-
Pertussis is an acute and highly contagious respiratory disease caused by Bordetella pertussis (B. pertussis), which occurs in people of all ages and is most dangerous for young children, especially infants. The introduction of whole-cell pertussis vaccines (wPVs) in the 1950s dramatically reduced the incidence of pertussis worldwide[1]. However, over the past two decades, many studies have reported the resurgence of pertussis in different countries[2]. Epidemiological surveillance in Hubei Province over the last 3 years revealed a clear increasing trend in the incidence of pertussis during the coronavirus disease 2019 (COVID-19) epidemic. B. pertussis mainly invades infant respiratory tracts, triggering acute respiratory infectious diseases. B. pertussis infection can also cause various complications, the most serious being pertussis encephalopathy. However, no obvious change was observed in the CSF during pertussis encephalopathy, because of which, B. pertussis has rarely been directly detected in the CSF of patients.
Neisseria meningitidis (N. meningitidis) is the chief pathogen responsible for infecting the central nervous system. Most cases of invasive meningococcal disease (IMD) are caused by one of the six meningococcal serogroups: A, B, C, W, X, or Y. Outbreaks of IMD are occurred worldwide, with major serogroups varying geographically and temporally in response to antigenic changes or the use of vaccines. According to surveys between 2010 and 2020, the most prevalent N. meningitidis serogroups in China was NmC (49.7%), followed by NmB (30.2%) and NmW (23.8%), while the most prevalent N. meningitidis serogroups in Hubei Province were NmB and NmC and no NmW was reported at present[3].
In this study, we firstly report the co-infection case in China wherein B. pertussis and NmW were together detected directly in the CSF of a child. This rare case presented a challenge to our current vaccination strategy in the context of high coverage of N. meningitidis and B. pertussis containing vaccines.
The patient was a female, 4-years-and-9-months-old, without any recognized immunodeficiency. Before the infection, she had received four doses of acellular pertussis-combined vaccine (DTaP) at 25th December 2017, 24th January 2018, 26th February 2018 and 8th April 2019, two doses of group A meningococcal polysaccharide vaccine (MPV-A) at 28th March 2018 and 4th July 2018, and one dose of group AC meningococcal polys accharide vaccine (MPV-AC) at 2th September 2020.
On June 5, the patient had headache and recurrent fever, which lasted for 5 days. The highest axillary temperature was 39 °C and it could be reduced to normal with antipyretics. On the day of onset, the patient had red rash on both lower extremities and two episodes of vomiting. The vomit was a yellow fluid and the abdomen was uncomfortable after vomiting. On Day 5 (June 9), the patient developed frequent cough, expectoration, chills, poor appetite, abdominal pain, and one episode of vomiting. The vomit was non-ejecting, constituted by dark-brown stomach contents.
The patient was hospitalized on Day 5. Routine blood tests revealed that the white blood cell count had elevated to 11.37 × 109/L and the percentage of lymphocytes had increased to 68.5%. CSF assays indicated that both karyocytes (930 × 106/L) and red blood cells (300 × 106/L) were detected, the protein content increased to 610 mg/L, and the chloride content increased to 133.8 mmol/L (Table 1). Computed tomography (Day 5) and magnetic resonance imaging scans (Day 9 and Day 21) showed that the bilateral cerebral hemispheres were symmetrical, without any obvious abnormal density shadow in the brain parenchyma. The brain cistern, sulcus, and ventricular systems were not dilated (Figure 1B–D). The electroencephalogram showed a slightly slowed background activity. After hospitalization, tests for influenza A/B antigens, Mycoplasma pneumoniae and Chlamydia pneumoniae antibodies, and COVID-19 nucleic acids all were negative. No pathogens were detected in CSF, sputum, and blood cultures on Days 5 and 15 (Figure 1A).
Items Value in this case Normal reference value (Children aged 4–5) Blood testing Red blood cells (×1012/L) 4.88 3.9–5.3 White blood cells (×109/L) 11.37 4.0–10.5 Hemoglobin (g/L) 131 110-150 Lymphocyte (%) 68.5 30–50 Neutrophil (%) 26.0 22–55 Platelet (×109/L) 305 100–320 CSF assay Protein qualitative Positive Negative Karyocyte count (×106/L) 930 0-8 Red blood cells (×106/L) 300 0–15 Glucose, mmol/L 2.89 2.8–4.5 Protein, mg/L 610 80–430 Chloride, mmol/L 133.8 120–130 qPCR test based on CSF sample N. meningitidis CtrA Ct = 26.87, sodc Ct = 27.25 Negative B. pertussis IS481 Ct = 23.11 Negative N. meningitidis W serogroup Ct = 34.57 Negative Nanopore 16S rRNA sequencing based on CSF sample N. meningitidis (reads/%) 11,912/72.34 Negative B. pertussis (reads/%) 2,408/14.62 Negative Other (reads/%) 2,148/13.04 Note. CSF, cerebrospinal fluid; Mp, mycoplasma pneumoniae; Cp, chlamydia pneumoniae; IgM, immunoglobulin M; IgG, immunoglobulin G. SARS-CoV-2, C. pneumonia, M. pneumonia, S. pneumonia, S. pyogenes, S. agalactiae, M. tuberculosis, and H. influenza were not detected by qPCR test. Additionally, Mp-IgM &Mp-IgG, Cp-IgM&Cp-IgG and Influenza A/B Antigen tests were negative. No bacterium was isolated from CSF, blood and sputum samples. Table 1. Results of blood testing, CSF assay, q-PCR and nanopore 16S rRNA sequencing
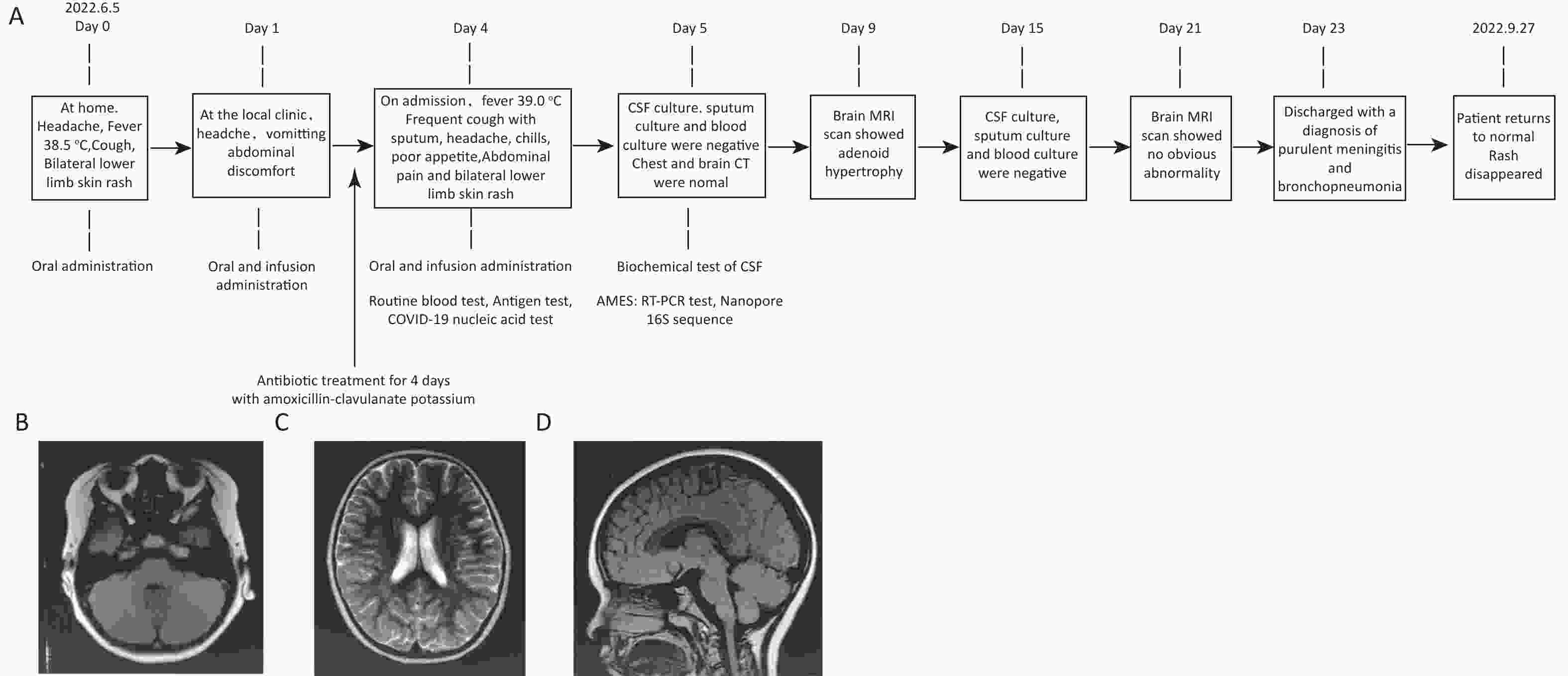
Figure 1. The patient's disease progression and MRI examination results. (A) Disease progression flow diagram of the patient; (B–D) Brain MRI scan on Day 9.
On the day of onset, the patient orally took child Ganmao Ning granules, Lianhua Qingwen granules, amoxicillin and clavulanate potassium tablet, and Ambroxol Hydrochloride for 1 day at home. On Days 2–4, the patient was treated with oral and infused oseltamivir and amoxicillin–clavulanate potassium at a local clinic (Figure 1A). On Day 5, the patient was hospitalized and treated with cefotaxime, mannitol, acetylcysteine, and compound ipratropium bromide with budesonide for bronchial and intracranial infections. After 19 days of hospitalization, the patient recovered and was discharged with a diagnosis of purulent meningitis and bronchopneumonitis. The patient reverted to normal health with a return visit on September 27 (Figure 1A).
Later, quantitative PCR performed on CSF samples collected on Day 6 revealed that the patient had been co-infected with N. meningitidis (ctrA Ct = 26.87/sodc Ct = 27.25) and B. pertussis (IS481 Ct = 23.11) (Innowavedx Suzhou Co., Ltd., China, CLRO22A248). The N. meningitidis serogroup was W (Ct = 34.57), determined according to the World Health Organization’s protocol (Table 1) (https://stacks.cdc.gov/view/cdc/11632 and https://www.who.int/publications/i/item/WHO-IVB-14.03). To verify the authenticity of the co-infection, full-length bacterial 16S rRNA gene was amplified using BK-16S096 (16sF: CCTTATCATTTAGAGGAAGGAG, 16sR: TCCTCCGCTTATTGATATGC, Hangzhou Baiyi Technology Co., Ltd., China), followed by nanopore sequencing using the GridION platform (Oxford Nanopore Technologies, UK). Genomic sequencing was then performed on the GridloN sequencing platform (Oxford Nanopore Technologies, UK). The sequencing results were imported into the BAIYI MicroGeno Platform (v 4.1, Hangzhou Baiyi Technology Co., Ltd., [http://www.baiyi-tech.cn/]). Barcode and adapter sequences were trimmed from the reads using porechop [v0.2.4 https://github.com/rrwick/Porechop]. Seqkit [https://github.com/shenwei356/seqkit] was used to retain reads between 1.3-1.6k and with an average phred quality score above 12. Subsequently, the filtered sequences were aligned to the MirrOr database using minimap2 [2.6.1 2.24-r1122 https://github.com/lh3/minimap2], retaining only the best alignment result for each sequence. The identified species from the matched sequences were recorded as the species of the sequenced reads. A total of 16,468 reads were obtained, of which 72.34% belonged to N. meningitidis and 14.62% to B. pertussis, consistent with the quantitative PCR results. The nanopore sequencing reads have been submitted to the Genome Sequence Archive under accession number CRA008593.
Combined hypoxia is known to cause encephalopathy in about 0.3% of children with pertussis. Pertussis toxin (PT) can also directly affect pertussis encephalopathy, the fatality rate of which is extremely high, possibly making it a lethal factor for pertussis[4]. B. pertussis is a Gram-negative pathogen usually obtained from respiratory specimens. The few cases of invasive infections caused by B. pertussis mostly involve immunocompromised patients. However, B. pertussis has been detected in the CSF in only a few cases; especially, convincing numbers of B. pertussis reads have been difficult to obtain using high-throughput sequencing methods[5]. In this study, more than 2,400 B. pertussis reads were detected in the patient’s CSF using full-length 16S rRNA gene nanopore sequencing, providing high-confidence results. Moreover, this process took only 6 hours, from nucleic acid sampling to report.
In previous studies, full-length 16S rRNA gene nanopore sequencing has proven to be a reliable and efficient option for identifying bacterial infections[6]. However, this technology lacks well-established guidelines in clinical applications and uniform standards for interpreting the sequencing results. The limitations need to be overcome by researchers in future. Additionally, it could identify multiple pathogens at once and provide more information than traditional RT-PCR test.
The qPCR and nanopore 16S rRNA sequencing test based on CSF sample suggested that this case might involve an N. meningitidis and B. pertussis co-infection. That was also supported by the clinical symptoms. N. meningitidis and B. pertussis infection usually resulted in the symptoms of fever, headache, vomiting, cough and increase of white blood cell count and lymphocytes, which had been found in this case. Moreover, the immune function declines due to the N. meningitidis infection and the action of PT might give B. pertussis the opportunity to breach the blood–brain barrier. Similarly, Kugler et al. found that barrier integrity, organelle organization and transmigration of monocytes were affected by pertussis toxin in a human brain microvascular endothelial cell barrier model[4].
Vaccination is considered as the optimal strategy to prevent IMD[3]. In this case, the patient had received one dose of MPV-AC. The bivalent MPV-AC vaccine is widely available in China. In addition, the tetravalent MPV-ACWY vaccine has been successfully administered for many years, but it is a part of the non-immunization program vaccine management in China, which requires self-payment. Although the development, production, and quality management of MPV-ACWY has greatly progressed in China, it still faces challenges, such as the underestimated burden of IMD and the underdeveloped immunization strategies for meningococcal vaccines.
Over the past decade, the incidence of pertussis has increased in many countries, mostly due to the replacement of whole-cell pertussis vaccine with acellular pertussis vaccine (APV). APV fails to provide long-lasting immunity and establish adequate protection against B. pertussis[7]. APV could induce anti-PT immunoglobulin G, and the level induced by the component-purified DTaP-IPV/Hib (Pentaxim, Sanofi) vaccine was significantly higher than that induced by the co-purified DTaP vaccine. Nevertheless, anti-PT immunoglobulin G levels induced by either vaccine decrease over time[8,9]. Similarly, the incidence of B. pertussis showed an upward trend in Hubei in recent years according to our previous study[10] and unpublished data (epidemiology of pertussis in Hubei Province from 2016 to 2021). It suggested that the current immunization strategies should be further optimized in Hubei province. The need for a booster dose of pertussis-containing vaccine for children entering school and the feasibility of incorporating the tetravalent MPV-ACYW into the immunization program must be investigated. In addition, enhanced active surveillance of IMD and pertussis-associated cases will enable more targeted vaccine development. Interventions that are more appropriate and developed early will help prevent and control NmW infections and pertussis.
The First Reported Case of Co-Infection with Neisseria Meningitidis and Bordetella Pertussis in the Cerebrospinal Fluid Specimen of a Normal Four-Year-Old Child
doi: 10.3967/bes2024.048
- Received Date: 2023-11-20
- Accepted Date: 2024-02-21
| Citation: | XIA Xian, LI Guo Ming, TANG Yi, WANG Si Quan, HE Fei, JIANG Yong Zhong, YANG Hong Mei, JIANG Hong Lin, LYU Jing, MAO Ling Feng. The First Reported Case of Co-Infection with Neisseria Meningitidis and Bordetella Pertussis in the Cerebrospinal Fluid Specimen of a Normal Four-Year-Old Child[J]. Biomedical and Environmental Sciences, 2024, 37(4): 432-435. doi: 10.3967/bes2024.048 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: