-
Generally, a healthy immune system should be in dynamic balance, which can be maintained by both promoting and resisting inflammation. Lymphocyte apoptosis is indispensable for maintaining homeostasis[1] and participates in the entire process of lymphocyte differentiation, development, maturation, and immune effects. It has been reported that a large amount of lymphocyte apoptosis occurs in lymphoid organs during severe trauma[2]. Lymphocytes consist of T and B lymphocytes, among which CD4+T cells were the focus of this study. CD4+T lymphocytes play an important role in the innate immunity. Apoptosis of CD4+T lymphocytes is an important biological process that induces CD4+T cell depletion[3]. Numerous studies have shown that CD4+T cell apoptosis participates in many pathological processes of diseases such as HIV infection, cancer, and systemic sclerosis[4]. Classical apoptosis is induced by factors that can activate several pathways, including the mitochondrial, endoplasmic reticulum, and death receptor pathways[5]. The mitochondrial pathway is mainly activated by the Bcl-2 family[6]. The endoplasmic reticulum (ER) pathway is affected by endoplasmic reticulum disorders. Some external factors can trigger the death receptor pathway, such as the binding of TNF-TNFR and the combination of Fas-FasL[7]. Considering these pathways, it is feasible to study the specific mechanisms of lymphocyte apoptosis, primarily in CD4+T cells.
C6ORF120 is a novel secreted glycoprotein that has recently received considerable attention because of its involvement in autoimmune hepatitis (AIH), type I diabetes, and acquired immune deficiency syndrome (AIDS)[8]. The main function of C6ORF120 is predicted to regulate the function of CD4+T lymphocytes and glycolipid metabolism and participate in autoimmune hepatitis[9]. Previous studies have reported that C6ORF120 is differentially expressed in AIDS patients. Further studies show that C6ORF120 may promote CD4+T cell apoptosis[10], mainly during early apoptosis; however, the specific mechanism is unknown. The mitochondrial pathway plays a key role in early apoptosis, among which the Bcl-2 family of proteins are the main regulators controlling the release of mitochondria-related apoptosis factors. Therefore, it is speculated that C6ORF120 could promote the early apoptosis of CD4+T lymphocytes by activating the mitochondrial pathway.
Continuing our interest in the biological function of C6ORF120, we investigated mitochondria-mediated apoptosis. The aim of this study was to confirm that C6ORF120 promotes CD4+T cell apoptosis in rat models and to use a well-characterized in vitro model of human CD4+T cells, using the Jurkat cell line, to determine whether C6ORF120 could activate the mitochondrial apoptotic pathway in CD4+T cells.
C6orf120 knockout (C6orf120−/−) rats (SD, 6–8 weeks) were prepared by Cyagen Biotechnology Co. Ltd. based on the TALEN gene complete knockout technology. Wild-type (WT) rats of the same age were provided by genotype identification or purchased from SPF Biotechnology Co., Ltd. (Beijing, China). Both genotypes of rats were cohoused in the Animal Center of Peking University under specific pathogen-free conditions (4–6 rats per cage, 21 ± 2 °C, 12-h light–dark cycle). All animal experiments were approved by the Animal Care and Use Committee. Single-cell suspensions were separated from the blood, spleen, liver, and mesenteric lymph nodes by euthanizing the WT and C6orf120−/− rats. The frequencies of the expected subsets were detected using flow cytometry (FCM), with FACS Canto, BD Bioscience Company, USA.
Combined with the fact that the Jurkat cell line is extensively employed as a model of T cell signaling, the molecular mechanism of C6ORF120 in Jurkat cells was further studied. Jurkat (human acute T lymphoma leukemia cell line) cells were purchased from the Peking Union Cell Resource Center (Beijing, China) and cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% FBS (Gibco, Grand Island, NY, USA) and 1% P/S (Gibco, Grand Island, NY, USA), at 37 °C in the presence of 5% CO2. Jurkat cells were seeded into 96-well plates at a density of 1 × 104 cells/well. 12 h later, the final concentrations of rC6ORF120 (C6ORF120 recombinant protein) was adjusted to 0, 10, 100, 200, or 500 ng/mL. The 0 ng/mL group was used as the control. After 48 h, cell viability was measured using a CCK-8 Assay Kit (DOJINDO, Japan). The CCK-8 (10 µL) was added to each well and incubated for 4 h in the dark at 37 °C incubator. Absorbance of the solution was measured at 450 nm using a Varioskan Flash spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The cell viability was calculated using the following formula: Cell Viability = OD450 (treated group)/OD450 (control group).
Jurkat cells were seeded into 12-well plates at a density of 1 × 105 cells/well. After treated with rC6ORF120 for 48 h, apoptosis was detected using an APC Annexin V Apoptosis Detection Kit (BioLegend, San Diego, CA, USA), according to the manufacturer’s protocol. Cell fluorescence was immediately determined using a FACS Canto II flow cytometer (BD Biosciences, San Jose, CA, USA). The percentages of apoptotic cells were analyzed by Cell Quest Pro software (BD Biosciences, San Jose, CA, USA). Total protein (40 μg) extracted from Jurkat cells was used for western blot analysis. The following primary antibodies were used: rabbit anti-BAX polyclonal antibody (1:2,000, proteintech), mouse anti-Bcl2 monoclonal antibody (1:1,000, proteintech), mouse anti-Caspase3 monoclonal antibody (1:3,000, proteintech), mouse anti-PARP1 monoclonal antibody (1:40,000, proteintech) and mouse anti-β-actin monoclonal antibody (1:10,000, proteintech). After densitometric scanning, the results were quantified using ImageJ 1.43. RNA was extracted from Jurkat cells using the TRIzol reagent (Dakewe Biotech Co., 8034011) and reverse transcribed into cDNA using the Takara PrimeScriptTM RT Reagent Kit (Takara, RR037A). Primer sequences were obtained from PrimerBank (https://pga. mgh. harvard. edu/primerbank/) and synthesized by SYNBIO Technologies Ltd. (Suzhou, China) (Supplementary Table S1, available in www.besjournal.com). RT-qPCR was performed using the Applied Biosystems PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, A25776) on an ABI Applied Biosystems system.
Gene Primer Sequence (5’→3’) Bax Forward TTGCTACAGGGTTTCATCCAGG Reverse CACTCGCTCAGCTTCTTGGT Bcl2 Forward CTTTGAGTTCGGTGGGGTCA Reverse TAGTTCCACAAAGGCATCCCAG β-actin Forward AGCGAGCATCCCCCAAAGTT Reverse GGGCACGAAGGCTCATCATT Table 1. Primers for RT-qPCR
Data are presented as mean ± standard deviation (SD). Statistical differences between groups were analyzed by Student’s t-test and subsequent Bonferroni post-hoc test using the SPSS software (version 22.0; SPSS Institute, Chicago, IL, USA). The P-value was two-tailed and considered statistically significant or highly significant when P < 0.05 or P < 0.01, respectively.
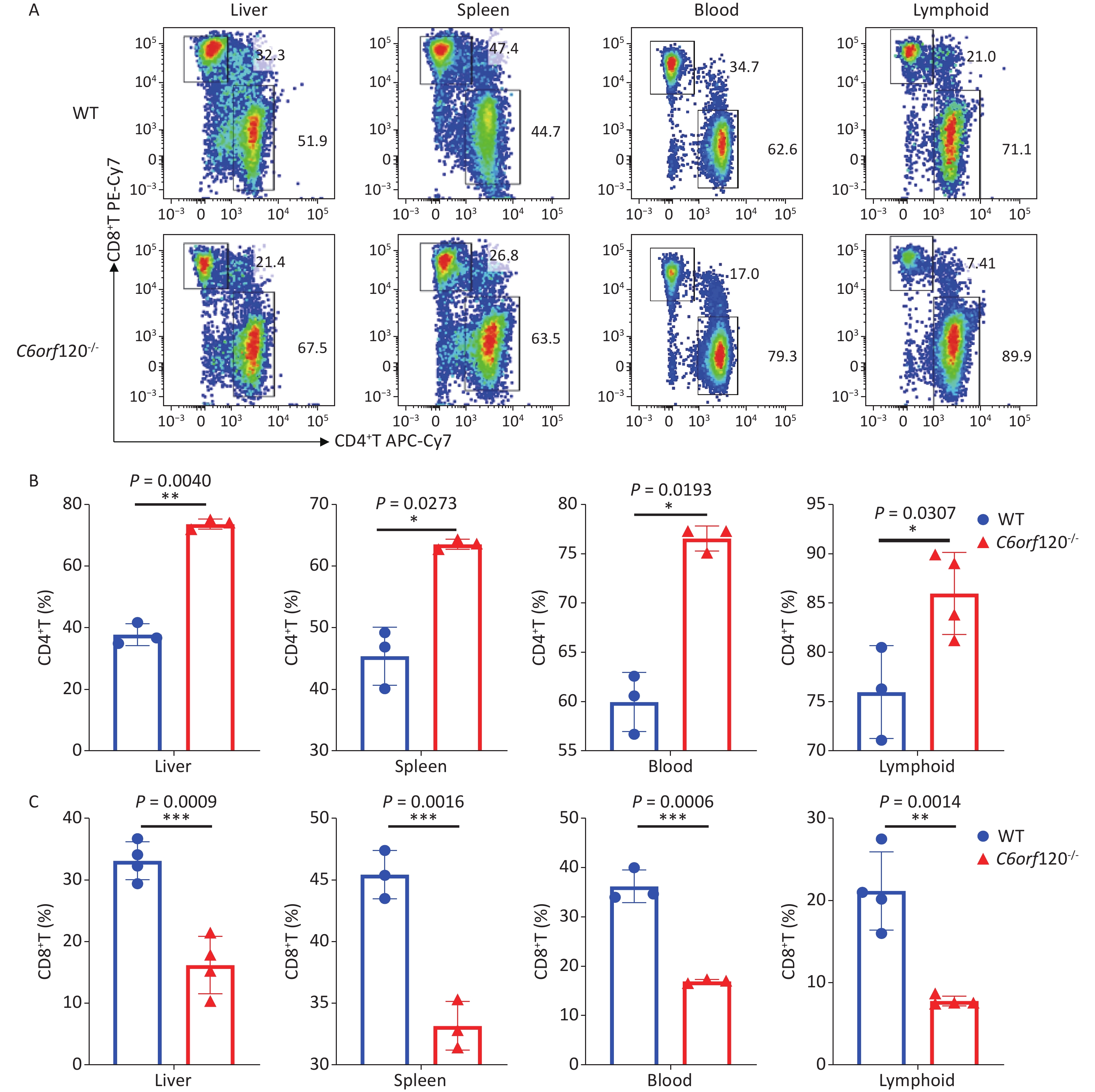
Our previous study showed that C6ORF120 could induce CD4+T cell apoptosis. To identify the mechanism of C6ORF120 in CD4+T cells, we detected various immune cell subsets in the liver, spleen, peripheral blood, and lymphoid tissues using FCM. The results showed that the proportion of CD4+ subsets in CD3+T cells was significantly elevated in C6orf120−/− rats, whereas that of CD3+CD8+T cells was reduced (Figure 1). It was also found that the primary CD4+T cells of C6orf120−/− rats showed stronger proliferative ability and fewer apoptotic cells. Furthermore, adequate quantity and quality of rC6ORF120 are essential for the functional investigation of C6ORF120. Thus, a total of 50 μg of high-quality rC6ORF120 (with a C-terminal hFC-tagged) with high purity (> 85%) and low endotoxin (0.125 EU/mg protein) was used by CUSABIO BIOTECH CO. Ltd (Wuhan, China). To evaluate the potential anti-cell growth activity of C6ORF120 which was synthesized according to the procedure previously described in our study, the inhibition rates of C6ORF120 against Jurkat cells were detected using the CCK8 assay (Figure 2A, B). The half-maximal inhibitory concentration (IC50) (IC50 = 191.4 ng/mL) is shown in Figure 2C. Microscopic images showed that the cell morphology was slightly altered compared to the control group in the presence of rC6ORF120 (Figure 2D). To further determine the extent of apoptosis in Jurkat cells treated with rC6ORF120, an AnnexinV-APC/7-AAD double staining assay was performed. Compared to the control group, the percentages of early and late apoptotic cells were significantly elevated after treated with 200 ng/mL rC6ORF120 for 48 h (Figure 2E, F). These results further verified the previous results, suggesting that C6ORF120 plays an important role in apoptosis.
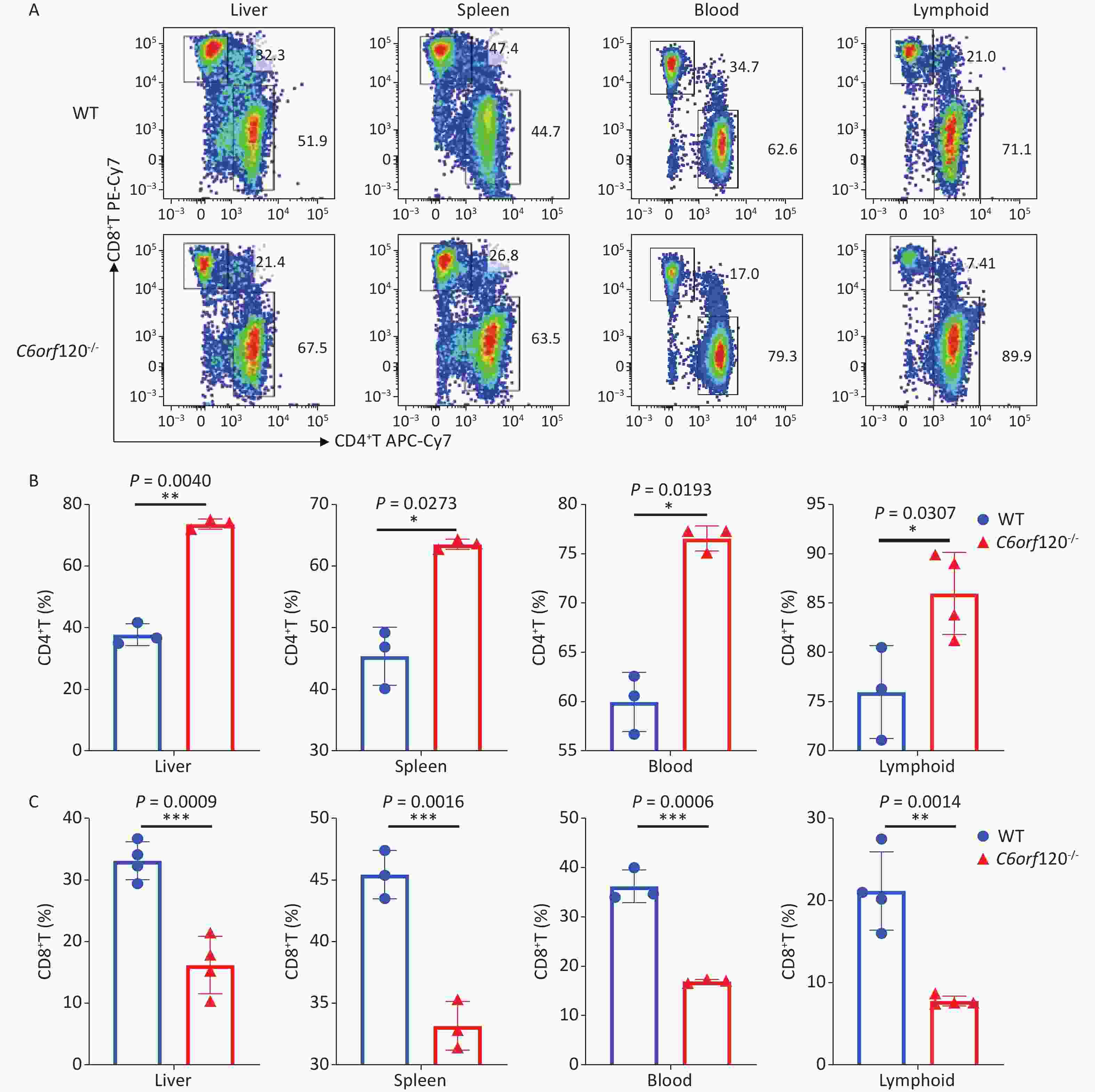
Figure 1. The percentage of CD4+T and CD8+T cells in C6orf120−/− and WT rats. (A) Changes in the level of CD4+T and CD8+T cells in C6orf120−/− and WT rats were determined by flow cytometer. (B, C) The histogram shows the percentage of CD4+T and CD8+T cells in C6orf120−/− and WT rats (n = 3–6). Data are presented as mean ± SEM of three independent experiments; *P < 0.05 and **P < 0.01 vs. the control group.
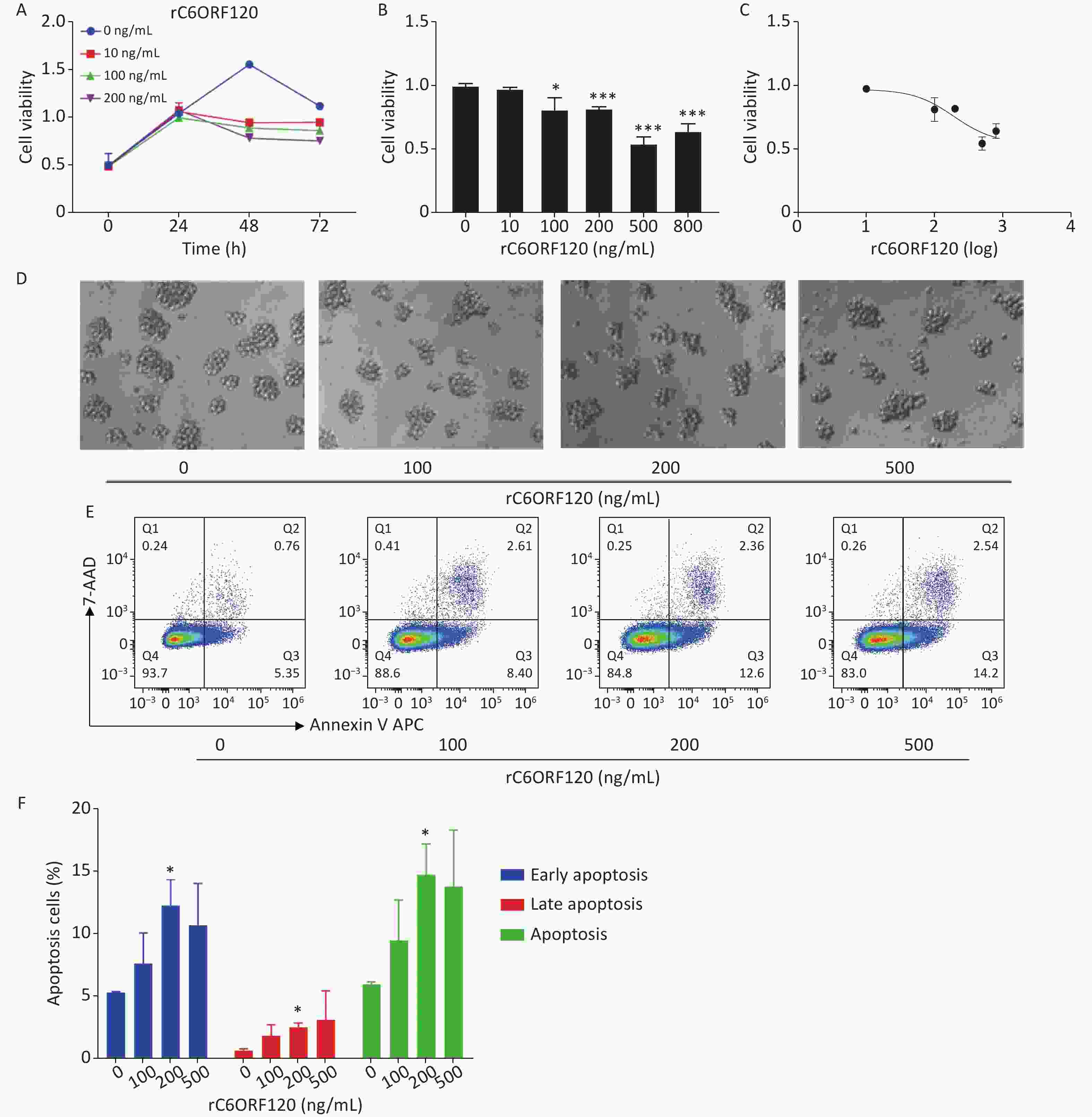
Figure 2. The effect of C6ORF120 on Jurkat cells. (A–C) The cell viability. (D) The changes in cell morphology in Jurkat cells treated by various concentrations of rC6ORF120. (E) Effects of rC6ORF120 at different concentrations for 48h on the apoptosis of Jurkat cells as detected by flow cytometer. (F) The sum of early and late apoptotic cells ratio was calculated. *P < 0.05 and **P < 0.01 vs. the control group.
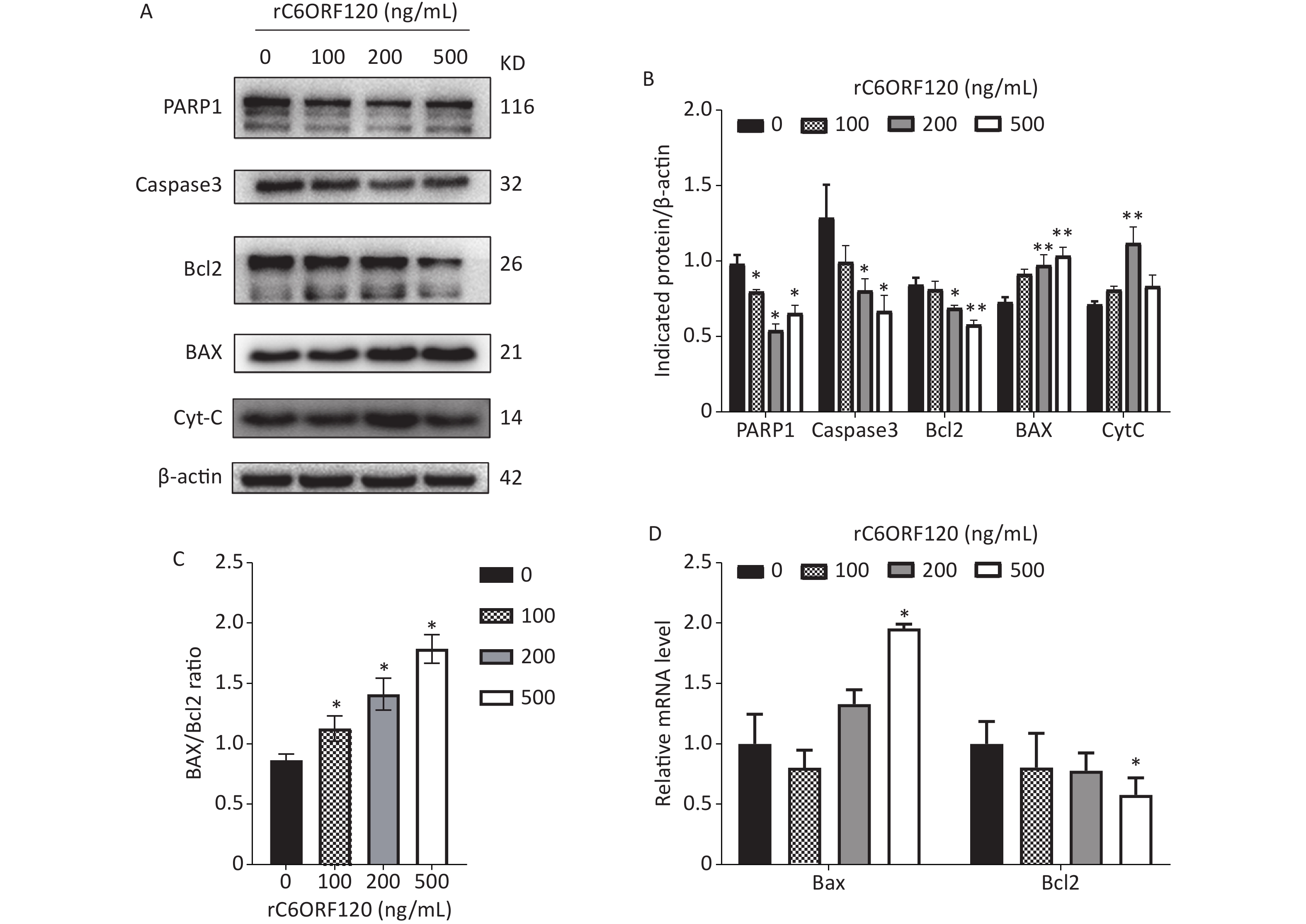
Apoptosis is autonomous programmed cell death controlled by genes that maintain the stability of the internal environment[1]. Generally, owing to the different start-up stages, the classic apoptosis pathway is divided into the mitochondrial, ER, and death receptor pathways. Numerous studies have shown that the mitochondrial pathway is the most common pathway and plays an essential role in the regulation of apoptosis[5]. Bcl-2 family proteins are usually involved in the mitochondrial apoptotic pathway, and the Bcl-2/BAX ratio is a critical indicator of apoptosis[6]. To further explore the mechanism underlying C6ORF120 induced apoptosis in Jurkat cells, the expression of apoptotic markers and anti-apoptotic factors was measured using western blot analysis and RT-qPCR. As shown in Figure 3, the expression of BAX and CytC increased significantly in a dose-dependent manner in Jurkat cells treated with rC6ORF120. Meanwhile, the expression of anti-apoptotic factors, such as Bcl2, Caspase-3, and PARP1, decreased in Jurkat cells treated with rC6ORF120. Furthermore, immunofluorescence images showed that the fluorescence intensity of BAX significantly increased in Jurkat cells treated with rC6ORF120, while that of Bcl2 decreased. The Bcl-2 family of proteins is a key regulator of mitochondrial pathway-mediated apoptosis. Therefore, the above findings not only confirmed that rC6ORF120 induced apoptosis, but also suggested that a mitochondria-dependent pathway might be involved.
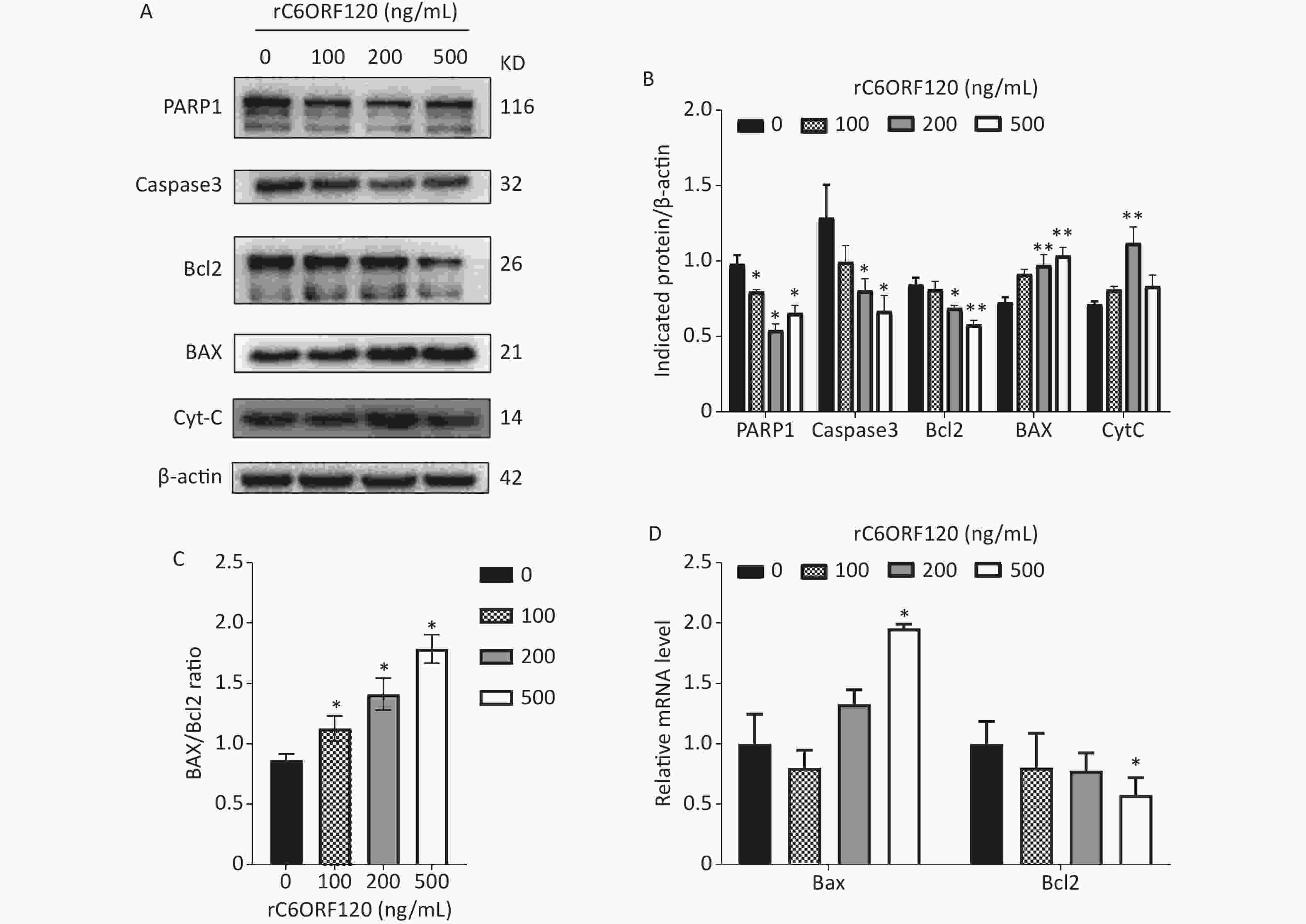
Figure 3. Apoptosis-associated genes in Jurkat cells treated by rC6ORF120 were analyzed by Western blot and RT-qPCR. (A) The expression of PARP1/Caspas3/ BAX/Bcl2/CytC in Jurkat cells treated by various concentration of rC6ORF120. β-actin was used as an internal control. (B) The histogram shows the relative protein levels in Jurkat cells treated by rC6ORF120. (C) The histogram showed BAX/Bcl2 ratio in Jurkat cells treated by rC6ORF120. (D) The mRNA level of Bax and Bcl2 in Jurkat cells treated by rC6ORF120. Data are presented as mean ± SEM of three independent experiments; *P < 0.05 and **P < 0.01 vs. the control group.
In summary, our results showed that C6orf120 deficiency markedly inhibited CD4+T cell proliferation but promoted apoptosis. Induction of apoptosis was associated with the presence of C6orf120. Mechanistically, C6ORF120 showed the anti-proliferation of Jurkat cells by inducing apoptosis through a mitochondria-dependent pathway mediated by the BAX/Bcl2 ratio. Based on these findings, C6ORF120 is a promising drug candidate for regulating CD4+T cells. Exploration of the effect of C6ORF120 on other cell types is underway.
HTML
 23039+Supplementary Materials.pdf
23039+Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: