-
Toxoplasma gondii is an obligatory intracellular protozoan widely distributed in nature and has a complex life cycle. Almost all endothermic organisms, including humans, are susceptible to T. gondii infection, resulting in toxoplasmosis, which can cause major damage. Notably, almost one-third of the global population is chronically infected with T. gondii[1]. T. gondii was first found in the tissues of the North African rodent gondii by Nicolle and Manceaux in 1908[2]. In the same year, Splendore[3] also found them in the tissues of rabbits. T. gondii has five stages in its life cycle: trophozoites (including bradyzoites and tachyzoites), gametophytes, cysts, schizonts, and oocysts, each with a unique shape.
Humans, pigs, and cows can act as intermediate hosts for T. gondii; however, cats and other felines are definitive hosts. Multiple stages of T. gondii infection could occur, and intermediate hosts can be infected via various routes, such as the ingestion of oocysts, cysts (bradyzoites), or tachyzoites. After penetrating the intermediate host and invading nucleated cells, the parasite prefers to multiply in the liver, lymph nodes, neurological system, and muscular tissue. T. gondii is a single parasitic species; however, different strains exist. The virulence and sensitivity of T. gondii vary when infecting different hosts. Using six polymerase chain reaction and restriction fragment length polymorphism markers, Howe et al. found that most of the 106 Tg strains from North America and Europe belonged to three clonal lineages, namely classical types I, II, and III[4]. Serotyping studies have shown that type I strains significantly increase the incidence of psychiatric illnesses[5,6]. In epidemiological studies of T. gondii in China, 12 genotypes have been isolated, among which ToxoDB#9 accounted for the highest proportion (65.5%) and was named Chinese1, followed by the classic type I lineage (18.3%)[7]. Notably, the Chinese1 type includes both virulent and attenuated strains, and its pathogenic mechanism requires further study[8]. These reports suggest that the pathogenic outcomes of T. gondii infection differ in different geographic regions.
-
Most of the time no significant clinical symptoms have been observed after humans become infected with T. gondii. When a parasite intracellularly invades an immunocompetent patient, the host may be stimulated to build protective immunity and develop tissue cysts[9]. The host often remains asymptomatic. Most patients with schizophrenia infected by the parasite are such healthy hosts. Immunosuppressed or immunocompromised patients, such as those with acquired immune deficiency syndrome, may experience extensive parasite proliferation and increased pathogenicity from the reactivation of tissue cysts to the tachyzoite stage, resulting in acute toxoplasmosis, which can cause serious pathological injury or even death[10]. Moreover, the parasite may be transmitted from the placenta to the fetus if the immunocompetent mother is infected during pregnancy or within three months before conception[11]. Blood transfusions (whole blood or white blood cells) or organ transplants can also transmit the parasite if the donor has T. gondii infection.
A growing number of reports have confirmed that central nervous system (CNS) dysfunctions, including toxoplasmic encephalitis, may occur in immunocompetent hosts infected with T. gondii[12-14]. Parasites can migrate to the host brain within seven days of infection. Experimental studies in mouse models of T. gondii infection have shown that approximately 10% of neurons and astrocytes and 30% of microglia may be infected with tachyzoites[15].
Toxoplasmosis poses a serious risk to humans, particularly developing fetuses. Pregnant women infected with T. gondii may experience abortion, stillbirth, and intrauterine growth retardation; infants may develop congenital toxoplasmosis, leading to problems in the CNS, vision, and hearing[16]. In recent decades, there has been increasing interest in the potential connection between T. gondii infection and mental diseases. However, the exact mechanism by which this parasite causes mental illnesses remains unknown.
-
Schizophrenia is a heterogeneous disorder characterized by the following three features: positive symptoms, such as hallucinations and delusions; negative symptoms, such as disordered thought processes, social withdrawal, and cognitive and mnemonic impairments. Although the exact etiology of schizophrenia remains unknown, it is a serious mental illness affecting 1% of the population. Recent studies have shown that some cases of schizophrenia may be related to T. gondii infections. To date, many researchers have demonstrated this correlation using serological studies, animal models, and other methods. Currently, the most commonly used technique for investigating the connection between T. gondii infection and schizophrenia is serological research.
Chen et al. studied psychopaths in the southeastern region of Zhejiang Province, China, using a case-control methodology. Through enzyme-linked immunosorbent assays, they found that the seroprevalence of anti-T. gondii IgG and IgM levels was significantly higher in patients with schizophrenia than healthy controls[17]. Similarly, Veleva et al. found that compared to people with schizophrenia who were serum-negative for T. gondii, patients who were positive for T. gondii had more severe damage in a range of cognitive domains, as well as more severe psychopathological damage[18]. In addition, Grada et al. conducted a case-control study in western Romania and found that patients diagnosed with schizophrenia had a significantly higher seroprevalence of IgG antibodies against T. gondii than healthy controls[19]. Liu et al. conducted a cross-sectional study on the status of T. gondii infection in patients with schizophrenia in Shandong Province, China. They found that the serum positivity rate for T. gondii infection in patients with schizophrenia was relatively high, and it was estimated that age was an influencing factor for this[20]. In recent years, similar studies have been conducted in Jiangsu Province, China[21].
However, a study by Ademe et al. revealed that the seroprevalence of toxoplasmosis in individuals with schizophrenia was only marginally higher than that in normal individuals, and the statistical data indicated no substantive difference[22]. Contopoulos et al. conducted a meta-analysis of 66 studies on the association between T. gondii infection and schizophrenia involving 11,540 patients with schizophrenia and 69,491 normal controls. Their findings demonstrated a robust statistical correlation between T. gondii infection and schizophrenia despite the presence of numerous methodological flaws in the included case-control studies[23]. Other studies have also suggested a strong association between T. gondii infection and schizophrenia[24-28].
Furthermore, an association between T. gondii infection and schizophrenia was demonstrated using animal models. Studies on the behavior of T. gondii-infected mice were conducted in 1978 to compare changes in their learning capacities[29]. In Morris water maze experiments, Daniels et al. found that latent toxoplasmosis alters neurocognitive symptoms, especially memory deficits, in infected rats[30]. High specificity for cat urine has also been demonstrated in mice with chronic T. gondii infection[31]. A study by Boillat et al. (2020) showed that infected mice not only have a specific tendency toward the smell of cats but also exhibit decreased fear and aversion to various predators. They also demonstrated that behaviors manipulated by T. gondii included increased activity, impulsiveness, and reduced fear, which correlated positively with the increased number of cysts in the cerebral cortex of the brain[32]. Rats and mice do not exhibit the entire phenotypic spectrum of schizophrenia. Schizophrenia manifests as a wide range of symptoms that differ across individuals. Although this complexity cannot be fully summarized in rodent models, specific symptom classes can be simulated using behavioral models[33].
Furthermore, other research demonstrated that T. gondii infection may only exacerbate schizophrenia rather than cause it. Holub et al. conducted a survey of 251 patients, of whom 77.3% were infected with T. gondii. The positive symptom assessment scores of the infected patients were significantly higher than those of the uninfected patients, whereas the negative scores were equivalent to those of the general population. Particularly in terms of irritability, cognitive disorders, hostility, and behavioral disorders, there were significant differences between infected patients and patients negative for anti-T. gondii antibodies. T. gondii-infected patients frequently exhibit erratic, impulsive, easily agitated, and irritable behavior[34]. Similarly, a survey by Arjen et al. that included young and patients with mild schizophrenia, patients with anti-T. gondii antibodies had significantly more severe positive symptoms, whereas negative symptoms remained unchanged[35].
-
According to evolutionary theory, some researchers have proposed evolutionary hypotheses, such as the behavioral manipulation effect of parasites, and they contend that T. gondii infection will prevent people from entering society, which will aid in the survival of the parasite[36]. However, it is difficult to verify specific aspects of this hypothesis. Therefore, we should focus on studying molecular biological mechanisms. Presently, the focus of this study was immunological responses, brain encapsulation, central neurotransmitters, and brain tissue structures. However, an increasing number of researchers have employed recently developed techniques to elucidate the genetic basis of T. gondii infection. Additionally, studies on drug use and metabolism have complemented the association between schizophrenia and T. gondii infection. Below is a summary of the review considerations regarding the methods mentioned above (Figure 1).
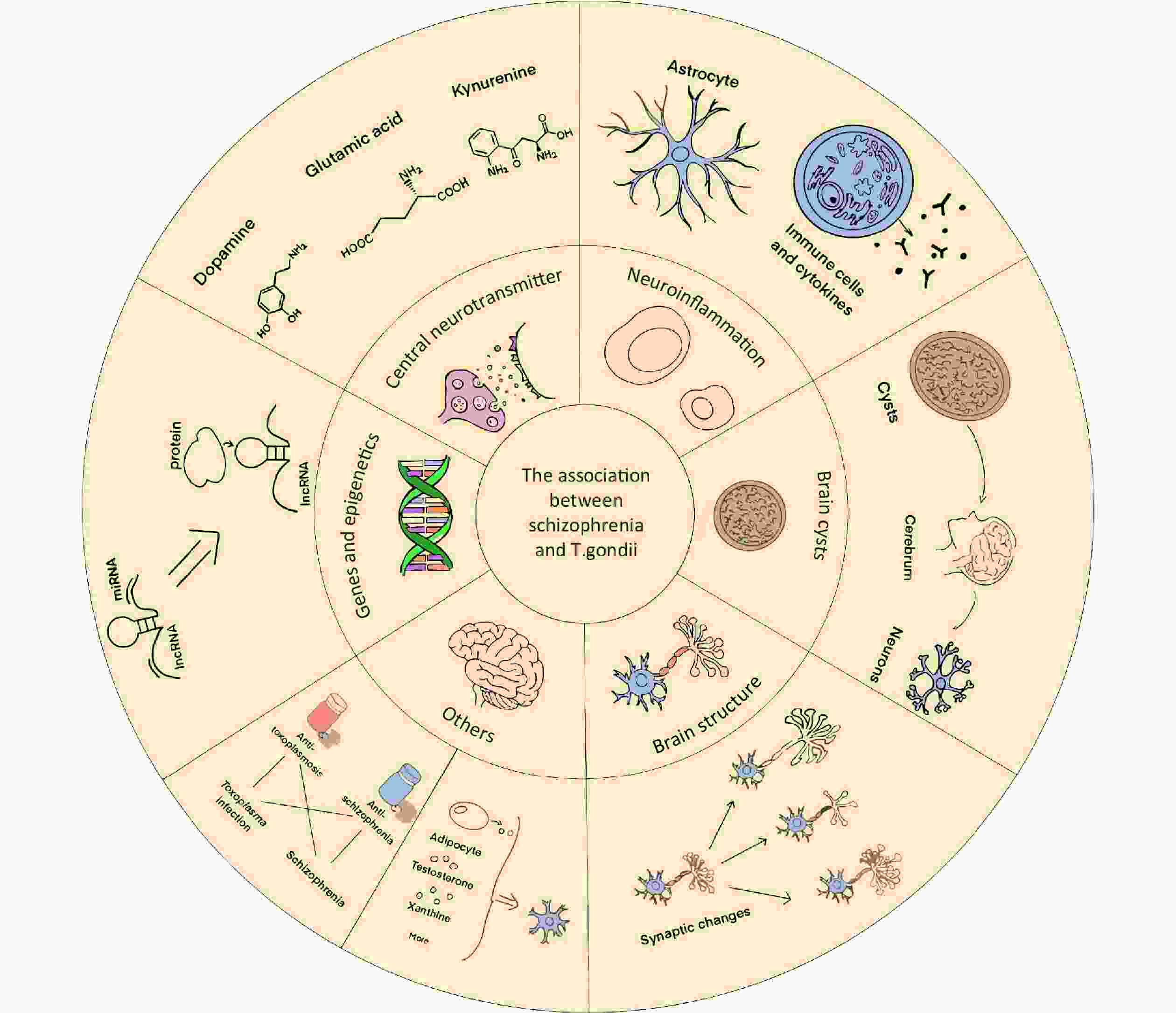
Figure 1. Summary of the association between schizophrenia and Toxoplasma gondii infection. This article reviews the association between schizophrenia and T. gondii infection from the perspective of molecular biological mechanisms, including (1) central neurotransmitters, (2) neuroinflammatory response and altered immune functions, (3) distribution of T. gondii cysts in the brain, (4) structural changes in the neural tissue in the brain, (5) gene regulation and epigenetics, (6) combination therapy (which suggests an association between schizophrenia and T. gondii infection), and (7) changes in other metabolic pathways. lncRNA, long noncoding RNA; miRNA, microRNA.
-
Current research has shown that the occurrence of schizophrenia is closely related to an imbalance in regulating central neurotransmitters, such as DA, glutamate, 5-hydroxytryptamine (5-HT), and gamma-aminobutyric acid (GABA); its core hypothesis is the dopamine hypothesis. Therefore, researchers have considered central neurotransmitters, such as dopamine, while examining the connection between T. gondii infection and schizophrenia. As early as 1985, Stibbs found that chronic infection with T. gondii caused a significant increase in DA in the brain of mice, but acutely infected mice had unchanged dopamine levels, elevated homovanulanic acid levels, and decreased norepinephrine levels; serotonin and 5-hydroxyindoleacetic acid levels were not altered in infected mice. These findings offer fresh insights into the connection between T. gondii infection and neurotransmitter systems and imply that T. gondii infection may affect neurotransmitter levels in mice, thereby affecting their behavior[37].
Several studies have linked chronic T. gondii infection to increased dopamine release, emotional problems, and aberrant behavior[38,39]. Moreover, some studies have found that drugs targeting the dopamine system can attenuate behavioral abnormalities that occur after T. gondii infection[40]. However, not all forms of schizophrenia can be explained by the dopamine hypothesis. Gaskell et al. used dual-active amino acid hydroxylase as a study subject and knocked out two aromatic amino acid hydroxylase genes, AAH1 and AAH2, in the T. gondii genome and found that the genes could encode levodopa (L-DOPA), a direct precursor protein for dopamine production. Thus, this enzyme plays an important role in T. gondii metabolism, growth, and development, suggesting that T. gondii affects DA production and synaptic transmission[41]. McFarland et al. found that the mechanism by which T. gondii infection affects the dopamine system might not be related to the AAH2 gene, which is not required for T. gondii to induce behavioral abnormalities[42]. Furthermore, Afonso et al. suggested that T. gondii infection produces behavioral changes in mice, such as aggression. However, the existence or lack of AaH2 tyrosine hydroxylase activity encoded by the Toxoplasma genome has little bearing on these behavioral alterations[43]. Dopamine plays a regulatory role in subsequent T. gondii infections, and there is an interaction between them. Some researchers have shown that dopamine stimulates the growth and spread of T. gondii trophozoites in cultures of neonatal rat astrocytes and infected human fibroblasts. This effect may be related to the capacity of the parasite to influence host cell immune responses and immune clearance[44].
David et al. examined the impact of T. gondii infection on glutamate metabolic homeostasis and neuronal function in the CNS. They discovered that T. gondii infection inhibits the expression and function of the glutamate transporter protein glutamate transporter-1 (GLT-1) in the CNS, which interferes with the uptake of glutamate by neurons, causing glutamate accumulation in the CNS and neurons and triggering neuronal death and neurotransmitter dysregulation. These results suggest that T. gondii infection may inhibit GLT-1 to promote neuronal cell death, causing pathological processes, such as dysregulation of neurotransmitter homeostasis and neuronal damage in the CNS[45]. Acquarone et al. found that mice infected with T. gondii were stunned by the amplitude of the startle, showing reduced glutamate and D-serine levels in the prefrontal cortex and hippocampus. Their results suggest that infection with the T. gondii ME49 strain could induce behavioral changes associated with psychiatric disorders and glutamatergic neurotransmission dysfunction[46]. Excitatory ionotropic glutamate receptors, including the N-methyl-D-aspartate receptor (NMDAR), are involved in synapse formation, neuronal development, and other processes. A previous study investigating the mechanism of immune cross-reactions proposed that the NMDA model was connected to the association between T. gondii and schizophrenia. Moreover, different NMDAR subunits are targeted at different exposure times, especially NMDA 2D, the main potential target[47]. Glutamate is decarboxylated by glutamic acid decarboxylase to produce GABA. Since GABA is the primary inhibitory neurotransmitter in the brain, an imbalance between excitatory and inhibitory neuronal activity could result from a disruption in GABA production. T. gondii infection increases the dispersion of glutamic acid decarboxylase 67, a key GABA synthesis enzyme, in the mouse brain, thereby affecting GABA synthesis[48].
Tryptophan is reportedly broken down by astrocytes, which subsequently produce a significant quantity of kynurenine (KYNA), which affects peripheral neurons. KYNA is an antagonist of NMDAR and elevated levels of KYNA can block the glutamate recognition sites of NMDAR. Pearce et al. found higher levels of tryptophan in patients with schizophrenia with longer onset and peak latency, contrary to the theory that tryptophan depletion leads to enhanced dopaminergic responses. Furthermore, they showed that the schizophrenia group had lower levels of KYNA in the blood and higher levels of KYNA in their cerebrospinal fluid[49]. Thus, they hypothesized that KYNA is an important link between T. gondii infection and schizophrenia. In addition, Mahmoud et al. found that T. gondii interferes with the synthesis of 5-HT through the KYNA pathway[50].
In summary, although there is a large amount of evidence that neurotransmitters such as dopamine and glutamate are strongly associated with schizophrenia and T. gondii infection, there are still some issues that need to be addressed. First, more studies on central neurotransmitter regulatory pathways are needed, as some signaling pathways have not yet developed into a fully functional system. Second, there is a need to investigate why the levels of neurotransmitters, such as dopamine, are elevated after T. gondii infection, for which we can try to start elucidating from the aspects of genetic and epigenetic regulation. Many research methods rely on animal studies; however, it is important to investigate how human neurotransmitter alterations in the brain in vivo align with the published results in other models like animal behavioral experiments. An overview of the modifications to the core system is provided below (Table 1).
Year Author Target Main findings* 1985 Stibbs et al.[37] Catecholamines and indoleamines In the acute phase: vanillic acid (↑), dopamine levels remain unchanged, epinephrine (↓). In the chronic phase: DA levels (↑), serotonin levels are not altered. 2009 Gaskell et al.[41] L-DOPA and DA The genome of the protozoan parasite Toxoplasma gondii was found to contain two genes encoding tyrosine hydroxylase that produce L-DOPA. T. gondii AaaH1 is constitutively expressed, while the other gene, T. gondii AaaH2, is induced during the formation of the bradyzoites of the cyst stages of the life cycle. 2011 Prandovszky
et al.[40]DA After infection: K+-induced release of DA (↑). T. gondii orchestrates a significant increase in DA metabolism in neural cells. 2012 Strobl et al.[44] DA Higher doses of dopamine significantly increased the number of T. gondii tachyzoites in human fibroblast host cells. DA antagonists had no significant effect on DA-stimulated tachyzoite production in human fibroblasts. DA significantly increased the number of intracellular tachyzoites in primary cultured neonatal rat astrocytes. 2015 Brooks et al.[48] GAD67 and GABA Seizures and other neurological complications seen in Toxoplasma-infected individuals are due, at least in part, to changes in GABAergic signaling. 2016 Mahmoud
et al.[50]Serotonin(5-HT), DA, and NE. With/without DXM at 60 dpi: DA turnover (↑), 5-HT turnover (↑). DA and norepinephrine levels are not associated with the observed behavioral changes. 2016 David et al.[45] Glutamate and GLT-1 After infection, the major astrocyte glutamate transporter GLT-1 (↓), extracellular glutamate concentration (↑), dendritic spines (↓), immune reactivity of VGlut1 and NeuN (↓) 2017 Afonso et al.[43] TH Parasite-secreted AaaH2 TH is neither necessary for the generation of risky behavior nor for the increased trappability observed during chronic Toxoplasma infection. 2017 Lucchese et al.[47] NMDAR The NMDA 2D subunit, which is mainly expressed in parvalbumin-positive interneurons, appears to be a hotspot for potential T. gondii-induced cross-reactive immune attacks. 2018 McFarland
et al.[42]DA, DAT and VMAT2 After infection of BALB/c mice: blunted response to amphetamine or cocaine, expression of DAT and VMAT2 (↓). AAH2 is not required for T. gondii infection-produced DA-dependent neurobehavioral abnormalities. 2020 Ibrahim et al.[38] IgG anti-Toxoplasma dopamine Dopamine levels in +ve IgG schizophrenia are higher than those in -ve IgG schizophrenia. Chronic T. gondii infection can lead to elevated DA levels, which can lead to schizophrenia. 2020 Pearce et al.[49] Kynurenine KYN pathway markers predict a slowing of startle latency in SCZ subjects and in those with chronic T. gondii infection; this was not seen in CON or T. gondii-seronegative subjects. 2022 Acquarone
et al.[46]Glutamate and D-serine Chronic infection: glutamate and D-serine (↓), but behavioral and cognitive aspects are maintained. 2023 Omidian et al.[39] Dopamine Serum DA on the first to the fourth day after T. gondii inoculation was the same as the negative control, but increased on the fifth day. DA production is low in acute toxoplasmosis; chronic disease increases DA production. Note. *(↑), upregulation; (↓), downregulation. L-DOPA, levodopa; GABA, gamma-aminobutyric acid; NE, norepinephrine; DXM, dexamethasone; GAD67, glutamate decarboxylase 67; GLT-1, glutamate transporter-1; 5-HT, 5-hydroxytryptamine; DA, dopamine; GLT-1, glutamate transporter-1; TH, Tyrosine hydroxylase; NMDAR, N-methyl-D-aspartate receptor; DAT, dopamine transporter; VMAT2, vesicular monoamine transporter 2; VGlut1, recombinant vesicular glutamate transporter 1; NeuN, neuronal nuclei; KYN, kynurenine; SCZ, schizophrenia; CON, chronic Toxoplasma gondii infection. Table 1. A brief summary of the results of existing studies on the changes in central neurotransmitters
-
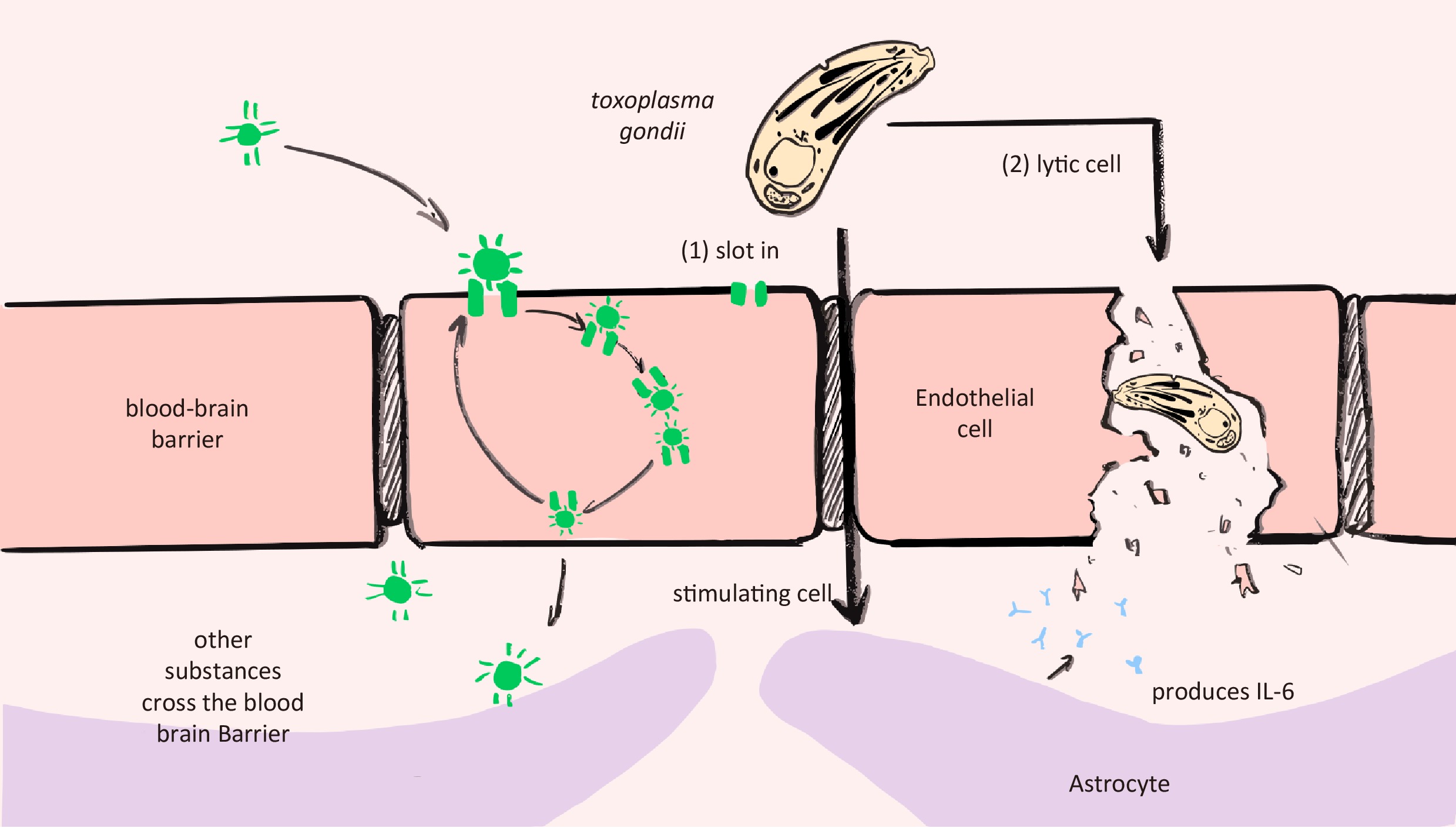
T. gondii infection typically triggers a robust immune response, particularly a neuroinflammatory response, because T. gondii is viewed as a foreign agent that invades the organism. It is believed that the blood-brain barrier protects the brain and is not easily affected by immune responses. However, T. gondii could infect the brain rapidly and break through the blood-brain barrier. Konradt et al. found that T. gondii replicates in endothelial cells (ECs) and then lyses them, as evidenced by in vivo imaging in acutely infected mice[51]. Kim et al. constructed an in vitro microfluidic model of acute T. gondii infection and transendothelial migration. They found that T. gondii could rapidly infect ECs in the acute phase and then pass through the extracellular matrix to neurons. However, it is difficult to experimentally demonstrate whether tachyzoites directly infect neurons[52]. Moreover, from the perspective of neuroinflammation, the destruction of the blood-brain barrier may be an important pathogenic mechanism (Figure 2).
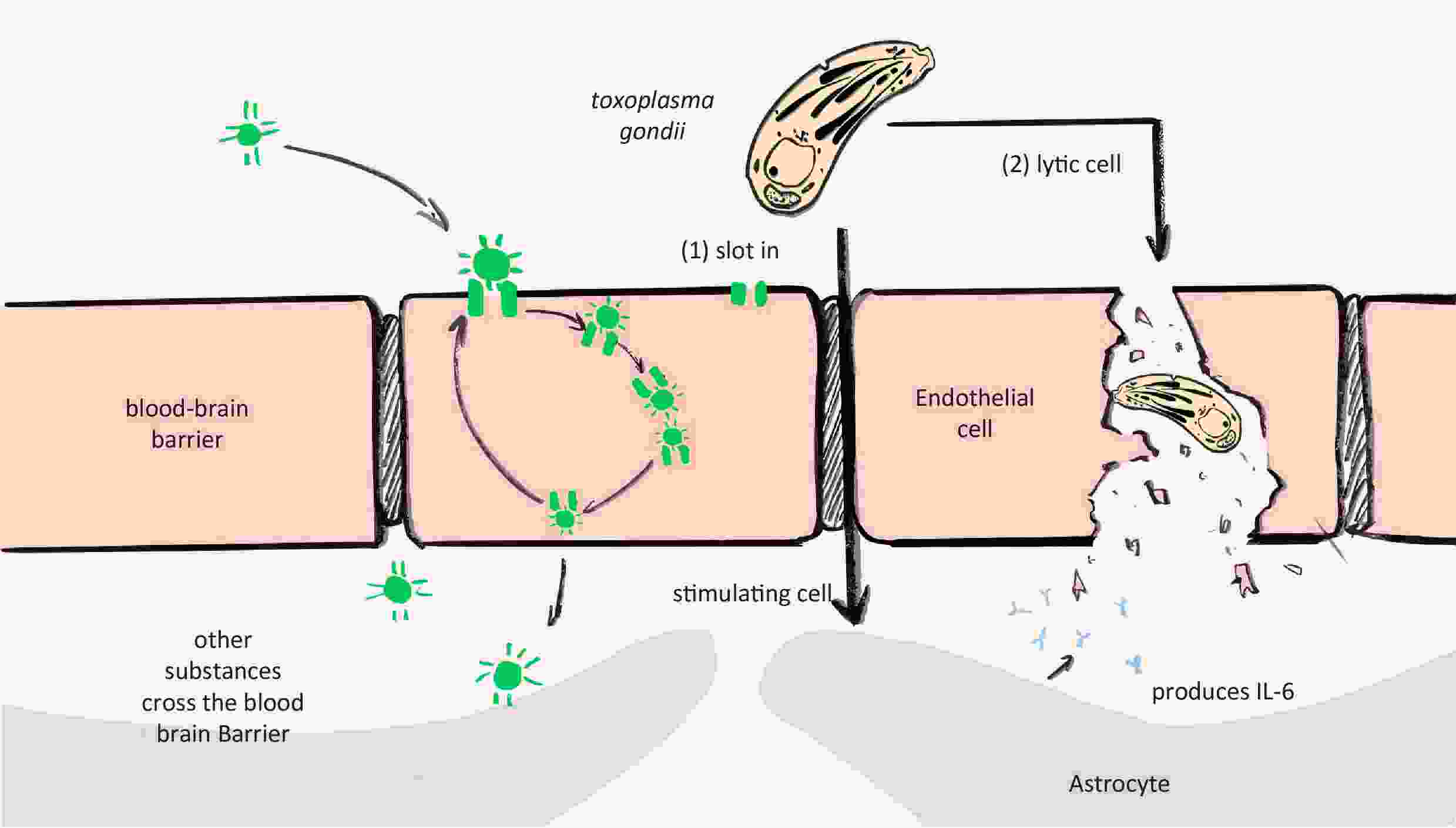
Figure 2. Hypothesis: Toxoplasma gondii infection disrupts the blood-brain barrier, causing schizophrenia. Endothelial cells lyse upon the invasion, spread, and proliferation of T. gondii. In contrast, when the permeability of the blood-brain barrier increases and restriction decreases, T. gondii easily crosses the barrier and enters the brain parenchyma. IL-6, interleukin-6.
In addition to the acute phase, the chronic phase and latent T. gondii infection are also intimately linked to the inflammatory response. Estate et al. found that T. gondii infection leads to a reduction in the number, length, and branching of mouse brain capillaries, accompanied by neuroinflammation, microglia activation, and an increase in the number of white blood cells in the brain capillaries. Therefore, it can be inferred that T. gondii infection activates neuroinflammation, changes the permeability of the blood-brain barrier, reduces angiogenesis, and causes microcirculation dysfunction. The study of Estate et al. was the first to show that even during the asymptomatic phase of the disease, T. gondii infection causes long-term changes in the cerebral microvascular structure that are linked to endothelial dysfunction and persistent leukocyte-endothelium interactions, suggesting elevated vascular inflammation[53]. Hamdani et al. found that patients with schizophrenia who were infected with T. gondii were more likely to develop changes in the immune-inflammatory process by testing their antibody and immunoglobulin levels[54]. Fond et al. discovered that latent T. gondii infections were three times more common in patients with schizophrenia than in the controls and that latent infections in these patients were linked to specific mental symptoms and peripheral inflammation[55]. Moreover, several studies have demonstrated an important association between the schizophrenic course of T. gondii infection and neuroinflammation, from the antibody level to the brain imaging, including cytokines such as interleukin-6 (IL-6), transforming growth factor-β1 (TGF-β1), interferon-gamma (IFN-γ), and various other immune proteins, even complement expressed by astrocytes, microglia, and infiltrating monocytes[56-59].
Neuroinflammation may also be associated with brain cysts. Using infected C57BL/6 mice as models, Barrios et al. discovered a positive correlation between the number of T. gondii cysts in the brain and the upregulation of TNF and CC-chemokine expression in the brain tissues. Additionally, elevated levels of IFN-γ, TNF, and CCL5/MCP-2 in the peripheral blood suggested that the persistence of parasite cysts may result in persistent neuroinflammation[58]. The presence of parasitic cysts may also lead to the disruption of the blood-brain barrier, allowing many cytokines to enter the brain tissue. Xiao et al. found that immunotherapies targeting the PD-1 pathway alleviated neuroinflammation induced by chronic T. gondii infections and suggested that this was associated with a reduction in the numbers of T. gondii tissue cysts during treatment[60].
Numerous studies have investigated the various impacts when a mother is infected with T. gondii during pregnancy. Romero et al. explored the mechanisms of maternal transmission to the offspring and found that pre-pregnancy exposure to T. gondii induces the production of maternal pathogenic antibodies that recognize and bind to fetal brain protein mimics, leading to neurostructural, cognitive, and social alterations in the offspring[61]. According to the observations of early pregnancy infection with T. gondii by Bi X. et al., the immune escape of T. gondii at the maternal-fetal interface may be linked to the aberrant expression of CD47 and its ligands, signal regulatory protein- alpha (SIRPα) and thrombin-sensitive protein 1 (TSP-1), in the mother and fetal mice during mid-to-late gestation[62].
In conclusion, the prevailing consensus is that T. gondii disrupts the blood-brain barrier before alterations occur in the CNS. However, questions remain regarding whether tachyzoites directly infect neurons, how the systemic inflammatory environment affects the brain environment through cytokines and inflammatory mediators, evidence of the lytic infection cycle of bradyzoites after cyst formation, and the mechanism of maternal infection. Better research models and extensive research are required to answer these questions.
-
Cysts play a major role in the pathogenicity of T. gondii. Following infection, T. gondii produces cysts in the brain that remain in the human body for a long time, causing T. gondii encephalopathy. T. gondii cysts are common in the brain, particularly in the hippocampus and amygdala. Several studies have explored the sites of cyst distribution and their influence on the immune system. Haroon et al. identified neurons as the only cyst-harboring cell population in the CNS[63]. According to Galvan-Ramirez et al., mice with chronic infections harbor more cysts than animals with acute infections, and the hippocampus and telencephalon are the primary sites of cyst distribution in chronically infected mice[64]. Barbosa et al. demonstrated through an in vitro model study that T. gondii cysts can disrupt neuronal function, changing the concentration of neurotransmitters in chronically infected mice; encystment also causes enlargement of the neurites and neurite network impairment[65]. Wu et al. performed a pathological analysis of the distribution of T. gondii cysts in mouse brains and concluded that there was a correlation between the localization and distribution of T. gondii cysts in the brains of infected mice, which displayed dynamic changes over time and induced behavioral and neuropsychiatric symptoms during chronic T. gondii infections[66].
However, other studies have yielded contradictory findings. According to Ingram et al., after early acute infection with T. gondii, mice may permanently lose their intrinsic fear of cats even without long-lasting parasitic cysts or ongoing brain inflammation[67]. Xiao et al. studied antibody types and found that the distribution of cysts in mouse brains infected with type I T. gondii was directly related to the antibody spectrum, and mice with high levels of the MAG1 antibody showed increased cyst volume and changes in the tendency toward schizophrenia[68].
In general, it remains controversial whether the distribution and existence of cysts in the brain are necessarily related to schizophrenia, which may also be related to differences in the strains of T. gondii and the age of the host.
-
More studies suggested that the effects of T. gondii infection on the nervous system are not limited to the behavioral performance commonly used in the laser swimming test but also involve other underlying mechanisms such as nerve cells and tyrosine hydroxylase. The effects of T. gondii infection on neuronal structure and tyrosine hydroxylase expression were investigated by Barbosa et al. (2020). Studies have found that T. gondii infection can cause spontaneous cyst formation in neurons and changes in neuronal structure and tyrosine hydroxylase expression. Specifically, T. gondii infection reduced the length and nuclear size of nerve fibers, the number and total length of dorsal synapses, and the total length and length distribution of neurites. T. gondii infection also significantly reduces the expression of tyrosine hydroxylase. Based on these findings, researchers have suggested that T. gondii infection causes changes in neuronal structure and function and involves the regulation of multiple molecular pathways[65]. Microglia-neuron interactions and perisomatic inhibitory synapses have also been studied in relation to T. gondii infection. Carrillo et al. showed that microglia and neurons can also become infected with T. gondii, which can cause a loss of peripheral synaptic inhibition, interaction, and contact.Such aberrant neuronal stimulation can cause other diseases. For example, T. gondii infection was found to regulate the interaction between microglia and neurons by activating specific signaling pathways. The study by Carrillo et al. provides important information for further understanding of the mechanisms by which T. gondii affects the nervous system and provides new ideas for the treatment of related diseases[69].
Changes in brain structure may also be linked to immunological responses and neurotransmitters. Chronic inflammatory substances, for example, excite nerve cells, resulting in structural changes, and long-term alterations in neurotransmitters can feedback control synapses and other structures, while the precise mechanisms are unknown, suggesting that when studying intricate mechanisms, it is important to consider the effects of several different factors rather than just one.
-
With the rapid development of epigenetics, many researchers have attempted to explain the association between T. gondii infection and schizophrenia from an epigenetic-regulatory perspective. TEEGR, the effector of T. gondii, was reported by Braun et al. to stimulate the EZH2 gene to restrict cell expression, reducing NF-κB signaling and fostering a robust immune response following T. gondii infection[70]. Rovira et al. demonstrated that the catechol-O-methyltransferase (COMT) genotype increases the risk of developing schizophrenia after T. gondii infection; the risk is higher in individuals with a Met/Met phenotype, intermediate in heterozygotes, and lower in individuals with a Val/Val phenotype. It has also been proposed that T. gondii infection decreases COMT activity, increasing dopamine synthesis[71].
Glutathione S-transferases (GSTs) are a class of isoenzymes encoded by glutathione S-transferase mu-1 (GSTM1), glutathione S-transferases θ-1 and θ-2 (GSTT1 and GSTT2, respectively), glutathione S-transferase pi-1 (GSTP1), and glutathione S-transferase ω-1 (GSTO1). Deleting the entire gene results in insufficient enzyme activity and may cause an increase in the oxidative stress response. Previous studies have linked GST expression to vascular disorders and cancers. Ansari-Lari et al. found an additive effect of T. gondii and the GSTT1-active genotype as risk factors for schizophrenia in an Iranian population. However, further research with larger patient groups is needed to elucidate these findings because this was only a small pilot trial[72].
Epigenetic long noncoding RNAs (lncRNAs) and microRNAs (miRNAs) have been the focus of many studies. These studies have indicated that lncRNA and miRNA expression levels changed dramatically in T. gondii-infected hosts. The sperm of male mice infected with T. gondii showed notable alterations in the miRNA lineage, according to Tyebji et al. Of the total miRNAs mapped (585), 174 were differentially expressed in the Toxoplasma-infected group, with 35 downregulated and 75 upregulated significantly. They showed that compared to non-infected mice, infected mice had higher anxiety and aversion behaviors, with abnormal social interaction patterns that can span generations. In addition, these effects differed between male and female offspring, suggesting that infection in male mice may have caused sex-specific generational changes[73]. Based on the results of an lncRNA and mRNA co-expression microarray in the brains of mice infected with T. gondii Chinese1 type, Sun et al. revealed that the expression of lncrNA-11496 was downregulated in mice chronically infected with T. gondii, and its dysregulation could further affect cell proliferation, differentiation, and apoptosis by targeting Mef2c[74]. Wang et al. demonstrated that lncRNA102796 regulates the proliferation of nerve cells by regulating the transcription and expression of oprd1 (a protein that can bind to lncRNA102796) in mouse neuroglial cells (BV-2). These findings indicate that T. gondii-infected nerve cells with downregulated lncRNA102796 expression have an inhibitory effect on nerve cell proliferation, which affects the neurological system[75]. The results of some studies on epigenetic regulation are summarized in Table 2.
Year Author Target Main findings 2019 Braun et al.[70] The dense granule-resident effector TEEGR TEEGR binds with the E2F/DP transcription factors to promote EZH2 activation, which eventually antagonizes the positive regulation of NF-κB activity by tachyzoites. 2020 Tyebji et al.[73] Sperm small RNA profiles of male mice chronically infected with Toxoplasma gondii Toxoplasma infection alters small RNA profiles in the F0 sperm and may affect the behavior of F1 and F2 progeny. 2020 Sun et al.[74] The expression of lncRNAs and mRNAs by microarray assay The expression of lncRNA-11496 is significantly downregulated and the lncRNA11496/Mef2c/HDAC2 pathway was proposed. 2021 Ansari-Lari
et al.[72]GSTT1 and GSTM1 An additive effect for T. gondii and GSTT1-active genotype as risk factors for schizophrenia in an Iranian population. 2022 Wang et al.[75] The expression of lncRNA102796 in the brains of mice chronically infected with T. gondii The expression of lncRNA102796 was downregulated; lncRNA102796 can regulate the proliferation of nerve cells by regulating the transcription and expression of the oprd1 gene. 2022 Rovira et al.[71] COMT Val105/Met158 polymorphism COMT variation and exposure to T. gondii interact and modify the risk for schizophrenia. Note. GSTT1 encodes glutathione S-transferase T1; GSTM1 encodes glutathione S-transferase M1; COMT, catechol-O-methyltransferase. Table 2. Current studies regarding epigenetic regulation of Toxoplasma gondii infection
-
Numerous researchers have discovered correlations between schizophrenia and T. gondii infection in studies of combination and cross-medications for the treatment of both diseases. The findings of several studies support the relationship between T. gondii infection and schizophrenia.
Castano et al. found that sulfadiazine plus pyrimethamine treatment could reverse multiple behavioral and neurocognitive changes in long-term chronic toxoplasmosis by reducing brain cysts and inflammation-related changes, providing a new idea for combination therapy and a basis for the association between schizophrenia and T. gondii infections or other diseases[76]. Valproic acid is a widely used antipsychotic drug, and trimethoprim-sulfamethoxazole tablets (TMP-SMZ) are commonly used as anti-T. gondii drugs. Using qPCR, Enshaeieh et al. found reduced copy numbers and the transcription level of the bradyzoite-specific protein BAG1, the parasite repetitive DNA fragment REP529, and the highly expressed tachyzoite-specific surface protein SAG1 in the brains of chronically infected mice treated with valproic acid. Additionally, histological analysis of the mouse brain showed that cyst development, lymphocyte perivascular infiltration, and glial nodules were greatly reduced after valproic acid treatment, which showed the same therapeutic level as that in the TMP-SMZ group[77]. Martynowicz demonstrated that guanabenz is an effective treatment for neuroinflammation and can significantly reverse the hyperactivity induced by latent toxoplasmosis in C57BL/6 mice. This supports the hypothesis that behavioral disorders in infected hosts may result from dopamine dysfunction or a dysregulation of the GABA pathway[78].
-
According to a cross-sectional study conducted by Sagud et al. (2018), for the first time, the ratio of serum triglycerides to high-density lipoprotein cholesterol (HDL-C) was found to be lower in patients with T. gondii antibodies than in seronegative patients. No association was observed between T. gondii seropositivity and serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), glucose levels, waist circumference, body mass index, or the MetS ratio. Of note, patients with schizophrenia were taking antipsychotic medications (clozapine, olanzapine, risperidone, and quetiapine) more frequently, which theoretically cause weight gain and metabolic abnormalities, suggesting that T. gondii infection affects lipid metabolism[79]. In 2022, a survey conducted in East China reached the same conclusion[80]. In terms of sex hormone metabolism, Borraz-Leon et al. suggested that chronic alterations associated with latent T. gondii infection that affect testosterone feedback regulation may also be risk factors for the development of schizophrenia[81]. Osman et al. conducted an untargeted metabolomic analysis using liquid chromatography-mass spectrometry to examine plasma metabolomics. They discovered that individuals with schizophrenia or toxoplasmosis had lower levels of xanthine and adenosine monophosphate and higher levels of α-hydroxyglutarate than the controls. These results suggest that purine catabolism is a common metabolic alteration in toxoplasmotic infections and schizophrenia[82]. Since vitamin D deficiency can increase the risk of various chronic mental diseases, Kankova et al. attempted to link vitamin D levels with toxoplasmosis and schizophrenia. However, their findings refute the theory that a decrease in vitamin D levels could be linked to toxoplasmosis[83].
The function of the metabolites investigated in these studies in the pathophysiology of T. gondii infection-related schizophrenia requires more research due to the complexity and heterogeneity of schizophrenia. Further elucidation regarding the underlying pathophysiological mechanisms will assist in clarifying the relationship between T. gondii and schizophrenia.
-
Human nerve cells infected with T. gondii can multiply and induce cyst formation, nerve cell death, changes in neural gene expression, and the release of various inflammatory mediators. These findings have been verified using BrainSpheres, a human 3D CNS model derived from human pluripotent stem cells, strengthening the validity of earlier research findings[84]. A better understanding of host-parasite interactions of T. gondii in human neurons and the effects of the parasite on neuronal function is limited by the need for a suitable in vitro model of human neurons[85]. The advent of cell reprogramming technology, in which somatic cells can be used to generate functional human neurons in vitro, either by using induced pluripotent stem cells (iPSCs) or by directly converting somatic cells into induced neurons, provides an opportunity to develop in vitro human neuronal culture systems. In addition to producing two-dimensional neuronal monolayer cultures that facilitate cellular and molecular mechanistic investigations, induced and iPSC-derived neurons can also be used to create 3D brain organoids, which will aid in disease modeling investigations of the neurological effects of T. gondii in the brain.
-
Genome-wide association studies (GWAS) have regularly linked polymorphisms in various major histocompatibility complex (MHC) regions in schizophrenia. In humans, the MHC region (known as the HLA system) is approximately 40,000 bp long and comprises more than five genes, many of which are involved in the immune system by encoding HLA proteins[86]. Prior research on the MHC region and schizophrenia, which demonstrated the association between schizophrenia and specific HLA class I and class II alleles, as well as the connection between several autoimmune disorders and schizophrenia, were confirmed by current GWAS investigations.
-
Lori et al. used the Psychiatric GWAS Consortium Polygenic Risk Score for Schizophrenia (SCZ-PRS) to assess T. gondii-positive and T. gondii-negative individuals, with schizophrenia diagnosis serving as an outcome variable[87]. SCZ-PRS assessment does not include MHC variants. Polygenic risk scores (PRS), which assess the genetic liability of several variants into a single individual score, have been used to capture the genetic effects of genome-wide markers that may not individually reach significance in GWAS. Markers in the first training samples were chosen at various p-value threshold levels, and the relevant alleles within each subject were weighted and summed to create scores for the independent replication samples. A significant association between polygenic scores and traits in the replicated samples implies the presence of genetic signals in the selected markers, establishing a common polygenic basis for both datasets. Summary statistics obtained from these datasets are beneficial for PRS analysis because of the polygenic characteristics of schizophrenia and the vast number of samples included in the Schizophrenia Psychiatric Genomics Consortium.
-
The C-terminus-truncated form of the disrupted-in-schizophrenia-1 (DISC1) protein is generated by a translocation associated with several major psychiatric disorders, including schizophrenia, depression, and bipolar disorder. To mimic a possible gene-environment interaction (G × E) model associated with T. gondii-related psychiatric disorders in mice exposed to T. gondii, Kannan et al. developed a novel mouse model that induced the expression of DISC1. They used a C-terminus-truncated form of the full-length protein as a dominant-negative molecular tool (DN-DISC1) to alter the expression of endogenous full-length DISC1 to elucidate the role of DISC1. In this gene-environment interaction model, T. gondii exposure caused DN-DISC1-expressing mice to exhibit sex-dependent prepulse suppression of acoustic startle[88]. This provides a new experimental system and scheme for future research.
-
In addition to uncovering novel therapeutic targets and diagnostic indicators, research on T. gondii infection and schizophrenia contributes to our understanding of the pathophysiology of Mental disease. It also offers a pertinent theoretical framework for forensic psychology, sociology, psychiatry, and other applicable fields. Many studies have demonstrated a strong association between T. gondii infection and schizophrenia, supported by serological and animal experimental data. Many researchers have attempted to explore the association between T. gondii infection and schizophrenia in terms of pathological anatomy, imaging, behavior, immune molecules, and genes. Their findings demonstrate that researchers need to continue studying this direction, especially the underlying mechanisms at the genetic and epigenetic levels. It is important to note that schizophrenia is a complex illness. While numerous studies have demonstrated the null hypothesis of a relationship, only a small percentage have refuted the theory that T. gondii infection causes schizophrenia. Future studies should pay more attention to related factors or confounders, such as the course of acute or chronic T. gondii infection, whether a patient is in the stable phase or experiencing an episode of schizophrenia during the statistical investigation, age, sex, drug use, and the type of subject used in animal experiments. In particular, researchers should pay attention to the different strains of T. gondii that caused the infection because the virulence and tendency of T. gondii may have important effects on the experimental results. For example, the type I strain is associated with mental illness[5,6]. Furthermore, the dominant strain is dispersed throughout many regions; for example, the dominant strain in Chinese1 was found in China[7].
Research Progress on the Association between Schizophrenia and Toxoplasma gondii Infection
doi: 10.3967/bes2024.071
- Received Date: 2023-09-29
- Accepted Date: 2024-03-11
-
Key words:
- Toxoplasma gondii /
- Schizophrenia /
- Neurotransmitters /
- Neuroinflammation /
- Immunity /
- Epigenetics
Abstract: Abstract: Toxoplasma gondii (T. gondii or Tg), is an obligatory intracellular parasite with humans as its intermediate hosts. In recent years, significant correlations between T. gondii infection and schizophrenia have been reported, including the possible mediating mechanisms. Currently, mechanisms and hypotheses focus on central neurotransmitters, immunity, neuroinflammation, and epigenetics; however, the exact underlying mechanisms remain unclear. In this article, we review the studies related to T. gondii infection and schizophrenia, particularly the latest research progress. Research on dopamine (DA) and other neurotransmitters, the blood-brain barrier, inflammatory factors, disease heterogeneity, and other confounders is also discussed. In addition, we also summarized the results of some new epidemiological investigations.
The authors declare that they have no competing interests.&These authors contributed equally to this work.
| Citation: | Yiting Zhu, Xiaohui Yang, Miaoru Chen, Yu Hu, Yunfeng Chang, Xiang Wu. Research Progress on the Association between Schizophrenia and Toxoplasma gondii Infection[J]. Biomedical and Environmental Sciences, 2024, 37(6): 647-660. doi: 10.3967/bes2024.071 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: