-
Gas explosion injury is a compound injury caused by a shock wave, high-temperature flame, or toxic gas directly or indirectly acting on the human body, resulting in multiple organ damage. As a hyperaerated tissue, the lung is the primary organ that experiences an early injury. The pathological manifestations of gas-explosion-induced acute lung injury (ALI) include pulmonary tissue edema, pulmonary capillary rupture, airway wall congestion, edema, alveolar hemorrhage, and infiltration and accumulation of inflammatory cells[1]. Symptomatic supportive treatments such as antibacterial and anti-inflammatory agents, fluid-replacement therapy, and tracheotomy are mainly used in the clinic to treat gas-explosion-induced ALI. However, mature treatment systems and targeted treatment drugs are lacking, which impedes effective judgment on the therapeutic effect and prognosis of patients with gas explosion-induced clinical ALI. Accordingly, gas explosion-induced ALI has become one of the main causes of early death[2]. Gas explosion models are difficult to simulate because the explosion is complex, and subsequent biological material may not be available. Although some simple animal models have been developed, these models are mostly at the numerical simulation stage, and they do not reflect the complex and diverse biological responses to gas explosions. As per our previous study, 24 h after exposure to a gas explosion, severe lung pathological injuries were observed in rats, with significantly reduced minute volume (MVb), peak inspiratory flow (PIFb), and peak expiratory flow (PEFb), significantly increased enhanced pause (Penh), excessive activation of autophagy, and aggravation of lung injury[3]. However, the detailed molecular mechanism of gas explosion-induced ALI is unclear. This study simulated the complex and multifaceted biological reactions occurring during underground roadway gas explosions. It provided the possibility to further clarify the pathogenesis of gas-explosion-induced ALI and search for early diagnostic biomarkers and therapeutic targets.
Based on the data-independent acquisition (DIA) strategy, mass spectrometry has been extensively used in proteomics owing to its repeatability and comprehensiveness [4]. Currently available proteomic techniques are effective tools for medical research, and they have been extensively applied in studies of several illnesses to detect changes in the expression patterns of different signaling pathways and identify diagnostic biomarkers and therapeutic targets. Quantitative proteomics has been used to analyze the lung tissues of four patients with coronavirus disease 2019 (COVID-19), resulting in the identification of 641 differentially expressed proteins (DEPs) associated with the activation of the NF-κB pathway leading to upregulated expression of inflammatory factors [5]. These findings have helped us understand the pathological process of COVID-19 pneumonia. Therefore, DIA proteomics also provides a new method and technique for studying the molecular mechanism of gas explosion-induced ALI.
In this study, we attempted to explore the potential mechanisms of gas explosion-induced ALI at different time points and identify early biomarkers and therapeutic targets for the disease using DIA proteomic techniques.
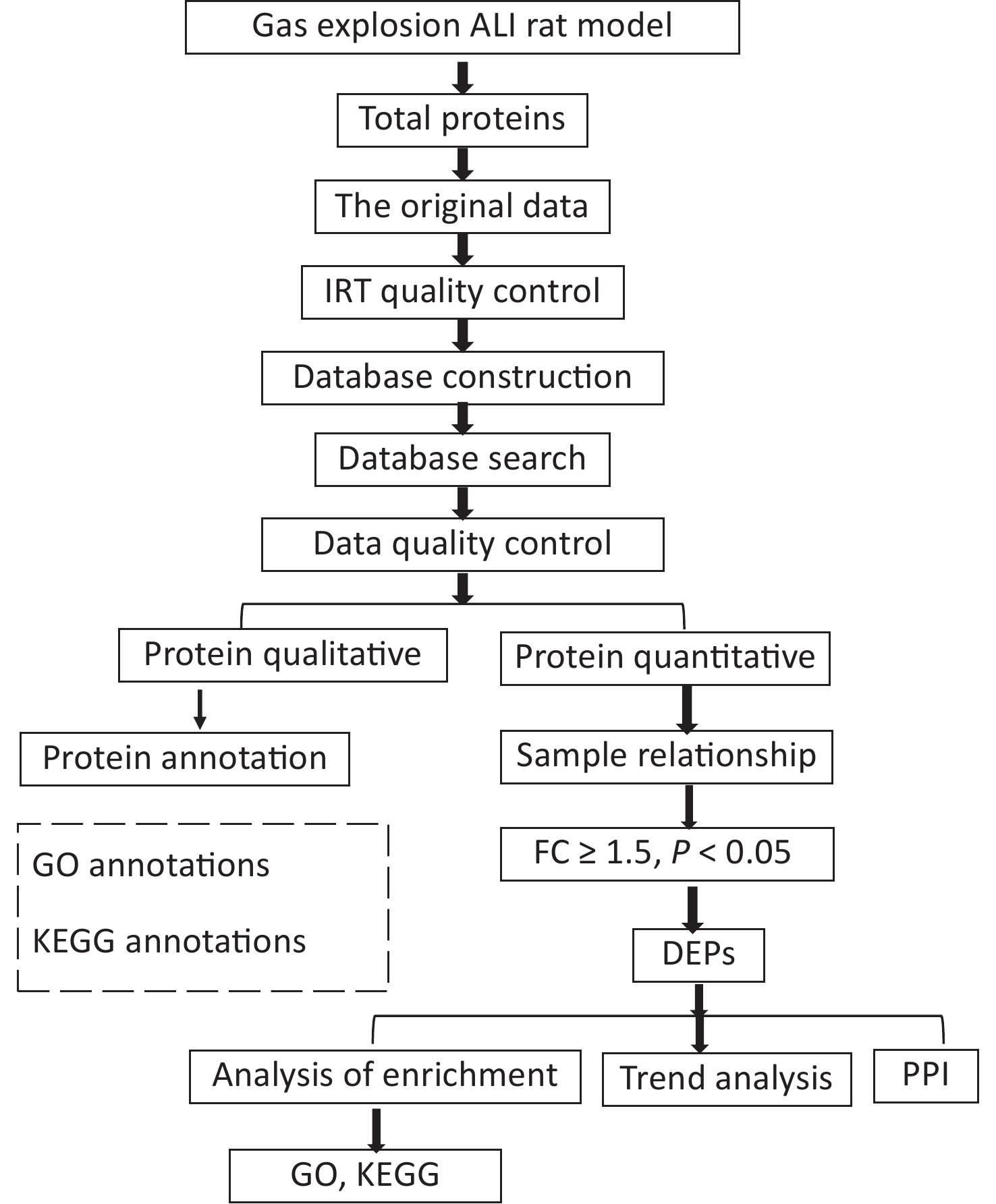
This study was reviewed and approved by the Animal Ethics Committee of Xinxiang Medical University (No. XYLL-2019001). A total of 50 male specific-pathogen-free rats (aged 6–8 weeks, weighing 180–220 g) were purchased from Beijing Sipeifu Biotechnology Co., Ltd. After weighing, the rats were randomly divided into control (n = 10) and gas-explosion-induced ALI groups (n = 10). Rats in the model group were divided into four observation periods, namely, 2 h, 24 h, 72 h, and 1 week after the gas explosion, with 10 rats in each group. At the gas explosion experiment tunnel at Chongqing Research Institute of China Coal Science & Engineering Group, two special four-layered rat cages, with one cage per layer, were fixed on both sides of the tunnel 160 m away from the explosion point. A 100 m3 mixture of methane gas and air containing (9 ± 0.5)% gas was detonated with 2 × 10 J of energy, leading to the successful establishment of a rat model of gas-explosion-induced ALI. The control group received no treatment and was housed outside the underground roadway. On the day of the pulmonary function test, the rats were placed in the monitoring room 30 min in advance to acclimatize. The air tightness of the pulmonary function monitor (EMKA Technologies, Paris, France) was confirmed before testing. The parameter settings were as follows: adaptation time, 10 min; atomization time, 1 s; reaction time, 10 min; and recovery time, 1 min. Pulmonary function tests were performed with six rats simultaneously. The control and model groups were tested at the corresponding time points. Pulmonary function tests and hematoxylin and eosin (H&E) staining were used to detect lung histological changes. The histopathological changes in the lung tissue were evaluated by Mikawa’s standard from four aspects: inflammatory factor exudation, significant swelling, hemorrhage, and alveolar wall thickening [6]. Three lung tissue samples were selected from each group. DIA proteomics was used to detect proteins in the lung tissue samples. Data-independent acquisition proteomic raw data were obtained, quality control of proteomic data identification of DEPs and bioinformatics analysis (Supplementary Figure S1, available in www.besjournal.com). The DEPs were verified by immunofluorescence, quantitative polymerase chain reaction (qPCR), and Western blotting.
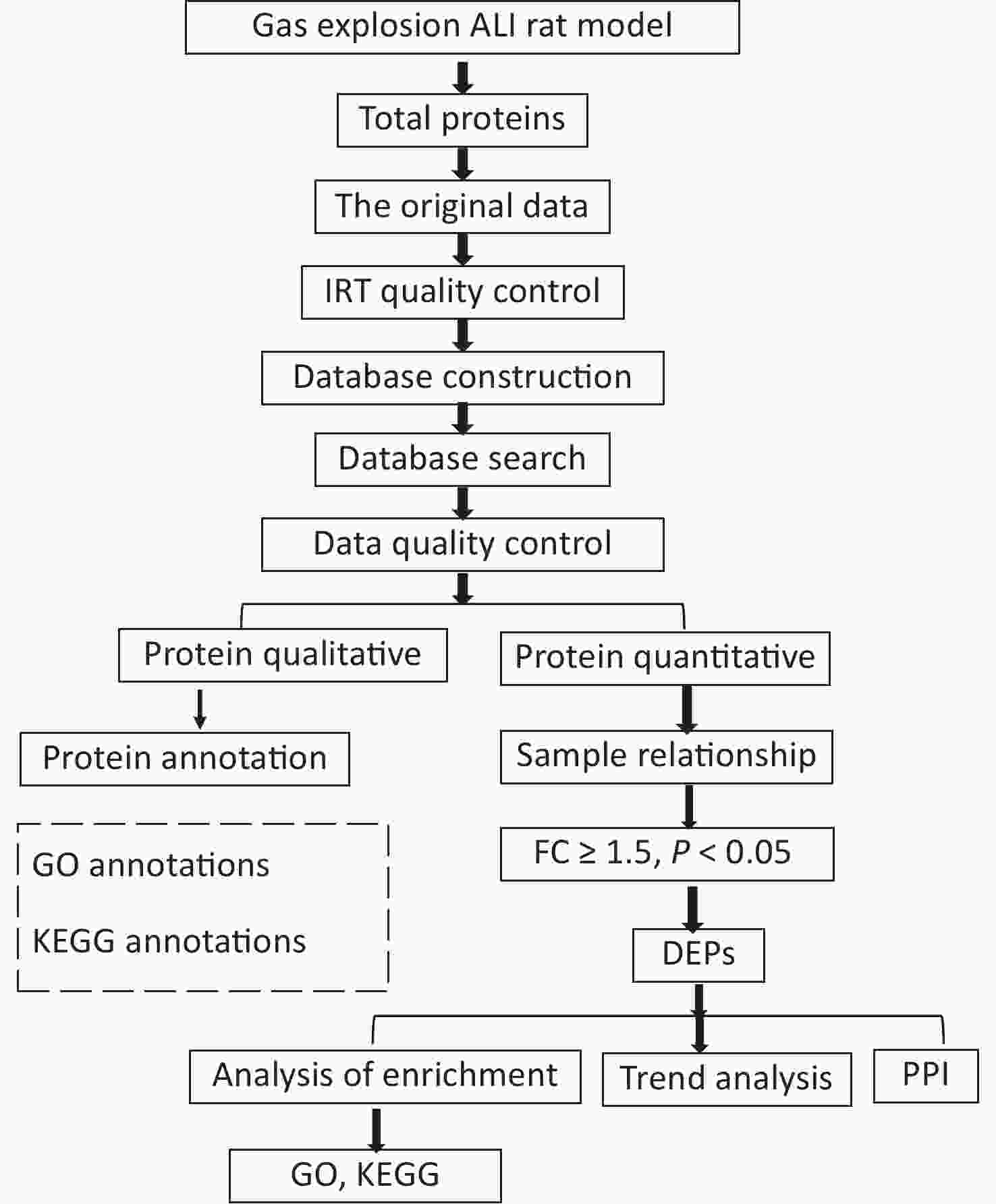
Figure S1. Flowchart of the proteomics analysis. Data-independent acquisition proteomic raw data were obtained, quality control of proteomic data identification of differentially expressed proteins and bioinformatics analysis.
All statistical analyses were performed using IBM SPSS Statistics for Windows version 20.0 (IBM Corp., Armonk, NY, USA). Student’s t-test or a one-way analysis of variance was used for between-group comparisons. All raw data are presented as the mean ± standard deviation. A P-value < 0.05 indicated statistical significance.
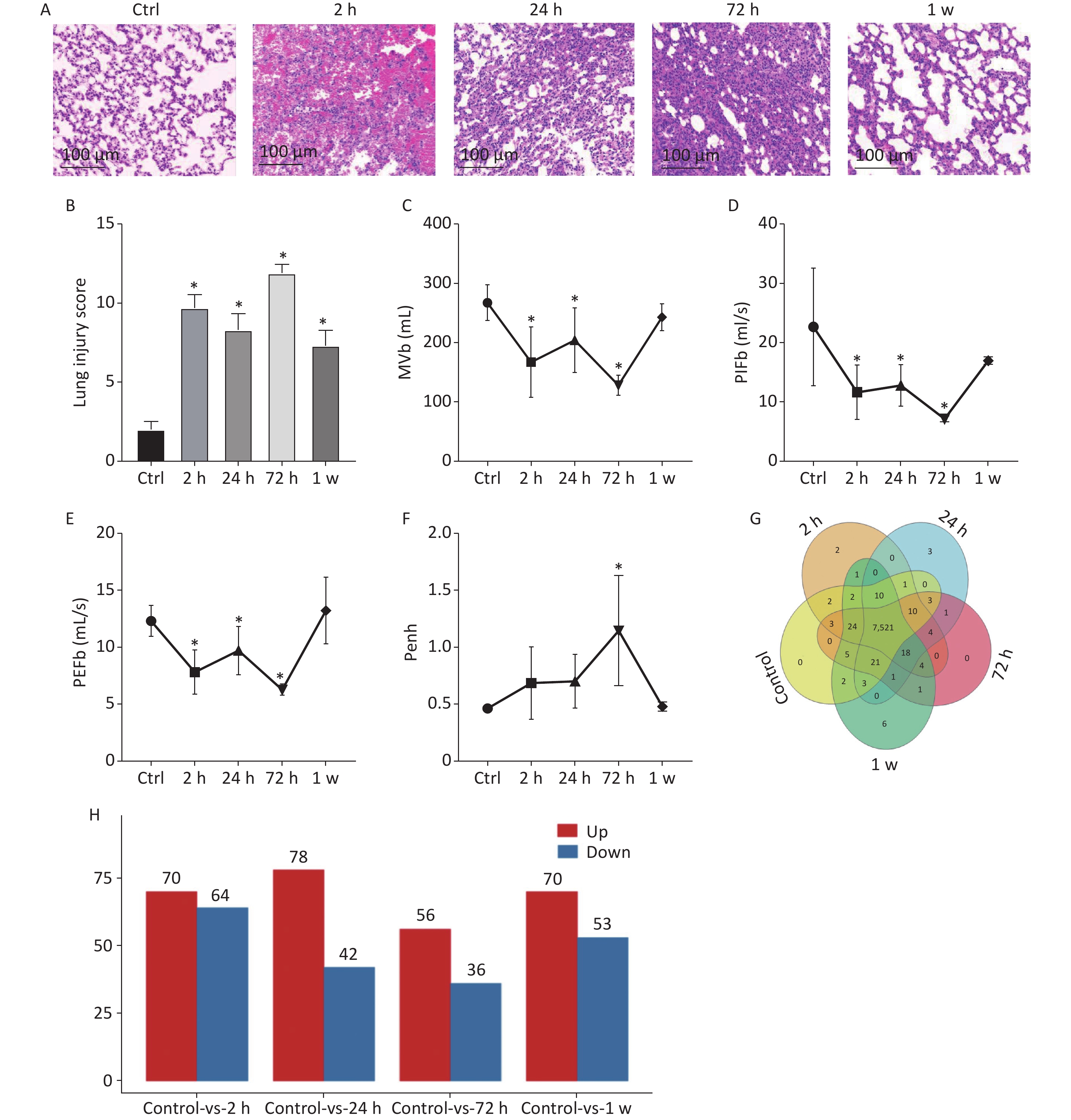
In this study, to observe lung histological changes by H&E staining, we established the rat model of gas explosion-induced ALI based on the gas explosion experiment tunnel at Chongqing Research Institute of China Coal Science & Engineering Group. Compared with the control group, the model group had a ruptured alveolar basement membrane, incomplete alveolar structure, and obvious hemorrhage in the alveolar cavity and interstitial lung 2 h after the gas explosion. Moreover, 24 h after the gas explosion, the alveolar epithelial cells appeared significantly swollen, the alveolar wall was thickened, and inflammatory cell infiltration in the alveolar cavity and lung interstitial increased. From 24 h to 72 h after the gas explosion, inflammatory cell infiltration significantly increased, but hemorrhage was reduced. Subsequently, hemorrhagic and edematous lung tissue returned to normal within 1 week after the gas explosion (Figure 1A). To further quantify the pathological lung injuries in the model group, Mikawa’s standard was used, and the results are shown in Figure 1B. In addition, we examined the change in pulmonary function. Compared with the control group, the model group had significantly decreased MVb, PIFb, and PEFb after the gas explosion, which slightly increased 24 h compared with 2 h after the explosion, significantly decreased 72 h, and then gradually recovered to normal levels. Compared with the control group, the model group had gradually increased Penh value after the gas explosion, which significantly increased after 72 h and then gradually decreased and returned to its normal level (Figure 1C–F). These results suggested that the lung tissue volume decreased, airway resistance increased significantly, and lung ventilation capacity decreased, which were consistent with the pathological injury scores. The most serious injury occurred 72 h after the gas explosion.
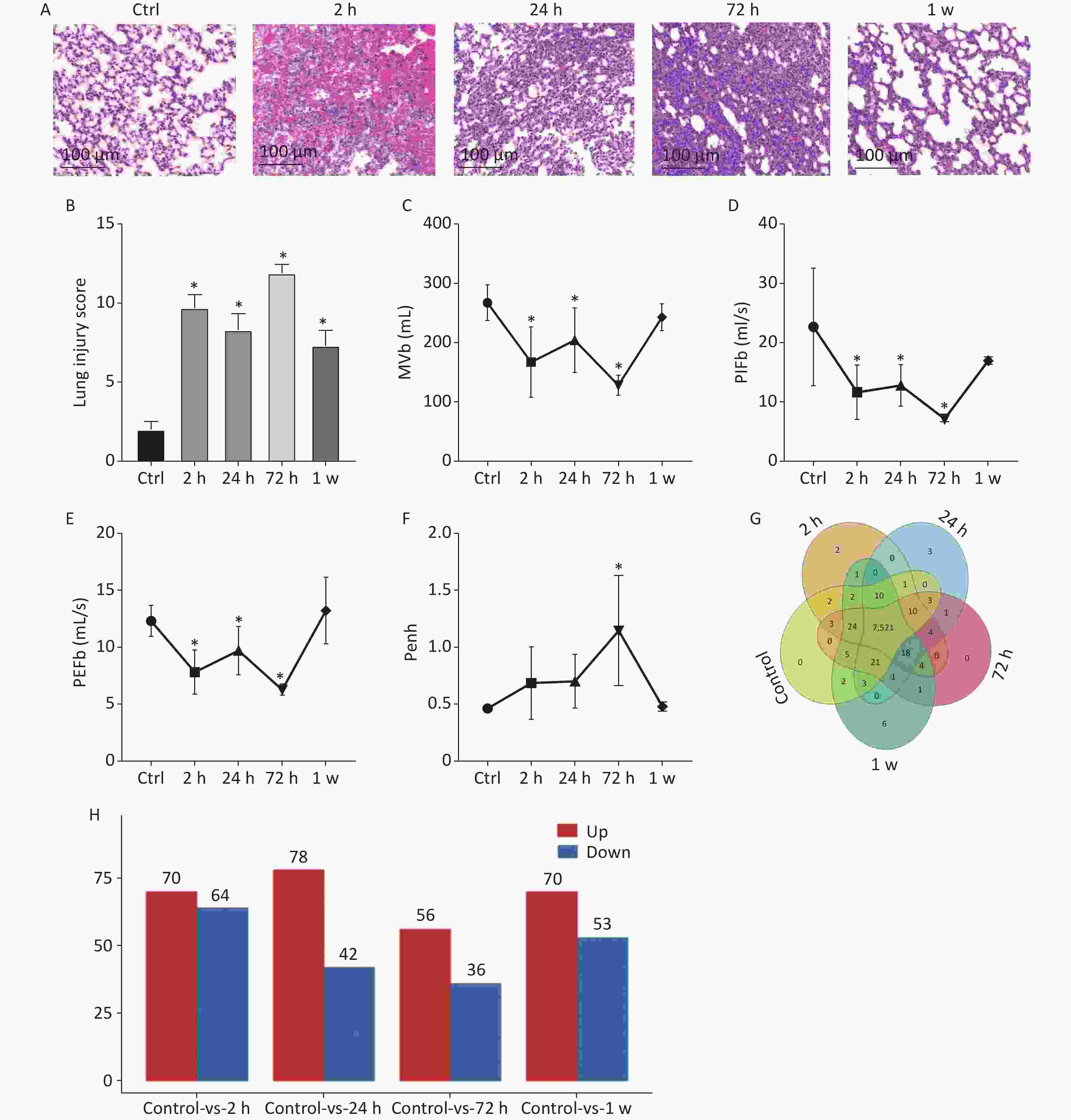
Figure 1. (A) Histology of lung tissue calculated using H&E staining (200×). (B) Lung injury score (n = 9). (C)–(F) Changes in respiratory function parameters, MVb, PIFb, PEFb, and Penh value in rats with gas explosion-induced ALI. (Pulmonary function tests were performed with six rats simultaneously, n = 6). (G) Venn diagram of all expressed proteins in rat lung tissue at different time points after the gas explosion. The overlapping parts of the Venn diagram represent the number of proteins shared between groups, and the non-overlapping parts represent proteins unique to each group (protein abundance > 1). (H) Histogram of the DEPs identified in the gas explosion rat model at different time points. The abscissa represents the model groups at different time points, and the ordinate represents the number of DEPs. Red indicates the number of upregulated DEPs, whereas blue indicates the number of downregulated DEPs. *Indicates the P < 0.05 model compared with the control (Ctrl) group. ALI, acute lung injury; DEPs, differentially expressed proteins; H&E, hematoxylin and eosin; MVb, minute volume; PIFb, peak inspiratory flow; PEFb peak expiratory flow.
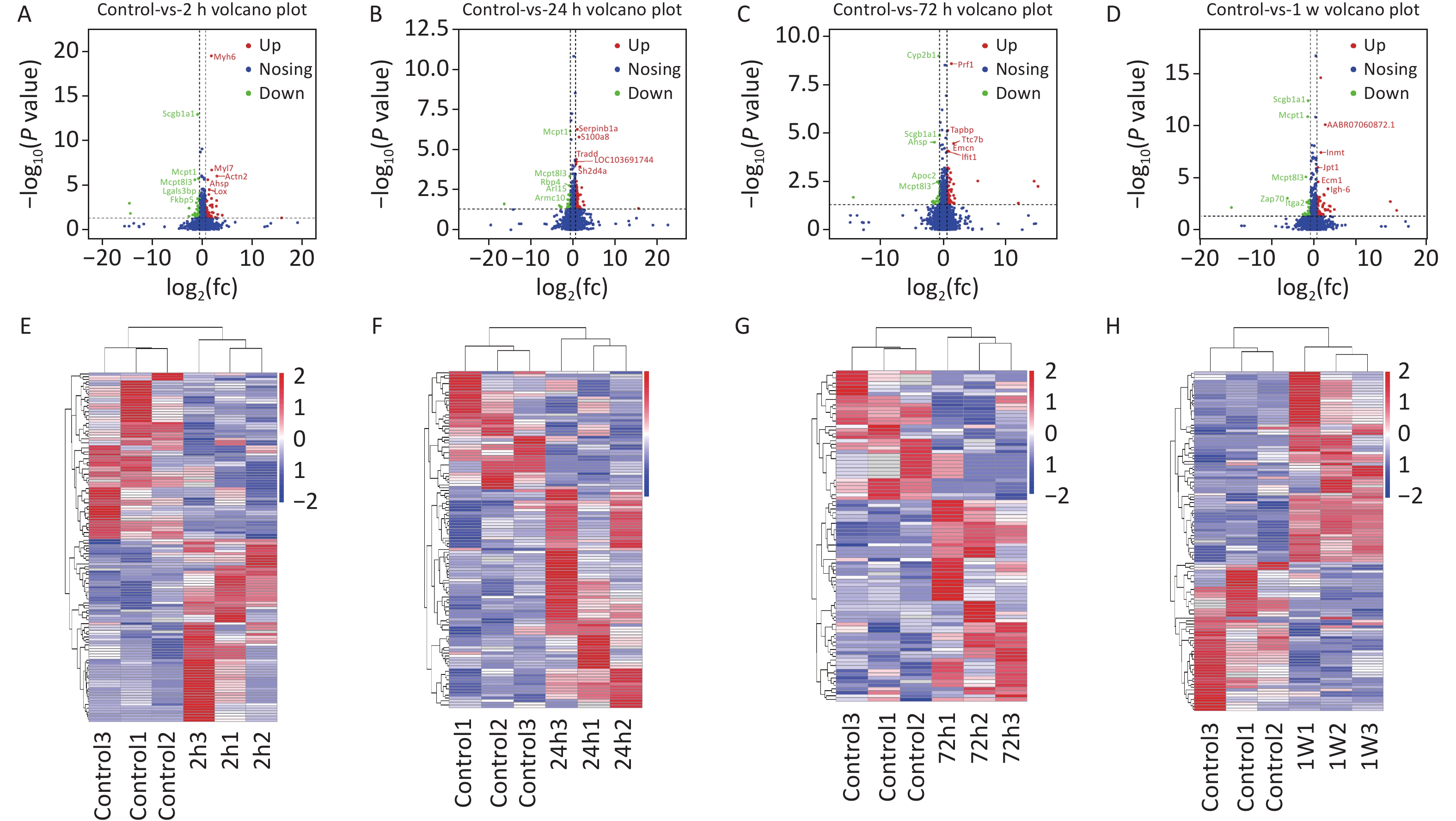
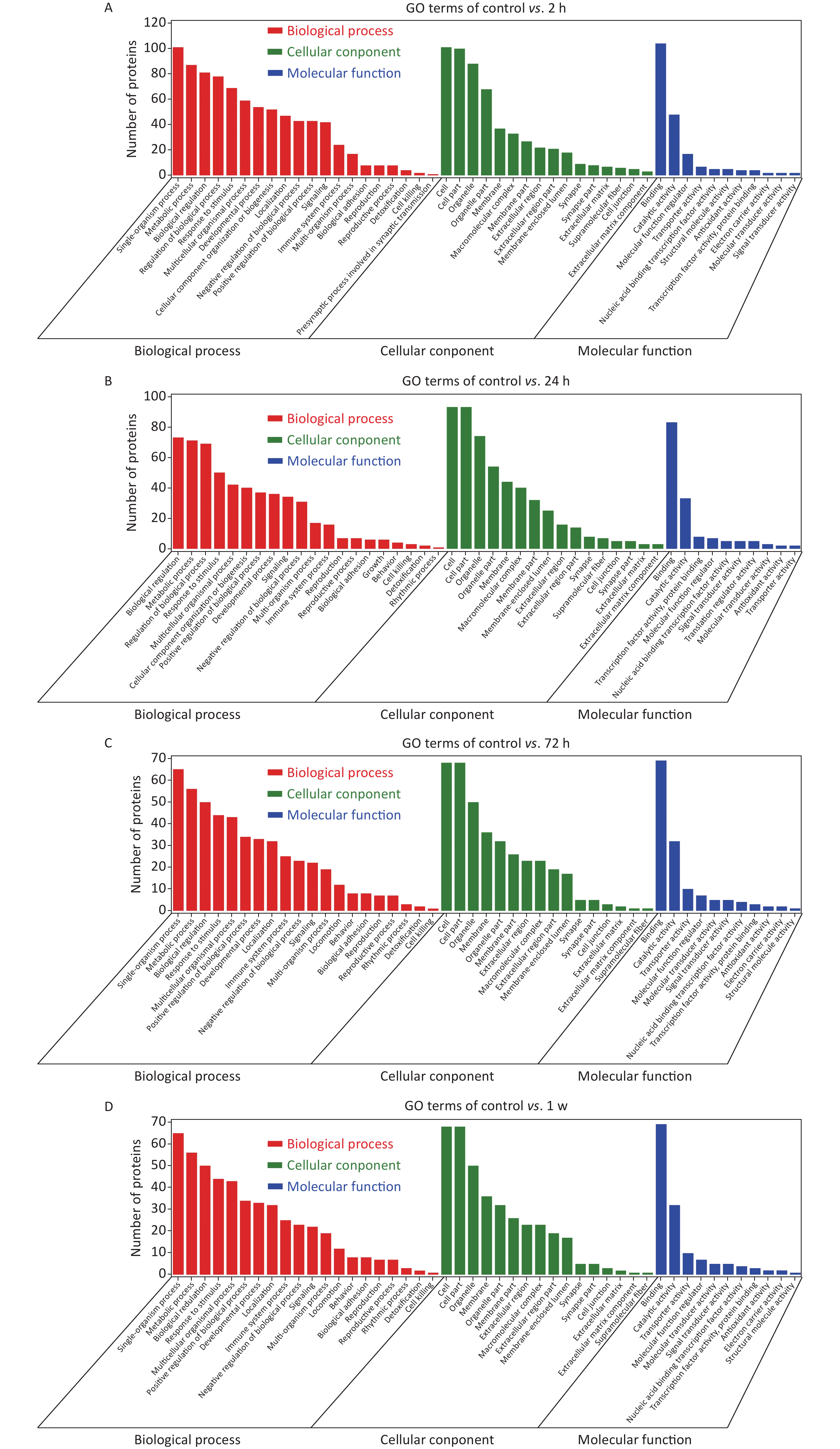
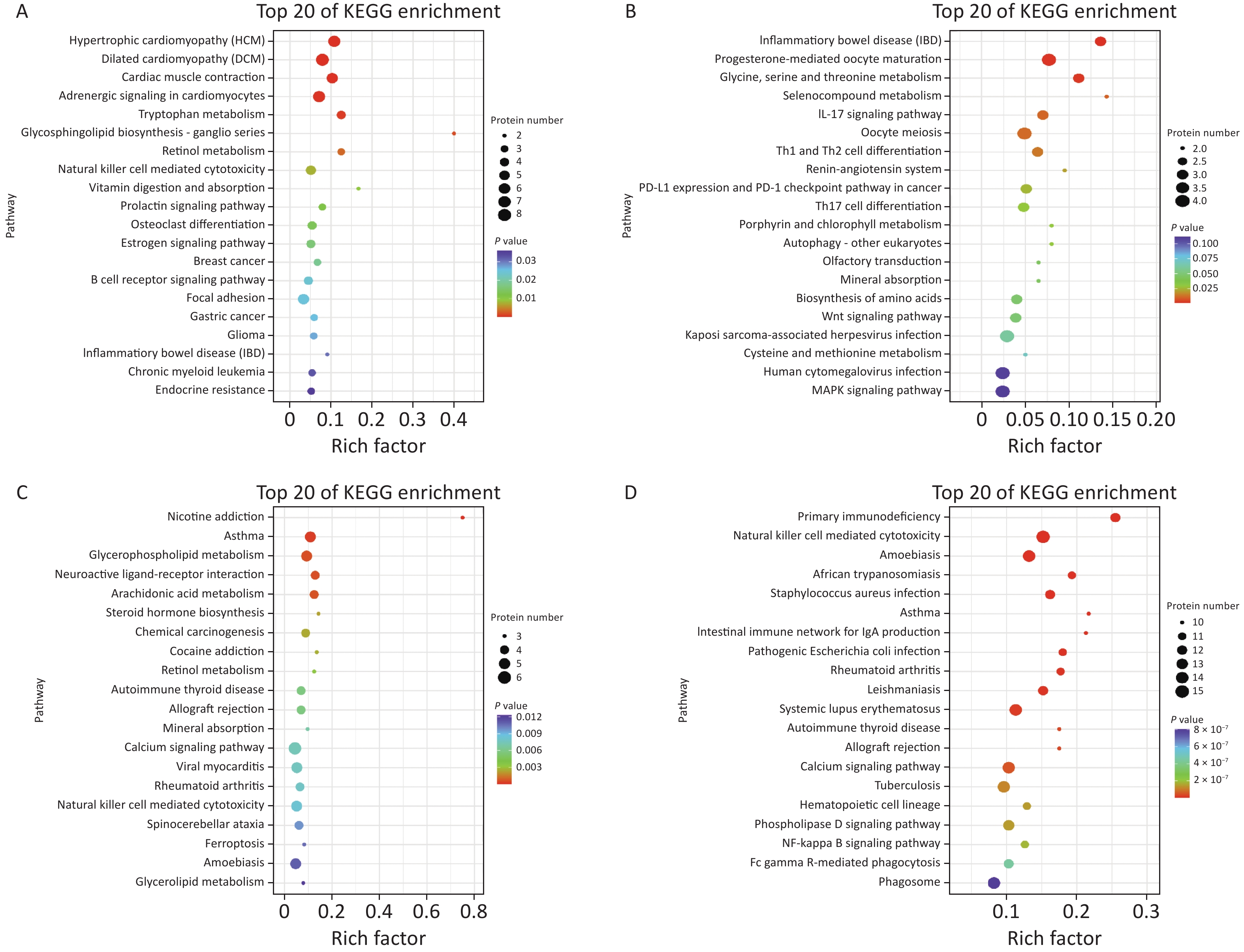
To identify the differential protein expression profile and further explore the pathological mechanism of gas explosion-induced ALI, DIA quantitative proteomics technology was used to test rat lung tissue samples after the gas explosion. A total of 7,648 proteins (protein abundance > 1) were identified using quantitative proteomics technology (Figure 1G), and 404 DEPs were detected in the model group compared with the control group, with a fold change of > 1.5 and P < 0.05. A total of 134 DEPs were also identified 2 h after the gas explosion (70 and 64 DEPs were upregulated and downregulated, respectively) compared with their expression in the control group. Moreover, 24 h after the gas explosion, 120 DEPs were identified, including 78 upregulated DEPs and 42 downregulated DEPs. At 72 h after the gas explosion, 92 DEPs were identified, including 56 upregulated DEPs and 36 downregulated DEPs. One week after the gas explosion, 123 DEPs were identified, including 70 upregulated DEPs and 53 upregulated DEPs. The histograms for the control and model groups are shown in Figure 1H. The volcano plots and heatmap of DEPs in gas explosion-induced ALI are provided in Supplementary Figure S2 (available in www.besjournal.com). To further investigate the biological functions of the DEPs related to gas-explosion-induced ALI, Gene Ontology (GO) analysis was used to annotate the biological functions of the DEPs in rats at 2 h, 24 h, 72 h, and 1 week (Supplementary Figure S3, available in www.besjournal.com). Through Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis, autophagy-animal, autophagy-other eukaryotes, mitophagy-animal, PI3K-Akt signaling pathway, mammalian/mechanistic target of rapamycin (mTOR) signaling pathway, mitogen-activated protein kinase signaling pathway, apoptosis, NOD-like receptor signaling pathway, and tumor necrosis signaling pathway, and other signaling pathways were found to be involved in gas-explosion-induced ALI (Supplementary Figure S4, available in www.besjournal.com). Inflammation plays a vital role in autophagy. After the pathogen is phagocytosed by cells, it is transferred to the lysosome and destroyed, and autophagy occurs, leading to the reduction of severe inflammation. In mammalian cells, the invasion of Acinetobacter baumannii depends on septin SEPT2 and SEPT9 and induces the activation of the AMPK/ERK/mTOR pathway through the downregulation of the p62 protein, leading to excessive autophagy [7]. Autophagy, also known as type II apoptosis, plays an essential role in the pathogenesis of ALI and has attracted a lot of attention. The protein-protein interaction (PPI) networks were constructed 2 h, 24 h, 72 h, and 1 week after the gas explosion using the top 10 significant DEPs as key proteins (Supplementary Figure S5, available in www.besjournal.com).
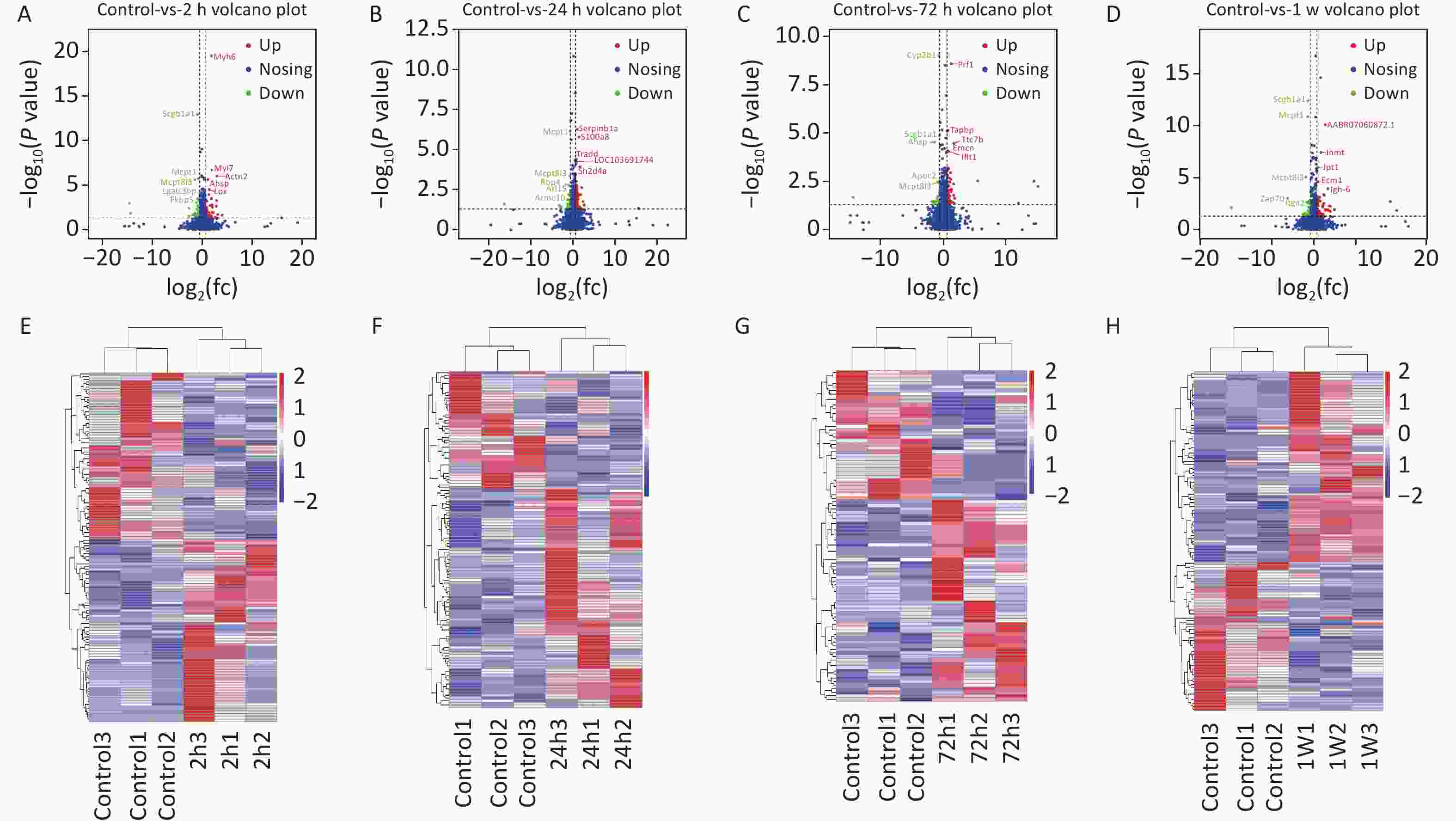
Figure S2. (A)–(D) Volcano plots of DEPs at 2 h (A), 24 h (B), 72 h (C), and 1 week (D) for the rat model of gas-explosion-induced ALI. The x-axis indicates the protein expression level differences (log2-transformed fold changes; gas explosion/control ratios), and the y-axis shows the P-value for each of the identified proteins (-log10-transformed). The red dots represent significantly upregulated proteins, and the green dots represent significantly downregulated proteins in the control group compared with the model group. The blue dots represent proteins with no significant change between groups. (E)–(H) Heatmaps based on the cluster analysis of DEPs identified at 2 h (E), 24 h (F), 72 h (G), and 1 week (H) compared with the control group. Each line indicates a protein, and each column depicts a sample. A deeper red or blue color indicates more significant upregulation or downregulation, respectively, of the protein abundance. DEPs, differentially expressed proteins.
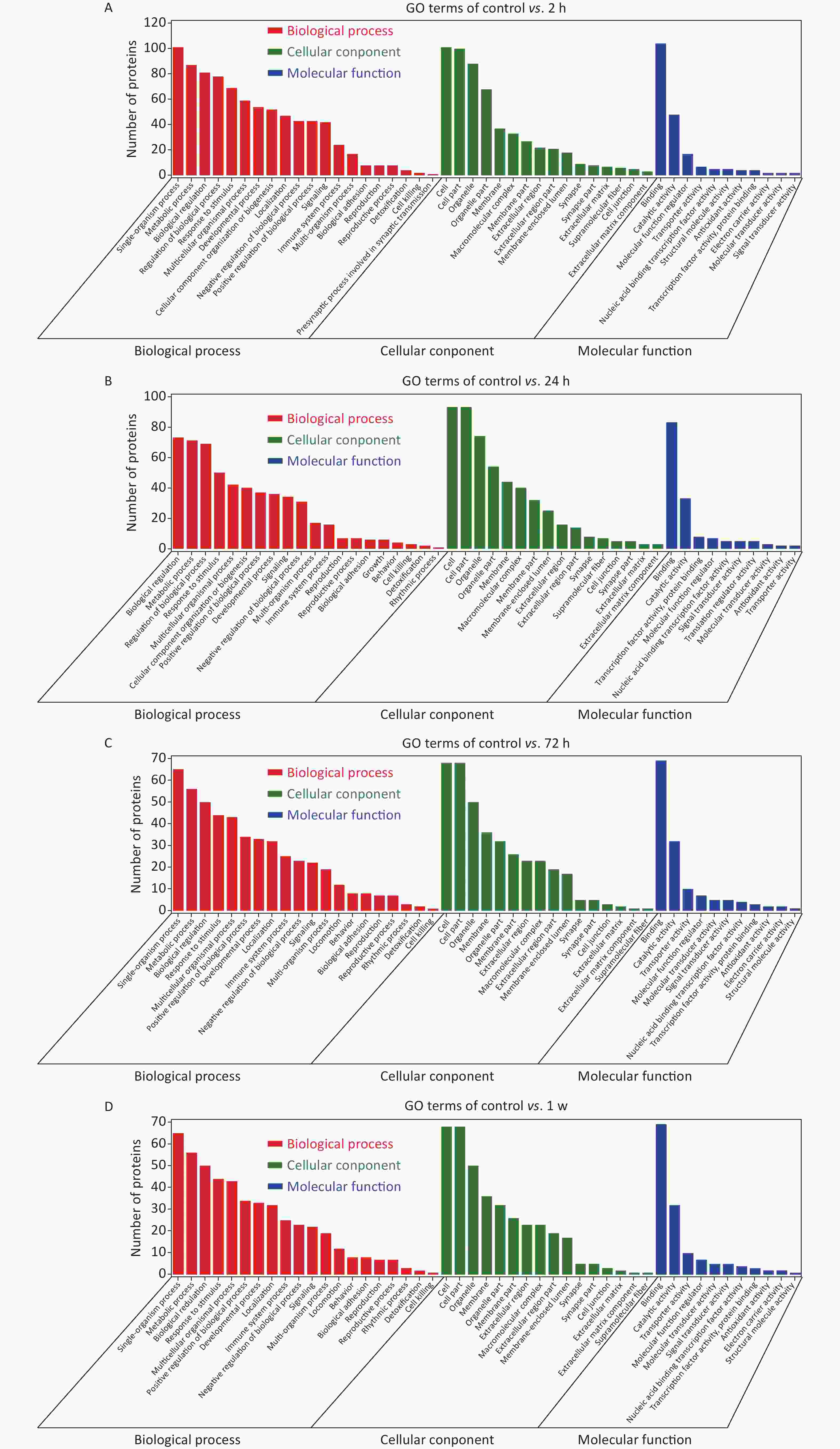
Figure S3. GO enrichment analysis. Bar charts based on functional GO classification enrichment analysis for the DEPs at 2 h (A), 24 h (B), 72 h (C), and 1 w (D) in the rat model of gas-explosion-induced ALI. DEPs, differentially expressed proteins; GO, Gene Ontology.
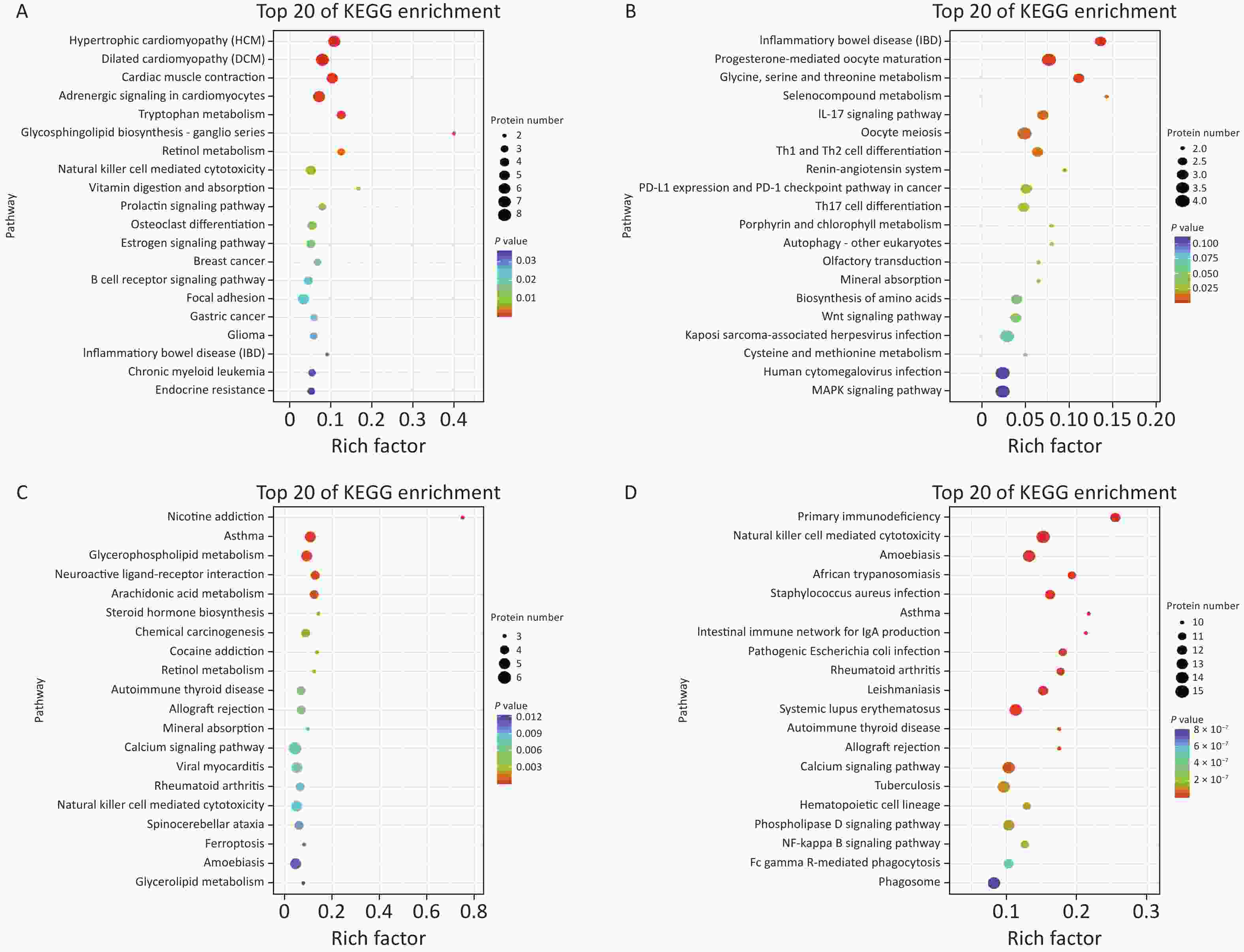
Figure S4. KEGG pathway analysis of DEPs. (A)–(D) Air bubble diagram displaying the top 20 pathways with significant enrichment based on KEGG enrichment analysis of DEPs at 2 h (A), 24 h (B), 72 h (C), and 1 week (D) after the gas explosion. DEPs, differentially expressed proteins; KEGG, Kyoto Encyclopedia of Genes and Genomes.
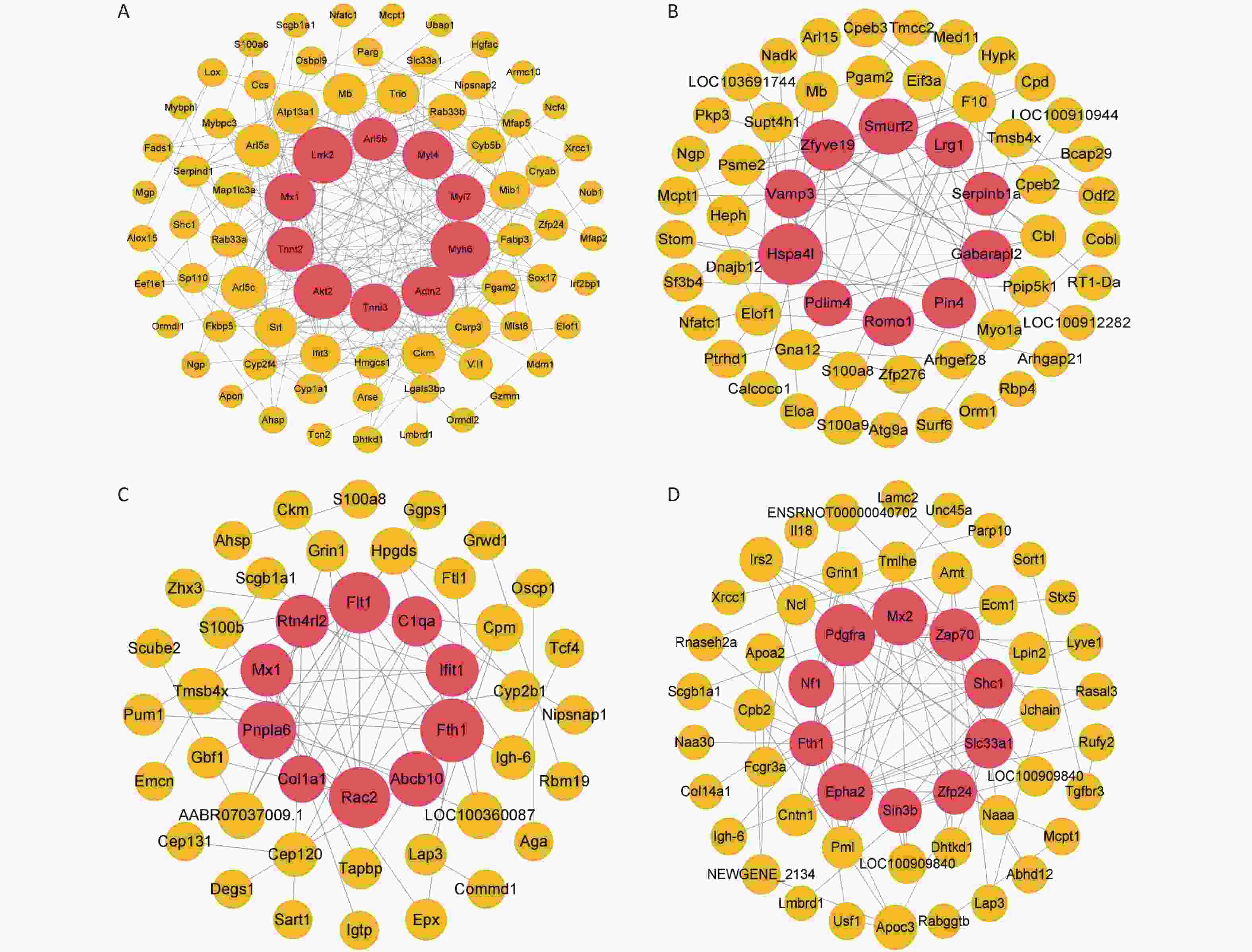
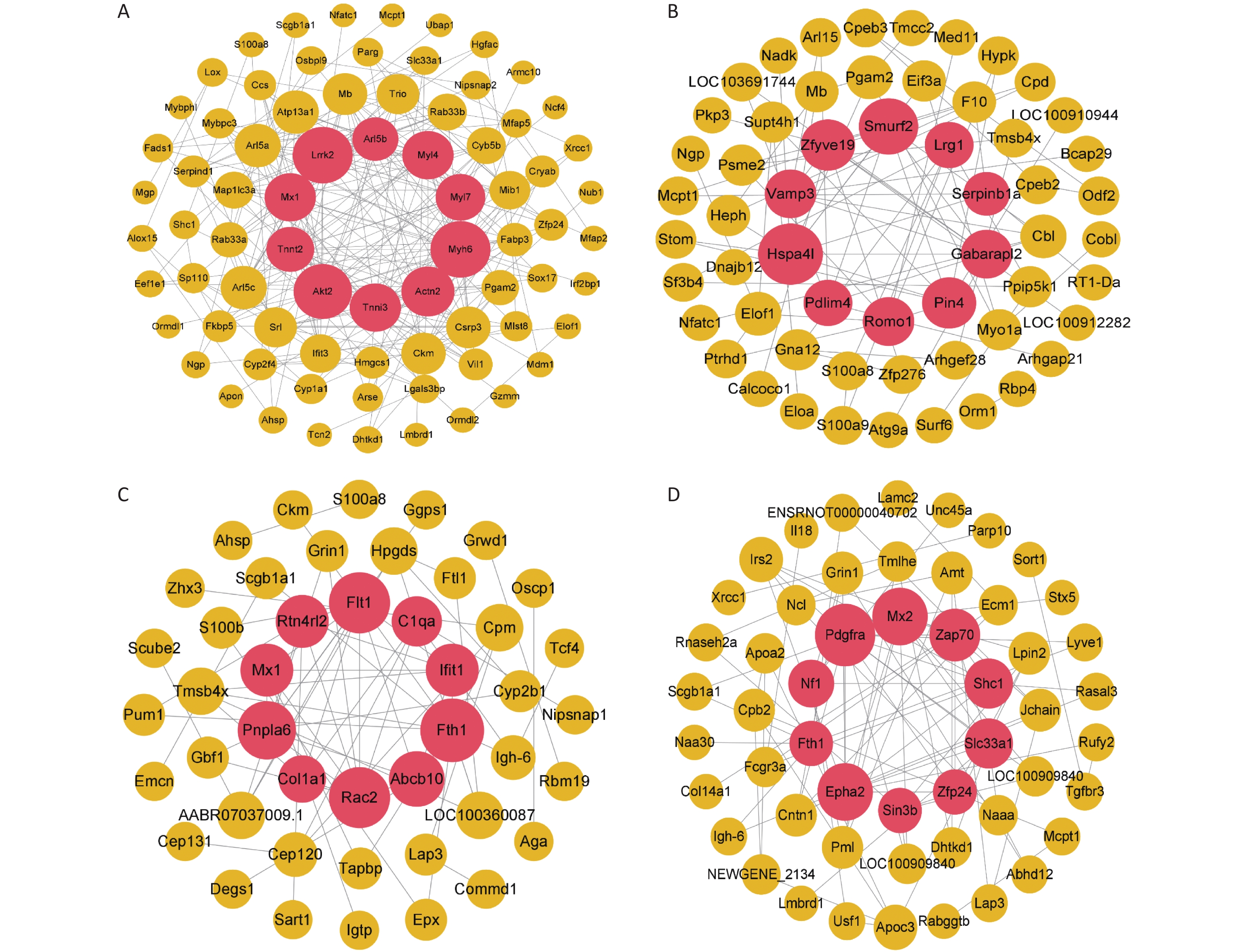
Figure S5. Construction of the protein-protein interaction network for different time points of the rat model of gas-explosion-induced ALI. (A)–(D) PPI network of DEPs at 2 h (A), 24 h (B), 72 h (C), and 1 week (D) after the gas explosion. The red sphere in the middle contains the top 10 DEPs in the PPI network, which are the key proteins. PPI, protein-protein interaction; DEPs, differentially expressed proteins.
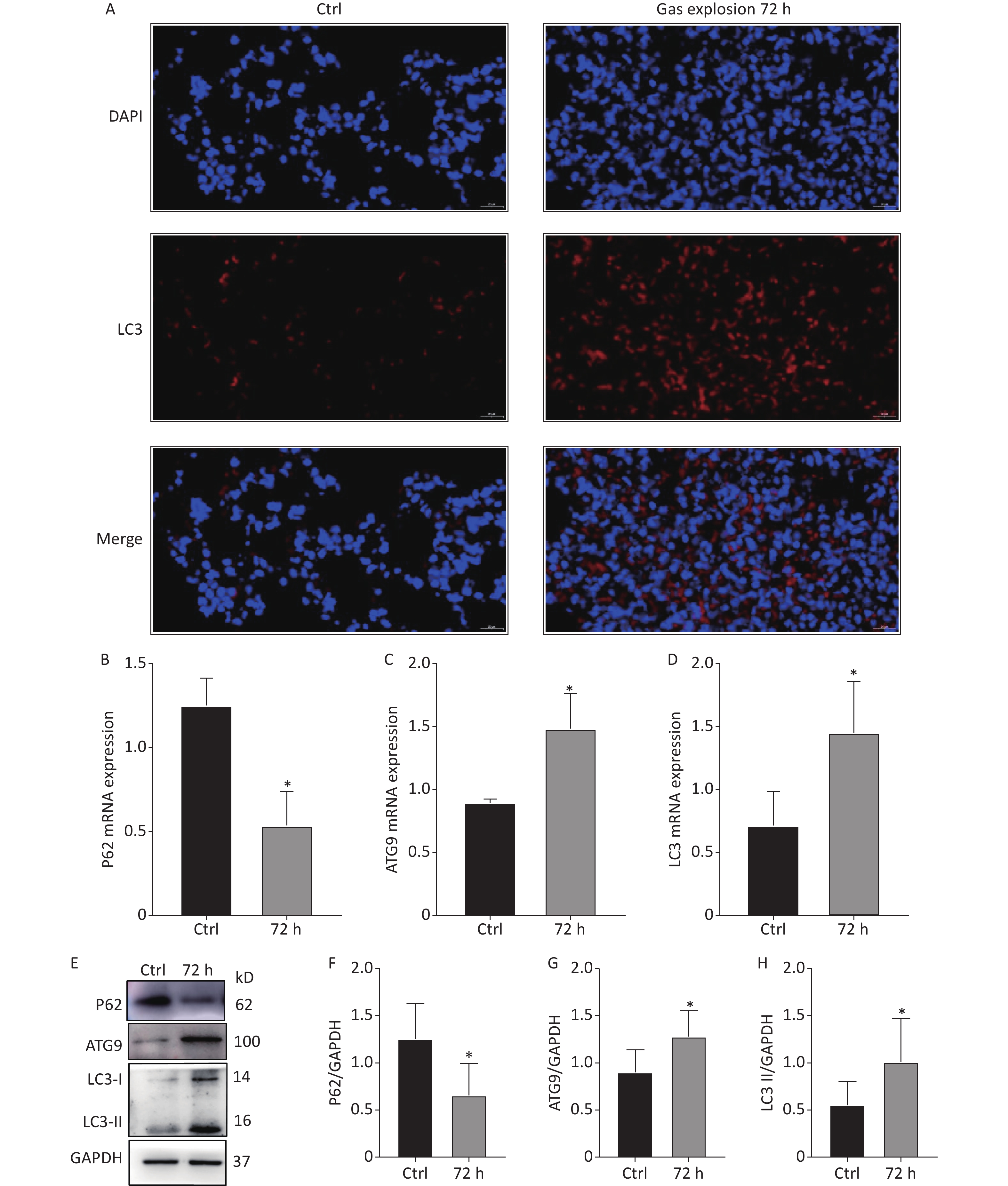
Autophagy occurs in nearly all eukaryotic cells, and it plays an important role in maintaining homeostasis. The overactivation of autophagy aggravates lipopolysaccharide-induced ALI, and the inhibition of autophagy reduces inflammation and lung injury [8]. The 404 DEPs related to gas-explosion-induced ALI at different time points were clustered into 20 profiles (Figure 2A), and most of the differential proteins were clustered in profiles 16 and 18. Compared with the DEPs clustered in profile 18, the 36 DEPs clustered in profile 16 had a closer aggregation degree (Figure 2B, C). Therefore, we focused on the trend of the DEPs in profile 16. To further investigate the biological function of the DEPs in profile 16, GO and KEGG enrichment analyses of the pathway were performed. A total of 36 DEPs in profile 16 were annotated with GO functions (including biological process, cellular component, and molecular function ontologies) and categorized into 179 GO terms (Figure 2D). The GO enrichment analysis annotation of the top 20 DEPs mainly identified the functions, laminin-2 complex, dendritic growth cone, perinuclear region of the cytoplasm, extracellular region, and extracellular matrix component. The KEGG pathway enrichment analysis revealed that 36 DEPs in profile 16 were mapped in 44 pathways (Figure 2E), in which 20 pathways were associated with oxidative stress-mediated programmed cell death, including the peroxisome proliferator-activated receptors signaling pathway, PI3K-Akt signaling pathway, mTOR signaling pathway, autophagy-other eukaryotes, autophagy-animal, mitophagy-animal, necroptosis, chemokine signaling pathway, and NOD-like receptor signaling pathway. In a recent study, Liu et al. found that lung tissue inflammation, oxidative stress, and apoptosis occurred 24 h after blast-injury-induced ALI in mice, suggesting that gas-explosion-ALI is related to blast injury [9]. The results of that related study were in accordance with our findings, and they demonstrated that oxidative stress is closely related to ALI onset. Oxidative stress could cause the release of inflammatory factors and increase endothelial and epithelial cell permeability during lung injury. The release of inflammatory factors stimulates the production of excessive reactive oxygen species, which further exacerbates ALI [10]. The pathways enriched by differential proteins and trend analysis were interleaved to obtain five key pathways related to autophagy, including autophagy-animal, mitophagy-animal autophagy-other eukaryotes, PI3K-Akt signaling pathway, and mTOR signaling pathway. The five signaling pathways in profile 16 of the trend analysis, identified by KEGG pathway enrichment analysis, confirmed the occurrence of excessive autophagy in gas explosion-induced lung injury. The results showed the trend pattern of protein expression, thus providing insights into the molecular mechanism of gas explosion-induced ALI. Lung tissue injury occurred after the gas explosion and was most severe 72 h after the gas explosion based on the lung histopathological scores and pulmonary function tests at different time points after the gas explosion. Moreover, KEGG analysis results showed upregulated expressions of autophagy-related DEPs, indicating excessive autophagy. The trend analysis showed that the DEPs enriched in autophagy-animal and autophagy-other eukaryotes were all upregulated 72 h after the gas explosion. Therefore, we chose 72 h after the gas explosion as the time point to verify changes in the expressions of autophagy-related proteins and mRNA in the lung tissue of rats with gas explosion-induced ALI. As shown in Figure 3A, immunofluorescence results showed that the number of LC3 dots increased in the gas explosion group. Western blotting and qPCR were used to examine the expression levels of autophagy-associated DEPs (LC3 and ATG9) and the autophagy substrate protein P62. Compared with the control group, the model group had increased LC3 and ATG9 expression levels and decreased P62 expression levels. The Western blotting and qPCR results were in agreement with the protein abundance changes found in proteomics data (Figure 3B–H). These data indicated that gas explosion treatment induced excessive autophagy in rat lung tissues. In conclusion, autophagy plays a key role in regulating the molecular mechanism of gas-explosion-induced ALI. Notably, LC3 and ATG9 may be candidate biomarkers for the occurrence and development of gas explosion-induced ALI.
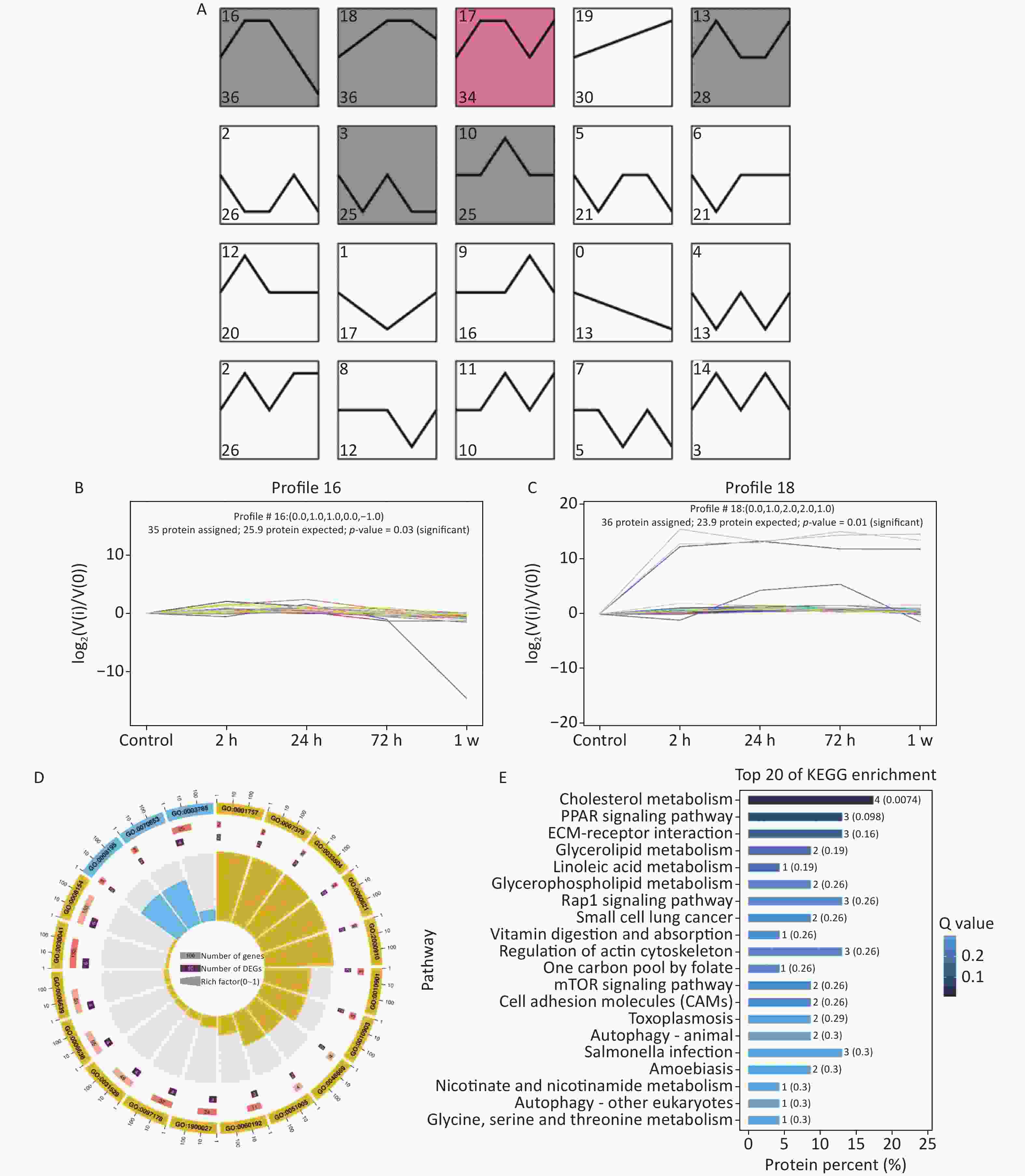
Figure 2. Trend analysis of DEP enrichment at different stages of gas-explosion-induced ALI. (A) The 404 DEPs identified at different time points in the model of gas-explosion-induced ALI were clustered into 20 Profiles. The significantly enriched trend analysis of profile 16 (B) and profile 18 (C). (D) Top 20 GO classifications for the DEPs in profile 16. (E) Top 20 KEGG pathways for the DEPs in profile 16. The length of the bar plot represents the protein percent in DEPs, and the bar plot color represents the Q value. ALI, acute lung injury; DEP, differentially expressed protein; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
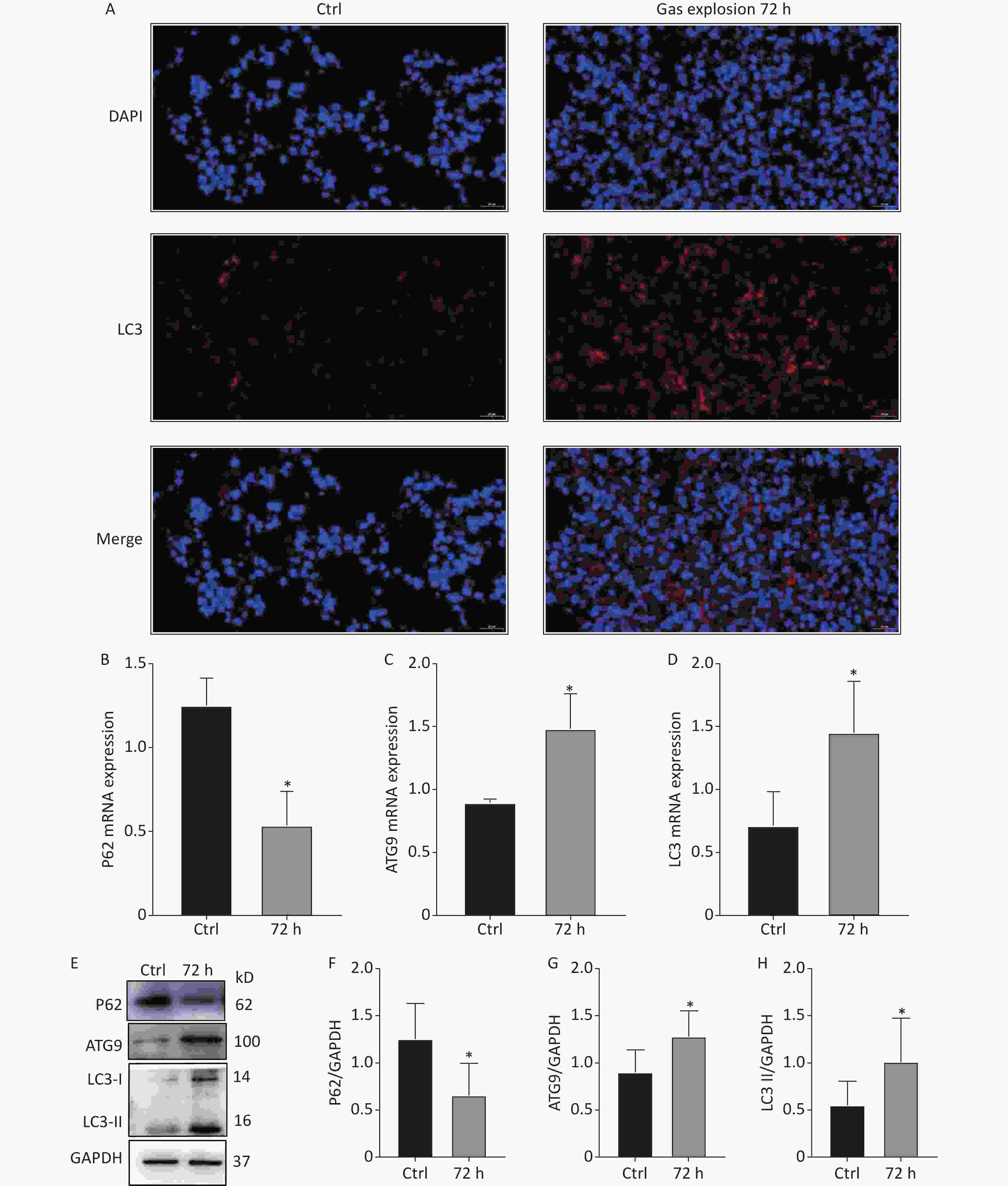
Figure 3. Autophagy-related protein and mRNA expression changes in the lung tissue of rats after exposure to a gas explosion. (A) Quantification of LC3 punctate formation in lung tissue by immunofluorescence. (B)–(D) Expression of autophagy-related mRNAs, P62, ATG9, and LC3, in lung tissues of rats with gas-explosion-induced ALI (n = 5). (E)–(H) Expression of autophagy-related proteins, P62, ATG9, and LC3, in lung tissues of rats with gas-explosion-induced ALI. All experiments were repeated at least 3 times (n = 5). *Means, P < 0.05 for the model group compared with the control (Ctrl) group. ALI, acute lung injury.
Conflicts of Interest The authors declare no conflict of interest.
CRediT Authorship Contribution Statement HONG Shan: Implemented the scheme and wrote the original draft. DING Chun Jie and ZHOU Qiang: Collected the data. SUN Yun Zhe and ZhANG Miao: analyzed the data, LI Ning and DONG Xin Wen: Software. GUAN Yi and ZHANG Lin: reviewed the manuscript. TIAN Lin Qiang: provided helpful discussions. REN Wen Jie: supervised the study; wrote, reviewed, and edited the manuscript. YAO San Qiao: conceived and designed the research methods; wrote, reviewed, and edited the manuscript.
Acknowledgments We thank the Army Medical University for providing the experimental site and Guangzhou Gene Denovo Biotechnology Co., Ltd. (https://www.omicsmart.com) for providing technical support for the DIA proteomics assay and the data analysis.
HTML
 22339Supplementary Materials.pdf
22339Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: