HTML
-
Serpiginous choroiditis (SC) is a recurrent vision-threatening, progressive, intraocular, inflammatory disease that is characterized by a geographic lesion, usually in both eyes, that originates in the peripapillary region. Lesions involve the choroid and overlying retinal pigment epithelium (RPE) and outer retina. The exact etiology of SC remains unknown, but some have suspected that the condition is autoimmune. Systemic corticosteroids and immunosuppressive agents are generally recommended to treat SC[1, 2]. Presumed tuberculous serpiginous-like choroiditis (Tb-SLC, also called multifocal SC) is an intraocular inflammatory condition associated with tuberculosis that progresses in a similar serpiginoid pattern as classic SC[2]. Some have suggested that Tb-SLC is a distinct entity from classic SC. However, differentiation between Tb-SLC and classic SC can be challenging. Patients with Tb-SLC are predominantly young to middle-aged men and tend to present with multifocal choroiditis. Unfortunately, this is not always the case[2, 3], but differentiating between Tb-SLC and classic SC is crucial because the two diseases are treated in markedly different ways. Immunosuppressive treatments often worsen a tuberculosis infection, which can be catastrophic to patients. In contrast, anti-tuberculosis agents have significant adverse effects, particularly in older patients with classic SC.
Optical coherence tomography (OCT) as a non-invasive imaging method that plays an important role in assessing fundus diseases, including choroiditis. However, a rigorous examination of SC and Tb-SLC features on OCT has not yet been performed. Some case reports in the literature have reported outer retinal hyper-reflections at active lesion sites and tiny hyper-reflective spots in the choriocapillaris in eyes with SC and Tb-SLC[1, 3]. China is a tuberculosis endemic area[4], but very few studies have examined ocular conditions associated tuberculosis in China. Our previous study reported characteristics of tuberculosis-associated uveitis in the Chinese population[5]. However, the literature lacks studies on Tb-SLC in China and how Tb-SLC is represented on OCT. It would be extremely helpful to have a simple, noninvasive method to differentiate between Tb-SLC and classic SC. Therefore, the current study examines OCT findings in eyes with active Tb-SLC and SC to characterize how each disease is represented on OCT. The usefulness of OCT in differentiating between the two conditions is also assessed.
-
This retrospective study was conducted in Beijing Tongren Hospital, a Capital Medical University Hospital (Beijing, China). The Ethics Committee (EC) of Beijing Tongren Hospital reviewed and approved this research protocol and all study conduct adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants before invasive examinations (e.g., fluorescein angiography and blood tests) were performed.
This study included consecutive patients with SC or active Tb-SLC lesions who presented to Beijing Tongren Hospital between November 2015 and June 2017. All patients underwent a detailed medical history, systemic work-up, and comprehensive ophthalmic examination. A history of tuberculosis or tuberculosis contact was of particular interest. The systemic work-up included tubercular etiology tests [e.g., tuberculin skin test (TST) and interferon gamma release test (Oxford Immunotec Co, Oxford, UK)], chest computed tomographic scans (to identify pulmonary tuberculosis and rule out sarcoidosis), urinalysis, complete blood count, and blood immunological testing [syphilis, hepatitis B and C, human immunodeficiency virus, and TORCH tests (i.e., toxoplasmosis, rubella, cytomegalovirus, and herpes)]. This testing was performed to rule out infections other than tuberculosis. Blood angiotensin-converting enzyme levels were examined to rule out sarcoidosis. Rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies were examined to rule out other connective tissue and autoimmune diseases.
-
Ophthalmic examinations included best-corrected visual acuity (BCVA) assessment, slit-lamp biomicroscopy, and dilated funduscopic examination. Fundus photography and fundus autofluorescence (FAF) imaging were also performed to determine lesion activity. Lesions were considered active if creamish-yellow lesions observed on clinical exam corresponded to an ill-defined, diffuse hyperautofluorescent zone on FAF images[3, 6-7]. Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) images focused on the edge of each active patch were also obtained, along with enhanced depth imaging (EDI) OCT scans. Fluorescein angiography (FA) and indocyanine green angiography (ICGA) were performed when clinical findings suggested the presence of choroidal neovascularization (CNV) and choroidal granulomas.
-
The SC or Tb-SLC disease was defined as active when ill-defined patches of grayish- or creamish-yellow subretinal infiltrates with central healing and active edges were present. Active edges were hyperautofluorescent and healed areas were hypoautofluorescent (Figure 1). All imaging evaluations were performed by two retinal specialists (WXN and YQS). During the first grading, evaluators recorded descriptions of OCT findings, summarizing several prominent OCT signs (e.g., intraretinal edema and choriocapillaris point-like hyper-reflection[8-9]). During the second grading, evaluators assessed the frequency of each OCT sign in each included patient. Image analysis data recorded by the first two evaluators were then adjusted by a senior retinal specialist (PXY).
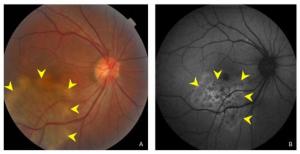
Figure 1. (A) Color fundus photograph showing ill-defined grayish or creamish subretinal infiltrate patches with active edges. (B) A fundus autofluorescent image showing hyperautofluorescent patches in active edges.
The distinction between Tb-SLC and classic SC was based upon ophthalmic findings and the results of the TST or interferon gamma release test. The TST was considered positive if an induration of at least 20 mm in diameter was noted. Patients with Tb-SLC were administered anti-tuberculosis therapy and patients with SC were administered corticosteroid or immunosuppressive therapy. Patients with Tb-SLC also received corticosteroids (1 mg/kg) 3 weeks after anti-tuberculosis treatments to prevent Kocha's phenomenon. When fundus lesions were properly controlled, oral corticosteroids were tapered. All included patients were in clinical remission following treatment, confirming that all patients had been correctly diagnosed with SC or Tb-SLC.
All statistical analyses were performed using SPSS for Windows (Version 25.0, SPSS, Inc., Chicago, IL, USA). The frequency of OCT signs were compared between the Tb-SLC and SC groups using chi-squared tests. All statistical tests were two-sided and statistical significance was defined as P < 0.05.
Subjects
Ophthalmic Examinations
Imaging Analysis
-
This study included 14 eyes of 9 patients (5 men) with Tb-SLC and 12 eyes of 8 patients (5 men) with SC. All patients were followed from the acute disease stage through final healing. The mean patient age was 44.0 ± 7.4 years (range: 26-58 years) in patients with Tb-SLC and 54.6 ± 9.8 years (range: 46-66 years) in patients with SC (P = 0.03). Demographic and clinical characteristics of included patients are summarized in Table 1.
Group Males Females Eyes Bilateral Age (years) BCVA LogMAR BCVA Lesion Location SC 6 2 12 6.00 54.6 ± 9.8 0.3 0.52 9 disc, 3 macula centered Tb-SLC 5 4 14 6.00 44.0 ± 7.4 0.1 1.00 2 disc, 12 macula centered P 0.40 0.71 0.03 < 0.01 < 0.01 Note. SC: serpiginous choroiditis; Tb-SLC: tuberculous serpiginous-like choroiditis; BCVA: best corrected visual acuity. Table 1. Clinical Features of Serpiginous Choroiditis and Tuberculous Serpiginous-like Choroiditis
-
The following eight OCT findings were most commonly observed in our study patients: (1) vitreal irregular, spheroid hyper-reflective spots (discrete, > 20 µm), (2) outer nuclear layer (ONL) hyper- reflection (small or punctiform hyper-reflective spots within the ONL), (3) intraretinal edema (retinal swelling, inner nuclear layer and outer plexiform layer cystoid cavities), (4) hyporeflective, wedge-shaped band within the outer plexiform layer, (5) outer retinal tubulation (round or ovoid hypo-reflective spaces with hyper-reflective borders in the ONL) of the retina), (6) sub-RPE conical, drusenoid deposits (sub-RPE deposition of drusenoid materials with a conical appearance), (7) choriocapillaris point-like hyper-reflections, (point-like hyperreflection appeared within choriocapillaris); and (8) choroidal granulomas (round or lobulated choroidal lesions on EDI-OCT scans, FA, and ICGA as hypofluorescence changes); Table 2, Figures 2-4).
No. Total Eyes SC (n = 12) Tb-SLC (n = 14) PValue 1 Vitreal hyper-reflective spots 0 5 0.02 2 Outer nuclear layer hyper-reflection 12 13 0.35 3 Intraretinal edema 3 11 0.01 4 Hyporeflective, wedge-shaped band 9 5 < 0.05 5 Outer retinal tabulation 4 7 0.40 6 Sub-RPE drusenoid deposits 2 11 < 0.01 7 Choriocapillaris point-like hyper-reflection 10 13 0.45 8 Choroidal granulomas 2 8 0.03 Note. SC: serpiginous choroiditidis; Tb-SLC: tuberculous serpiginous-like choroiditis; RPE: retinal pigment epithelium. Table 2. Frequency of Optical Coherence Tomography Signs in Patients with Serpiginous Choroiditidis and Tuberculous Serpiginous-like Choroiditis
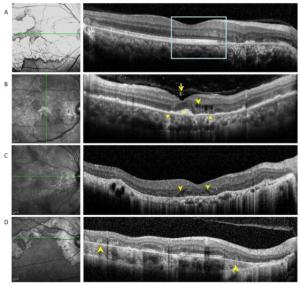
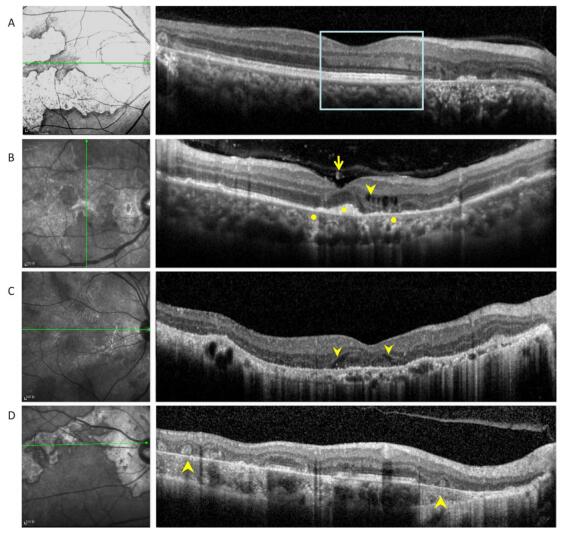
Figure 2. (A) Optical coherence tomography scans passing through active lesion edges show outer nuclear layer thinning and Henle fiber layer hyper-reflection. The underlying ellipsoid zone and RPE remained relatively intact (boxes). (B) Optical coherence tomography scans of a 49-year-old patient with tuberculous serpiginous-like choroiditis. Discrete vitreal hyper-reflective spots (arrows) are visible. Intraretinal edema (arrow heads), elevated sub-RPE drusenoid deposits (square), and choriocapillaris point-like, hyper-reflective lesions (circle) are also apparent. (C) Optical coherence tomography scans of a 47-year-old patient with serpiginous choroiditis. Optical coherence tomography scans show an intraretinal, wedge-shaped hyporeflection (arrow heads). No vitreal hyper-reflective spots or elevated sub-RPE drusenoid deposits were observed. (D) Optical coherence tomography scans of a 62-year-old patient with serpiginous choroiditis. No vitreal hyper-reflective spots are visible. Outer retinal tubulation and choroidal atrophy are also visible within a relatively stable lesion (arrowheads).
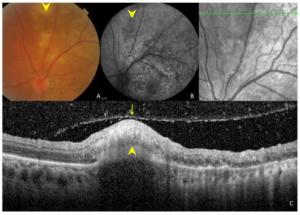
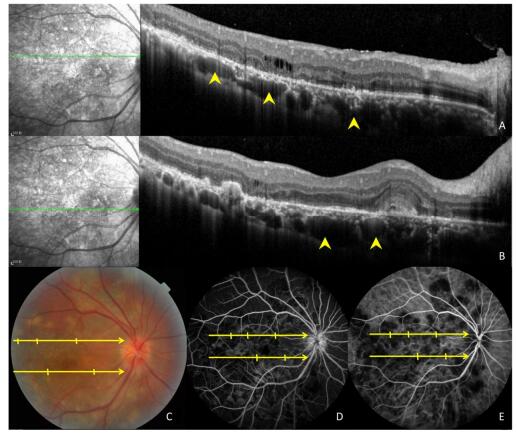
Figure 3. Ocular images of a 35-year-old patient with tuberculous serpiginous-like choroiditis. (A) A color fundus photograph shows grayish-yellow active lesions superior to the disc (arrowhead). (B) A fundus autofluorescence (FAF) image showing diffuse hyperautofluorescence in the same location as the active lesion. (C) An optical coherence tomography scan showing a hyper-reflective lesion (arrow head) corresponding to fundus photograph and FAF image anomalies. The entire retina and the preretinal vitreous cortex (arrow) are involved.
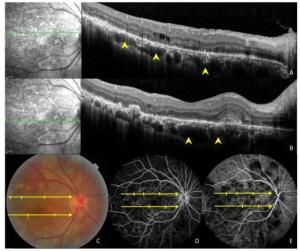
Figure 4. Images of a 47-year-old patient with tuberculous serpiginous-like choroiditis. (A, B) Optical coherence tomography scans reveal the presence of choroidal granulomas (arrow heads) beneath active lesions. (C) A color fundus photograph showing grayish-yellow active lesions that correspond to granuloma locations. (D, E) Fundus fluorescein and indocyanine green angiography images show hypofluorescent regions corresponding to lesion locations.
The frequency of each OCT sign in patients with SC and Tb-SLC is summarized and compared in Table 2. Some OCT signs were commonly detected in both SC and Tb-SLC eyes, including ONL hyper-reflections [13 Tb-SLC eyes (93%), 12 SC eyes (100%); P = 0.35], choriocapillaris point-like hyper-reflections [13 Tb-SLC eyes (93%), 10 SC eyes (83%); P = 0.45], and outer retinal tubulation [7 Tb-SLC eyes (50%), 4 SC eyes (33%), P = 0.40]. However, some OCT signs were observed significantly more often in patients with Tb-SLC than with SC. These included vitreal hyper-reflective spots [5 Tb-SLC eyes (36%), 0 SC eyes (0%); P = 0.02], intraretinal edema [11 Tb-SLC eyes (79%), 3 SC eyes (25%); P = 0.01], sub-RPE drusenoid deposits [11 Tb-SLC eyes (79%), 2 SC eyes (17%); P < 0.01], and choroidal granulomas [8 Tb-SLC eyes (57%), 2 SC eyes (17%); P = 0.03]. In contrast, a hyporeflective, wedge-shaped band was observed more often in eyes with SC than Tb-SLC [5 Tb-SLC eyes (36%), 9 SC eyes (75%); P < 0.05].
Basic Clinical Features
Optical Coherence Tomography Signs
-
Very few studies have examined Tb-SLC, although tuberculosis has an endemic prevalence in China. It was reported that serpiginous-like choroiditis can clinically present as choroidal tuberculosis and that it responds well to anti-tuberculosis treatment[10, 11]. Therefore, it is highly likely that Tb-SLC prevalence has been underestimated in China. It is clinically difficult to differentiate between Tb-SLC and SC without tuberculosis etiology testing and many attempts have been made to determine characteristic differences between these distinct diseases. Previous studies have shown that Tb-SLC commonly affects young to middle-aged healthy men and is frequently bilateral. Lesions generally begin as discrete, multifocal lesions that spread in a serpiginoid pattern and involve the macula[12, 13]. Lesions associated with Tb-SLC and SC appear similarly on angiographic and autofluorescent images[3]. However, the current study examines and compares OCT features of SC and Tb-SLC lesions. Some OCT features identified here have been previously reported in eyes with other disorders [e.g., diabetic retinopathy and age-related macular degeneration (AMD)][14-20], but not in eyes with SC or Tb-SLC. Additionally, the current study shows that OCT may be useful for identifying subtle anatomical differences between SC and Tb-SLC lesions. Vitreous hyper-reflective spots, intraretinal edema, sub-RPE drusenoid deposits, and choroidal granulomas occurred significantly more often in eyes with Tb-SLC than SC.
Previous case studies that included OCT scans[3, 21, 22] have shown that both SC and Tb-SLC lesions have a localized, fuzzy, hyper-reflective region in the outer retina and point-like hyper-reflections in the choriocapillaris. We also observed these findings. In addition, some patients had a thinned ONL and a hyper-reflective Henle fiber layer (inner ONL). However, the underlying ellipsoid zone and RPE were relatively intact (Figure 2A). Intraretinal OCT hyper-reflections most likely reflect focal capillary ischemia in eyes with retinal artery occlusion or acute macular neuroretinopathy[23, 24]. Therefore, the Henle fiber layer hyper-reflection observed here may also indicate focal ischemia caused by choriocapillaris microvasculitis, which would impair choroidal oxygen and nutrition delivery to the outer retina. Moreover, many OCTA and ICGA studies on eyes with SC or Tb-SLC have shown active lesion hyporeflection and hypofluorescence. This also suggests focal ischemia in both the outer retina and choriocapillaris caused by inflammatory cell accumulation[3, 25].
The current study found that OCT vitreal hyper-reflective spots, presumably created by inflammatory cell infiltration, were commonly found in eyes with Tb-SLC [5 of 14 eyes (36%)], but never in eyes with SC (0 of 12 eyes). Figure 3 shows a special case in which a grayish-yellow node was visible on color fundus photographs. On OCT scans, hyper-reflective lesions that involved the vitreous cortex, entire retina, and choroid were also observed. Altogether, these suggest a strong local inflammatory reaction. Our findings are in agreement with those of Rifkin et al.[21], who described an elevated choroidal lesion in eyes with Tb-SLC. The lesion was accompanied by focal elevation of the overlying neurosensory retina and RPE-Brucha's membrane complex. Notably, the current study found that patients with SC (54.6 ± 9.8 years) were significantly older than those with Tb-SLC (44.0 ± 7.4 years, P = 0.03; Table 1). It may be that older patients have a more rapid clearing of cells from the vitreous because they are more likely to have a posterior vitreous detachment (PVD) than younger patients. Therefore, fewer cells would be present, which would lead to fewer vitreal hyper-reflective spots on OCT scans. However, the incidence of PVD did not significantly differ between the SC [8 of 12 eyes (75%)] and Tb-SLC [11 of 14 eyes (79%)] groups (P = 0.67). Therefore, the difference between groups in vitreal hyper-reflective spot prevalence cannot be explained by differences in the PVD rate. This finding more likely reflects different pathogenic mechanisms of Tb-SLC and classic SC.
Eyes with Tb-SLC had a higher incidence of sub-RPE drusenoid deposits than eyes with SC. The origin of this sub-RPE material remains unknown. However, based on clinical and histological observations, a possible mechanism can be proposed. The RPE has an altered polarization in eyes with AMD[26]. Hageman et al.[27] postulated that cellular debris accumulated in Brucha's membrane and stimulated a chronic inflammatory reaction. This debris may serve as a nucleation site for drusen formation. Therefore, the observed cellular debris in the sub-RPE space may be related to abnormal photoreceptor function[28]. In Tb-SLC patients, the highly-raised, sub-RPE drusenoid deposits in the presence of intraretinal edema may indicate a strong RPE or cellular immune reaction to tuberculosis inflammation. Unfortunately, this inflammatory process may lead to vision loss.
Previous studies have illustrated that, compared with sarcoidosis and Vogt-Koyanagi-Harada disease, Tb-related choroidal granulomas are more likely to have a lobulated shape and a nonhomogeneous internal pattern. These differences in lesion appearance, as visualized by OCT, may be helpful in differentiating posterior granulomatous uveitis[20] from other conditions. The current study showed that eyes with Tb-SLC have choroidal changes that include both choriocapillaris point-like hyper-reflections and choroidal granulomas. Although the literature does not contain pathological studies of SC or Tb-SLC active lesions, clinicopathological findings in long-standing inactive lesions showed moderate choroidal mononuclear inflammatory cell infiltration with focal lymphocyte aggregation[29]. Considering these pathological findings and the differing OCT features of SC and Tb-SLC, it may be that Tb-SLC causes a different choroidal granulomatous inflammatory reaction than SC, possibly resulting in different choroidal OCT anomalies.
Eyes with SC had an intraretinal wedge-shaped hyporeflection on OCT scans more frequently than eyes with Tb-SLC. This hyporeflective structure is also commonly found in eyes with GA secondary to AMD or another chorioretinal atrophy (e.g., pathologic myopia)[16]. The wedge-shaped structure observed here appeared to be within the boundaries of the outer plexiform layer. Therefore, this wedge most likely represents photoreceptor apoptosis or Henle fiber layer atrophy. This type of inflammation differs from the granulomatous type of inflammation that occurs in eyes with Tb-SLC. Therefore, RPE and photoreceptor apoptosis may be a characteristic feature of active SC lesions.
Our study had several limitations, including the small number of included cases and lack of clinicopathological correlations. However, both SC and Tb-SLC are relatively rare, making our sample size reasonable. Additionally, OCT representation of active lesions have not yet been histologically correlated with lesion structures. Therefore, our interpretations of OCT anomalies are based on documented abnormalities on various image modalities (e.g., fundus photography, FA, and ICGA) and are largely presumed. However, because of the clear differences noted here, further OCT studies may lead to novel methods of identifying Tb-SLC and differentiating it from other diseases in a more timely manner. Additionally, the serial OCT scans presented here show ultrastructural changes over time and may lead to a better understanding of both Tb-SLC and SC pathology. In conclusion, OCT scans in eyes with Tb-SLC were significantly more likely to have vitreal hyper-reflective spots, intraretinal edema, sub-RPE drusenoid deposits, and choroidal granulomas than eyes with SC. Therefore, OCT is likely useful in differentiating between the two diseases.
-
No conflict of interest to declare.








 Quick Links
Quick Links
 DownLoad:
DownLoad: