-
A new variant of SARS-CoV-2, B.1.1.529, was reported to the World Health Organization (WHO) by South Africa on November 24, 2021. It was classified as Omicron and designated as the fifth variant of concern (VOC) after Alpha, Beta, Gamma, and Delta by WHO on November 26. The first case of the Omicron variant in Europe was confirmed in Belgium on November 26. A patient with a history of recent travel to Egypt tested positive for COVID-19 on November 22[1]. On November 26, 2021, the European Center for Disease Prevention and Control classified this variant as a VOC due to concerns regarding immune escape and potentially increased transmissibility compared to the Delta variant[2]. On November 26, 2021, the UK Health Security Agency designated Omicron as the variety being monitored (VUI‐21‐NOV‐01). On November 27, two cases of VOC Omicron were first detected in the United Kingdom[3]. On November 29, 2021, 3 days after the announcement by the WHO, Omicron-infected cases were detected in many countries like Austria, Australia, Belgium, Canada, the Czech Republic, Denmark, France, Germany, Italy, and the Netherlands[4]. On November 30, 2021, the Centre for Disease Prevention and Control (CDC) in the United States designated Omicron as a VOC[5]. On December 1, 2021, the first case attributed to the Omicron variant was identified in the United States in a traveler who had recently returned from South Africa[6]. On December 2, 2021, a second case was reported in a person with no international travel history but had attended a convention in the days prior to the onset of symptoms[6]. Within a few months, the Omicron variant became the dominant VOC in many countries and brought severe challenges to the prevention and control of novel coronavirus outbreak worldwide. On January 8, 2022, the Omicron variant was first discovered in Tianjin, Chinese mainland. The virus has shown the characteristics of high infectivity, rapid transmission, short incubation period, and a high proportion of asymptomatic infections, which greatly increased the difficulty of control and quickly attracted attention nationwide. The persistence of the human body’s immunity to the coronavirus has been a hot topic of global concern. This concerns whether the people who have been infected with SARS-CoV-2 can generate protection ability against reinfection, the protection durability provided by the SARS-CoV-2 vaccine, and if there is a tendency for COVID-19 to become a disease similar to influenza. Therefore, this study analyzed and evaluated the immune efficacy and persistence of immunity in the vaccinated population infected with Omicron BA.1 from two perspectives of humoral immunity and cellular immunity.
-
The case group was selected from Omicron BA.1 patients aged 3–79 years old in Jinnan District, Tianjin, from January 8, 2022, to February 2, 2022. The healthy control group was selected to match by age, gender, and vaccination profile and assigned at a 1:1 ratio to the case group. All of the subjects live in Tianjin with good compliance. The SARS-CoV-2 vaccines used in this study mainly included the inactivated vaccine developed by Beijing Institute of Biological Products Co, Ltd., Beijing, China; Sinovac Biotech Ltd., Beijing, China; and the adenovirus-vectored vaccine developed by CanSino Biologics Inc, Tianjin, China. Excluding those people who used blood products or immune-suppressants after basic immunization, infected with Omicron variant during this study, left the local area, or failed to follow-up and requested withdrawal, 124 patients with Omicron BA.1 infection and 124 controls were eventually included in this study.
-
This study was approved by the Tianjin Municipal Health Commission and the Ethics Committee of the Tianjin Center for Disease Control and Prevention (Ethics Committee Archival No. TJCDC-R-2022-001). All patients/participants provided written informed consent for their clinical information and blood samples to be collected for study purposes and data generated from the study.
-
Demographic data for the case group were obtained from the Chinese Disease Control and Prevention Information System. Clinical information was collected by trained investigators from hospital records. Vaccination data were obtained from the Tianjin Immunisation Planning Information Management System. In the control group, demographic data were collected by trained investigators. Vaccination information was collected from the Tianjin Immunisation Planning Information Management System.
-
After 3 months (90 + 7 days) and 6 months (180 + 7 days) of Omicron BA.1 infection, 3 mL procoagulant blood and 5 mL anticoagulant blood was collected from each subject. Serums were separated from 3 mL procoagulant blood and cryopreserved at −20 °C for neutralizing antibody assays, whereas 5 mL anticoagulant blood was stored at room temperature (approximately 20−25 °C) for cellular immunoassay and tested within 72 hours.
-
A neutralization assay of live SARS-CoV-2 variants, including the WT, Gamma, Beta, Delta, and Omicron variants, was performed by observing cytopathic effects (CPEs). Five SARS-CoV-2 strains were isolated from samples of COVID-19 patients at different times and in different regions using Vero cell isolation, culture, and purification. The specific information on the five strains of SARS-CoV-2 is as follows: WT strain, January 2020, from Zhejiang Province, China; Beta strain, December 2020, from Guangdong province, China; Gamma strain, December 2020, from Brazil; Delta strain, April 2021, from Zhejiang Province, China; and Omicron BA.1 strain, December 2021, from Hong Kong, China. Serum samples were collected and separated from the case and control groups. Each serum sample was serially diluted 2-fold with Dulbecco’s modified Eagle’s medium. Diluted sera were mixed with 100 CCID50 of live SARS-CoV-2 virus and incubated at 36.5 °C with 5% CO2 for 2 hours. Vero cell suspension was then added and cultured for 5 days. Serum neutralization antibody titers were examined by observing CPEs. Neutralizing antibody titers of serum samples ≥ 4 were considered positive. Neutralizing antibodies were obtained from Sinovac Biotech Ltd. All the experiments were performed in a Biosafety Level 3 facility.
-
CD3/CD4/CD8/CD45 T-lymphocyte cell counts were determined using a Becton-Dickinson FAcsCanto II flow cytometer (MultiTEST CD3 FITC/CD8 PE/CD45 PerCP/CD4 APC Reagent). The entire process was performed according to the manufacturer’s instructions. Cellular immunity was tested at the Pathogenic Microorganism Detection Institute of the Tianjin Center for Disease Control and Prevention.
-
The antibody levels are described using geometric mean titers (GMT) and 95% confidence intervals. The antibody titers were logarithmically transformed and normality tests were used. When the data were normally distributed, a paired t-test was used; otherwise, the data were analyzed using the Paired Wilcoxon signed-rank test. Categorical variables were compared by Pearson’s χ2 test or Fisher's Exact Test. Statistical analyses were performed using STATA software version 17.0. Statistical significance set at P < 0.05 were considered significant.
-
The average age of all participants was 43.0 ± 16.3 years; less than 9.0% were under 18 years, and 17.7% were over 60 years. Overall, 58.1% were female and 41.9% were male. All 124 patients and controls were inoculated with the domestic Chinese COVID-19 vaccine. Fifty-four were inoculated with the inactivated vaccine from Beijing Institute of Biological Products Co., Ltd. (BBIBP-CorV), 57 pairs were inoculated with the inactivated vaccine from Sinovac Biotech Ltd. (CoronaVac), and 13 were inoculated with the adenovirus-vectored vaccines from CanSino Biologics Inc. (Ad5-nCoV) (Table 1).
Characteristics Case
(n = 124)Control
(n = 124)Age (years) 43.0 ± 16.3 43.3 ± 16.1 Age group, n (%) < 18 11 (8.9) 10 (8.1) 18– 15 (12.1) 15 (12.1) 30– 27 (21.8) 25 (20.2) 40– 22 (17.7) 23 (18.5) 50– 27 (21.8) 29 (23.4) 60– 22 (17.7) 22 (17.7) Gender, n (%) Male 52 (41.9) 52 (41.9) Female 72 (58.1) 72 (58.1) Vaccine, n (%) BBIBP-CorV 54 (43.5) 54 (43.5) CoronaVac 57 (46.0) 57 (46.0) Ad5-nCoV 13 (10.5) 13 (10.5) Table 1. Demographic characteristics and vaccination condition of case group and control group
-
The most common complaints of the 124 patients were fever (33.9%), sore throat (27.4%), and dry cough (24.2%). Two cases were clinically diagnosed as asymptomatic infections, and 64 cases had mild disease, with slight clinical symptoms and no signs of pneumonia on imaging. Fifty-eight cases had moderate disease, including fever, respiratory symptoms, and pneumonia by radiology. The clinical characteristics of the patients are shown in Table 2. The average hospitalization duration was 13 days, and the average Ct values of the N and ORF genes were 26.9 and 27.8, respectively.
Characteristic Asymptomatic (n = 2) Mild (n = 64) Moderate (n = 58) Age group, n (%) < 18 1 (50.0) 9 (14.1) 1 (1.7) 18– 1 (50.0) 10 (15.6) 4 (6.9) 30– 0 (0.0) 15 (23.4) 12 (20.7) 40– 0 (0.0) 10 (15.6) 12 (20.7) 50– 0 (0.0) 12 (18.8) 15 (25.9) 60– 0 (0.0) 8 (12.5) 14 (24.1) Gender, n (%) Male 0 (0.0) 34 (53.1) 18 (31.0) Female 2 (100.0) 30 (46.9) 40 (69.0) Vaccine, n (%) BBIBP-CorV 2 (100.0) 33 (51.6) 19 (32.8) CoronaVac 0 (0.0) 27 (42.2) 30 (51.7) Ad5-nCoV 0 (0.0) 4 (6.2) 9 (15.5) Table 2. The clinical characteristics distribution of case group
-
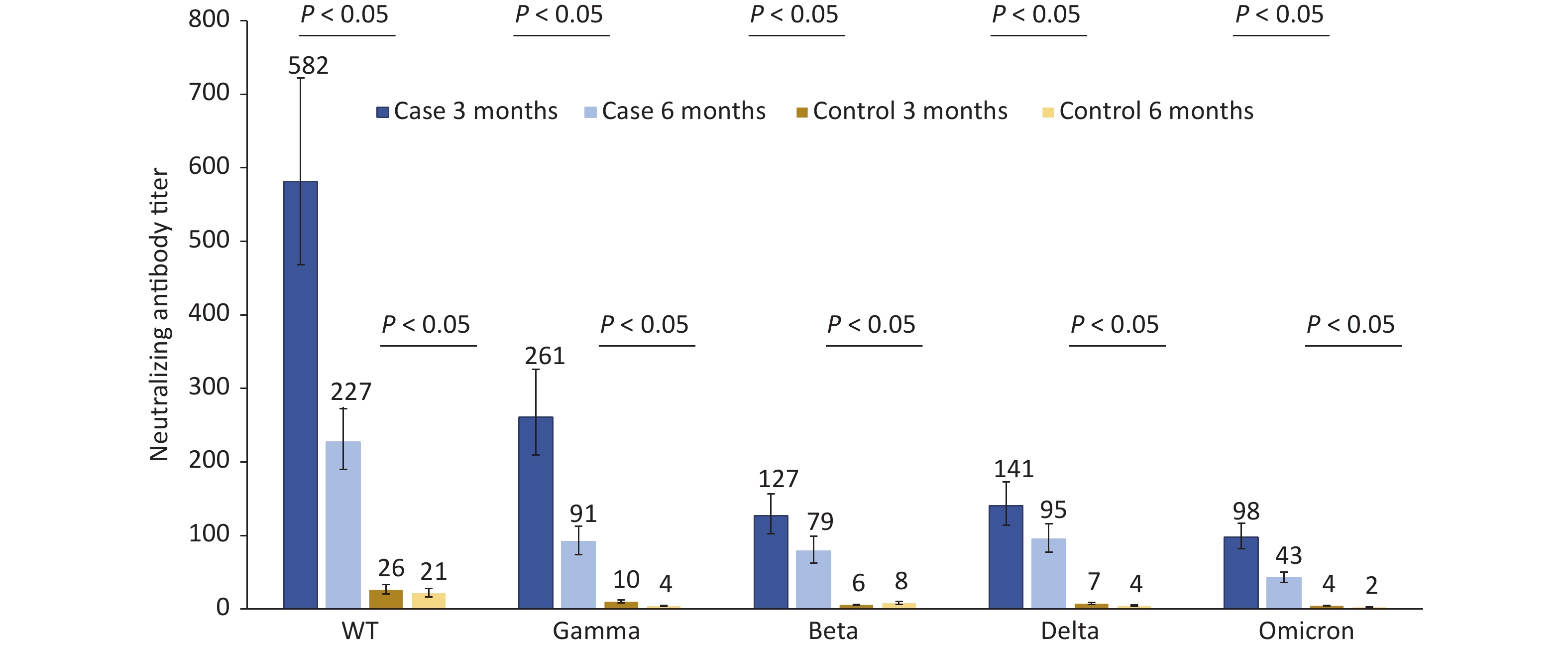
The neutralizing antibody titers of 124 patients infected with Omicron BA.1 against the WT, Gamma, Beta, Delta, and Omicron strains were 581.6, 261.1, 126.7, 140.5, and 97.9, respectively, at 3 months after infection, which were 22.37, 25.85, 22.63, 19.25, and 21.76 times those to the matched control group. Compared to 3 months after infection, the neutralizing antibody titers of the cases against the abovementioned strains decreased by 60.95%, 65.03%, 37.96%, 32.53%, and 56.59% respectively at 6 months after infection. The neutralizing antibody titers in the control group were very low and significantly lower than those in the case group 3 and 6 months after infection (Figure 1).
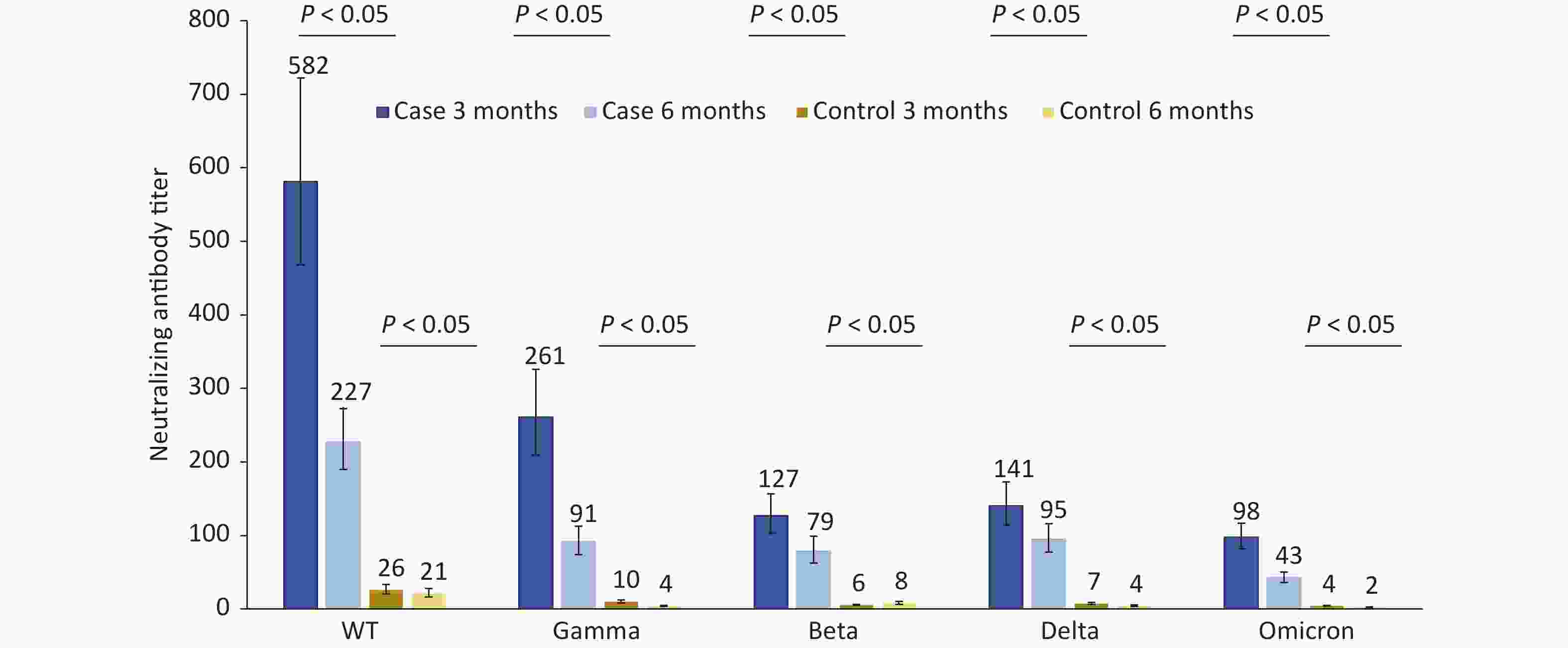
Figure 1. Changes in neutralization antibody levels against different SARS-COV-2 strains in case group and control group at 3 and 6 months after infection.
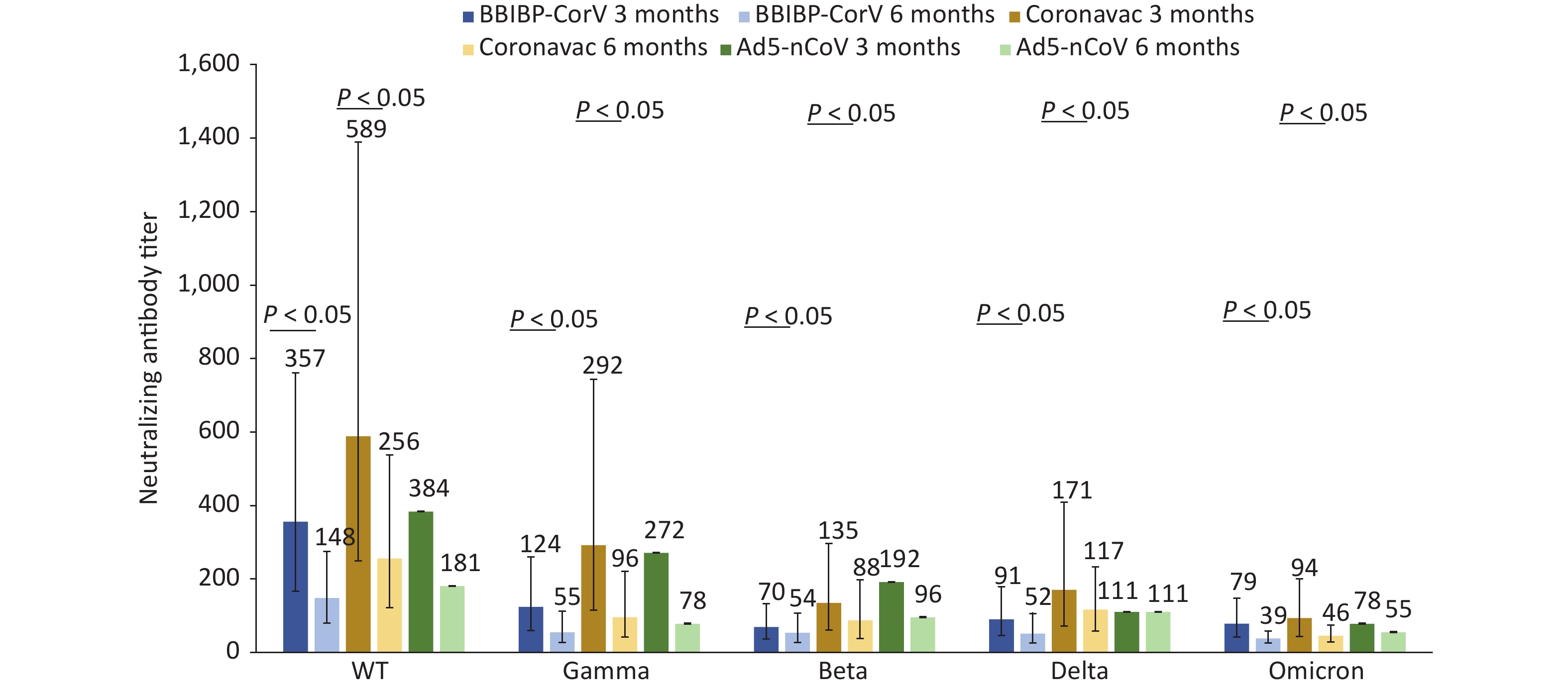
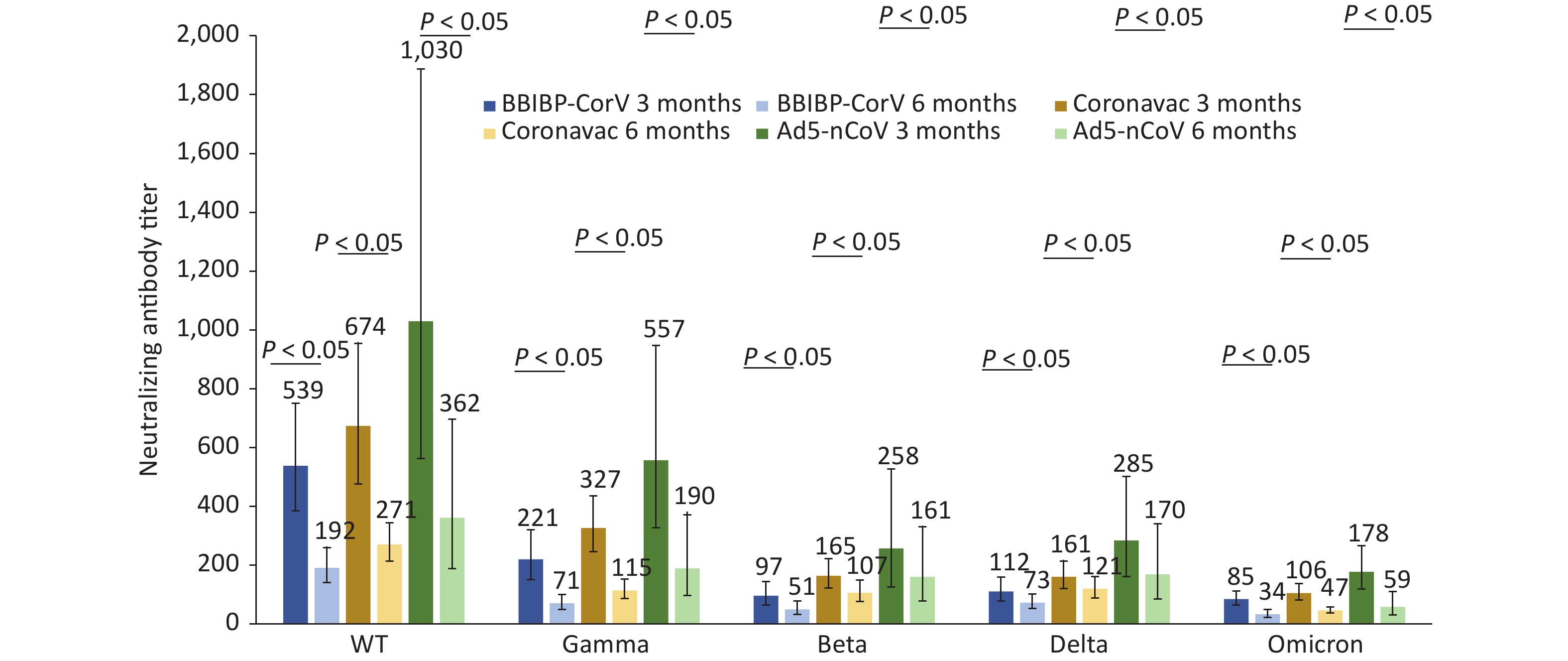
We compared neutralizing antibody titers against the WT, Gamma, Beta, Delta, and Omicron strains of convalescent Omicron BA.1 infected patients who were vaccinated with different doses of BBIBP-CorV, CoronaVac, and Ad5-nCoV. For 87 patients who had received three doses of inactivated vaccines, such as BBIBP-CorV, CoronaVac, or two doses of Ad5-nCoV, the median interval from the last vaccination to the onset of disease was 52 days (IQR: 35–75). For 37 patients who received two doses of inactivated vaccines, such as BBIBP-CorV, CoronaVac, or one dose of Ad5-nCoV, the median interval was 190 days (IQR: 135–211). For those vaccinated with the same dose of vaccine, regardless of the type of vaccine they received, the neutralizing antibody titers against WT and Omicron strains were the highest and lowest, respectively, at 3 and 6 months after infection with Omicron BA.1 (Figures 2–3). Booster immunization with three doses of BBIBP-CorV, three doses of CoronaVac, or two doses of Ad5-nCoV generally produced higher neutralizing antibody titers against different strains than basic immunization with two doses of BBIBP-CorV, two doses of CoronaVac, or one dose of Ad5-nCoV. This difference was statistically significant (P < 0.05) (Figures 2–3).
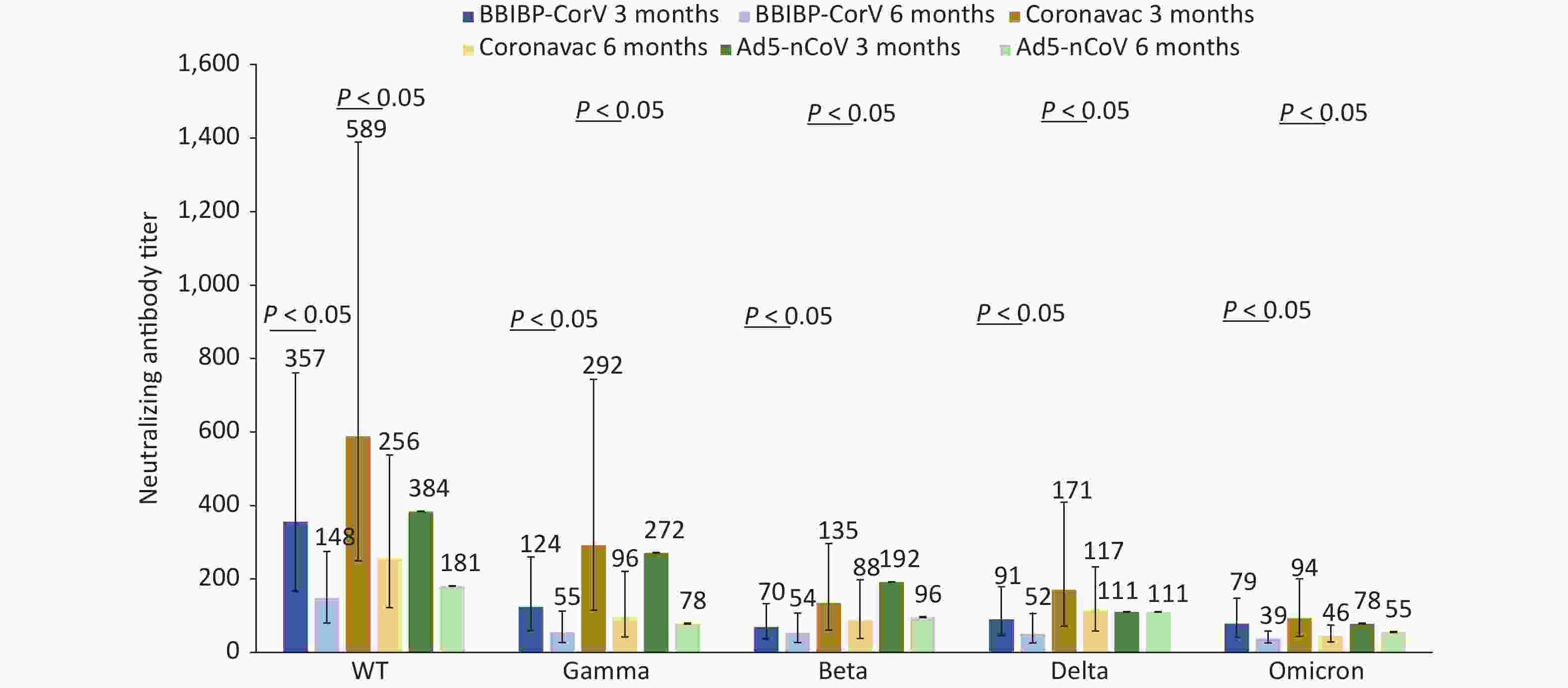
Figure 3. Changes in neutralization antibody levels against different SARS-COV-2 strains in case group vaccinated with 2 doses of inactivated vaccine or 1 dose of adenovirus-vectored vaccine at 3 and 6 months after infection.
For Omicron BA.1 breakthrough infected patients at 3 months after infection, the neutralizing antibody titers of individuals vaccinated with 2 doses of Ad5-nCoV (n = 11) against the Omicron variant were significantly higher than those of 3 doses of CoronaVac (n = 40) or 3 doses of BBIBP-CorV (n = 36), and the differences were statistically significant (P < 0.05) (Figure 2).
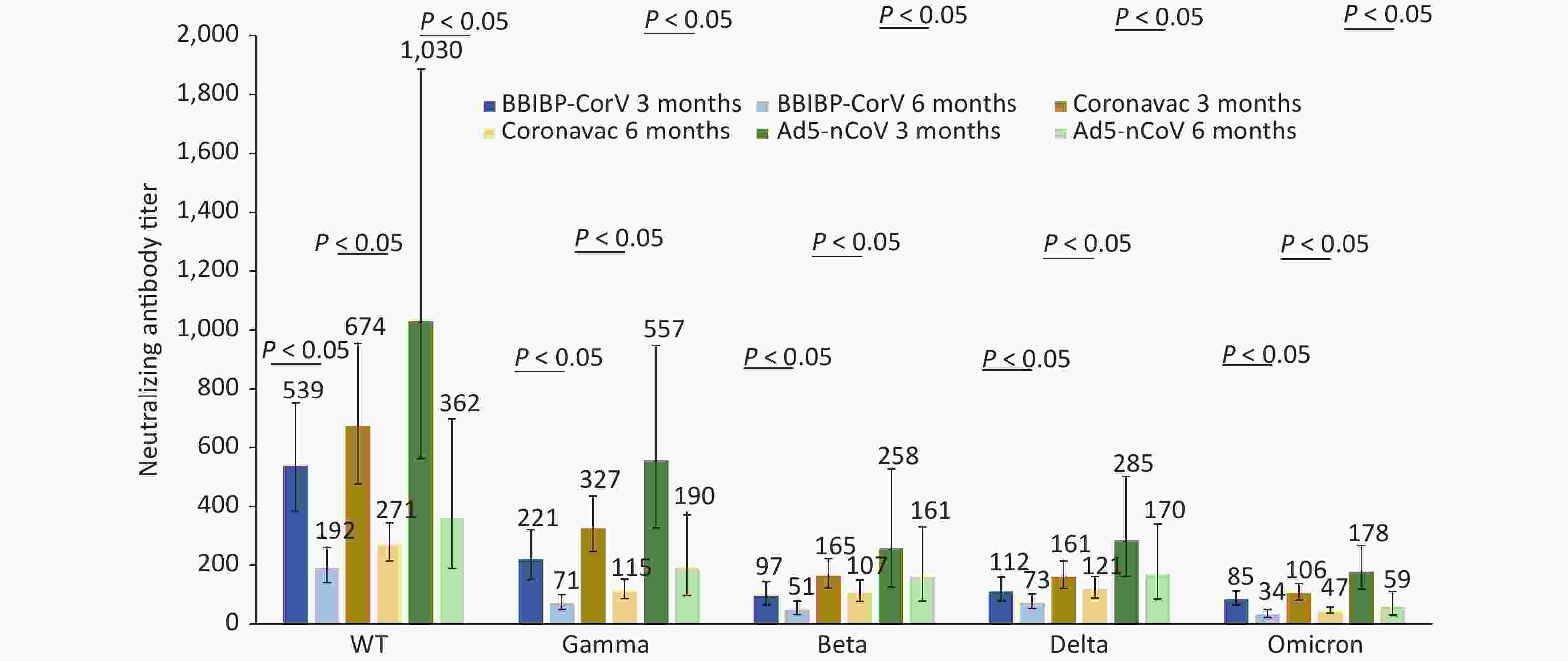
Figure 2. Changes in neutralization antibody levels against different SARS-COV-2 strains in case group vaccinated with 3 doses of inactivated vaccine or 2 doses of adenovirus-vectored vaccine at 3 and 6 months after infection.
The neutralizing antibody titers against five different strains in the convalescent Omicron BA.1 patients who received two doses of CoronaVac (n = 17) were higher than those who received two doses of BBIBP-CorV (n = 18); however, the differences were not statistically significant (Figure 3). Although only two cases were vaccinated with one dose of Ad5-nCoV and statistical comparisons could not be made, the neutralizing antibody titers were still not worse than those of the above two inactivated vaccines (Figure 3).
Compared with 3 months after infection, the neutralizing antibody titers against the gamma strain showed the largest drop in the patients with Omicron BA.1, who received three doses of BBIBP-CorV and CoronaVac at 6 months after infection, the declines were 67.65% and 64.85%, respectively, and the drop in Delta strain was smallest by 34.23% and 25.22%, respectively. The neutralizing antibody titers against the Omicron strain fell by more than 50% (the decline was 60.12% and 55.56%, respectively). The neutralizing antibody titers against the Omicron strain displayed the largest decrease (66.82%) and the smallest decrease (37.32%) in patients vaccinated with two doses of Ad5-nCoV (Figure 2).
The neutralizing antibody titers against the WT strain and Beta strain showed the largest drop (58.36%) and the smallest drop (22.35%) in patients vaccinated with 2 doses of BBIBP-CorV, respectively. The immune serum neutralizing antibody titers against gamma strain and delta strains displayed the largest decrease (67.07%) and the smallest decrease (31.36%) among patients vaccinated with 2 doses of CoronaVac, respectively. The neutralizing antibody titers in the convalescent Omicron BA.1 patients who received two doses of BBIBP CorV vaccine and two doses of CoronaVac vaccine against the Omicron strain both fell by more than 50%.
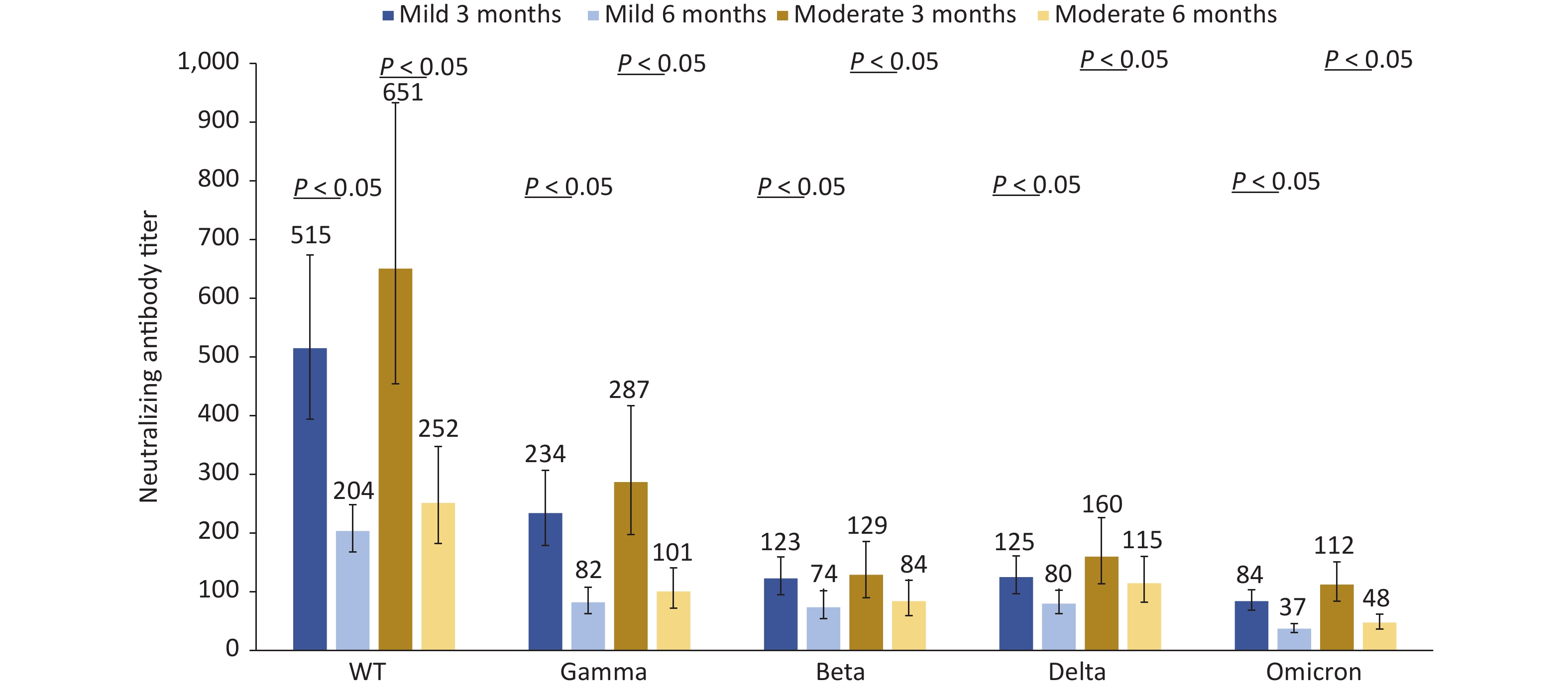
The neutralizing antibody titers against the five different strains in the 58 patients with moderate disease were higher than those in the 64 patients with mild disease at 3 and 6 months after infection (P < 0.05). Compared with 3 months after infection, the neutralizing antibodies against five different strains decreased by 60.46%, 64.92%, 39.92%, 36.08%, and 55.53%, respectively in the mild disease group at 6 months after infection and decreased by 61.33%, 64.90%, 34.73%, 28.42%, and 57.56%, respectively in the moderate disease group at 6 months after infection. The neutralizing antibody titers against the Omicron strain decreased by more than 55% (Figure 4). For the two asymptomatic cases, the neutralizing antibody titers against five different strains were 1,086.1, 543.0, 192.0, 135.8, 221.7, and 384.0, 156.8, 78.4, 90.5, 90.5 at 3 and 6 months after infection, respectively. Two cases of asymptomatic infection were not included in the statistical analysis because of the small sample size.
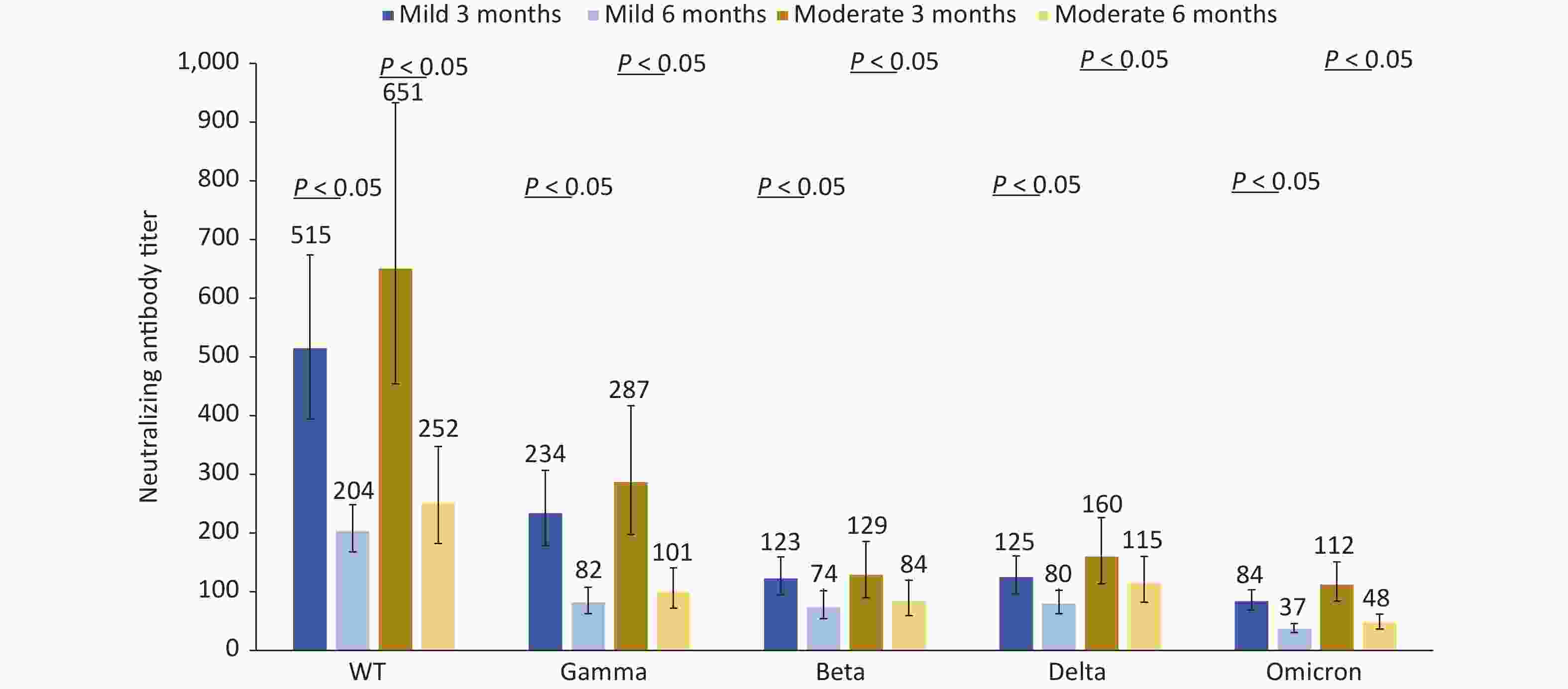
Figure 4. Changes in neutralization antibody levels against different SARS-COV-2 strains in mild patients and moderate patients at 3 and 6 months after infection.
To investigate the association of age with the quantity of Omicron BA.1 antibody responses, the neutralizing antibody titers were compared between patients over 60 years and those under 60 years. Among the group aged < 60 years, 56 cases manifested with mild disease symptoms, 44 manifested with moderate disease symptoms; Among the group aged ≥ 60 years, eight cases manifested with mild disease symptoms and 14 cases manifested with moderate disease symptoms. There were no statistically significant differences between the two groups (P = 0.095). Two asymptomatic patients were included in the group aged < 60 years and statistical comparisons could not be made because of the small sample size. The neutralizing antibody titers in the group aged < 60 years were generally higher than those ≥ 60 years at both 3 months and 6 months after infection; however, the differences were not statistically significant (Table 3).
Genotype 3 months after infection 6 months after infection < 60 GMT ≥ 60 GMT P value < 60 GMT ≥ 60 GMT P value WT 589.0 234.4 0.8674# 238.1 88.1 0.4674# Gamma 289.2 69.9 0.1825# 96.9 32.3 0.7717 Beta 133.4 44.5 0.4717# 81.6 32.3 0.9580 Delta 138.2 68.9 0.2192 96.8 38.7 0.6541 Omicron 103.1 40.0 0.3721# 45.2 20.5 0.0637 Note. P value labeled # means using paired t test, and P value unlabeled # means using paired Wilcoxon signed rank test. GMT, geometric mean titers; WT, wild type. Table 3. The comparison of neutralizing antibody titers between the group over 60 years old and the group under 60 years old
-
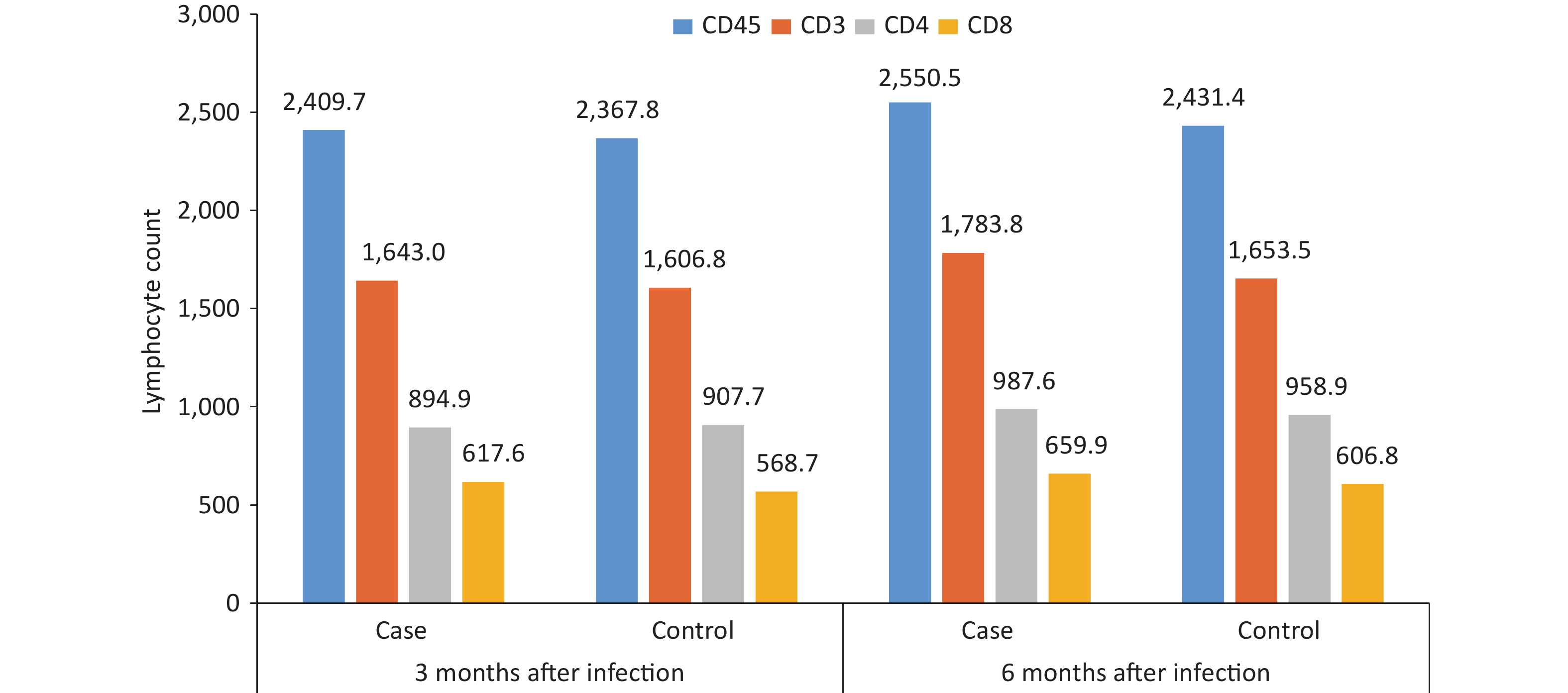
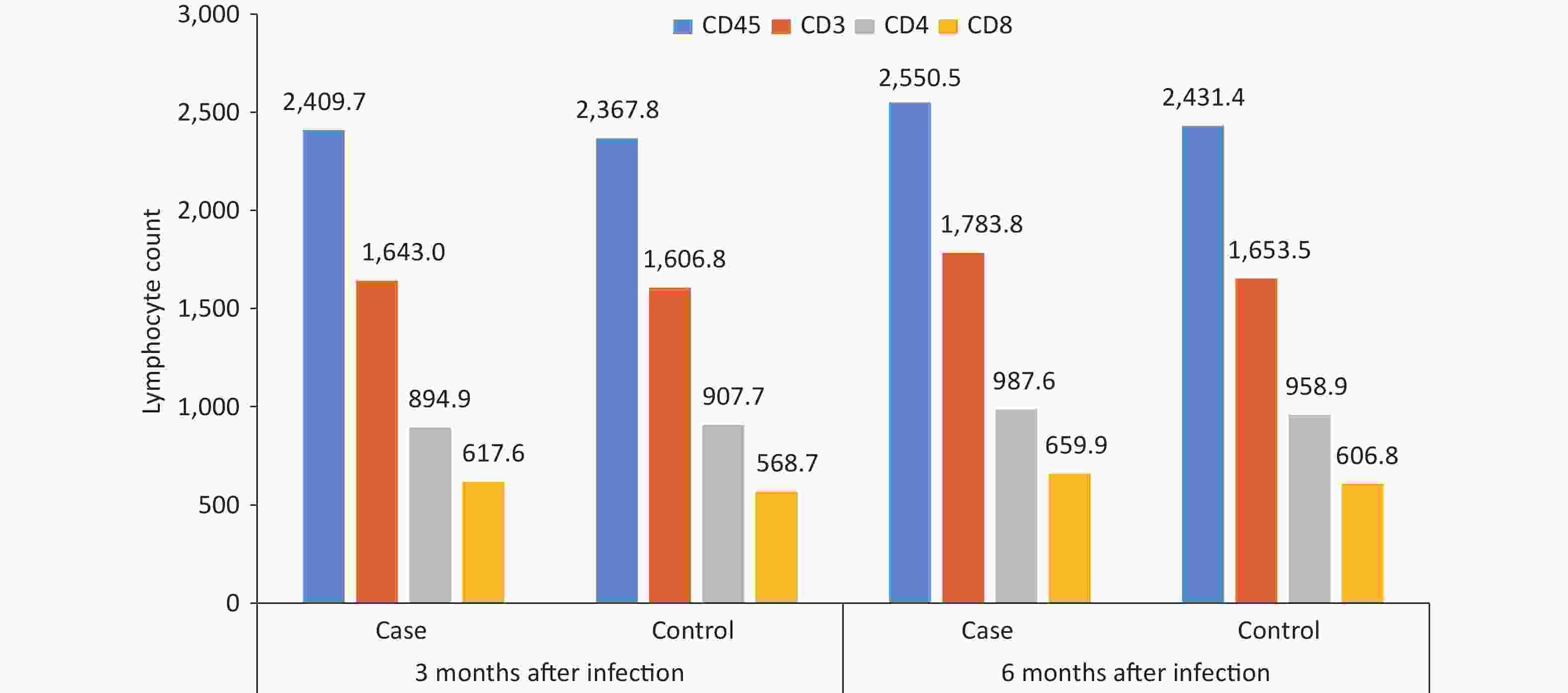
By comparing and analyzing the absolute counts of CD45+, CD3+, CD4+, and CD8+ T lymphocytes, and CD4+/CD8+ ratios in the peripheral blood of the case group and the control group, it was found that the CD4+/CD8+ ratio in the case group was 1.6 at 3 months after infection, which was slightly lower than that in the control group, and the difference was statistically significant (P = 0.047), while other cellular immune indexes were not found to be significantly different between cases and controls at 3 or 6 months after infection (P < 0.05) (Figure 5). The CD3+, CD4+, CD8+, and CD4+/CD8+ ratios in the case group were lower at 3 months after infection than those at 6 months after infection (P < 0.05).
-
Current serological studies have shown that SARS-CoV-2-specific antibodies can be induced in people infected with SARS-CoV-2, regardless of the severity of the infection. However, it remains unclear whether these antibodies are sufficient to provide long-term sterilizing immunity, that is, to stop the reproduction of the virus in the body so that the person can no longer become contagious and prevent reinfection[7]. This study found that the neutralizing antibody titers against the above-mentioned variants of SARS-CoV-2 were significantly increased in the vaccinated population infected with the Omicron BA.1 variant, but mainly increased the neutralizing antibody titer against the WT strain; the neutralizing antibody against the Omicron strain was the lowest, which was consistent with the research results of the initial outbreak of Omicron in Tianjin[8]. Reynolds CJ and his colleagues also found that in people who were triple vaccinated and had no prior SARS-CoV-2 infection, Omicron infection provided an immune boost against previous variants (Alpha, Beta, Gamma, Delta, and the original ancestral strain), but less so against Omicron. Those infected during the first wave of the pandemic and later with Omicron lacked the immune boost[9]. Related studies have also shown that Omicron BA.1 breakthrough infection mainly evokes humoral immune memory of the original strain produced after vaccination in the human body[10,11]. However, Omicron BA.1 infection has the phenomenon of immune imprinting; that is, Omicron BA.1 infection mainly evoked memory B cells induced by the previous original strain vaccine, and it was difficult to produce specific neutralizing antibodies against Omicron BA.1[9,12].
The neutralizing antibody titers against the WT, Gamma, Beta, Delta, and Omicron strains decreased rapidly at 6 months after infection compared with those at 3 months after infection. The neutralizing antibody titers against the WT, Gamma, and Omicron strains all decreased by more than 50%, and the antibody level against each strain was very low, which is likely to be insufficient to resist each type of SARS-COV-2 infection in the future.
In the early days of the epidemic, some scientists thought that infection with Omicron was equivalent to a natural booster vaccination based on its reduced virulence. However, according to our research results, the antibody levels of vaccinated and infected individuals can only be maintained for approximately half a year, although a newly published systematic review and meta-regression analysis on the duration of protection with hybrid immunity showed up to 12 months of protection following infection and vaccination[13]. This indicates that the effect of this natural booster vaccination is limited and that COVID-19 will become increasingly similar to an infectious disease like influenza in the future. Omicron vaccines based on the Omicron BA.1 variant are probably not suitable for use as a booster dose with the current immune background, and some recent studies indicate that the antibodies induced by the Omicron BA.1 variant do not have broad-spectrum protective efficacy against new variants such as BA.2, BA.2.11, BA.2.12.1, BA.2.13, BA.4, and BA.5[12,14]. In addition, the Omicron BA.1 variant has the phenomenon of immune imprinting and can rapidly evolve to evade immune responses; therefore, it is extremely difficult to achieve herd immunity through Omicron BA.1 infection, which may also be an important reason for the repeated infections with SARS-COV-2 in the population.
The neutralizing antibody titers against different virus strains after three doses of inactivated vaccine or two doses of adenovirus-vectored vaccine were generally higher than those after two doses of inactivated vaccine or one dose of adenovirus-vectored vaccine. A recent study found that people who first had an infection and then were vaccinated were comparable to those who had first vaccination and then infection, and the sequence of vaccination and infection did not influence the level of protection[15]; therefore, it is still necessary for Omicron BA.1-infected people who only received primary vaccination to get a booster vaccination. For Omicron BA.1 breakthrough infected patients at 3 months after infection, the neutralizing antibody against the Omicron strain in convalescent patients vaccinated with two doses of Ad5-nCoV was higher than that in patients with three doses of inactivated vaccine, indicating that the neutralizing immunity against SARS-CoV-2 Omicron BA.1 variant induced by Ad5-nCoV was better than that of CoronaVac or BBIBP-CorV; however, the antibody level also dropped the fastest at 6 months after infection. Analyzing those who received two vaccine doses, the neutralizing antibody titers against different virus strains in convalescent Omicron BA.1 patients vaccinated with CoronaVac were higher than those vaccinated with BBIBP-CorV. However, the differences were not statistically significant, indicating that the humoral immunity induced by Omicron BA.1 after inoculation with the CoronaVac vaccine was quite similar to that after inoculation with the BBIBP-CorV vaccine. However, a limited number of samples were included in this study, and further research is required.
The neutralizing antibody levels against different strains were higher in 58 patients with moderate disease than in 64 patients with mild disease at 3 and 6 months after Omicron BA.1 infection, which may be because the more severe the clinical symptoms, the higher the viral antigen load, and thus higher the stimulated neutralizing antibodies[16-18]. This phenomenon has also been observed in severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS)[19]. However, the neutralizing antibody titers against the WT, Gamma, Beta, Delta, and Omicron strains were significantly decreased at 6 months after Omicron BA.1 infection both in mild and moderate patients, and the decline was similar.
The neutralizing antibody titers of patients aged < 60 years were similar to those aged ≥ 60 years, and there were no significant statistical differences. This result suggests that we do not need to make age-targeted vaccination strategies in the future.
The neutralizing antibody level significantly decreases with time and may eventually lose its protective effect, especially when new SARS-COV-2 variants become dominant; therefore, it is particularly important to understand the changes in cellular immunity after Omicron BA.1 infection. COVID-19 is an acute inflammatory disease caused by SARS-COV-2. The correlation between the stage of infection, severity and prognosis of the disease, and the level of cellular immunity has attracted wide attention in academic circles. The adaptive immune response plays a crucial role in the maintenance of immune function, control and clearance of pathogens, and the generation of specific immune memories. Lymphocytes, particularly CD4+ and CD8+ T-lymphocytes, are important cellular components of the adaptive immune response. CD4+ T-cells can differentiate into a series of helper and effector cells, guide B-cells, induce naïve cells, assist CD8+ T-cells, exert direct antiviral activity, and promote tissue healing. CD8+ T-cells can kill infected cells and are critical for clearing many viral infections; thus, they are associated with COVID-19 prognosis[20,21].
A recent study showed that both CD4+ and CD8+ T-lymphocyte counts significantly decreased after SARS-CoV-2 infection[22]. Flow cytometry results also revealed that the lymphocytes, total T-cells, CD4+, and CD8+ T-lymphocytes in patients who were infected with acute SARS-COV-2 were significantly decreased compared to those in the healthy control group[23]. Liu Z and his colleagues found that the CD4 + T lymphocytes, CD8 + T lymphocytes, and CD4/CD8 ratios in patients with severe COVID-19 were significantly decreased compared to those with mild COVID-19[24]. It was also found that low levels of CD4 + and CD8 + T lymphocytes were associated with a poor prognosis in patients with SARS; however, with symptom relief, the numbers of CD4+ and CD8+T lymphocytes quickly returned to normal levels[25]. Wang et al. found that the CD8+ T lymphocyte counts gradually increased, whereas the CD4+ T lymphocyte counts did not change significantly with the relief of symptoms and improvement of radiographic abnormalities one week after COVID-19 infection[26].
This study results indicated that CD3+, CD4+, and CD8 + cell counts and the CD4 +/CD8 + ratio were significantly lower in the patients at 3 months after infection than at 6 months after infection. The CD4+/CD8+ ratio in the case group was slightly lower than that in the control group at 3 months after infection, indicating that the number of lymphocytes in the patients recovered to normal levels at 3 months after infection but continued to increase and returned to normal levels at 6 months after infection. The difference in the CD4+/CD8+ ratio may be associated with an imbalance of CD4+ and CD8+ T cells during the recovery process, and the mechanism needs to be further elucidated.
The COVID-19 pandemic has lasted for more than three years. Due to the strong immune escape ability of the virus, rapid decline of neutralizing antibodies, large number of asymptomatic infected people, unknown natural host, and the risk of transmission from animals to humans, the infectivity of symptomatic patients at the end of the latent period increases the difficulty of global eradication or elimination associated with this kind of infectious disease. Therefore, it is highly likely that SARS-CoV-2 will coexist with humans and may eventually become an endemic disease, similar to seasonal influenza. In such a scenario, the world will need to strengthen surveillance and adjust vaccine composition according to the latest circulating dominant strains to prevent seasonal epidemics of COVID-19 in the future until an effective universal vaccine is successfully developed. The adjustment of vaccine composition needs to be emphasized and evaluated in many aspects. Whenever a dominant SARS-CoV-2 variant is detected, testing for neutralizing antibody titer responses to the infection is necessary to evaluate its immune escape ability. However, we still lack definite neutralization antibody level criteria to evaluate the effectiveness of the vaccine or its ability to prevent infection. Adjustment of vaccine composition or vaccination strategy requires more scientific evidence, such as the immunogenicity of new vaccines, protection ability against mutated virus strains, and attenuation rate of neutralizing antibodies.
An obvious strength of our study is the ability to ensure that the controls remained uninfected throughout the study period because it was conducted during China’s dynamic zero-COVID period, and the case group and the control group were strictly matched by age, sex, and vaccination profile, which helped to improve the comparability between the two groups and statistical efficiency. A limitation is that there is no agreed-upon laboratory correlation of protection against COVID-19 outcomes, especially for inactivated COVID-19 vaccines; therefore, the relationship between neutralizing antibody levels and protection from infection and serious illness is not clear. Another limitation was that the study did not include virus-specific cellular immunity testing. The third limitation was its relatively small sample size. Because patients in mainland China have been recently infected with either Omicron BA.5.2 or BF.7, it is difficult to find an uninfected control population; therefore, referring to the design of this study, similar studies on the persistence of the neutralizing antibody response after different Omicron breakthrough infections can be carried out in the future, which will help to provide further scientific basis for the development and adjustment of SARS-COV-2 vaccination strategies.
HTML
Data Source
Ethical Statements
Personal and Clinical Information Collection
Blood Samples Collection and Storage
Laboratory Test
Live Virus SARS-CoV-2 Neutralization Test
Lymphocyte Cell Count Test
Statistical Method
Basic Information
Clinical Characteristics of the Case Group
The Neutralizing Antibody Level in the Case Group and Control Group
T Lymphocyte Cell Count Testing Results
DECLARATION OF INTERESTS Sinovac Biotech Ltd. only helped conduct the live SARS-CoV-2 virus neutralization test in this study, and they did not have specific information about each subject, particularly vaccination information.







 Quick Links
Quick Links
 DownLoad:
DownLoad: