-
Kashin-Beck disease (KBD) is a chronic, endemic, degenerative osteoarthropathic condition that is predominantly found in mainland China. KBD is a progressive disease, and the signs and symptoms become more severe in adulthood owing to the continuous development of lesions in the joint cartilage[1]. Although childhood KBD has largely been controlled, adult KBD is an important health concern in rural regions. Unfortunately, in the last century, many adult KBD patients still remain during serious epidemics.
Metabolomics, an important subdiscipline of systematic biology, is a research field of systematic biology in the class of genomics, proteomics, and transcriptomics. Metabolites are defined as the final products of cellular regulatory progression, and their levels can be regarded as the ultimate response of biological systems to genetic or ecological alterations[2]. As such, metabolomics has the potential to identify associations between metabolism and phenotype, and to further support studies on related metabolic pathways and networks in organisms.
Urine has a considerable value as a diagnostic biofluid, and previous studies have shown that the pathological changes associated with KBD are reflected in the biological changes in urine. Furthermore, urine detection is an acceptable method for KBD investigation in the Qinghai-Tibet Plateau and its neighboring regions, and has a non-invasive nature, enables easy sampling, and good operability. Thus, metabolomic detection of urine is necessary for an in-depth study of KBD and its prevention and control.
The present study was carried out in compliance with the ethical principles outlined in the world medical association Declaration of Helsinki. This study was approved by the ethics committee of the Qinghai institute for endemic disease prevention and control (Ethics Protocol number: 2017-002). All patients and controls signed an informed consent form for participation.
According to the national diagnostic criteria for KBD in China (WS/T207-2010), female patients were diagnosed with KBD based on epidemiological investigation, clinical symptoms, and radiographic findings. Controls were women with no clinical symptoms or radiographic findings. Individuals who suffered from osteoarthritis (OA), rheumatoid arthritis (RA), other osteoarticular diseases, or joint lesions or those who underwent joint operation within one year or had taken therapeutic drugs for arthritis were excluded. Women diagnosed with chronic systemic or acute inflammatory diseases were also excluded. Finally, 60 female participants (30 patients and 30 controls) were included in this study. The morning urine samples were collected from the target population after fasting for 10 h. Ultra-performance liquid chromatography and Q-TOF mass spectrometry (UPLC/Q-TOF MS) (Beijing Mass Spectrometry Medical Research Co., Ltd) were used to analyze all the urinary samples. The chromatographic and mass spectrometry conditions were as follows: Mass spectrometry full-scan data were acquired in positive and negative electrospray ionization (ESI) modes from 50 to 1,200 D. The parameter conditions were as follows: 2.5 kV capillary voltage, 350 °C desolvation temperature, 35 V sample cone voltage, 50 L/h cone gas flow, 700 L/h desolvation gas flow, and 350 °C source temperature. A Waters Xevo G2 Q-TOF (Quatropde Time-of-Flight) mass spectrometer (Waters Corp.) was connected to the UPLC system via an ESI interface. Chromatographic separation was performed on a Waters UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm). Analytes were eluted from the column using a gradient of water (A) and acetonitrile containing 0.1% formic acid (B) as the mobile phase. The gradient conditions were: 0 min, 60% A and 40% B; 2 min, linear from 60% to 57% A and linear from 40% to 43% B; 2.1 min, linear from 57% to 50% A and linear from 43% to 50% B; 12 min, linear from 50% to 46% A and linear from 46% to 54% B; 12.1 min, linear from 46% to 30% A and linear from 54% to 70% B; 18 min, linear from 30% to 1% A and linear from 54% to 99% B; 18.1–20 min, linear from 1% to 60% A and linear from 99% to 40% B. Each run time was of 20 min, with a flow rate of 0.30 mL/min.
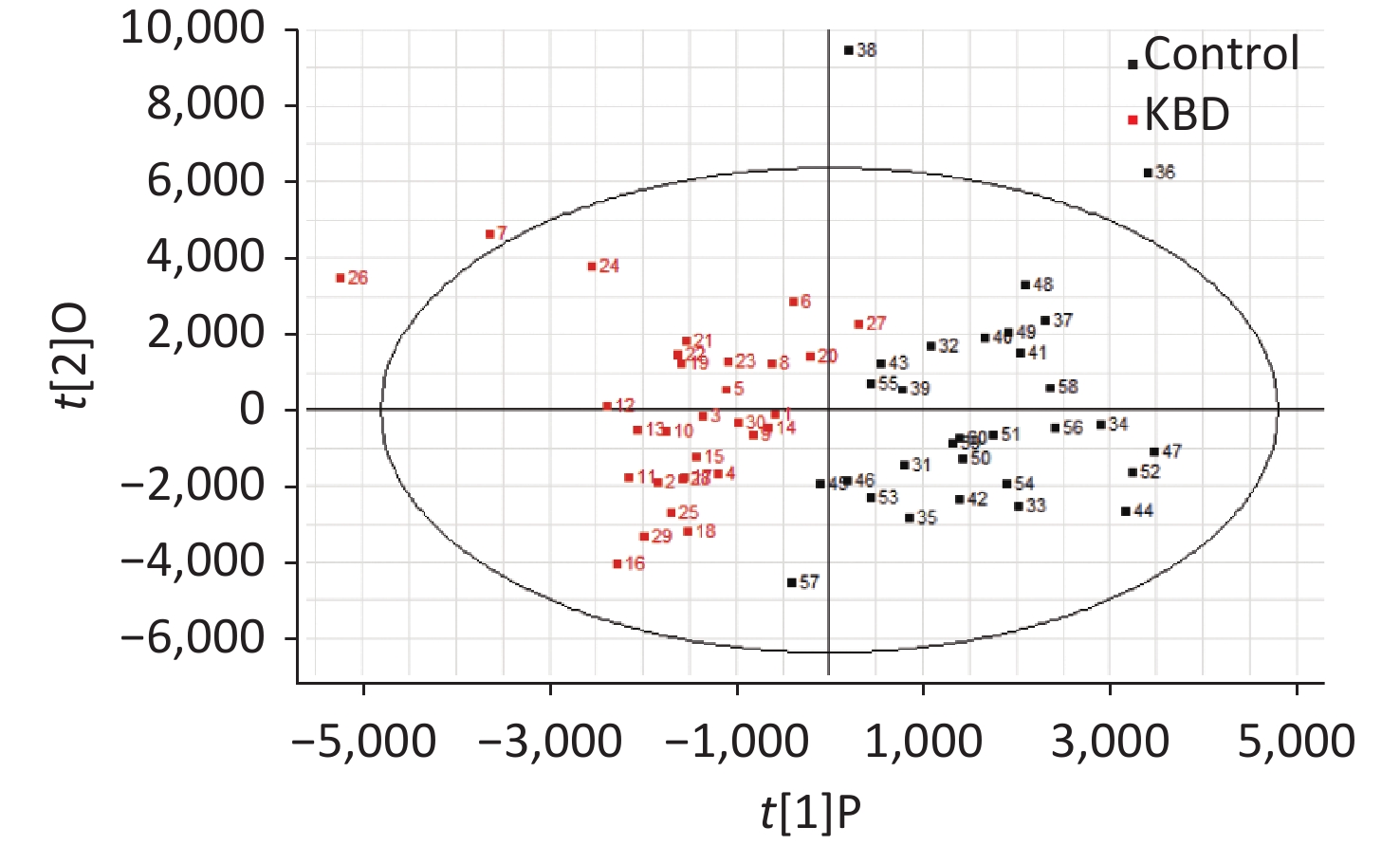
UPLC/Q-TOF MS data were processed using the Micromass Marker Lynx application version 4.1 (Waters Corporation, MA, USA). After denoising, deconvolution, peak extraction, alignment, and merging, the data matrix containing assigned peak numbers (retention time-m/z pairs), sample names, and normalized ion intensities was exported into SIMCA-P 11.5 software (Umetrics, Umea, Sweden) for partial least squares discriminant analysis (PLS-DA) and principal component analysis (PCA), which were used to obtain the greatest variable importance in projection (VIP) values, as well as to visualize the score plot. Variables with VIP values above 1.0 in the model were considered potential biomarkers. Goodness of fit was quantified by R2
, and predictive ability was indicated by Q2. Independent-samples t-test and nonparametric statistical test were used for statistical analysis using SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA), and a P value < 0.05 was considered statistically significant. The databases HMDB (http://www.hmdb.ca) and METLIN (http://metlin.scripps.edu/) were used to identify the potential metabolic marker candidates based on their tandem mass spectrometry (MS/MS) spectra.
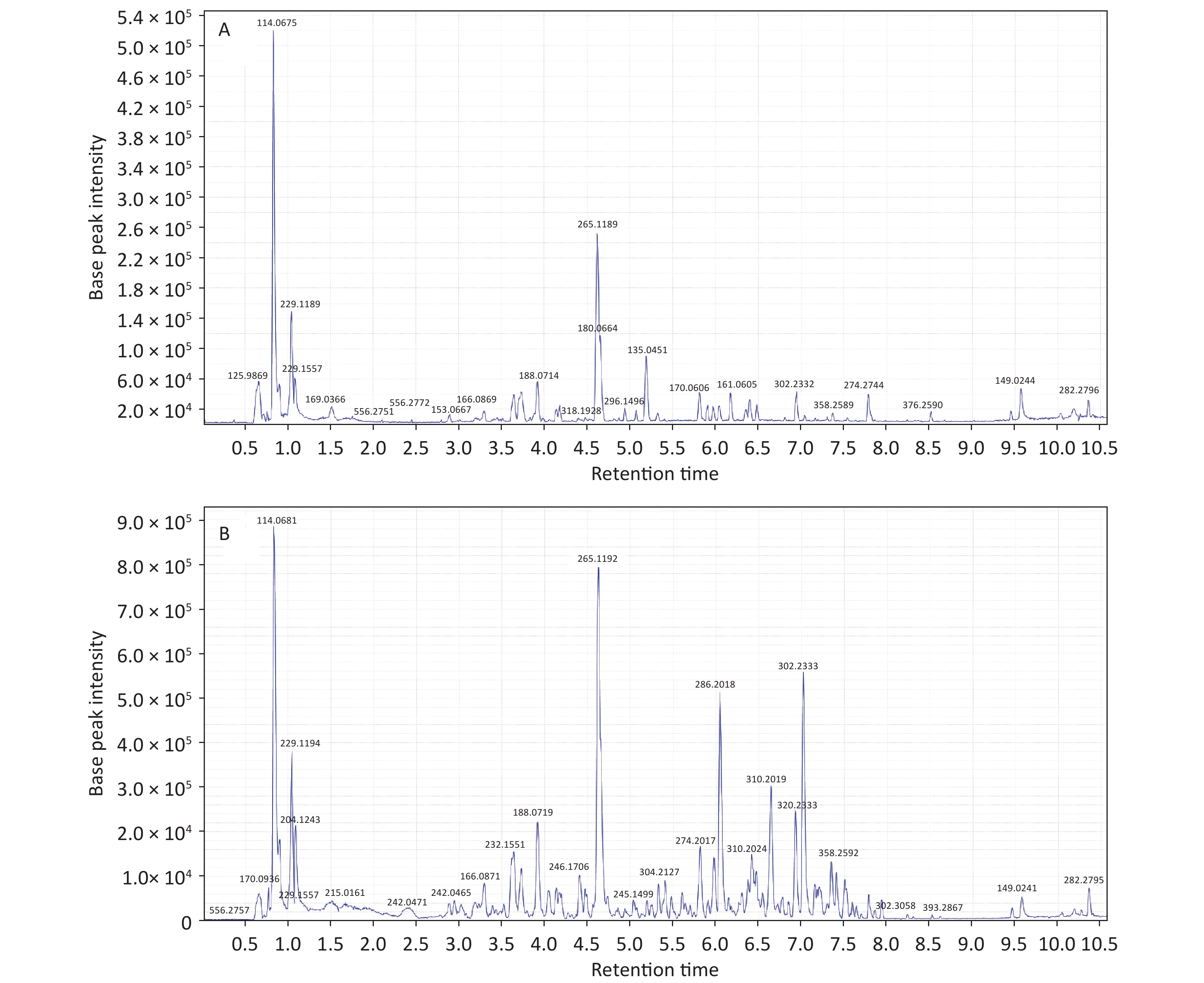
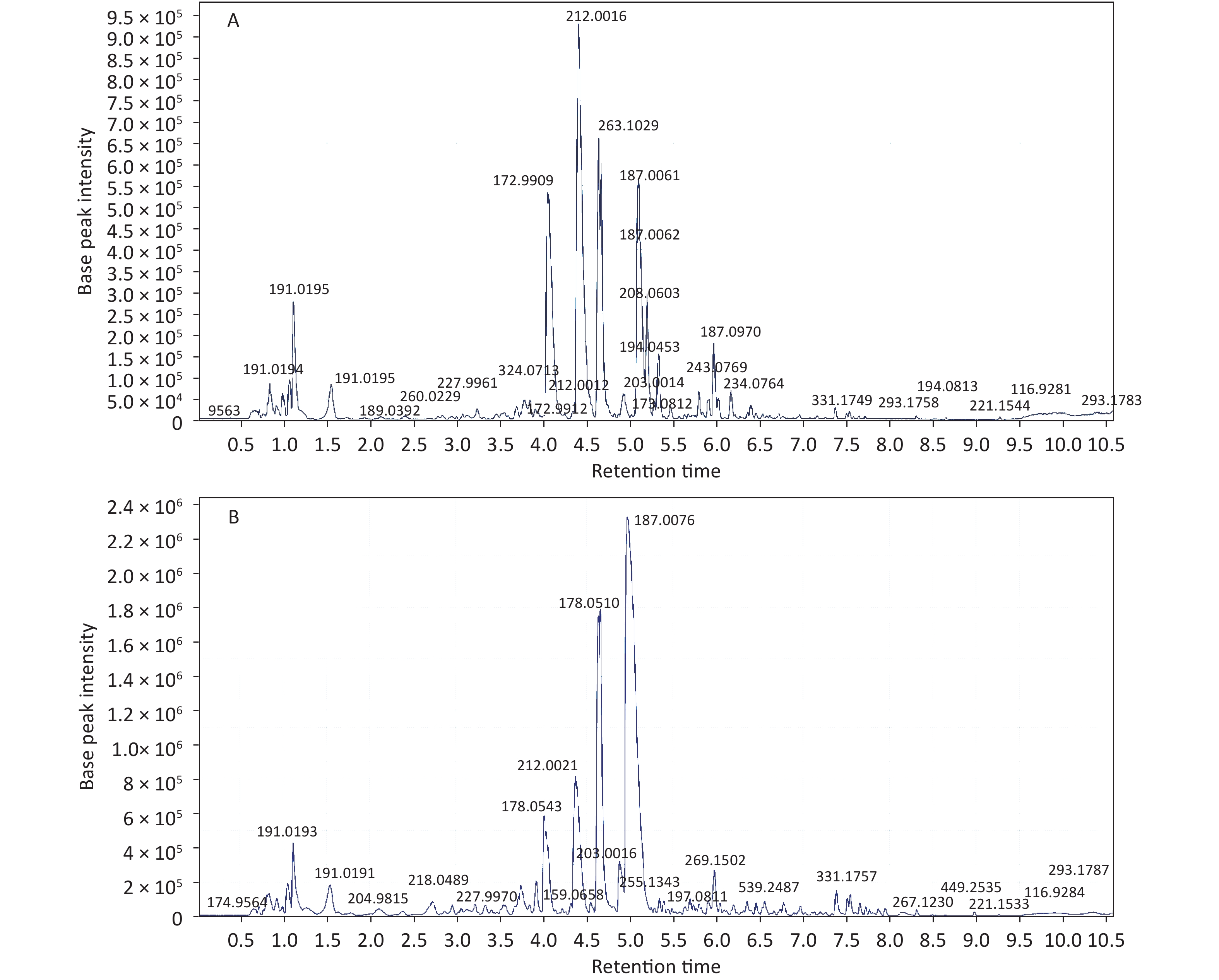
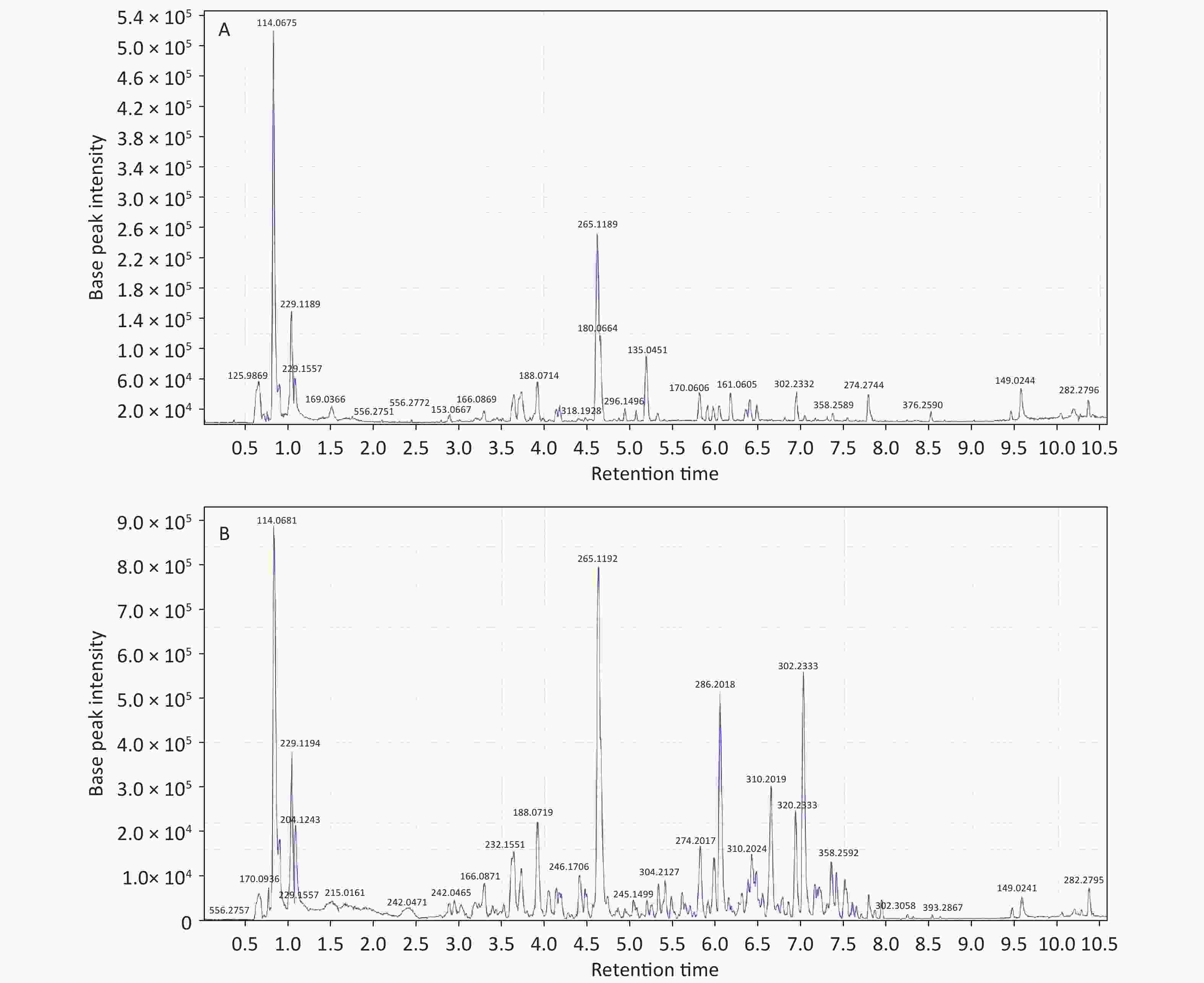
Initial analysis revealed no statistically significant differences in age, height, weight, or body mass index between patients with KBD and controls (P > 0.05) (Supplementary Table S1, available in www.besjournal.com). Urinary samples were analyzed by UPLC/QTOF-MS in both the positive and negative ionization modes, and the primary data of the mass spectrograph were obtained. The total ionization graphs of patients with KBD and controls are shown in Figure 1 and Supplementary Figure S1 (available in www.besjournal.com).
Group Number Age mean (years) Heights (cm) Weights (kg) BMI KBD patients 30 47.57 ± 9.97 157.45 ± 6.04 57.14 ± 7.24 23.07 ± 3.16 Controls 30 47.27 ± 13.06 158.76 ± 7.15 58.26 ± 8.64 23.08 ± 3.07 t 0.099 0.754 0.541 0.007 P 0.921 0.454 0.592 0.994 Table S1. General conditions of the female KBD patients and controls
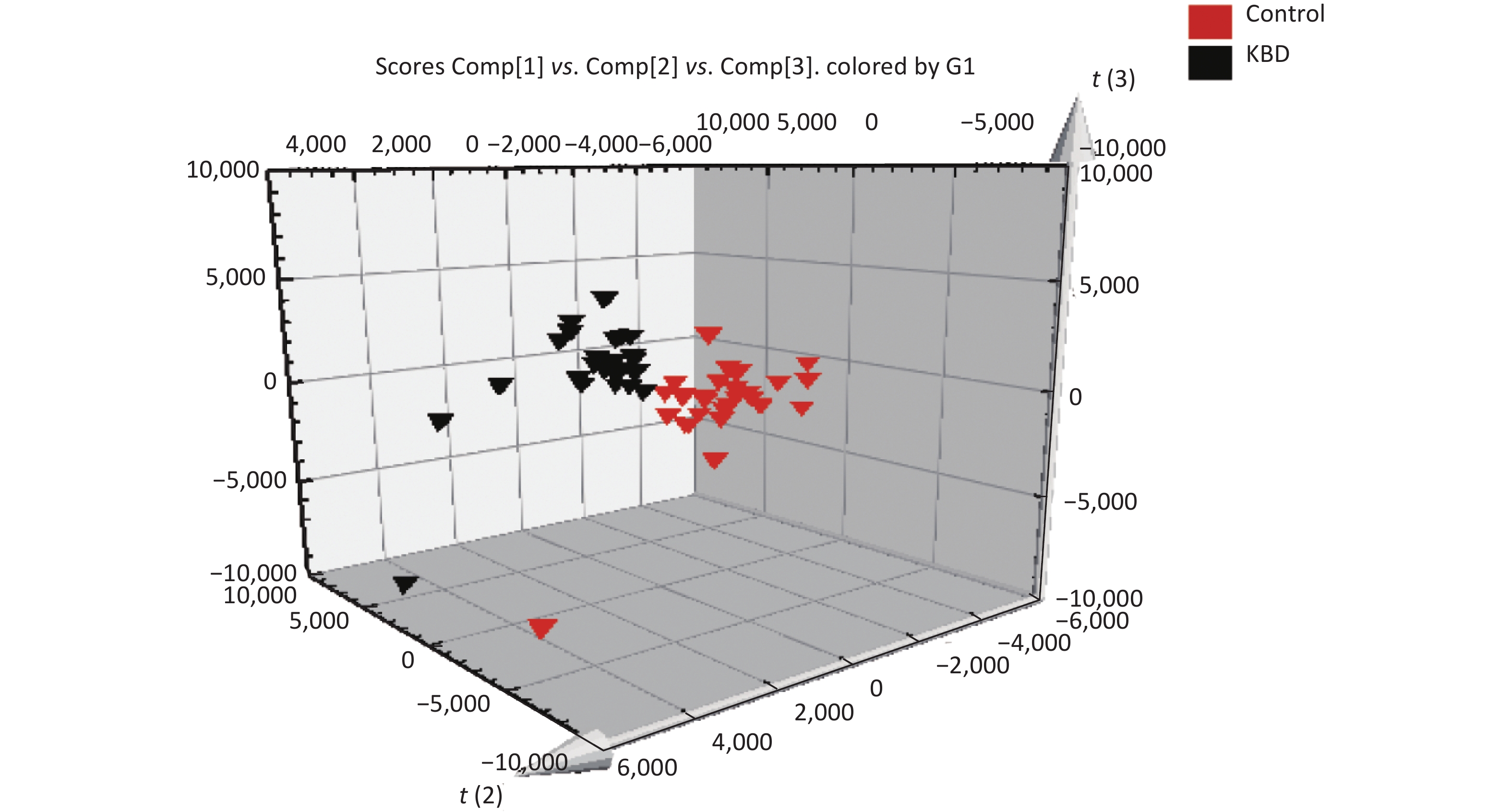
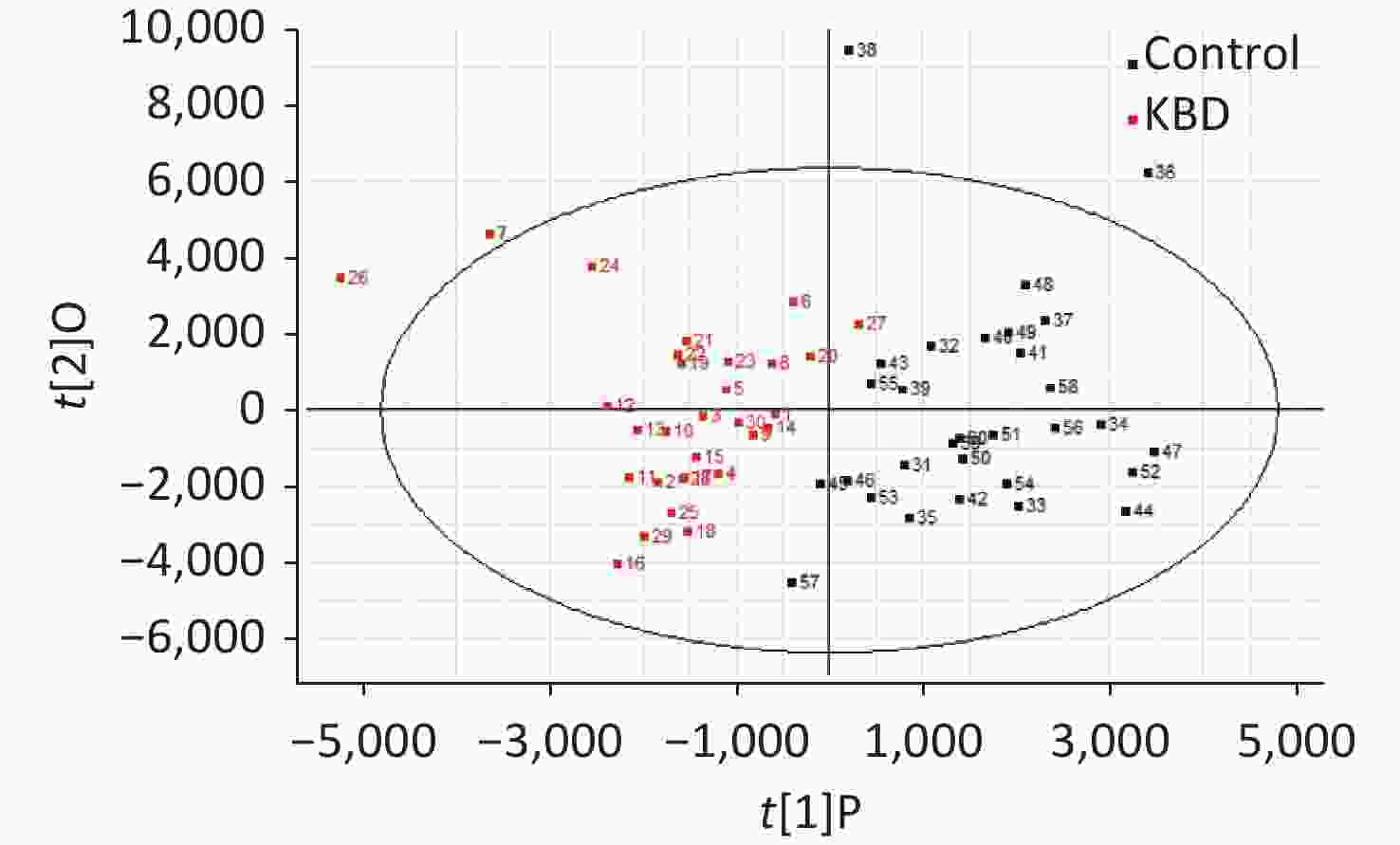
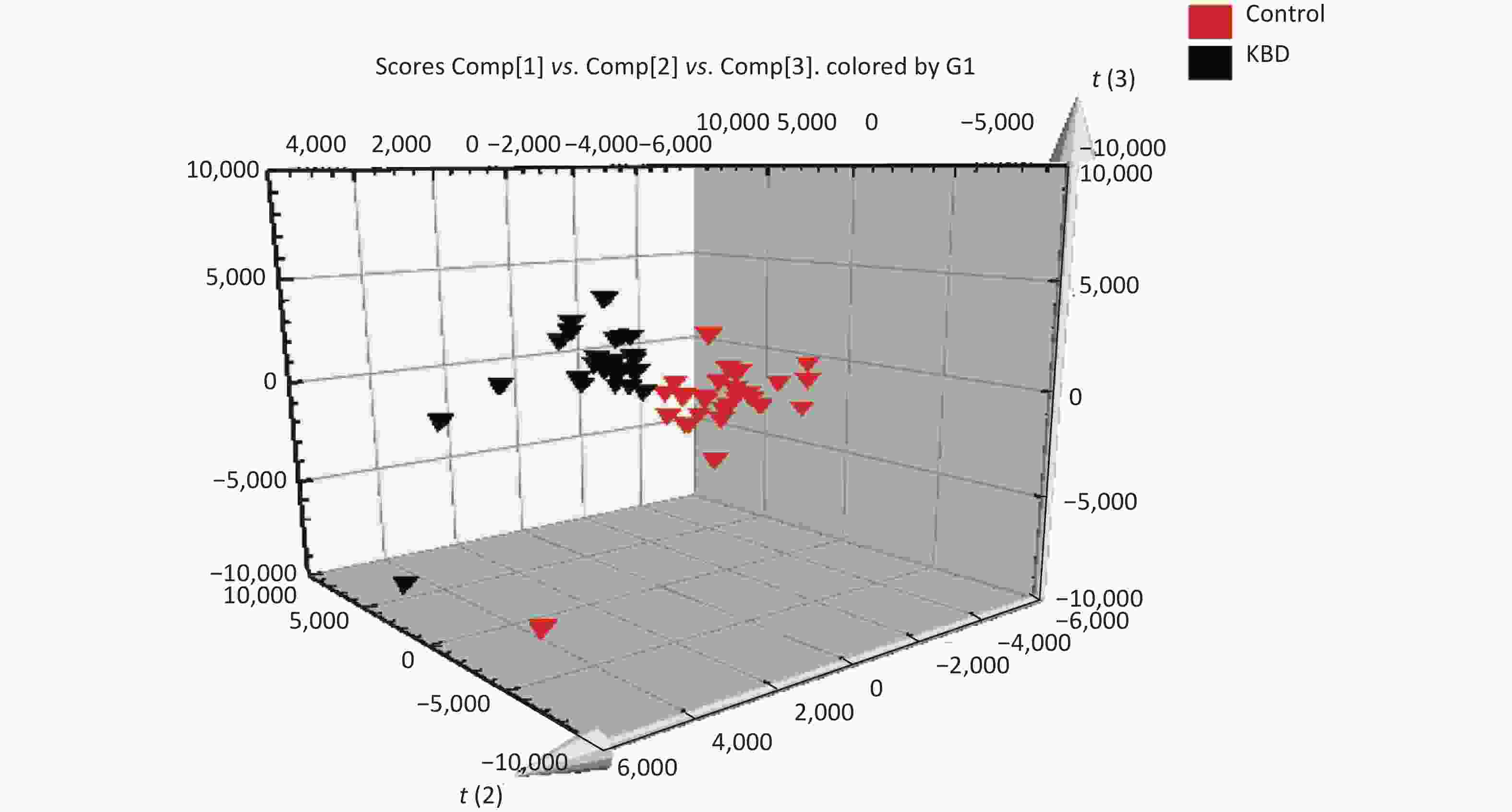
The mass-to-charge ratio ranged from 85.029–835.29. A total of 1,663 metabolites with VIP values above 1.0 were selected for subsequent analysis. After t-test or nonparametric statistical test, a statistically significant difference in 586 metabolites was found between patients with KBD and controls (P < 0.05). PCA was performed through multivariate data analysis using Simca-p software. PLS-DA was further carried out on the module (R2 = 0.802, Q2 = 0.731), which showed stable and significant clusters (Supplementary Figure S2 and Supplementary Figure S3, available in www.besjournal.com).
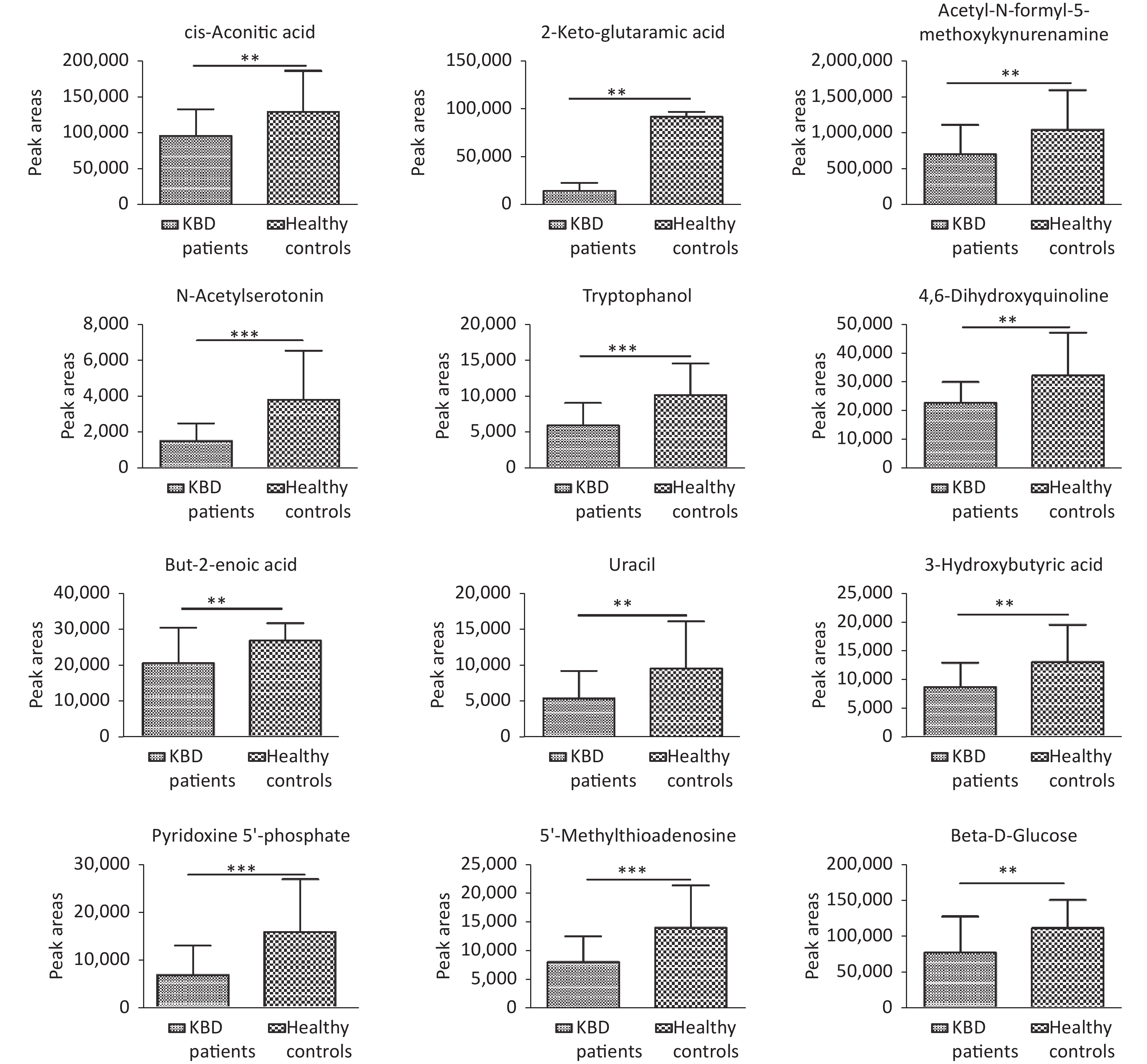
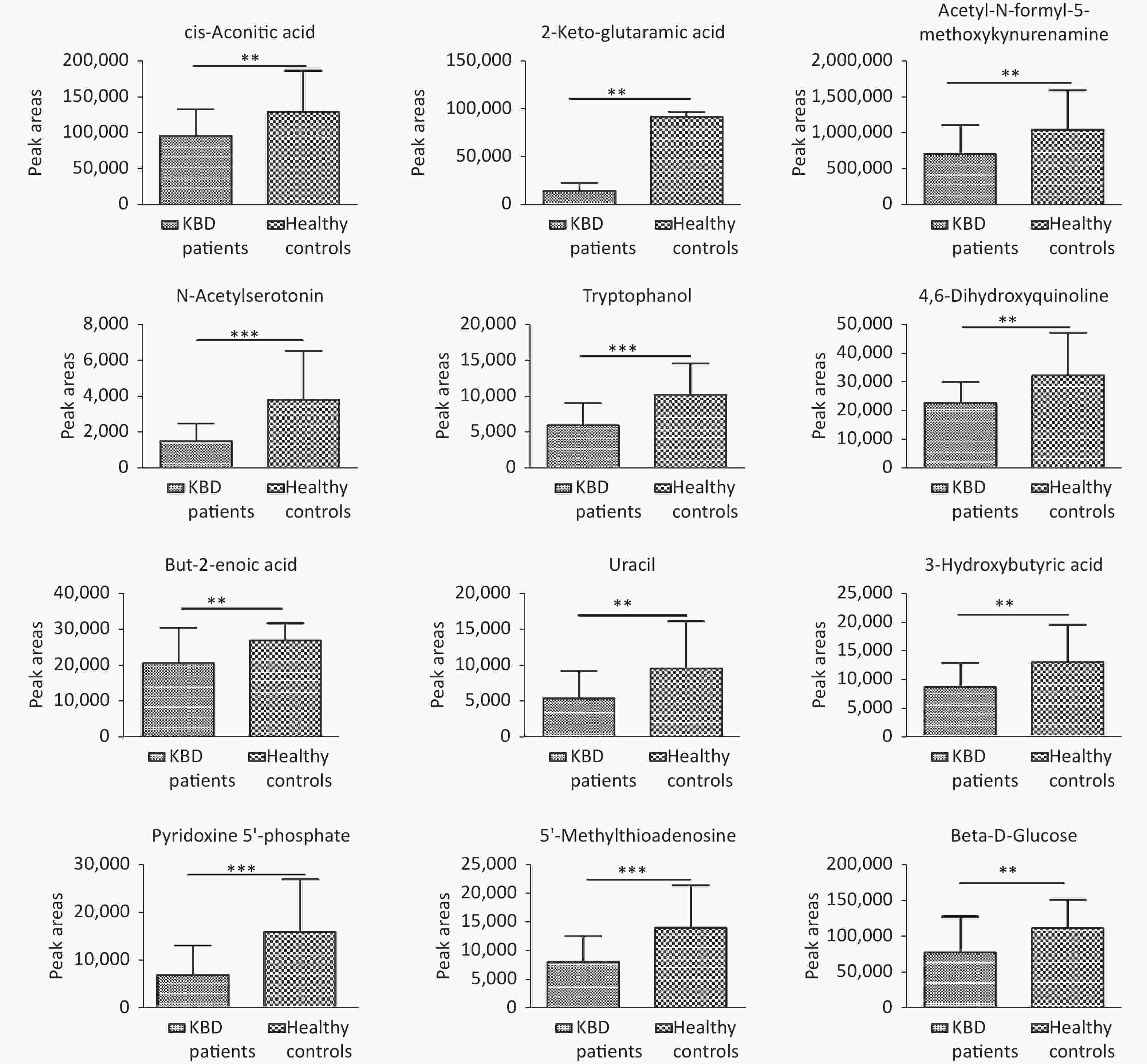
Structural identification of the selected substances was performed by comparing the exact mass data, retention times, and corresponding MS/MS fragments with those of reference standards in the HMDB and METLIN databases. Metabolites identified in the negative ionization mode included cis-aconitic acid, but-2-enoic acid, and acetyl-N-formyl-5-methoxykynurenamine. Metabolites identified in the positive ionization mode included beta-D-glucose, uracil, 4,6-dihydroxyquinoline 2-keto-glutaramic acid, tryptophanol, N-acetylserotonin, 3-hydroxybutyric acid, pyridoxine 5'-phosphate, 5'-methylthioadenosine, and acetyl-N-formyl-5-methoxykynurenamine. Overall, 12 differential metabolites were associated with KBD (Tables 1–2, and Supplementary Figure S4, available in www.besjournal.com).
No. Metabolite Formula Retention time/min Meraure mass VIP Related
pathway1 cis-aconitic acid C6H6O6 1.08 174.017 6.2721 Tricarboxylic acid cycle 2 But-2-enoic acid C4H6O2 1.12 86.037 2.1377 Fatty acid metabolism 3 Acetyl-N-formyl-5-methoxykynurenamine C13H16N2O4 4.64 264.154 13.7420 Trptophan metabolism 4 2-keto-glutaramic acid C5H7NO4 1.07 145.020 1.6551 Tricarboxylic acid cycle 5 Uracil C4H4N2O2 5.87 112.032 1.7565 Beta-alanine metabolism 6 Tryptophanol C10H11NO 4.27 161.084 3.1972 Trptophan metabolism 7 N-acetylserotonin C12H14N2O2 3.06 218.103 1.5789 Trptophan metabolism 8 4,6-dihydroxyquinoline C9H7NO2 4.94 161.048 2.2091 Trptophan metabolism 9 3-hydroxybutyric acid C4H8O3 4.03 104.050 1.5387 Ketone body metabolism 10 Pyridoxine 5'-phosphate C8H12NO6P 6.40 249.040 2.8699 Vitamin B6 metabolism 11 5'-Methylthioadenosine C11H15N5O3S 3.87 297.090 2.3111 Cysteine and methionine metabolism 12 Beta-D-glucose C6H12O6 4.16 180.053 3.9685 Glycolysis Note. VIP, variable importance in projection. Table 1. Differences in urinary metabolite between patients and controls
Differences metabolites KBD patients Controls P N Mean ± SD N Mean ± SD Cis-aconitic acid 30 95,423 ± 37,912 30 130,128 ± 56,621 0.007 But-2-enoic acid 30 20,461 ± 10,010 30 26,779 ± 4,969 0.003 Acetyl-N-formyl-5-methoxykynurenamine 30 702,743 ± 410,729 30 1,042,346 ± 554,197 0.009 2-Keto-glutaramic acid 30 14,297 ± 8,504 30 91,952 ± 5,072 0.007 Uracil 30 5,517 ± 3,823 30 9,651 ± 6,589 0.005 Tryptophanol 30 5,897 ± 3,137 30 10,156 ± 4,402 < 0.001 N-acetylserotonin 30 1,492 ± 997 30 3,806 ± 2,739 < 0.001 4,6-dihydroxyquinoline 30 22,739 ± 7,228 30 32,261 ± 14,914 0.003 3-hydroxybutyric acid 30 8,628 ± 4,216 30 12,971 ± 6,496 0.003 Pyridoxine 5'-phosphate 30 6,798 ± 6,107 30 15,767 ± 10,993 < 0.001 5'-Methylthioadenosine 30 8,042 ± 4,482 30 14,029 ± 7,407 < 0.001 Beta-D-glucose 30 77,254 ± 50,143 30 111,332 ± 39,204 0.004 Note. KBD, Kashin-Beck disease. Table 2. Comparison of the peak areas of twelve differences metabolites among the two groups (x ± S)
KBD is predominantly distributed in the diagonal broad belt ranging from southeastern Siberia in Russia to southwest China. The main pathological changes are chondrocyte necrosis, apoptosis, cartilage degeneration, and matrix degradation[3]. Environmental risk factors, such as selenium deficiency, grain contamination by mycotoxin-producing fungi, and high fulvic acid levels in drinking water, are closely associated with KBD[4]. As the etiology of KBD is uncertain, further analysis of the progression of OA could provide many new clues and opinions for KBD research.
Many studies have found that OA may be associated with differences in amino acid and collagen metabolism, the tricarboxylic acid cycle (TCA), and fatty acid metabolism. A prior metabolomics study examining serum samples from controls and patients with OA, RA, ankylosing spondylitis, and gout found that patients with arthritis could be differentiated from controls, and that each form of arthritis had a distinct metabolite profile[5]. Similarly, another study found that the progression of joint space narrowing in adults with OA was associated with a different metabolite pattern[6]. Metabolomics is a promising tool for identifying biomarkers for OA diagnosis, stratification, and treatment.
Metabolomic studies have been used to probe biomarkers and the pathogenesis of KBD. NMR revealed that serum metabolite changes in patients with KBD were involved in glucose metabolism, while eight metabolites were related to the metabolism of phospholipids and amino acids in Wistar rats with KBD induced by T-2 toxin [7].
In the present study, we identified 12 metabolites involved in TCA, amino acid, fatty acid, energy, and glycolytic metabolism. Of these, cis-aconitic acid and 2-keto-glutaramic acid are related to TCA, which is involved in energy metabolism. Because the key enzyme in TCA is located in the mitochondrial matrix, changes in cis-aconitic acid and 2-keto-glutaramic acid indicate functional disorders of the mitochondria in chondrocytes. Liu et al. found that mitochondrial dysfunction may play an important role in chondrocyte apoptosis, which is involved in the pathophysiology of KBD[8]. In the present study, the relative levels of cis-aconitic acid and 2-keto-glutaramic acid in patients were lower than in controls, indicating mitochondrial dysfunction.
Tryptophanol, acetyl-N-formyl-5-methoxykynurenamine, 4,6-dihydroxyquinoline, and N-acetylserotonin were related to tryptophan metabolism, which is a key pathway in melatonin biosynthesis and degradation. The metabolites are also associated with beta-alanine metabolism, while 5'-methylthioadenosine was associated with cysteine and methionine metabolism, together indicating a disorder of amino acid metabolism.
But-2-enoic acid is associated with fatty acid metabolism. Active processing of fatty acids occurs in the cytosol, whereas further oxidation of fatty acids occurs in the mitochondria matrix. Similarly, 3-hydroxybutyric acid is associated with ketone body metabolism[9]. In the present study, the relative level of 3-hydroxybutyric acid in patients was lower than that in controls, indicating a disorder in energy metabolism.
Beta-D-glucose is a metabolite associated with glycolysis. Pathological changes in KBD include focal chondrocyte death (necrosis) and associated proteoglycan (PG) depletion, which indicates risk factor-induced disruption of PG metabolism and subsequent abnormal biomechanical strain distribution in chondrocytes[10]. In the present study, the relative level of beta-D-glucose in patients was lower than that in controls, indicating a glycolysis disorder.
Vitamin B6 deficiency is also involved in the pathogenesis of KBD, and in this study, the relative level of pyridoxine 5'-phosphate in KBD patients was lower than that in controls, indicating a disorder of vitamin B6 metabolism.
Overall, in the present study, we applied UPLC/Q-TOF MS technology to conduct urinary metabolomics research on women in a KBD-endemic region. This analysis revealed 12 metabolites associated with KBD. These were involved in TCA metabolism, fatty acid metabolism, energy metabolism, glycolysis metabolism, and amino acid metabolism. Further metabolomics research is required to verify these metabolites and to screen for biomarkers of KBD.
HTML
 23005+Supplementary Materials.pdf
23005+Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: