-
Asthma is one of the most common respiratory diseases in the world, affecting nearly 300 million people across all ages[1]. Although the mortality of asthma is decreasing owing to improvements in diagnostic techniques and medications, the burden of asthma continues to increase owing to its unique characteristics and suboptimal treatment coverage. Additionally, ideal control of asthma has not yet been achieved. Therefore, the need to control asthma remains to be high.
Asthma is closely associated with diabetes and obesity. It has been suggested that the incidence of asthma and the exacerbation rate of asthma are especially increased in patients with type 2 diabetes mellitus (T2DM) or obesity[2–7]. It was also indicated that insulin resistance and low-grade systemic inflammation in patients with T2DM were closely associated with asthma[8,9]. Moreover, it was demonstrated that among patients with diabetes, those with asthma experienced longer hospitalization periods and had a higher risk of readmissions[10,11].
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have demonstrated notable efficacy in enhancing glycemic control and facilitating weight management[12,13]. GLP-1RAs are divided into short- and long-acting agonists based on their duration of action. Structurally, they can be distinguished into exendin-4 and human GLP-1 analogs. Furthermore, regarding route of administration, they are available in injectable and oral formulations.
The GLP-1 receptor-based agonists in our study included GLP-1RA, GLP-1 based twincretins and GLP-1 based triple receptor agonists. Twinretins are known as dual agonists for dual glucagon-like peptide-1 receptor (GLP-1R) and glucose-dependent insulinotropic peptide receptor (GIPR)[14,15]. Triple receptor agonist is defined as an agent stimulating GLP-1R, GIPR, and glucagon receptor simultaneously[16].
GLP-1RAs were found to have the potential to reduce the rate of asthma exacerbations[17]. It was also revealed that GLP-1 based twincretins were related to the inhibition of airway inflammation in an asthma model in obese mice[18]. However, previous meta-analyses with limited studies did not demonstrate the protective effects of GLP-1 receptor-based agonists on asthma[19]. To date, the relationship between GLP-1 receptor-based agonists and the incidence of asthma remains uncertain in patients with T2DM or obesity.
With the rapid development of GLP-1RA-based drugs, an increasing number of randomized controlled trials (RCTs) investigating GLP-1RAs, GLP-1 based twincretins and GLP-1 based triple receptor agonists have been conducted in populations with T2DM or obesity. It is necessary to re-evaluate the association between the use of GLP-1 receptor-based agonists and the risk of asthma using updated data from a more comprehensive perspective. Therefore, we designed and performed a meta-analysis of RCTs to assess the potential effects of GLP-1 receptor-based agonists on asthma in patients with T2DM and obesity.
-
The protocol for this meta-analysis has been registered in the International Prospective Register of Systematic Reviews (CRD42022378295). This study strictly adhered to the preferred reporting criteria stated in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines.
-
We searched PubMed, Web of Science, Embase, the Cochrane Central Register of Controlled Trials, and Clinicaltrial.gov for studies published between the date of inception and July 2023. The following search terms were used: albiglutide, dulaglutide, efpeglenatide, exenatide, liraglutide, lixisenatide, semaglutide, cotadutide, tirzepatide, taspoglutide, GLP-1RAs, dual receptor agonists, triple receptor agonists, co-agonism, twincretins, obesity, T2DM, and RCT. These terms were searched for subject headings and free words.
-
The inclusion criteria for this meta-analysis were as follows: 1) RCTs of GLP-1 receptor-based agonists conducted in T2DM; 2) RCTs of GLP-1 receptor-based agonists conducted in obese patients; 3) studies reporting asthma events; 4) no limits on follow-up duration; and 5) either placebo or active agent-controlled.
The exclusion criteria were as follows: 1) non-randomized controlled clinical trials; 2) literature reviews, meta-analyses, and observational studies; 3) studies conducted on type 1 diabetes, gestational diabetes, and pre-diabetes; and 4) trials that did not report asthma events.
According to the inclusion and exclusion criteria, two investigators (Mengqing Zhang and Chu Lin) screened for eligible RCTs and extracted relevant data, including the first author, publication year, study design, number of participants, age, duration of diabetes, drug exposure, asthma events, and available efficacy endpoints (changes in glycosylated hemoglobin [HbA1c], body weight, and blood pressure). If data on asthma events were absent in the original articles and supplementary materials, the data were retrieved from Clinicaltrial.gov website with a unique registered NCT number.
All relevant data were recorded in a well-designed data chart. The investigators double-checked data accuracy. Any disagreement regarding data extraction was resolved through joint discussions with a third investigator (Xiaoling Cai).
-
Sensitivity analyses were analyzed according to the following predefined subgroups: age (≥ 60 years old or < 60 years old), male predominance (male percentage ≥ 50% or < 50%), T2DM or obesity, duration of diabetes (≥ 10 years or < 10 years), follow-up durations (≥ 52 weeks or < 52 weeks), study control (placebo or active agent), drug type (GLP-1RA or twincretins), drug structure (exedin-4 derived or human GLP-1 based), duration of action (long-acting or short-acting), molecular weight (light or heavy), therapy mode (monotherapy or combination therapy), and different GLP-1RA subtypes (Supplementary Tables S1–S2, available in www.besjournal.com).
Drugs Molecular weight [Da] Duration of action Lixisenatide[29] 4858.6 Short-acting Albiglutide[40] 72970.4 Long-acting Dulaglutide[41] 59671 Long-acting Efpeglenatide[42] 3400 Long-acting Exenatide[43] 4186.6 Short-acting Exenatide [weekly][44] 4186.6 Long-acting Liraglutide[45] 3751.3 Long-acting Semaglutide[46] 4113.6 Long-acting Semaglutide [oral][47] 4113.6 Long-acting Taspoglutide[48] 3339.7 Long-acting Cotadutide Tirzepatide Table S1. Exposure-response related parameters
Subgroups Drugs Duration of action Short Exenatide, Lixisenatide Long Albiglutide, Dulaglutide, Efpeglenatide, Exenatide (weekly), Liraglutide, Semaglutide, Semaglutide (oral) Molecular weight Light Efpeglenatide, Exenatide, Exantide (weekly), Liraglutide, Lixisenatide, Semaglutide, Semaglutide (oral) Heavy Albiglutide, Dulaglutide, Cotadutide, Tirzepatide Note. *Cut-off points of molecular weight of GLP-1RA is 50,000 Da. Table S2. Subgroup classification based on duration of action and molecular weight of GLP-1RA
-
Continuous variables were evaluated using weighted mean differences with 95% confidence intervals (CIs). Categorical variables were evaluated as relative risks (RR) with 95% CIs. Higgins I² statistics were used to evaluate between-study heterogeneity, with an I² value of < 50% indicating a low level of heterogeneity. Additionally, a fixed-effects model was used when there was a low level of heterogeneity. Publication bias was assessed using funnel plots and the Egger’s test. The quality of RCTs was evaluated using the Cochrane risk of bias tool[20]. A P value less than 0.05 was considered statistically significant for all analyses. All statistical analyses were performed using STATA (version 16.0; StataCorp LLC, College Station, TX, USA).
-
There were 39 RCTs included in this meta-analysis, with 47,499 participants in the GLP-1 based treatment group and 38,256 in the control group (Figure 1). Among these, there were 34 trials with GLP-1RA treatment and five trials with GLP-1 based twincretin. And 32 trials were conducted in patients with T2DM, whereas seven trials were conducted in patients with obesity. Notably, during literature screening, because the RCTs conducted with GLP-1 based triple receptor agonists did not report asthma events, they were excluded from this meta-analysis. Detailed baseline characteristics of the included RCTs are summarized in Supplementary Table S3 (available in www.besjournal.com).
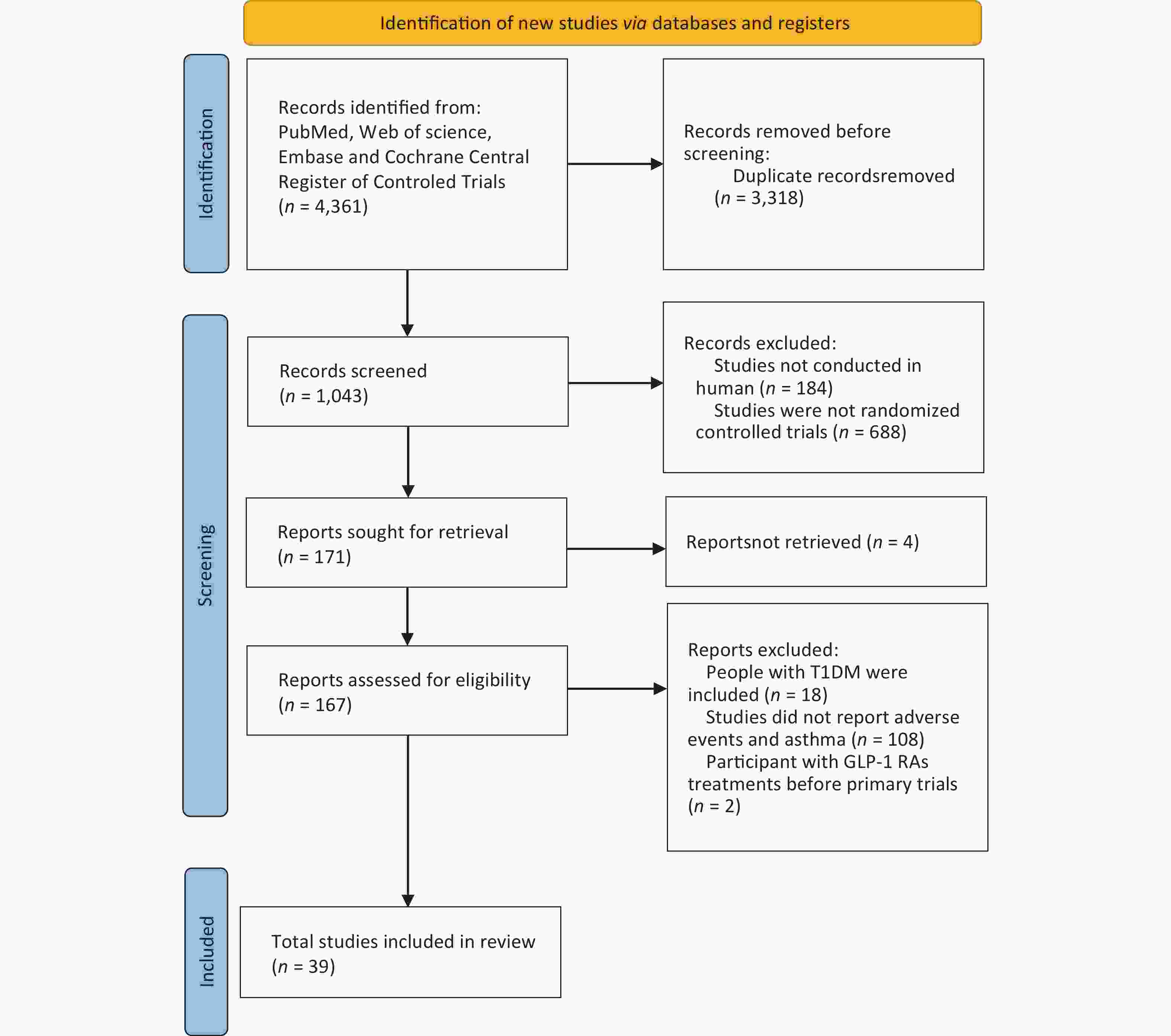
Figure 1. Flowchart of the included studies. GLP-1, glucagon-like peptide-1; GLP-1RA, glucagon-like peptide-1 receptor agonist; T1DM, type 1 diabetes mellitus.
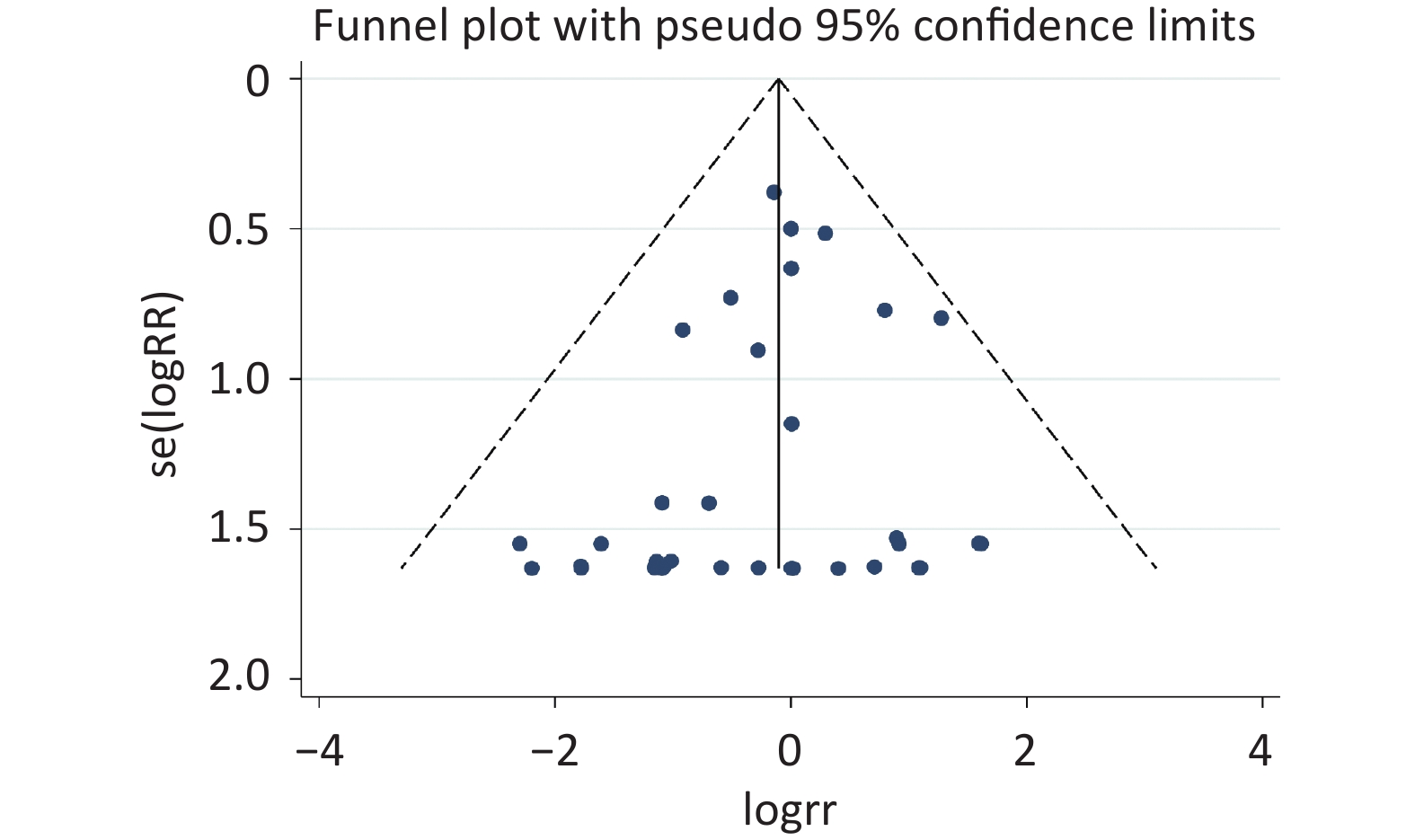
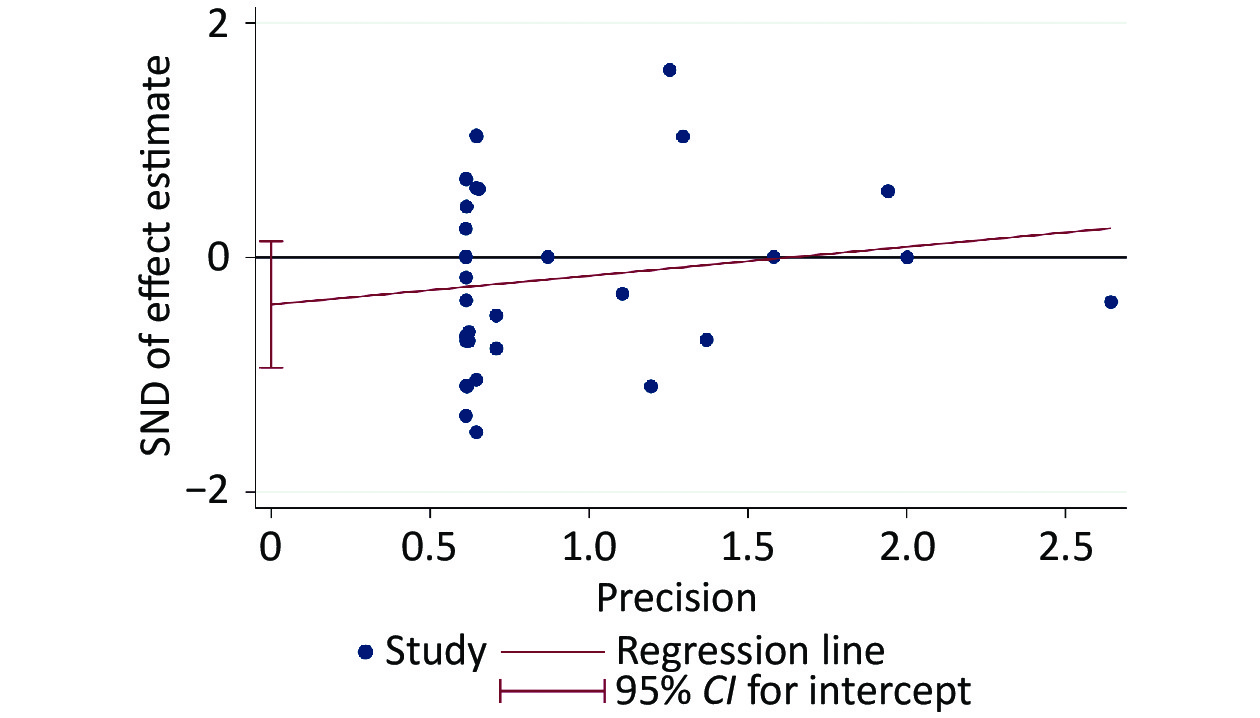
The risk of bias evaluation revealed a low level of selective reporting, which were summarized in Supplementary Table S4 (available in www.besjournal.com). The even distribution in the funnel plot indicated no sign of publication bias (Supplementary Figure S1, available in www.besjournal.com), which was consistent with the results of Egger’s test (slope = 0.2456, P = 0.142) (Supplementary Figure S2, available in www.besjournal.com).
Author, year Adequate randomization sequence generation Adequate allocation concealment Blinding
of participants and caregiversBinding of
outcome assessors
and adjudicatorsFree of incomplete outcome data Free of selective outcome reporting Free of other bias Albiglutide versus active drugs or placebo Home, 2015[1] Definitely yes
Interactive voice-response systemDefinitely yes
Interactive voice-response systemDefinitely yes Definitely yes Probably yes
Data from 14/101 (13.9%) participants of experimental group and 22/101 (21.8%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedNauck, 2016[2] Definitely yes
Interactive voice-response systemDefinitely yes
Interactive voice-response systemDefinitely yes Definitely yes Probably yes
Data from 32/102 (31.4%) participants of experimental group and 47/104 (45.2%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedHernandez, 2018[3] Definitely yes
Interactive voice-response systemDefinitely yes
Interactive voice-response systemDefinitely yes Definitely yes Probably yes
Data from 111/4731 (2.4%) participants of experimental group and 154/4,732 (3.3%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedWeissman, 2014[4] Definitely yes
A sequestered fixed scheduleDefinitely no
open-labelDefinitely no
open-labelDefinitely yes Probably yes
Data from 107/516 (20.7%) participants of experimental group and 39/263 (14.8%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedRosenstock, 2014[5] Definitely yes
A sequestered fixed scheduleDefinitely yes
Interactive voice-response systemDefinitely yes Definitely yes Probably yes
Data from 1,896/2,539 (74.7%) participants of experimental group and 643/2,539 (25.2%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedDulaglutide versus active drugs or placebo Gerstein, 2019[6] Definitely yes
Computer-generated random codeDefinitely yes
Interactive web response systemDefinitely no
Single-blindDefinitely yes Probably yes
Data from 132/4,949 (2.7%) participants of experimental group and /4,952 (3.2%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedWeinstock, 2015[7] Definitely yes
Computer generated random sequenceDefinitely yes
Interactive voice-response systemDefinitely yes Definitely yes Probably yes
Data from 112/304 (36.8%) participants of experimental group and 129/315 (41.0%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedSkrivanek, 2014[8] Definitely yes
Computer generated random sequenceDefinitely yes
Interactive voice response systemDefinitely yes Definitely yes Probably yes
Data from 18/104 (17.3%) participants of experimental groupDefinitely yes Probably yes
Baseline characteristics were generally balancedWysham, 2014[9] Definitely yes
Computer generated random sequenceDefinitely yes
Interactive voice response systemDefinitely yes Definitely yes Probably yes
Data from 19/279 (6.8%) participants of experimental group and 17/141 (12.1%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedEfpeglenatide versus active drugs or placebo Gerstein, 2021[10] Definitely yes
Interactive web response systemDefinitely yes
Interactive web response systemDefinitely yes Definitely yes Probably yes
Data from 82/2,717 (3.0%) participants of experimental group and 53/1,359 (3.9%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedFrias, 2022[11] Probably yes
Randomized, double-blindedProbably yes
Randomized, double-blindedDefinitely yes Definitely yes Probably yes
Data from 65/304 (21.4%) participants of experimental group and 22/102 (21.6%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedExenatide versus active drugs or placebo Davies, 2009[12] Probably yes
Randomized, double-blindedProbably yes
Randomized, double-blindedDefinitely yes Definitely yes Probably yes
Data from 19/118 (16.1%) participants of experimental group and 12/116 (10.3%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedGallwitz, 2011[13] Probably yes
Randomized, open-labelDefinitely no
Randomized, open-labelDefinitely no
open-labelDefinitely yes Probably yes
Data from 47/182 (25.8%) participants of experimental group and 44/181 (24.3%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedGallwitz, 2012[14] Definitely yes
Computer generated random sequenceDefinitely no
Randomized, open-labelDefinitely no
open-labelDefinitely yes Probably yes
Data from 174/515 (33.8%) participants of experimental group and 128/514 (24.9%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedJaiswal, 2015[15] Probably yes
Randomized, open-labelDefinitely no
Randomized, open-labelDefinitely no
open-labelDefinitely yes Probably yes
Data from 3/22 (13.6%) participants of experimental group and 0/24 (0%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedWysham, 2015[16] Definitely yes
Computer generated random sequenceDefinitely yes
Interactive voice response systemDefinitely yes Definitely yes Probably yes
Data from 26/276 (9.4%) participants of experimental group and 17/141 (12.1%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedHolman, 2017[17] Definitely yes
Computer generated random sequenceDefinitely yes
Interactive voice response systemDefinitely yes Definitely yes Probably yes
Data from 262/7,356 (3.6%) participants of experimental group and 303/7,396 (4.1%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedInagaki, 2012[18] Definitely yes
Computer generated random sequenceDefinitely yes
Interactive voice response systemDefinitely no
Open-labelDefinitely yes Probably yes
Data from 22/215 (10.2%) participants of experimental group and 11/212 (5.2s%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedFrias, 2016[19] Definitely yes
Computer generated random sequenceDefinitely yes
Interactive voice response systemDefinitely yes Definitely yes Probably yes
Data from 228/458 (49.8%) participants of experimental group and 230/458 (50.2%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedLiraglutide versus active drugs or placebo Rubino, 2022[20] Definitely yes
Interactive web/voice response systemDefinitely no
Open-labelDefinitely no
Open-labelDefinitely yes Probably yes
Data from 9/127 (7.1%) participants of experimental group and 4/85 (4.7%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedWadden, 2019[21] Definitely yes
Computer generated random sequenceDefinitely yes
Interactive voice response systemDefinitely yes Definitely yes Probably yes
Data from 9/100 (9.0%) participants of experimental group and 4/50 (8.0%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedWhicher, 2021[22] Definitely yes
Computer generated random sequenceDefinitely yes
Interactive voice response systemDefinitely yes Definitely yes Probably yes
Data from 2/24 (8.3%) participants of experimental group and 1/23 (4.3%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedBuse, 2014[23] Definitely yes
Interactive web/voice response systemDefinitely yes
Interactive web/voice response systemDefinitely yes Definitely yes Probably yes
Data from 32/207 (15.5%) participants of experimental group and 35/206 (17.0%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedNahra, 2021[24] Definitely yes
Computer generated random sequenceDefinitely no
Open-labelDefinitely no
Open-labelDefinitely yes Probably yes
Data from 5/110 (4.5%) participants of experimental group and 4/112 (3.6%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedDavies, 2015[25] Probably yes
Randomized, double-blindedDefinitely yes
Using an interactive voice response systemDefinitely yes Definitely yes Probably yes
Data from 47/211 (22.3%) participants of experimental group and 72/212 (34.0%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedGough, 2015[26] Probably yes
Randomized, open-labelDefinitely no
Open-labelDefinitely no
Open-labelDefinitely yes Probably yes
Data from 28/415 (6.7%) participants of experimental group and 28/414 (6.8%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedMarso, 2016[27] Probably yes
Randomized, double-blindedProbably yes
Randomized, double-blindedDefinitely yes Definitely yes Probably yes
Data from 139/4,668 (2.9%) participants of experimental group and 159/4672 (3.4%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedPi-Sunyer, 2015[28] Probably yes
Randomized, double-blindedProbably yes
Randomized, double-blindedDefinitely yes Definitely yes Probably yes
Data from 2,487/3,731 (66.7%) participants of experimental group and 1,244/3,731 (33.3%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedLixisenatide versus active drugs or placebo Pfeffer, 2015[29] Definitely yes
Randomization is performed with the use of a centralized assignment systemProbably yes
Randomized, double-blindedDefinitely yes Definitely yes Probably yes
Data from 833/3,034 (27.5%) participants of experimental group and 727/3,034 (24.0%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedRiddle, 2013[30] Definitely yes
The
centrally generated randomized treatment kit number listDefinitely yes
Interactive voice response systemDefinitely yes Definitely yes Probably yes
Data from 29/223 (13.0%) participants of experimental group and 12/223 (5.4%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedSeino, 2012[31] Definitely yes
An interactive voice/Web
response systemDefinitely yes
An interactive voice/Web
response systemDefinitely yes Definitely yes Probably yes
Data from 21/154 (13.6%) participants of experimental group and 13/157 (8.3%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedSemaglutide versus active drugs or placebo Rubino, 2022[20] Definitely yes
Interactive web/voice response systemDefinitely no
Open-labelDefinitely no
Open-labelDefinitely yes Probably yes
Data from 6/126 (4.8%) participants of experimental group and 4/85 (4.7%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedHusain, 2019[32] Probably yes
Randomized, double-blindedProbably yes
Randomized, double-blindedDefinitely yes Definitely yes Probably yes
Data from 244/1,591 (15.3%) participants of experimental group and 156/1,592 (9.8%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedWilding, 2021[33] Definitely yes
an interactive web-response systemDefinitely yes
an interactive web-response systemDefinitely yes Definitely yes Probably yes
Data from 66/1,306 (5.1%) participants of experimental group and 46/655 (7.0%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedMarso, 2016[34] Probably yes
Randomized, double-blindedProbably yes
Randomized, double-blindedDefinitely yes Definitely yes Probably yes
Data from 25/1,648 (15.2%) participants of experimental group and 40/1,649 (24.3%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedDavies, 2021[35] Probably yes
Randomized, double-blindedProbably yes
Randomized, double-blindedDefinitely yes Definitely yes Probably yes
Data from 805/1,208 (66.6%) participants of experimental group and 403/1,208 (33.4%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedCotadutide versus active drugs or placebo Nahra, 2021[24] Definitely yes
Computer generated random sequenceDefinitely yes
An interactive web response systemDefinitely yes Definitely yes Probably yes
Data from 5/110 (4.5%) participants of experimental group and 18/356 (5.1%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedMedImmune, 2019[36] Probably yes
Randomized, double-blindedProbably yes
Randomized, double-blindedDefinitely yes Definitely yes Probably yes
Data from 6/50 (12.0%) participants of experimental group and 5/24 (20.8%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedTirzepatide vs active drugs or placebo Bernhard, 2021[37] Definitely yes
Computer generated random sequenceDefinitely yes
An interactive web response systemDefinitely no
Open-labelDefinitely yes Probably yes
Data from 85/1,079 (7.9%) participants of experimental group and 34/365 (9.3%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedStefano, 2021[38] Definitely yes
Computer generated random sequenceDefinitely yes
An interactive web response systemDefinitely no
Open-labelDefinitely yes Probably yes
Data from 41/997 (4.1%) participants of experimental group and 52/1,005 (5.2%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedJastreboff, 2022[39] Definitely yes
Computer generated random sequenceDefinitely yes
An interactive web response systemDefinitely no
Open-labelDefinitely yes Probably yes
Data from 1,896/2,539 (74.7%) participants of experimental group and 643/2,539 (25.3%) participants of control group missedDefinitely yes Probably yes
Baseline characteristics were generally balancedTable S4. Risk of bias for included studies in this meta-analysis
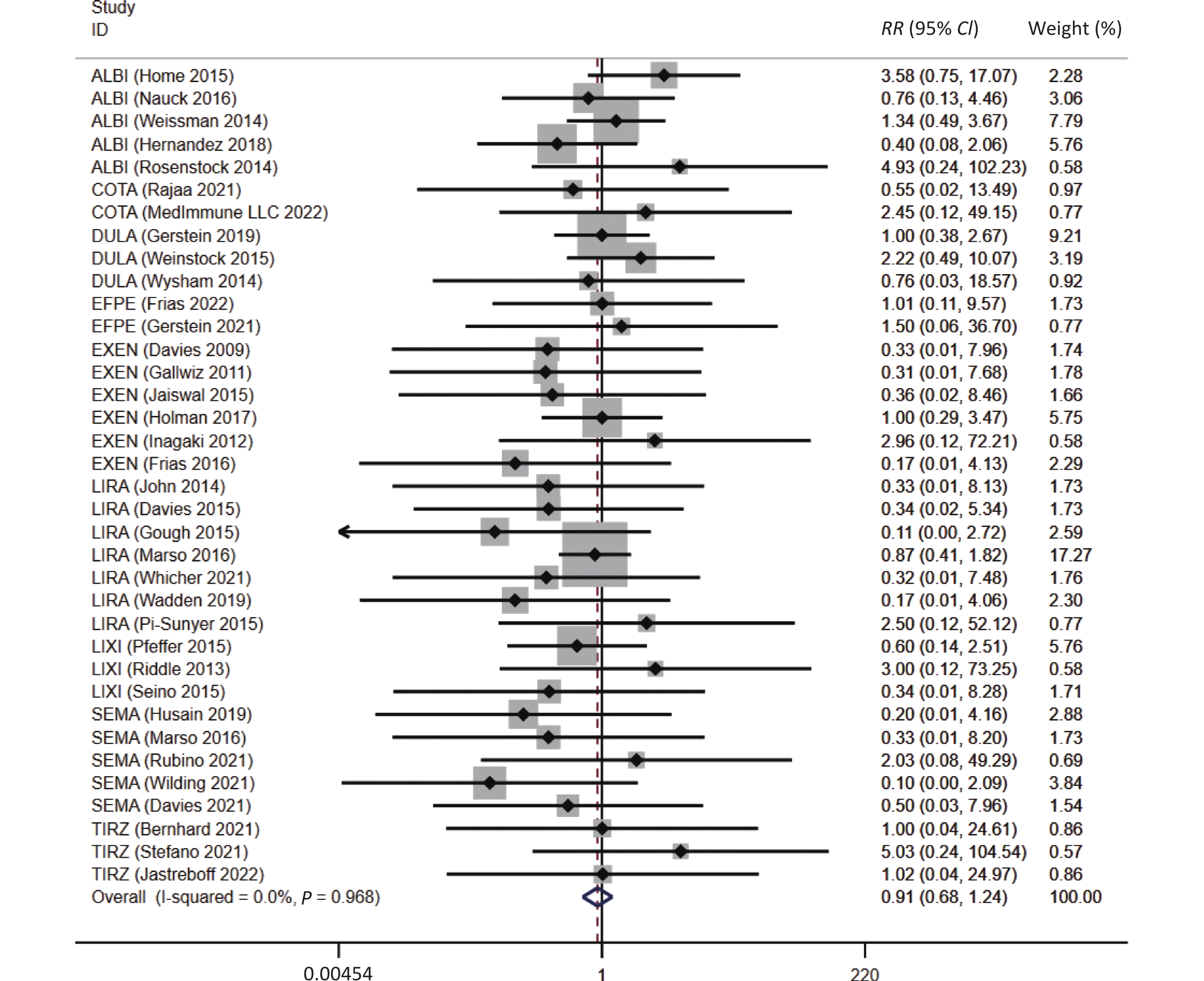
Overall, a trend of reduced risk of asthma were observed in patients with GLP-1 receptor-based agonists treatment (RR = 0.91, 95% CI: 0.68 to 1.24), which was consistent with the result after excluding the twincretins (RR = 0.88, 95% CI: 0.64 to 1.20), although a statistical significance was not reached (Figure 2).
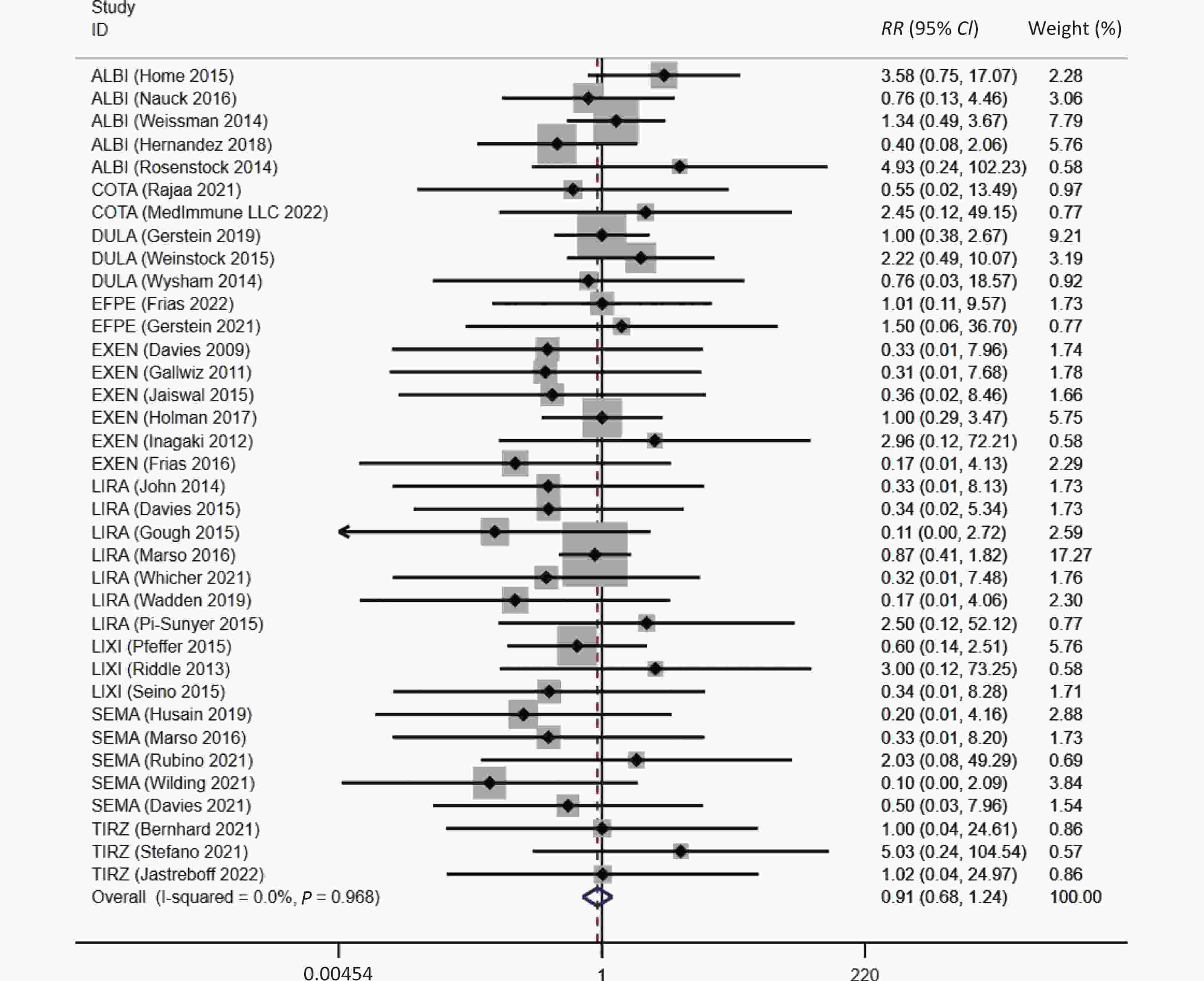
Figure 2. The association between GLP-1RAs and the risk of asthma. ALBI, albiglutide; COTA, cotadutide; DULA, dulaglutide; EFPE, efpeglenatide; EXEN, exenatide; LIRA, liraglutide; LIXI, lixisenatide; SEMA, semaglutide; TIRZ, Tirzepatide; RR, risk ratio; CI, confidence interval.
Sensitivity analyses revealed that age and sex did not significantly affect the association between GLP-1 receptor-based agonist use and asthma risk (Table 1). Moreover, the incidence of asthma were comparable between GLP-1 receptor-based agonists users with T2DM and GLP-1 receptor-based agonists users with obesity. Similar associations were observed in patients with a diabetes duration of ≥ 10 years (Table 1).
Subgroup No. event/No. at risk RR 95% CI P I² Experimental groups (n) Control groups (n) Age, years ≥ 60 31/28,624 36/27,303 0.85 (0.53, 1.36) 0.50 0 < 60 55/18,875 30/10,953 0.96 (0.65, 1.43) 0.85 0 Sex Male ≥ 50% 59/39,678 56/34,003 0.81 (0.57, 1.15) 0.23 0 Male < 50% 27/7,821 10/4,253 1.31 (0.72, 2.37) 0.38 0 Conditions T2DM 80/41,389 62/35,449 0.94 (0.69, 1.30) 0.72 0 Obesity 6/6,110 4/2,807 0.68 (0.26, 1.76) 0.43 0 Duration of diabetes, years ≥ 10 34/30,845 41/28,788 0.81 (0.53, 1.24) 0.33 0 < 10 47/12,127 24/7,509 1.03 (0.66, 1.63) 0.88 0 Study control Placebo 50/40,870 53/34,334 0.76 (0.53, 1.09) 0.21 0 Active drugs 35/5,907 13/3,698 1.44 (0.81, 2.55) 0.14 0 Duration of study, weeks ≥ 52 81/44,971 60/36,554 0.95 (0.69, 1.32) 0.77 0 < 52 5/2,528 6/1,702 0.67 (0.28, 1.61) 0.37 0 Therapy mode Monotherapy 18/14,208 13/9,487 0.75 (0.40, 1.41) 0.37 0 Combination therapy 68/33,291 53/28,769 0.97 (0.69, 1.37) 0.85 0 Drug type GLP-1RAs 79/42,869 66/36,117 0.88 (0.64, 1.20) 0.42 0 Twincretins 7/4,630 0/2,139 1.75 (0.47, 6.49) 0.41 0 Drug structure (without twincretins) Human GLP-1 analogs 65/27,243 50/22,409 0.92 (0.65, 1.32) 0.66 0 Exendin-4 analogs 14/15,165 16/13,708 0.75 (0.39, 1.43) 0.38 0 Molecular weight (without twincretins) Light molecular weight 32/30,680 42/25,097 0.65 (0.43, 0.99) 0.04 0 Heavy molecular weight 54/16,819 24/13,159 1.36 (0.86, 2.16) 0.19 0 Duration of action (without twincretins) Long-acting 75/38,285 57/31,683 0.93 (0.67, 1.29) 0.66 0 Short-acting 4/4,584 9/4,434 0.57 (0.22, 1.48) 0.25 0 GLP-1RA subtype Albiglutide 28/5,977 14/5,615 1.34 (0.71, 2.51) 0.36 15.1 Dulaglutide 19/6,212 10/5,405 1.28 (0.58, 2.80) 0.54 0 Efpeglenatide 4/3,022 1/1,457 1.16 (0.19, 7.25) 0.88 0 Exenatide 6/9,196 9/8,839 0.70 (0.29, 1.66) 0.41 0 Liraglutide 16/9,578 20/7,007 0.69 (0.37, 1.26) 0.22 0 Lixisenatide 4/3,408 6/3,412 0.72 (0.23, 2.26) 0.57 0 Semaglutide 2/5,476 6/4,382 0.35 (0.10, 1.17) 0.09 0 Cotadutide 3/662 0/136 1.39 (0.17, 11.24) 0.76 0 Tirzepatide 4/3,968 0/2,003 2.02 (0.37, 11.00) 0.42 0 Note. No, number; GLP-1, glucagon-like peptide-1; GLP-1RA, glucagon-like peptide-1 receptor agonist; RR, risk ratios; CI, confidence interval; I², Higgins I² statistics; T2DM, type 2 diabetes mellitus. Table 1. Subgroup analyses of the association between GLP-1 receptor-based agonists and the risk of asthma
No significant differences were observed between the groups stratified by follow-up duration or comparator type (Table 1). The incidence of asthma also seemed to be comparable between trials that used monotherapy and combination therapy (Table 1).
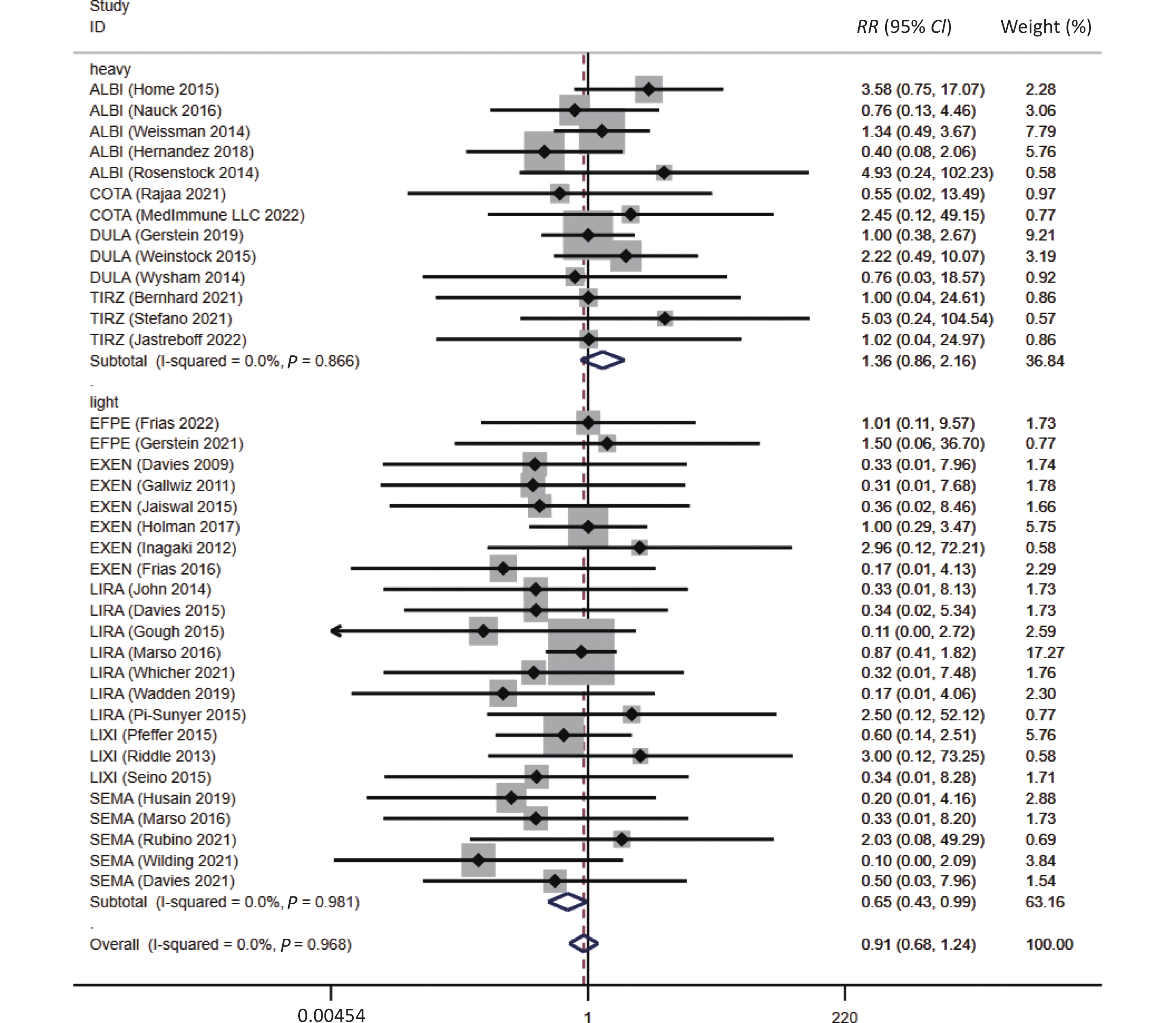
The risk of asthma in GLP-1RA users appeared to be independent of the drug structure (human GLP-1-based or exendin-4-derived) and duration of action (long- or short-acting) (Table 1). In assessing the disparities among the GLP-1RA subtypes, no signs of a reduced risk of asthma were observed in each GLP-1RA. However, the use of light-molecular-weight GLP-1RAs might be associated with a reduced risk of asthma when compared with non-users (RR = 0.65, 95% CI: 0.43–0.99, P = 0.045) (Figure 3).
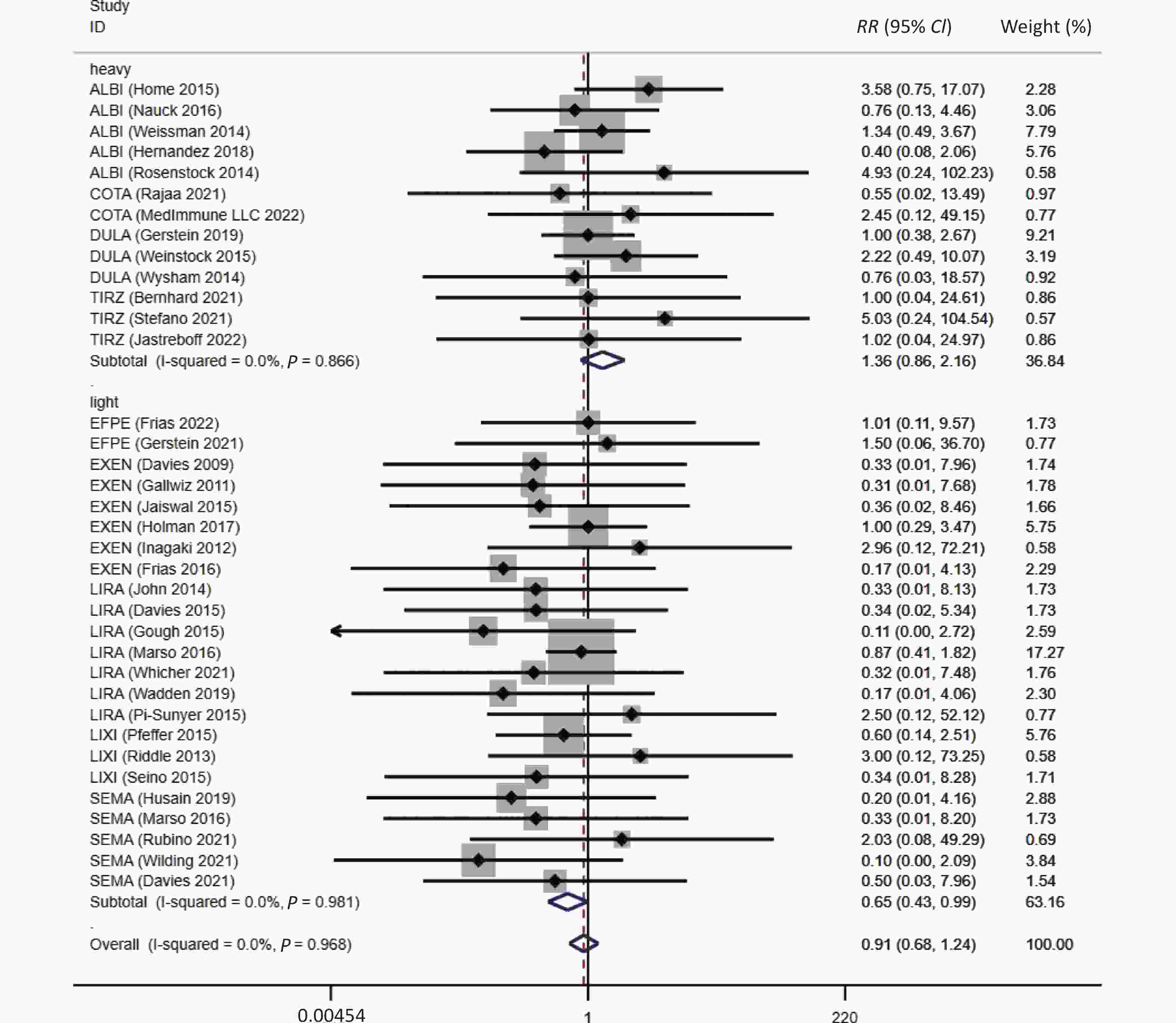
Figure 3. Molecular weight subgroup analyses between GLP-1RAs and the risk of asthma. ALBI, albiglutide; COTA, cotadutide; DULA, dulaglutide; EFPE, efpeglenatide; EXEN, exenatide; LIRA, liraglutide; LIXI, lixisenatide; SEMA, semaglutide; TIRZ, Tirzepatide; RR, risk ratio; CI, confidence interval.
Further meta-regression analyses suggested that change differences in HbA1c (β = 0.3700, P = 0.299) and body weight (β = 0.0600, P = 0.244) between the active and control groups were not associated with the risk of asthma in GLP-1 receptor-based agonist users. Moreover, no significant confounding effects were unveiled for the associated factors including age (β = –0.0005, P = 0.986), sex (β = –0.0110, P = 0.503), body mass index (β = –0.0850, P = 0.318) and duration of follow-up (β = –0.0031, P = 0.174).
-
To our knowledge, this meta-analysis comprehensively evaluated the association between the risk of asthma events and GLP-1 receptor-based agonists in patients with T2DM and obesity. In our study, fewer asthma events were observed in GLP-1 receptor-based agonist users than in non-users; but the difference was not statistically significant. However, the subgroup analysis showed that light-molecular-weight GLP-1 receptor-based agonists might be associated with a reduced risk of asthma.
It was reported that lower incidence of asthma and less frequent exacerbation were observed in patients with diabetes using GLP-1RAs when compared with those using other hypoglycemic medications[17,21]. Furthermore, it was assumed that GLP-1RA-based drug might modify asthma via indirect anti-inflammatory effects by decreasing glucose levels, weight reduction, and direct immune regulatory effects on crucial immune cells or cytokines involved in the development of asthma.
The relationship between hyperglycemia and asthma is reciprocal. Moreover, hyperglycemia was common in hospitalized patients with asthma exacerbations[22]. The inflammatory state of asthma disrupts the carbohydrate metabolism and led to immune system dysfunction, which might result in hyperglycemia[23]. However, hyperglycemia in vivo could also cause changes in intracellular calcium ion concentration, which affected airway smooth muscle relaxation[24]. Therefore, patients with diabetes were at a higher risk of developing asthma when compared with normal people[25]. Whether GLP-1 receptor-based agonists could reduce the incidence of asthma via their hypoglycemic effect remain unclear. However, according to our meta-regression analysis, HbA1c reduction was not associated with reduced risk of asthma in patients receiving GLP-1 receptor-based agonist treatment.
GLP-1 receptor-based agonists may alleviate obesity-related inflammation and, in turn, relieve the hyperreactivity of the airway through their weight-loss effect[26]. The relationship between obesity and asthma may be unidirectional. Obesity and asthma have similar inflammatory states. Moreover, obesity and weight gain are important factors in the development of asthma[27–31]. In some asthma phenotypes, such as female asthma or metabolic asthma, obesity profoundly increases the risk of asthma[32–34]. Furthermore, overconsumption of saturated fatty acids, increased adipocytes, and decreased adiponectin levels could promote nitric oxide synthase-2 decoupling and mitochondrial dysfunction, and therefore increase the risk of airway remodeling and corticosteroid resistance[35]. A clear benefit of weight loss for asthma has been demonstrated[36]. Whether GLP-1 receptor-based agonists could affect the incidence of asthma through their weight loss ability is unclear. However, our meta-regression analysis did not validate any association between the incidence of asthma and weight loss in patients receiving GLP-1 receptor-based agonist treatment.
GLP-1 receptor-based agonists were reported to regulate the immune pathways, which would further repress the occurrence and progression of asthma[37]. GLP-1 could activate phosphatidylinositol 3-kinase signaling pathway (PI3K/Akt) and tracheal submucosal gland receptors, which could weaken endoplasmic reticulum (ER) stress response and relax the airway smooth muscle[38–46]. The PI3K/Akt signaling pathway demonstrated an effect on ER stress upstream and ER stress associated proteins. This led to a decrease in reactive oxygen species production, prevention of airway mucosal tissue damage or cell signal imbalance, and ultimately alleviated airway inflammation and smooth muscle spasm[47–49]. Additionally, GLP-1 could also regulate the formation of arginine and glycation metabolites to inhibit airway inflammation and promote bronchial dilation[50]. L-arginine promotes the production of nitric oxide (NO), a free radical molecule involved in physiological processes, whose subtype NOS-3 acts on smooth muscle to promote bronchiectasis[51]. Moreover, GLP-1 receptor-based agonists was also able to inactivate the nuclear factor kappa-B pathway (NF-κB) suppressing the release of inflammatory factors[43,52]. At the site of inflammation, these inflammatory factors stimulated antibody production, promoted allergic reactions, improved activation of fibroblast function, and increased the secretion of airway mucus[53]. Furthermore, GLP-1 receptor-based agonists could suppress the recruitment of airway eosinophils, which alleviates allergic and viral inflammation[54]. The immune regulatory effect of GLP-1 receptor-based agonists in asthma is considered a crucial mechanism to mediate respiratory benefits in patients with diabetes or obesity.
Although GLP-1 receptor-based agonists theoretically possess mechanisms that might repress the development and progression of asthma, our study only indicated a trend of a reduced incidence of asthma in patients receiving GLP-1 receptor-based agonist treatment, without statistical significance. The possible reasons for this result are as follows. First, the included studies did not consider asthma a primary outcome. Therefore, asthma events were extracted from reports on registered websites, which may require further adjudications. Second, potential influencing factors, including patient occupation, concomitant medication, and allergy history, were rarely reported in the included studies. Thus, the corresponding sensitivity analyses could not be conducted using the current data. Third, we were unable to rate the severity of asthma events or distinguish patients during the acute exacerbation or the chronic stable period. Owing to the lack of explicit delineation in the original RCTs, we could not definitively ascertain the inclusion or exclusion of asthma exacerbations. Consequently, it remains unclear whether the risk of asthma recorded in the original RCTs corresponds to the inception of asthma or asthma exacerbation. Furthermore, there is a possibility that GLP-1 receptor-based agonists was able to reduce asthma exacerbation. Moreover, due to insufficient data, we could not assess the potential dose-effect relationship between the use of GLP-1 receptor-based agonist and asthma.
We also performed meta-regression analyses to assess the effects of HbA1c reduction and weight reduction mediated by GLP-1 receptor-based agonists on asthma. However, the HbA1c and weight reduction in GLP-1 receptor-based agonists users were not associated with the risk of asthma. The potential risk reduction of asthma in GLP-1 receptor-based agonist users may not be mainly driven by its hypoglycemic or weight-loss effect.
A previous meta-analysis found that GLP-1RAs showed a decreasing trend in the risk of nine respiratory diseases, but the use of GLP-1RA had no significant effect on asthma events, which is consistent with our conclusion[19,55]. However, owing to the limitations of the current evidence, the association between the use of GLP-1 receptor-based agonists and the risk of asthma remains controversial. To provide a more accurate evaluation, future clinical studies should focus on asthma as the primary endpoint and establish strict inclusion and exclusion criteria. The asthma events recorded in this study should be well-defined and widely accepted. Further studies should explicitly include the patients’ medical histories and record the severity or duration of adverse reactions in detail. Given the exclusive focus on adult participants in this study, it is essential to conduct additional research to ascertain the generalizability of the conclusions to pediatric and adolescent populations. In addition, attention should be paid to the therapeutic effects of dual or triple agonists on asthma, disparities among GLP-1 receptor-based agonists with different pharmacokinetic characteristics, and potential dose-effect relationships.
-
Compared with non-users, a modest reduction in the incidence of asthma was observed in patients with T2DM or obesity using GLP-1 receptor-based agonist treatments, but the difference was not statistically significant. Further investigations are warranted to assess the association between GLP-1 receptor-based agonists and the risk of asthma.
-
Linong Ji has received fees for lecture presentations and consultations with AstraZeneca, Merck, Metabasis, MSD, Novartis, Eli Lilly, Roche, Sanofi-Aventis, and Takeda. No support from any organization other than those described above was received for the submitted work. The funding agencies had no role in the study design, data collection or analysis, decision to publish, or manuscript preparation.
HTML
Searches
Data Extraction
Subgroups
Strategy for Data Synthesis
&These authors contributed equally to this work.








 Quick Links
Quick Links
 DownLoad:
DownLoad: