-
The intestine is the longest section in the digestive system and is associated with a series of intestinal diseases, such as intestinal obstruction, chronic inflammatory bowel disease (IBD), and colorectal cancer (CRC)[1-3]. Each part of the intestinal tract has a unique tissue structure and physiological function. Simulating the structure, function, pathogenesis, unique tissue structure, and the physiological functions of the intestinal tract in vitro is challenging. Previously, the biological research models were traditionally monolayer cell culture models and animal models. The monolayer cell model can only grow in a monolayer in the incubator and cannot simulate the complex living environment of normal cells. Compared with cell models, animal models can simulate the living environment of the body. However, their clinical applications are limited by their divergence from humans, long cultivation periods, and high costs.
In recent years, organoids, organ-specific cell collections formed by three-dimensional (3D) culture of stem cells and their derivatives in the microenvironment required to form specific organs, have been successfully constructed. Organoids have a physiological structure similar to that of real organs, and can partially mimic the physiological functions of the source tissue[4,5]. During organoid formation, two key events in the development of real organs can be simulated: cell sorting and spatially restricted lineage commitment. As a result, organoids contain various cell types, empowering them to simulate the functions of real organs better[4]. Since Clevers’s laboratory used small intestinal stem cells to generate organoids with small intestinal villi and crypts for the first time in 2009[6], organoids have been successfully constructed that simulate tissues, including the heart[7,8], brain[9,10], kidney[11], gastrointestinal[12,13], retina[14,15], lung[16,17], blood vessels[18,19], esophagus[20], breast[21], liver[22,23], and endometrium have been generated[24]. The rapid development of tissue organoid culture technology was rated as one of the top-10 advances and breakthroughs of the year by Science and Nature Methods journals in 2013 and 2017, respectively. These organoids not only maintain the original organ’s stable phenotype and genetic characteristics, but can also be cultured in vitro for a long time and can be cryopreserved. Compared to in vitro animal models and traditional two-dimensional (2D) cell culture models, organoid technology has apparent advantages in basic and preclinical studies of tumors and can maintain individual heterogeneity while retaining the stability of tissue genomes[25]. Additionally, the organoid model is more feasible and less ethically risky in terms of high-throughput research and is currently the best model for preclinical verification trials[26].
As one of the earliest organoid models, the research on intestinal organoids has progressed rapidly in recent years. Currently, intestinal organoids are primarily derived from LGR5+ adult stem cells and pluripotent stem cells (PSCs)[27]. Since their establishment, intestinal organoids have been widely used in research on various intestinal diseases, such as IBD and intestinal cancer. This review systematically discusses the construction and application of intestinal organoids, describes the similarities and differences between human and mouse intestinal organoid media, summarizes the challenges faced by intestinal organoids, and discusses their ethical issues faced by intestinal organoids. Overall, this review provides a systematic integration of knowledge on intestinal organoids and presents a complete picture of the field, which can help researchers to understand the latest advances and key findings in the field quickly.
-
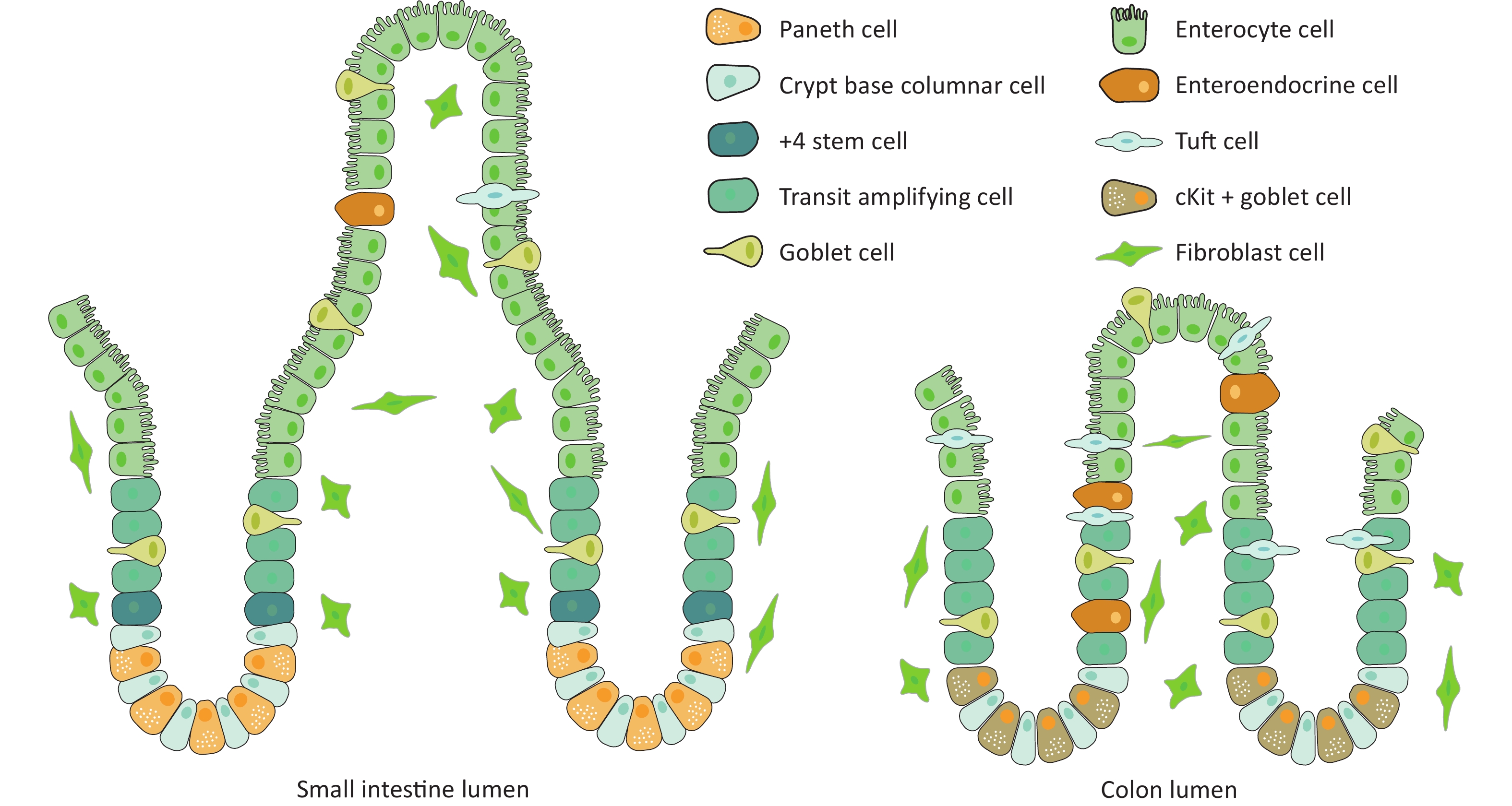
In recent years, intestinal stem cells have become the research focus for various intestinal diseases, such as intestinal injury, regeneration, and intestinal cancer. LGR5 is recognized as a marker of intestinal stem cells and plays a vital role in maintaining normal intestinal epithelial structure. The intestinal epithelium has the highest renewal rate of all adult mammal organs[28]. As shown in Figure 1, cryptvilli are the basic unit of the intestinal epithelium, with multiple crypts, which only exist in the intestine, present on the basal side[29]. Intestinal stem cells are located in the basal area of the crypts. These cells divide and proliferate to produce transition-amplifying (TA) cells[30]. TA cells migrate upwards along the longitudinal axis of the crypt to the upper part of the crypt, where they continue to proliferate and differentiate to form a crypt structure. After continuous migration and differentiation from the crypts to the villi, mature and fully differentiated intestinal functional cells are formed, including epithelial absorptive cells, Paneth cells, goblet cells, enteroendocrine cells, and tuft cells[31], which form the villi of the small intestine or the top of the crypts of the large intestine. After maturation, the Paneth cells move down to the bottom of the crypt, and other cells move up to the top of the villi along the crypt–villus axis, where they die and fall off into the intestinal lumen. The epithelium of the large intestine does not have Paneth cells, but has c-Kit-positive secreting cells that perform the same function. The remaining cell composition is the same as that of the small intestine. Dead cells are shed from the top of the crypts[32].
Current literature proposes two views regarding the specific location of intestinal stem cells in crypts. First, intestinal stem cells (crypt base columnar [CBC] cells) are located in the stem cell area at the bottom of the crypts and are adjacent with the Paneth cells. The local microenvironment of this area is unique, and cells differentiate when they migrate away from this environment[33]. Secondly, small intestinal stem cells are positioned in the crypt wall above the last Paneth cell[34]. This view holds that small intestinal stem cells are located at position +4 in the crypt region of the small intestinal epithelial tissue, based on the retention of DNA markers during cell division[35]. This is consistent with the hypothesis proposed by Cairns[36]. that adult stem cells contain immortalized DNA strands. Stem cells at +4 positions can be labeled with BMI1, HOPX, LRIG1, etc.[37-39], but the specificity of these markers remains controversial[40]. These +4 stem cells are believed to be a type of stem cell population that is in a relatively quiescent state and is somewhat tolerant to damage; however, their specific functions remain disputed. While no clear evidence is available supporting either of these two claim, the former was confirmed upon the discovery of the small intestinal stem cell marker LGR5. A classical study conducted by Clevers et al. found that CBC cells specifically express LGR5, and by using in vivo lineage tracing technology, they showed that various mature cells throughout the crypt are derived from these LGR5+-cells, including Paneth, goblet, and enteroendocrine cells[41], confirming that LGR5+-epithelial cells are actually intestinal stem cells. LGR5 is a transmembrane protein that can enhance the WNT–β-catenin signaling pathway when combined with the ligand R-spondin1[42]. LGR5+-stem cells and Paneth cells were staggered at the bottom of the crypt. CD24+-Paneth cells can express epidermal growth factor (EGF), WNT agonist (WNT3A), transforming growth factor (TGF-α), and the NOTCH ligand DLL4, which can promote the proliferation and differentiation of stem cells[43]. These studies largely unified the theories of intestinal stem cells, confirming that LGR5 is a surface molecule specific for intestinal stem cells. In addition to CBC cells, +4 cells have been described as another candidate stem cell population, and the term “+4” refers to their position from the progenitor location[44].
-
First, this review focuses on the composition of intestinal organoids. Intestinal stem cells can regenerate the entire crypt–villus structure and are capable of self-renewal and differentiation into six different types of intestinal cells. Absorptive cells account for 80% of all epithelial cells, and their primary function is to digest food and absorb nutrients[45]. Goblet cells are present in both intestinal crypts and villi, accounting for 4%–12% of the cells in these structures. The central role of goblet cells is to maintain the balance of intestinal flora and prevent microbial damage to epithelial cells[46]. Paneth cells are long-lived (2–3 months) differentiated cells compared to other cells that account for 3%–8% of all intestinal epithelial cells. The main function of Paneth cells is to produce antimicrobial peptides that disinfect the crypt cavity[46]. M cells are located in the intestinal crypts and primarily regulate immune responses through the transcytosis of antigens in the lumen. M cells account for less than 1% of epithelial cells[46]. Enteroendocrine cells are distributed in the crypt-villous shaft structure and regulate metabolism, insulin secretion, food intake, and nutrient assimilation by releasing a variety of hormones; however, they account for less than 1% of intestinal epithelial cells[45].
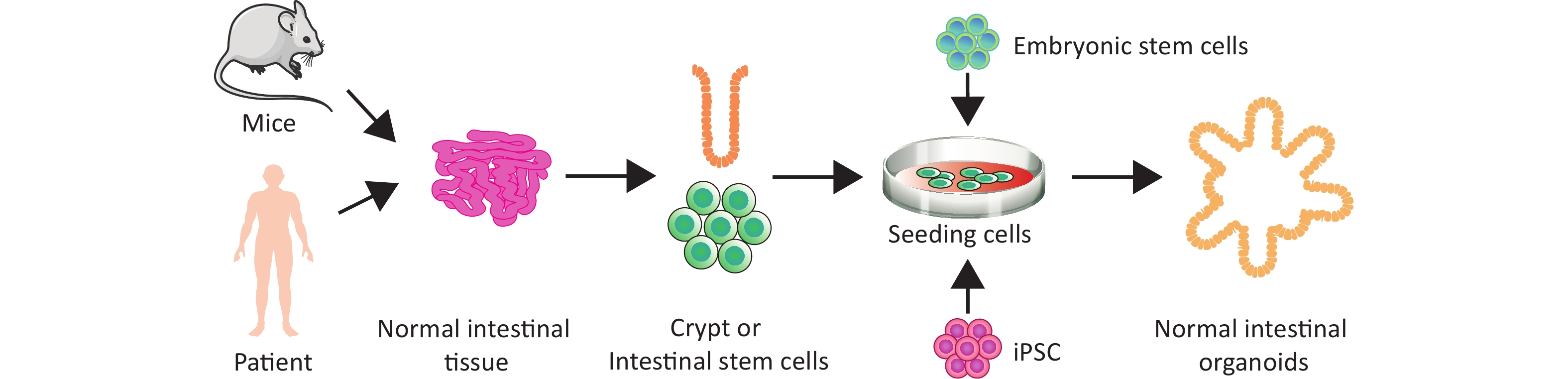
Next, we briefly discuss the history of intestinal organoids. Scientists first proposed a human cell colony culture method in 1975[47], In 2009, a significant breakthrough was made by Sato et al. in the successful induction of LGR5+-intestinal stem cells to form crypt villi[6]. In 2011, Li et al. adopted a two-step differentiation procedure for differentiating embryonic stem cells (ESCs) into endoderm tissue and found that the endoderm derived from ESCs could differentiate into intestinal organoids, which could be used for transplantation and repair of damaged bowel tissue in vivo[48]. Another group demonstrated for the first time that human induced PSCs (hiPSCs) could be efficiently differentiated into a structure very similar to that of fetal intestine in vitro in 2011[49].
Finally, we discuss the classification of intestinal organoids. Intestinal organoids can be divided into adult stem cell organoids, embryonic stem cell organoids, and induced PSC (iPSC) organoids, based on stem cell origin (Figure 2). Adult mouse intestinal stem cell organoids exhibit budding structures, whereas mouse embryonic intestinal stem cell organoids are mostly spherical. This tendency to form spheres gradually weakens with maturity, and mouse intestinal stem cell organoids no longer form spherical structures by 15 days after birth[50]. Notably, exogenous Wnt signaling promotes the transformation of spherical organoids formed by embryonic intestinal stem cells into budding structures[51]. Furthermore, LGR5 is weakly expressed in the early stages of embryonic intestinal stem cell organoid development, and is limited to the epithelial area after long-term culture[52]. Embryonic intestinal stem cell organoids grown under the renal capsule resemble intact intestinal tissue in vivo, exhibiting small intestinal villi, lamina propria, and intact crypt architecture[53]. According to the various components of the initial culture system, intestinal organoids can be divided into primary epithelial cell organoids, epithelial–mesenchymal tissue organoids, and PSC organoids[54]. One group cultured mouse small intestine or colon tissue fragments containing crypt stem cells and epithelial basement membrane fibroblasts at the air–liquid interface of a collagen matrix to obtain epithelial–mesenchymal organoids that could survive for 30 days to more than 350 days[55].
-
The basic principle behind culturing intestinal organoids is mimicking the microenvironment of intestinal stem cells. The stem cell niche at the bottom of the crypt is the environment in which intestinal stem cells grow, and activation of WNT and NOTCH signaling and inhibition of BMP signaling are necessary to maintain their proliferation. A growing number of studies have shown that IECs (including those of the small intestine, colon, and fetal intestine) from mice, humans, and other species can self-organize to form unique 3D tissue-like structures when a sufficient supply of protein factors and extracellular matrix scaffolds are available[56]. The culture conditions were identical for both primary tissues and stem cell-derived organoids, and the basal medium contained DMEM/F12, N2, B27, nicotinamide, and N-acetylcysteine. Moreover, additional growth factors, such as EGF, noggin, and gastrin, need to be added to the basal medium to regulate the growth of the intestinal mucosa and the proliferation of IECs. Additionally, the presence of WNT3a is critical for regulating intestinal stem cell self-renewal, proliferation, and differentiation, because LGR5 expression in intestinal crypt stem cells is dependent on the canonical WNT signaling pathway[49]. Currently, the most widely used extracellular matrix is Matrigel or basement membrane extract purified from Engelbreth–Holm–Swarm (EHS) mouse sarcoma, and its main components are laminin, type IV collagen, and cohesin. The composition of the culture medium required for normal human colon organoid culture is different from that needed for mouse intestinal organoid culture medium: human colon organoid culture medium requires additional supplementation with gastrin, nicotinamide, SB202190, and A83-01[57,58]. SB202190 is a p38 inhibitor that blocks the negative feedback of p38 mitogen-activated protein kinase on EGF. A83-01 is a TGF-β inhibitor that inhibits the antiproliferative effects of TGF-β. Ablation of WNT3a, nicotinamide, and SB202190 completes the differentiation of human colonic organoids into secretory and absorptive lineages to generate various types of mature intestinal epithelial cells[59]. Intestinal organoids derived from PSCs are treated with activin[60-63]. After endoderm induction, PSCs differentiate into midgut and hindgut tissues and germinate from a monolayer of epithelial cells attached to tissue culture dishes. They are then cultured in a matrix containing intestinal growth factors, thus forming various major intestinal cell types through proliferation and expansion. The detailed composition table of the intestinal organoid culture medium is presented in Table 1.
Type of organization Species Cytokines and small molecules Culture medium References Intestine Human Wnt 3a, EGF, R-spondin 1, Noggin, A8301, SB202190, FGF, Nicotineamide, N-Acetylcystein, Y-27632 CHIR-99021, PGE2, IL-22, DATP, Gastrin1, B27, Matrigel DMEM/F12 [58,133-135] Mice Wnt 3a, EGF, R-spondin 1, Noggin, A8301, SB202190, B27,GlutaMAX, Nicotineamide, Y-27632, Matrigel DMEM/F12 [6,49,136] Colon Human Wnt 3a, EGF, R-spondin 1, Noggin, A8301, SB202190, B27, N2, Gastrin1, Nicotineamide, HGF, Y-27632, Matrigel DMEM/F12 [137-139] Mice Wnt 3a, EGF, R-spondin 1, Noggin, A8301, Y-27632, B27, PGE2, Matrigel DMEM/F12 [60,140] Colorectal
cancer tissueHuman Wnt 3a, EGF, R-spondin 1, Noggin, A8301, SB202190, B27, N2, FGF, FGF-10, Nicotineamide, PGE2, HEPES, Glutamax, N-Acetylcysteine,Penicillin-Streptomycin IGF, Matrigel DMEM/F12 [13,134,141] Table 1. Composition of intestinal organoid media
Although Matrigel, the extracellular matrix most widely used in organoid culture, is commercially produced at an acceptable price, it has some disadvantages. Matrigel has complex components, including more than 1,000 proteins, and batch effects cannot be avoided[64], leading to poor repeatability of experimental results. To alleviate these issues, decellularized extracellular matrices, synthetic hydrogels, and engineered extracellular matrix proteins have emerged as technological advances that can replace Matrigel in intestinal organoid culture, and Matrigel-free organoid culture technology has been developed[65-67]. Lactide-co-glycolide (PLGA) is a synthetic biodegradable polymer with adequate mechanical strength and biosafety. Shaffiey et al. used matrigel-coated PLGA tubular scaffolds as carriers for intestinal-like organs, which were implanted into the rectum of dogs. Through transplantation, intestinal-like organs promote rectal mucosal regeneration[68]. Synthetic hydrogels based on polyethylene glycol have been used in intestinal-like organoid cultures. Polyethylene glycol a widely used synthetic polymer in life sciences. It is a hydrophilic polymer that is highly stable and biocompatible[69]. Hydrogel-based 3D intestinal organoid cultures restore the internal environment of the intestinal epithelium. Wang et al. prepared a collagen scaffold with micropatterned cylindrical protrusions and depressions, by which intestinal epithelial cells inoculated on the scaffold could be polarized to reconstruct the crypt–villus structure[70]. In addition, by combining an organoid microfluidic chip with a specific hydrogel, the monolayer culture of intestinal organoids could be successfully realized, allowing investigations of the effects of exogenous reagents and microorganisms on the crypt–villus structure[71]. In 2019, a group of decellularized porcine small intestine tissues was used to design an acellular extracellular matrix in which human intestinal stem cells could grow and eventually successfully form human intestinal organoids[72]. Another group used molecular engineering technology to recombine and synthesize an engineered extracellular matrix, hyaluronic acid elastin-like protein, in which intestinal epithelial organoids could grow, differentiate, and be passaged[73]. This engineered extracellular matrix has good reproducibility and is a promising extracellular matrix substitute for current organoids. Bioactive ceramic materials can modulate the mechanical properties of hydrogels and the released ions can regulate stem cell functions through multiple signaling pathways. A research team used bioceramic materials to improve the hydrogel system and developed a composite hydrogel consisting of silicate-bioactive ceramic calcium silicate nanowires and gelatin methacrylate, which was optimized by adding a certain proportion of matrigel, and could be used as a matrix material for the cultivation of intestinal and liver organoids. The composite hydrogel promoted the growth and functionalization of organoids, and the bioactive ions released by the silicate-bioactive ceramic calcium silicate nanowires were shown to be essential for maintaining stem cell homeostasis and self-renewal[74].
-
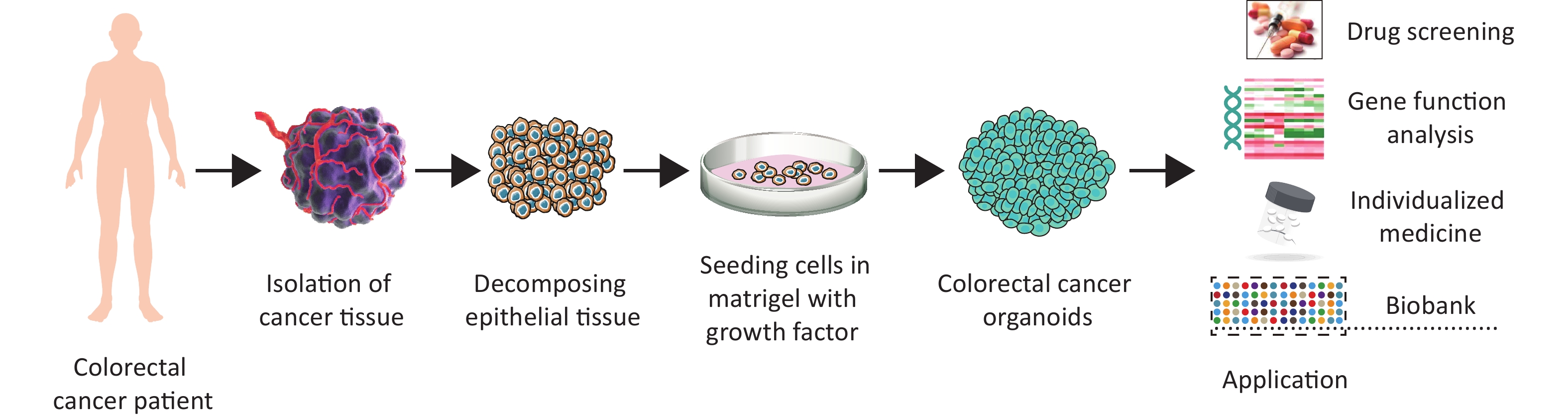
Organoid cultures can be derived not only from normal tissues, but also from tumor tissues, to generate tumor organoids (Figure 3). Cultivation of intestinal tumor organoids has received much attention, and cultivation methods and application models remain a research focus. Similar to normal intestinal organoids, tumor organoids are derived from tumor cells digested from tumor tissues and cultured in Matrigel. However, some studies have shown that cultured tumor organoids do not grow as fast as their corresponding normal organoids[75,76]. One possible reason for this is that tumor organoids have a higher rate of mitotic failure, which leads to tumor cell death. Therefore, a selective medium suitable for tumor-organoid culture needs to be developed. Specific growth factors may not be required, because of the abnormal activation of prosurvival signaling pathways in tumor tissues. For example, in most CRCs, WNT signaling is specifically activated; therefore, in the culture of these intestinal cancer organoids, WNT and R-spondin1 factors do not need to be added whereas they are essential for culturing normal tissue organoids[58]. Similarly, most colorectal adenocarcinoma organoids have persistent activating mutations in various oncogenes (such as those in the WNT pathway) and can therefore survive in a medium lacking WNT or R-spondin1[58]. Nutlin-3 stabilizes p53 by inhibiting the E3 ubiquitin ligase mouse double-minute 2 (MDM2), and can be used to generate p53-mutant colon cancer organoids[76]. Similarly, in CRC with mutations in the EGF receptor (EGFR) pathway, EGF does not need to be present in the culture medium[77]. If a p38 mutation is present, no addition of the p38 inhibitor SB202190 is required to maintain growth[78]. Intestinal cancer organoids with various mutations can be obtained by combining different growth factors and establishing different culture environments. Organoids cultivated using this method retain the histopathological characteristics and gene stability of the original tumor, making them useful for basic research and translational medicine.
-
Functional analyses of intestinal organoids have been performed in various studies. 3D-like intestinal organoids formed through the self-organization of cell clusters recapitulate important features of their native tissues in vivo, including highly folded epithelial structures composed of crypts and villi, which are similar to native intestinal epithelium[56]. Once embedded in Matrigel, cell clusters begin to self-organize, with the luminal surface of the epithelium facing the center of the organoid and the basal side in contact with Matrigel and the surrounding medium, gradually forming intestinal organoids. Intestinal organoids not only contain almost all cell types in native intestinal tissue in vivo, but also exhibit functions similar to these cells in vivo, such as mucus production, absorption, and secretion[53]. Additionally, intestinal organoids mimic epithelial regeneration in vivo. As apoptotic cells are gradually shed into the organoid lumen, LGR5+-cells in the crypts differentiate to form new cells that replenish these epithelial cells. Transport of water and electrolytes across the intestinal barrier is an essential function of enterocytes. One study demonstrated that intestinal organoids lacking the apical transporters SGLT-1 or PEPT1 could inhibit the transport of D-glucose, D-fructose, and peptides across the epithelial membrane, confirming their role in nutrient absorption[79].
-
The development of organoid technology has promoted in-depth studies on disease models, drug discovery, pathogenic microorganism–host interactions, toxicology, gene editing, precision medicine, and regenerative medicine[80,81]. The applications of intestinal organoids in various fields are summarized in Table 2 and are described below.
Type of organoids Species Application References Intestinal organoids Mice, Rat As a material for intestinal transplantation [51,82,83] Mice Genotoxicity and cytotoxicity research [91] Human As a research model for cystic fibrosis disease [94,95] Intestinal tumor organoids Human Drug resistance [75] Human Drug screening [78] Mice, Human Models for studying cancer formation, development and proliferation [76-78,106,109] Human Explored the effectiveness of drug combination [113] Human Recovery of drug sensitivity in drug-resistant cancer organoids [114] Human Predicting the effect of targeted drugs or chemotherapy [129] Human Guide the clinical formulation of precise treatment strategies [130] Table 2. Applications of intestinal organoids
-
Organ transplantation is currently the best treatment for many diseases. However, organ shortages and rejection after allogeneic transplantation remain challenges. The establishment of organoids can solve this problem. Using a patient’s stem cells for in vitro 3D culture to construct an autologous organoid from the original organ for transplantation can prevent immune rejection. Transplantation of many different types of organoids, including kidney, intestinal, and liver organoids, has been reported. In 2013, one group transplanted intestinal organoids into mice and found that these organoids could promote colon repair after injury. This confirmed that intestinal organoids can be used as a therapeutic option to replace the original colon after resection of injuries or lesions[51,82] and suggests that organoid transplantation is a possible approach for addressing the organ shortage problem.
The small intestine is the main organ for nutrient absorption, and massive resection of the small intestine can lead to short bowel syndrome (SBS) and severe malabsorption, characterized by diarrhea, dehydration, and weight loss. Patients often require total parenteral nutrition to compensate for nutritional deficiencies. However, when these strategies fail or when severe complications of parenteral nutrition occur, intestinal transplantation is the last treatment option for SBS. Moreover, in 2021, a study reported that transplanting small intestinal organoids into rats ameliorated the severity of SBS[83]. Owing to the similarities in the subepithelial structures of the small intestine and colon, ileal organoids were used to replace the native colonic epithelium, resulting in the generation of a highly vascularized and functional enterogenic colon, forming an intact small-bowel architecture that included vasculature and innervation, villous structures, and lacteal ducts. Transplantation of this enterogenic colon into SBS rats restored its absorptive function and significantly reduced intestinal failure while affecting intestinal flora remodeling. This study confirmed the feasibility of using intestinal organoids for gut regeneration. This method also has therapeutic potential in animals, providing a new strategy for the regenerative transplantation of other luminal organs. Therefore, the use of intestinal organoids derived from patient stem cells as transplant materials should be investigated in future.
The in vitro growth of organs has long been the goal of scientists in this field, and organoids are the first step. Cutting-edge scientific research is necessary to construct complex artificial organs that are closer to the advanced structures of the internal organs. In this context, researchers have employed 3D-printing technology or decellularized skeleton methods to construct artificial intestines[72,84]. By providing organoids with a culture environment close to the spatial structure of in vivo tissues, researchers have overcome the limitations of traditional Petri dish culture and have made breakthroughs in producing artificial intestines with the macroscopic anatomical structure of the intestines, in which the microbial environment of the intestines can be restored[85,86]. Further attention should be focused on the construction of advanced and complex artificial organs by integrating multiple tissue-engineering strategies and the use of organoids.
-
The digestion and absorption of food in humans is an extremely complicated process. The nutrients needed for body growth are transformed through food intake. Research on the digestion and absorption characteristics of food through in vivo experiments and animal models has expanded; however, current methods have disadvantages, such as high cost, complicated operations, and irreversibility. In contrast, in vitro models have the advantages of simplicity and ease of use. With the ongoing development of organoid technology, intestinal organoids have been developed that contain various cell types and that replicate some functions of intestinal organs, and their use has become a hotspot in nutrition research, where they are increasingly used to study the nutritional properties of different substances.
One group studied the effects of different dietary protein sources on the function of epithelial cells using organoid-derived monolayers of intestinal epithelial cells[87]. They found that proteins from different dietary sources were associated with unique biological expression processes in epithelial cells, suggesting that intestinal organoid models can be used to assess the complex interplay between dietary components and the intestinal epithelium[87]. Sittipo et al. used the intestinal organoid model to study the effect of vitamin D3 on intestinal epithelial cell differentiation and stem cell viability[88]. They showed that vitamin D3 promotes the differentiation of intestinal epithelial cells and induces apoptosis, and provided a basis for preventing rectal cancer with vitamin D3 supplementation. One group demonstrated that short-chain fatty acids, which are important for the proliferation and renewal of intestinal epithelial cells, promote the development of intestinal organoids[89]. Furthermore, Nesrin et al. used organoid technology to culture intestinal stem cells from persons who were healthy, lean, or had morbid obesity. The authors demonstrated that the increased glucose absorption and gluconeogenesis in the diet of people with obesity was due to the expression of glucose transporters, while expression of rate-limiting enzymes related to intestinal carbohydrate metabolism and gluconeogenesis enzymes was significantly higher than that in lean people with low glucose absorption and lower levels of gluconeogenesis enzymes[90]. Seiwert et al. evaluated the genotoxicity and cytotoxicity of heme iron in mouse intestinal organoids and revealed that heme iron promotes the formation of reactive oxygen species, thereby inducing DNA damage and reducing cell viability[91].
Currently, most researchers use intestinal organoids only as a technical tool for nutritional evaluation. Some researchers have begun to use intestinal organoids to study the mechanisms of nutrient absorption and have introduced intestinal microorganisms for co-cultivation. The emerging technology of intestinal organoids requires further exploration for its full utilization.
-
Intestinal organoid models can be used to study intestinal diseases. Cystic fibrosis (CF) is a rare disease characterized by mutations in the chloride channel of the CF transmembrane conductance regulator (CFTR) expressed in epithelial cells[92,93]. Simulating the numerous mutant phenotypes of CFTR using conventional cell lines and animal models is challenging, and effective drugs and clinical therapies available for CF are lacking. Intestinal organoids can be used as models to study such diseases. In 2013, Dekkers et al. developed a forskolin-induced swelling method using small intestinal organoids for the functional detection of CF[94]. We found that forskolin activated CFTR in organoids, which in turn caused organoid swelling. Swelling was reduced in samples lacking CFTR or expressing mutated CFTR. Another interesting study found that the phenotype of CFTR could be rescued when the defective allele of CFTR was repaired using the CRISPR/CAS9 genome editing system in the intestinal stem cells of patients with CF, resulting in normal expression of the repaired allele[95]. If such organoids were generated and reintroduced into the source patient, these repaired organoids may partially cure CF, opening a new avenue for gene therapy. Multiple intestinal atresia (MIA) is a rare disease characterized by intestinal obstruction, which may be caused by a repeat-sequence mutation in TTC7A. Intestinal organoids generated from patients with MIA exhibit reversed polarity of the epithelial apical and basement membranes, making them difficult to cultivate in the long term. Common approaches for normalizing polarity include TTC7A overexpression and pharmacological Rho-associated protein kinase inhibition[96]. Intestinal organoids can also be used to study IBD-related pathogenesis. Studies have found that the occurrence of IBD is related to local hypoxia, and that expression of hypoxia-inducible factor-2α in the intestinal epithelial cells of colitis model mice and of IBD patients was increased. Similarly, under hypoxic conditions, the expression of TNF in PSC intestinal organoids increased, suggesting that stem cell organoids are consistent with the results of human and animal studies[54]. Furthermore, IBD can lead to intestinal fibrosis, PSC intestinal organoids can be used to simulate the fibrotic response induced by transforming growth factor-β (TGF-β) agonists, and the addition of anti-fibrotic drugs can reverse this fibrotic-like change[97].
The intestinal tract is a significant site of infectious diseases caused by viral, bacterial, and parasitic infections. Intestinal organoids share many properties with the human intestinal epithelium and are ideal platforms for studying the pathogenesis and treatments of infectious diseases. In 2019, a study showed that norovirus can replicate in intestinal organoids, which is helpful for clinical work to determine whether the virus in patients is infectious[98]. In 2020, Pradhan et al. established a small intestinal organoid model infected with Shiga toxin to observe the biological response[99]. They revealed that Shiga toxin could induce necrosis and apoptosis of intestinal epithelial and mesenchymal cells. A previous study found that microinjection of Salmonella typhimurium into small intestinal organoids could invade the epithelial barrier and alter the transcriptional profile of the organoids[100]. Another group used intestinal organoids to establish cholera infection models and found that exposing human intestinal organoids to cholera toxin could inhibit the activity of the sodium–hydrogen antiporter 3, resulting in dilatation of the cyst cavity[101].
-
CRC is a common gastrointestinal malignancy[102-105]. Currently, surgery is the primary clinical treatment for CRC, and radiotherapy and chemotherapy are the immediate treatments used for some advanced CRCs, albeit with poor effect. Currently, two main preclinical models are used to study colorectal tumors: the CRC cell line and patient-derived tumor xenograft models. These two models have many advantages for exploring the mechanism of CRC; however, some unavoidable problems and defects remain. The organoid model not only induces the development and differentiation of intestinal stem cells and simulates intestinal diseases, but also plays a role in CRC drug screening and tumor signaling pathway research. Below, we review the application of intestinal organoids in CRC research (Figure 3).
-
CRC is caused by the accumulation of gene mutations in normal colonic epithelial cells[2]. However, the pathogenic mechanism of CRC is not fully understood, and various signaling pathways, such as those involving WNT, NOTCH, and AKT, are known to participate in this process. Compared to cancer cell lines, colorectal tumor organoids can mimic the pathology of real tumors as well as mutations in different signaling pathways. One group established an orthotopic transplantation model of the large intestinal mucosa by injecting CRISPR-CAS9 gene-edited mouse colon organoids with APC, P53, and KRAS mutations[106]. Fifteen tumors grew in the 10 mice used in this model after 6 weeks, and three mice developed liver metastases after 12 weeks. Next, human CRC-organoids were injected into the submucosa of mice, and eight of 26 mice developed liver metastases that maintained the morphology and differentiation features of the primary tumors. Although tumor cell lines can form tumors after injection, these formed tumors do not metastasize and do not demonstrate the histological features of the primary tumor. Drost et al. edited four genes commonly mutated in CRC (APC, p53, KRAS, and SMAD4), using CRISPR/CAS9 technology and found that organoids with mutations in all four genes could survive independently of exogenous growth factors. In addition, they demonstrated that the loss of APC and p53 is a key factor causing CRC[76]. After orthotopic transplantation of these mutated organoids into immunodeficient mice, organoids with only APC, KRAS, and TP53 mutations, but without SMAD4 mutations, formed adenomas, while organoids carrying mutations in all four genes successfully formed adenocarcinomas after transplantation. One group induced enteritis in mice to form a microenvironment to aid tumor settlement and then injected a gene-edited organoid suspension into the mouse intestine via enema, successfully establishing an orthotopic tumorigenesis model[107]. Tumors formed within 4–9 weeks, and liver metastases appeared by 21 weeks. To simulate colorectal carcinogenesis, Matano et al. mutated the tumor suppressor genes APC, SMAD4, and TP53 as well as the oncogenes KRAS and PIK3CA in normal human intestinal epithelial-derived organoids using CRISPR-CAS9 methodology to form tumors after transplantation into mice, and revealed that these genetic mutations underlie adenoma-to-adenocarcinoma progression[108]. One group cultivated an adenoma organoid biobank from patients with colon adenomas to demonstrate the pathological processes involved in the occurrence and development of colorectal tumors[78]. In addition, another group constructed a CRC-organoid library, and revealed a gradual loss of the requirement for niche-related factors during tumorigenesis as well as proliferation in the absence of exogenous EGF[77]. Similarly, the introduction of the BRAF V600E mutation in normal colon organoids allowed for the construction of a serrated colon carcinoma model, which was used to demonstrate that TGF-β signaling promoted the transformation of serrated colon carcinomas into the mesenchymal colon carcinoma subtype that is associated with a worse prognosis[109]. Furthermore, the combination of organoid orthotopic transplantation animal models with gene-editing technology markedly reduced the time required for model establishment, while maintaining the histological characteristics of the primary tumor and tumor microenvironment in the model. Based on these advantages, this method is expected to become a mainstream model for in vivo tumor research in future.
-
Organoids have a wide range of applications in the development of anticancer drugs. Researchers have established a biobank of related tumor organoids to study tumor heterogeneity using gene sequencing, thereby allowing high-throughput drug screening techniques for identification of potential therapeutic targets and drugs. Therefore, the development of organoid technology could help researchers screen drugs that explicitly target cancer cells and minimize drug toxicity. One study demonstrated a strong link between gene mutations and drug sensitivity in organoids, using a CRC-organoid sample bank for drug screening[78]. Inhibitor of WNT production-2, a small-molecule inhibitor of porcupine O-acyltransferase, was found to inhibit the growth of organoids with an RNF43 mutation, providing a new target for the treatment of colon cancer[78]. Schütte et al. explored the correlation between the sensitivity of CRC therapeutic drugs targeting EGFR and biomolecular markers, using 19 pairs of CRC-organoid models and traditional in vitro biomarkers. Nine pairs of models were observed to have the same sensitivity to afatinib, and 12 pairs of models had the same sensitivity to sapitinib, providing a basis for the application of organoid models in drug screening[110]. Another group used the genetic diversity of primary tumor-organoid banks to study the drug-resistance mechanism of KRAS mutations in CRC in vitro, and found that KRAS mutations were strongly associated with EGRF inhibitors[75]. Kondo et al. used patient-derived organoids to conduct screening tests for 2,427 drugs and found that CRC-organoids showed different sensitivities to different drugs[111]. Xinaris et al. conducted a sensitivity test on colon cancer organoids for 85 chemotherapeutic and targeted drugs. The authors indicated that colon cancer cells with loss-of-function mutations in TP53 were resistant to MDM2 inhibitors; KRAS-mutant cells were insensitive to EGFR inhibitors; and RNF43-mutant colon cancer organoids had marked sensitivity to WNT-secretion inhibitors, indicating a new treatment for RNF43 mutant colon cancer[112]. Pauli et al. explored the effectiveness of drug combinations using organoids based on gene mutation data from patients with colon cancer, and found that the combination of afatinib and vorinostat markedly inhibited tumor growth in mice transplanted with patient-derived APC-mutated tumor organoids[113]. Shen et al. used organoids derived from patients with CRC to conduct drug-screening experiments, and successfully screened a Kruppel-like factor 5 inhibitor, ML264, which could restore the sensitivity of drug-resistant CRC-organoids to oxaliplatin[114]. Taken together, these results demonstrated the potential of organoids for drug screening. As preclinical models, organoids accelerate the development of new drugs and reduce costs. CRC-organoid models can be used to test the effectiveness of drugs in clinical trials and to advance the development of new drugs.
Intestinal organoids also play an important role in high-throughput drug screening. Lukonin et al. used intestinal organoids for high-throughput drug screening to characterize intestinal organoid phenotypic profiles and to explore the regulatory effects of RXR receptor-inhibitory drugs on intestinal organoid proliferation and differentiation. For the first time, they demonstrated a functional gene interaction profile that regulates organoid development and self-organization[115]. Norkin et al. developed an organoid-based high-throughput screening system, “TORNADO-seq”, for the identification of small molecules capable of inducing differentiation of intestinal normal and cancer cells[116]. Luo et al. established a large-scale library of patient-derived high-risk colorectal adenoma-like organoid samples, performed a series of high-throughput, high-intensity, high-risk Colorectal Adenoma drug screening, and successfully validated the effectiveness of the screened compounds in subsequent samples[117]. Lin et al. combined organoid technology with high-throughput CRISPR genetic screening to create a CRISPR-screening platform, spanning the full range of transcription factors, in human intestinal organoids to identify the transcription factors that regulate human enteroendocrine cells differentiation[118]. Snipper et al. screened microtubule-targeting agents (MTAs) from 414 anticancer drugs using CRC patient-derived organoids (PDOs) and found that MTAs were able to convert induced cell growth inhibitory phenotypes into cytotoxic phenotypes when used in combination with pan-HER/MEK inhibitors[119]. Tang et al. established a drug screening method based on human tumor organoids combined with experimental screening and computational prediction and successfully screened and validated potential drug candidates for CRC treatment[120]. Moreover, the mechanism and mode of action of the drug candidates were revealed by transcriptome sequencing technology, which provided important evidence supporting the discovery of therapeutic drugs for CRC[120]. This study has potential application value for the clinical treatment of CRC[120].
Intestinal organoids also play an important role in drug-toxicity studies. Grabinger et al. exposed small intestine-like organs, the mouse colon cancer cell line MC38, and Caco-2 cells to cisplatin, 5-fluorouracil, or X-rays to study cytotoxic responses. The results showed that, while small intestinal organoids were very sensitive to cisplatin and irinotecan, Caco-2 and MC38 cells were much less responsive to drugs at the same drug concentration[121]. Sato et al. showed that organoid-derived intestinal epithelial cells have physiological properties similar to those of the intestinal epithelium and that organoid-derived intestinal epithelial cells are a suitable model for preclinical toxicology and absorption, distribution, metabolism, and excretion studies, and can be used as a tool to enhance the prediction of bioactivity in humans[122]. Using healthy intestinal organoids, researchers have assessed the safety of cancer immunotherapeutic drugs with T cells conjugated to bispecific antibodies as a model therapy. Organoid–immune cell co-cultures capture the clinical toxicity overlooked in animal models and can be used to elucidate the cellular mechanisms underlying these effects[123].
-
Targeted therapy refers to a targeted approach in which tumor cells are killed at specific cancer-causing sites, thereby significantly improving the disease-free survival rate of patients. In future, tumor samples from patients undergoing surgery or colonoscopy can be collected for in vitro organoid culture, and lesion types can be detected via sequencing or pathology to select targeted drugs for personalized treatment[124].
Following the trend for individualized therapy in cancer treatment, organoids derived from the patient’s own cells can be used to replicate some of the critical properties of the primary tissue. Compared with other systems, the responses of tumor organoids to drugs and therapies are closest to the actual tumor response in vivo. Moreover, organoid biobanks, which can capture genetic diversity among tumors and enable the identification of specific drug–genetic interactions, offer the possibility of personalized treatment for cancer patients. The time required to construct CRC-organoid models is relatively short, and drug test results are available within weeks. After analyzing the causes of drug resistance in CRC, an effective treatment strategy can be formulated. In 2015, a critical study performed whole-gene sequencing of organoids derived from patients with metastatic CRC and found that 90% of the somatic mutations were the same between CRC-organoids and the biopsy samples. This finding confirmed that CRC-organoids retain the genetic and phenotypic characteristics of the original tumor and suggests the feasibility of applying organoids in individualized treatment[125]. Another study has reported the successful application of organoids in CF clinical treatment[126]. As there are nearly 2,000 types of CFTR deficiencies, the curative effect of the same drug on different CF patients varies greatly[127]. Researchers compared responses to CFTR modulator drug combinations in an in vitro setting using a gut organoid approach and examined potential inter-individual differences in response to combination therapy[128]. Vlachogiannis et al. constructed an organoid model from patients with metastatic drug-resistant CRC[129] and achieved 100% sensitivity, 93% specificity, 88% positive-predictive value, and 100% negative-predictive value in predicting the effect of targeted drugs or chemotherapy. In addition, the BRAF inhibitor vemurafenib was found to inhibit growth of PDOs with BRAFV600E mutations, but failed to induce apoptosis, which was consistent with the lack of efficacy of single-agent BRAF inhibitors in metastatic CRC. While two cases of PDOs containing KRASG12D and BRAFV600E mutations did not respond to cetuximab, this finding was consistent with the clinical resistance to both drugs in the relevant patients. Based on these results, ineffective treatments can be avoided. In 2020, a research group applied organoid technology to guide the clinical formulation of precise treatment strategies for patients with CRC with peritoneal metastasis and achieved good results[130].
In summary, 3D cultured organoids are good tools for use in precision medicine because of their extensive and unique sources. These organoids have great potential for clinical application and are expected to be applied in prospective clinical trials.
-
Although significant progress has been made in organoid research, its limitations and challenges cannot be overlooked. First, the Matrigel used in organoid culture comes from the basement membrane matrix of EHS mouse sarcoma, which contains various proteins, such as laminin, nestin, and collagen, as well as multiple growth factors, such as TGF-β, EGF, and insulin-like growth factor. Additionally, animal-derived components cannot be removed, limiting their clinical application. As an essential material for 3D organoid culture, Matrigel must be optimized for future applications to meet the various requirements in different fields. Secondly, organoid models cannot fully reproduce the dynamic and complex microenvironment of the human body because of the lack of vascular, nervous, and immune systems. The current co-culture system is relatively simple. However, organoids remain unable to mimic the overall structure and function of organs comprehensively and can only simulate part of the disease process[131]. Third, organoid homogeneity is poor. As organoids are cultured in 3D Matrigel, a particular edge effect is noted in their growth; that is, the size of organoids on the periphery of the Matrigel is generally larger, whereas the size of organoids in the middle of the Matrigel is smaller, which causes difficulties in high-throughput organoid cell number quantification and statistical analysis of their size. In addition, various organoid culture conditions and methods may lead to significant changes in cell composition, resulting in different organ differentiation and affecting experimental reproducibility. Furthermore, compared to 2D cell culture, organoid establishment is costly and time-consuming, limiting its large-scale clinical application. In future, it will be necessary to formulate a unified and standardized culture process to improve repeatability and reproducibility in organoid experiments. Moreover, challenges in controlling the biophysical environment and simulating tissue–tissue connections in organoids remain. To simulate the internal environment of organs in vivo and the interaction between organs more accurately, researchers have developed organoid chips using microfluidic technology[132]. However, further progress is needed to achieve accurate simulation of the internal environment. In addition to the technical limitations, ethical issues have arisen because organoids are derived from stem cells. Although drug testing and genetic modifications have been validated in animal experiments, the transition to human organoid experiments has been hindered by ethical issues. At present, organoids remain in gray areas legally and ethically, and society and the relevant government departments will need to revise the current ethical principles and regulations governing this research before this technology can be implemented fully.
Currently, organoid technology is gradually developing towards the construction of more complex organ systems, realization of a higher degree of simulation, and deeper integration with other technologies. The ethical aspects of organoids are of paramount importance, considering their specificity, complexity, and sensitivity. Patentability of organoid technologies and their products is another pressing issue. The use of organoids of human cellular origin, and in particular, the manipulation of biological processes using molecular biology methods, will lead to greater debate. Additionally, the construction of specific organs that are highly ethically controversial (e.g., brain-like organs) raises a host of ethical and moral issues. The cultivation of brain-like organs in particular has raised moral concerns about potential consciousness. Currently, human brain-like organoids are not as developed as the human brain and do not exhibit cognitive or conscious abilities. However, scientists are working toward developing more complex brain-like organs, raising the pressing and controversial question of whether brain-like organs can develop human characteristics, which has ethical and legal implications. Whether brain-like organs exhibit human qualities is relevant not only to their ethical and legal status, but also to the protection of the donor's right to informed consent and privacy. The development of organoids is not only limited to in vitro culture, but also to transplanting them into animals to form "chimeras" to validate the function of the organoids, to study the complex mechanisms of organogenesis, or to model human diseases. However, cross-species fusion of tissues and organs raises further serious ethical issues. Although no conclusive evidence exists that such animals would demonstrated human-like intelligence, such experiments have fueled a debate about the boundaries between humans and animals as well as the moral status of animals. This debate is as much about the boundaries of science and technology as it is about human responsibility, animal welfare, and the dignity of life in general as well as human dignity. At present, a series of efforts has been made at the international level and in major developed countries to promote the ethical management of organoid research, with the aim of effectively preventing and resolving moral risks, while promoting scientific and technological progress. Currently, the ethical issues related to organoids are not well researched and regulated, partly due to their inclusion in the traditional research framework of “stem cells” and “embryos.” However, science and technology is a dynamic process that requires Ethics Review Committee and government health departments to adapt and respond appropriately in keeping with the times. In the process of organoid ethical governance, studying the differences and consensus in ethical governance in different cultural contexts is crucial. This requires the penetration of pluralistic ethical values into all aspects of ethical supervision, thus promoting the continuous improvement of the ethical management system in life sciences.
When organoids are applied in the field of nutritional research, the limitations of the co-growth of organoids and parietal membranes must be considered. Classical organs usually grow with the parietal membrane. On the one hand, the apical membrane provides physical support for organoids, enabling them to maintain a certain morphology and structural stability. For example, during the growth of intestinal organoids, the apical membrane helps to maintain the lumen structure. On the other hand, the apical membrane can also affect the exchange of nutrients and signaling molecules in organoids. They can regulate material exchange between cells and the external environment, providing the necessary nutrients and growth factors for organoid growth. However, the apical membrane is imperfect. In some cases, the presence of an apical membrane may limit full contact of organoids with the external environment, resulting in some cells being unable to obtain sufficient nutrients and signal stimuli, thereby affecting the overall growth and functional performance of the organoids.
Many factors restrict the co-growth of organoids and apical membranes. The source, state, and genetic variation of cells have significant limitations on the co-growth of organoids and apical membranes. Healthy cells are more likely to form stable organoid structures, whereas diseased cells may have functional defects that affect their synergistic growth with the apical membrane. The state of the cells is also crucial. If the cells are in a senescent or damaged state, their proliferation and differentiation abilities decline, hampering establishment of good interactions with the apical membrane. Genetic variations may lead to abnormal cell function, thereby affecting the normal development of organoids and their adaptability to the apical membrane. The balance between nutrients and additives in the culture medium is extremely important for the co-growth of organoids and apical membranes. If the proportion of nutrients is imbalanced or the concentration of additives is inappropriate, it may lead to slow growth and abnormal morphology of the organoids, affecting their synergistic development with the apical membrane. The selection of extracellular matrix material significantly influences organoid growth. Unsuitable matrix materials may lead to restricted cell growth and the inability to form effective connections and interactions with the apical membrane. Culture temperature, oxygen concentration, and pH can also significantly limit the co-growth of organoids and apical membranes. Microbial contamination has a destructive effect on the organoid culture system and affects the growth of the apical membrane. Improper passage timing and operation methods can have serious consequences. Physical damage during operations, such as excessive pipetting or vigorous stirring, destroys the structural integrity of organoids and affects their co-growth with the apical membrane. The heterogeneity of cells within the organoids has various effects. This heterogeneity may affect the stability and reproducibility of the co-growth of organoids and apical membranes, thereby increasing uncertainty in the experimental results. In addition, problems regarding vascularization and the infiltration of immune cells, the regulation of immune factors, and the mechanisms of immune recognition and tolerance during the co-growth of organoids and apical membranes remain.
-
Overall, organoids can simulate the 3D structure, cell composition, and functions of real organs in vitro, providing broad application prospects in biomedical research and regenerative medicine. In recent years, intestinal organoid technology has made significant progress due to the improved preservation of the original tissue structure, cell–cell interactions, and differentiation ability. Compared with 2D cultured cells, organoids were found to be closer to the physiological functions of in situ tissues, which could provide a more direct understanding of organ development, homeostasis, and pathogenesis. Compared to animal models, the handling of organoids is more straightforward and intuitive, and organoids provide an alternative method for experiments that cannot be accurately modeled in animals. Organoids derived from patient tissues can serve as preclinical models that may offer new translational avenues for clinical research.
Despite some limitations, the application prospects of organoids are extensive, and the development of organoids could be an indispensable cornerstone for future translational medicine. In future, the combination of intestinal organoids with emerging technologies, such as CRISPR/CAS9 gene-editing methods and 3D bioprinting technology, could be used to construct a more complex and precise research platform for intestinal diseases. These have great potential for gene therapy and transplantation in the treatment of intestinal diseases.
In current scientific research, organoids and artificial intelligence are two cutting-edge and highly promising technologies. These developments have contributed significantly to advances in medicine, biology, and drug development. When combined, the two can create an unprecedentedly powerful platform for a deeper understanding and treatment of diseases, and their synergy opens up revolutionary possibilities for the future of healthcare. A combination of these two technologies may have applications in the following areas:
Precision and personalized medicine: Personalized organoids are cultivated by obtaining stem cells from patients, and artificial intelligence can analyze the response of these organoids to different treatments and customize personalized and precise treatment plans for patients. This personalized approach can improve treatment effectiveness and reduce unwanted side effects.
Drug Screening: Organoids provide a model close to human physiological conditions for drug research and can be used to test the safety and efficacy of drugs. Artificial Intelligence can quickly analyze experimental data on the effects of thousands of compounds on organoids, thus enabling the prediction of drug activity, accelerating the drug screening process, reducing dependence on animal testing, and improving the efficiency and economy of new drug development.
Pathological mechanism research: Organoids can mimic the occurrence of tumors and neurodegenerative diseases. Artificial intelligence technology can help us understand the occurrence and development of diseases better, and can identify new therapeutic targets by processing and analyzing massive amounts of data.
Toxicological assessment: AI technology enables high-throughput toxicity testing of drugs or chemicals on organoids and predicts their likelihood of tissue toxicity before they enter clinical trials. This technology can be applied for early screening to ensure public health safety.
Regenerative medicine: AI technology can, to a certain extent, improve the growth and differentiation conditions of organoid organs, improve their quality, and facilitate their approximation of target organ function. AI can be used to design complex tissues and promote the development of tissue-engineering technology.
Image analysis and diagnosis: Cell images contain a large amount of information, AI technology can qualitatively and quantitatively analyze the morphology, function, and subcellular morphology of cells, obtaining a vast amount of information on cell characteristics, which will help us to understand changes in the structure and function of cells, and then facilitate understanding of cellular life activities and working mechanisms. By combining AI technology with organoids, AI technology, based on the ability to quickly process large amounts of image data, can provide researchers with quantitative analysis results, such as cell number, shape, and distribution, information that is critical to understanding the function of organoids. By using AI technology algorithms, researchers are able to quickly analyze images of organoids in a way that is more systematic and efficient than human judgment.
Gene editing and genetic disease treatment: Organoids can provide a test platform for gene editing, and AI can assist in the design and optimization of gene-editing programs, to enhance the success rate of genetic disease treatment. AI technology can be used to manage bioinformatics, big data analysis, and other processes efficiently, and to analyze the biological implications and associated principles in depth.
Although significant progress has been made in the use of AI in organoid research, many challenges remain. Difficulties in data acquisition, insufficient sample quality and size, and poor model interpretability have limited the generalization of the method. To address these problems, data consistency, model interpretability, and multimodal information fusion need to be improved for more comprehensive and reliable application of AI to organoid research. Multidisciplinary cross-collaboration is required to solve these problems and promote the in-depth application of AI technology in the field of organoids, which is expected to play an increasingly important role, thereby accelerating progress towards clinical and precision medicine.
With the rapid evolution of organoid cultivation technology, intestinal organoids provide inestimable value in research and regenerative tissue medicine related to human intestinal disease.
Research Advances in the Construction and Application of Intestinal Organoids
doi: 10.3967/bes2025.010
- Received Date: 2024-03-20
- Accepted Date: 2024-09-03
-
Key words:
- 3D culture /
- Colorectal cancer /
- Disease model /
- Intestinal organoids /
- Organ transplantation /
- Pluripotent stem cells /
- Regenerative medicine /
- Tumor organoids
Abstract: The structure of intestinal tissue is complex. In vitro simulation of intestinal structure and function is important for studying intestinal development and diseases. Recently, organoids have been successfully constructed and they have come to play an important role in biomedical research. Organoids are miniaturized three-dimensional (3D) organs, derived from stem cells, which mimic the structure, cell types, and physiological functions of an organ, making them robust models for biomedical research. Intestinal organoids are 3D micro-organs derived from intestinal stem cells or pluripotent stem cells that can successfully simulate the complex structure and function of the intestine, thereby providing a valuable platform for intestinal development and disease research. In this article, we review the latest progress in the construction and application of intestinal organoids.
The study concept was developed by Hongzhou Lu, Jun Xue, and Weizheng Liang; The “In vitro construction, culture, and function of intestinal organoids” part was written by Qingxue Meng with the help of Cuishan Chi; The “Application of intestinal organoids” section was written by Hongyang Yi with the help of Weiquan Liang; The “Application of intestinal tumor organoids” section was written by Peng Wang with the help of Chenyu Mao. The fourth section was written by Shan Liu. Figures was prepared by Hongyang Yi; Tables was prepared by Peng Wang; Publications was collected by Weiquan Liang, Cuishan Chi, and Chenyu Mao; Manuscript revision was participated in by Peng Wang. The manuscript was reviewed by Hongzhou Lu, Jun Xue, Weizheng Liang, and Qingxue Meng. All the authors have read and approved the final manuscript.d approved the final manuscript.The authors declare that they have no conflicts of interest.
Not applicable.
&These authors contributed equally to this work.
| Citation: | Qingxue Meng, Hongyang Yi, Peng Wang, Shan Liu, Weiquan Liang, Cuishan Chi, Chenyu Mao, Weizheng Liang, Jun Xue, Hongzhou Lu. Research Advances in the Construction and Application of Intestinal Organoids[J]. Biomedical and Environmental Sciences, 2025, 38(2): 230-247. doi: 10.3967/bes2025.010 |









 Quick Links
Quick Links
 DownLoad:
DownLoad: