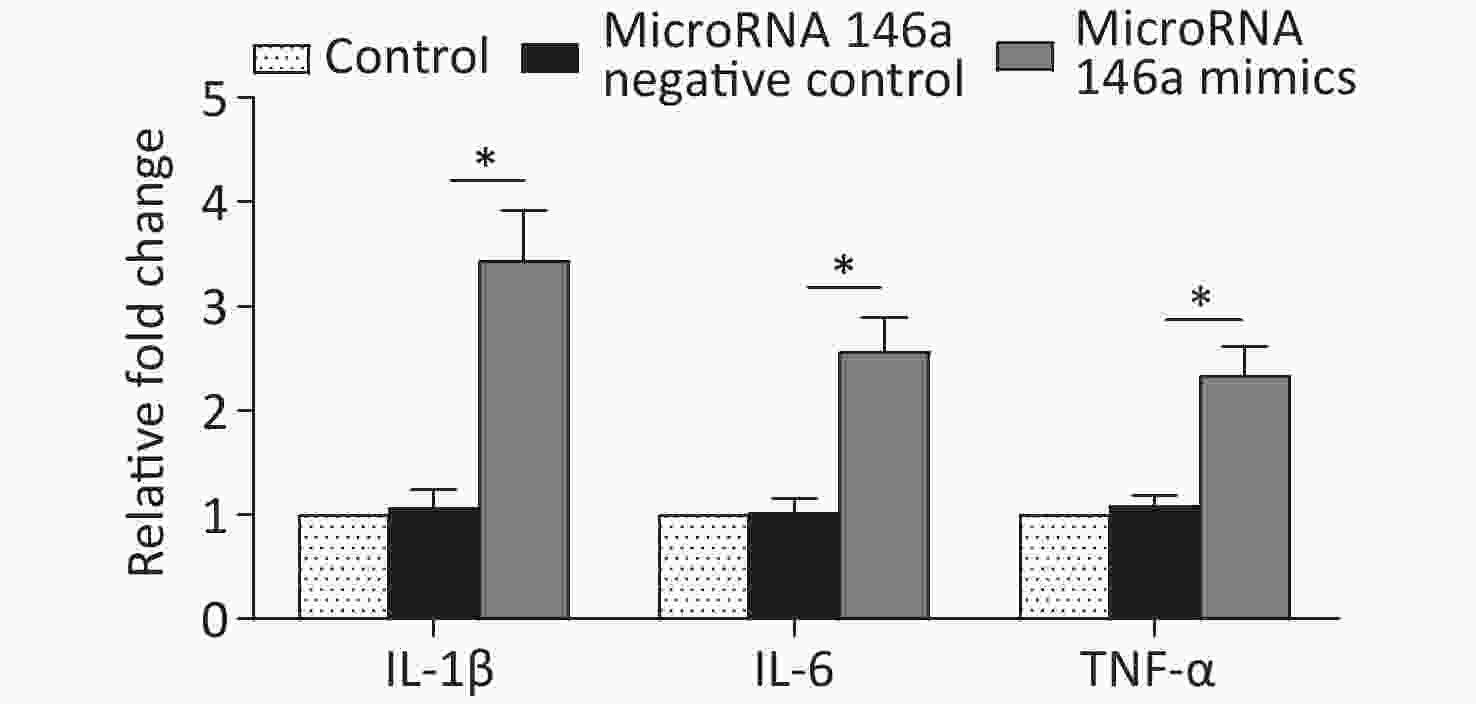
Figure S3. Transfection of microRNA 146a promoted the expression levels of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, compared with those in the group of untreated macrophages and group of untreated macrophages + microRNA 146a negative control. *Means statistically significant compared with other groups (P < 0.05). The experiments were repeated three times. control, macrophage culture alone. microRNA 146a negative control, macrophages + microRNA 146a negative control. microRNA 146a mimics, macrophages + microRNA 146a mimics.
Groups IL-1β IL-6 TNF-α IL-10 TGF-β Control group 673 ± 78 206 ± 18 117 ± 21 353 ± 38 495 ± 55 High glucose 3309 ± 198 483 ± 51 344 ± 45 128 ± 19 227 ± 26 High glucose+transfection
of negative control3255 ± 243 502 ± 44 328 ± 39 116 ± 22 234 ± 32 High glucose+transfection of microRNA 146a inhibitor 1539 ± 162* 336 ± 49* 247 ± 35* 235 ± 38* 396 ± 45* High glucose+PDLSCs 1259 ± 133 298 ± 37 217 ± 23 278 ± 36 443 ± 38 Note. *Means statistically significant compared with the high glucose group of the same item tested (P < 0.05). Table S3. ELISA indicated that microRNA 146a inhibitor reduced the secretion of IL-1β, IL-6, and TNF-α and increased the secretion of IL-10 and TGF-β, and these actions were comparable with the effects of PDLSCs
Periodontitis damages local periodontal tissues and is closely related to other systemic diseases, such as diabetes. Local inflammatory reactions may give rise to the damage of periodontal supporting tissues, and the uncontrolled inflammatory reaction is the basis of the relationship between diabetes and periodontitis. The levels of systemic inflammatory mediators play an important role in the association between diabetes and periodontitis. As highly plastic immune cells, macrophages have important functions in bacterial phagocytosis and the occurrence and development of inflammation. High glucose levels can interfere with the proliferation and apoptosis of cells in the body and affect the functions of macrophages.
Macrophages can produce a large number of factors to balance inflammatory responses. Affected by various internal and external influencing factors, macrophages can polarize into different sub-types and function differently in physiological and pathological conditions. M1 macrophages are involved in inflammatory responses, and M2 macrophages participate in the anti-inflammatory process. An extensive relationship exists between macrophages and periodontitis’ occurrence and development, and the M1/M2 status of macrophage polarization plays a key role in the pathogenesis of periodontitis. M1 and M2 macrophages are responsible for the destructive and reparative stages of periodontitis, respectively. Periodontitis can be cured by polarizing macrophages toward the M2 anti-inflammatory state.
In this study, our data showed that high glucose concentration (30 mmol/L) could increase the expression of CD80 (M1 marker) and pro-inflammatory cytokines IL-1β, IL-6, and TNF-α and decrease the expression of CD206 (M2 marker) and anti-inflammatory cytokines IL-10 and TGF-β, thus converting macrophages into M1 polarization. This phenomenon may lead to periodontitis onset or further aggravate periodontitis. High glucose levels induce macrophages to their inflammatory state by up-regulating pro-inflammatory cytokines and down-regulating anti-inflammatory cytokines[4], which is consistent with the findings of our study. Inflammatory cytokines, especially IL-1β, IL-6, and TNF-α, play crucial roles in periodontitis, including inducing macrophages and gingival fibroblasts to produce prostaglandins, promoting the activation of osteoclasts, inhibiting the synthesis of collagen, and enhancing bone resorption to increase the destruction of periodontal tissue and exacerbate periodontitis.
Non-surgical and surgical procedures and guided tissue regeneration are used for the treatment of periodontitis by removing pathogens and necrotic tissues to prevent disease progression and repair a small number of periodontal tissues. However, the overall results are not necessarily satisfactory and lack clinical predictability. Under appropriate induction conditions, PDLSCs can form multiple periodontal tissues, including peridontal ligament, alveolar bone, and cementum, which will be the mainstream strategy of periodontitis treatment in clinics. A recent study using a paracrine model showed that PDLSCs produce a large number of cytokines and participate in tissue regeneration[5]. MicroRNAs are small endogenous and ubiquitous RNAs that regulate gene expression post-transcriptionally and are regarded as key regulators in the occurrence and progression of diseases, including periodontitis.
Our results showed that the expression level of microRNA 146a in the high-glucose group was up-regulated statistically. Transfection with miRNA 146a inhibitor significantly suppressed IL-1β, IL-6, and TNF-α; up-regulated IL-10 and TGF-β; and converted the M1/M2 polarization of macrophages, all of which were reversed by high-glucose treatment to induce inflammation. Additionally, the effects of microRNA 146a inhibitor are similar to those of PDLSCs, which prompt the M2 polarization of macrophages and efficaciously contribute to the periodontal tissue regeneration.
MicroRNA 146a mediates inflammatory responses, antigen presentation, and cytokine secretion, thereby participating in immune and inflammatory reactions and playing a crucial role in inflammation. MicroRNA 146a expression level is significantly increased in a variety of inflammatory diseases, including acute pneumonia, coronavirus disease 2019 (COVID-19), and psoriasis, and is closely related to disease prognosis[6-8]. Our data showed that the up-regulation of microRNA 146a was involved in the shifting of macrophages into their pro-inflammatory M1 state, indicating that microRNA 146 has inflammation-promoting functions. MicroRNA 146a is the most widely studied microRNA in periodontal diseases, and its expression is higher in patients with periodontitis than in healthy controls; a positive correlation was found between microRNA 146a level and periodontitis severity in the assessment of clinical diagnostic indicators, including probing depth, clinical attachment level, and bleeding on probing[9]. Moreover, microRNA 146a may be a feasible biomarker to monitor and aid in the early diagnosis of periodontitis[9]. Subgingival plaque samples and serum samples of patients with periodontitis were subjected to quantitative PCR to assess the levels of circulating microRNA 146a and associated pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and to understand the detrimental effects of microRNA 146a in periodontal tissues; the data showed that elevated microRNA 146a expression was accompanied by the up-regulation of associated cytokines[10]. MicroRNA 146a is an inflammatory regulator that interacts with anti-inflammatory cytokines, such as IL-13, and PDLSCs transfected with microRNA 146a can remarkably reduce the proliferation rate compared with that in negative controls. Thus, microRNA 146a is possibly involved in periodontitis by down-regulating IL-13 and inhibiting PDLSC proliferation.
This study demonstrated that high glucose levels significantly inhibited the proliferation of macrophages and converted these cells to their pro-inflammatory M1 state by up-regulating microRNA 146a. This effect could be partially counteracted by PDLSCs.
Competing Interests The authors declare no competing interests.
HTML
 22078Supplementary Materials.pdf
22078Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: