-
Sudden sensorineural hearing loss (SSHL) is defined as > 30 dB sensorineural hearing loss at three contiguous frequencies within an interval of less than 3 days. This type of hearing loss is observed in 5‒160 individuals per 100,000 per year[1]. The cause of SSHL cannot be determined in most cases, and these are considered as idiopathic (I)SSHL.
Corticosteroids are the first-line treatment for ISSHL; other adjunctive therapies include hyperbaric oxygen treatment (HBOT), vasodilators, anticoagulants, antioxidants, and plasma expanders[2,3]. HBOT increases partial oxygen pressure to improve the blood oxygen profile and microcirculation. Accumulating evidence indicates that HBOT improves hearing levels in ISSHL patients[4,5] through mechanisms that are not well-understood.
The molecular basis of ISSHL is presumed to involve chronic inflammation, which can lead to microvascular injury and atherogenesis and increase the risk of ischemia[6]. Blood is supplied to the cochlea mainly through a single terminal (labyrinthine) artery; because cochlear hair cells have high oxygen consumption and poor tolerance of hypoxia, the inner ear is prone to changes in circulation. Thus, the pathophysiology of ISSHL is closely associated with the vascular state of the inner ear. Inflammation can result in endothelial dysfunction, which can cause a thrombotic event that alters the blood supply to the inner ear[7]. There is evidence suggesting that inflammation is involved in the development of ISSHL and affects prognosis[8].
Toll-like receptors (TLRs) are a family of signal transduction molecules that play a critical role in innate immunity and the inflammatory response through nuclear factor (NF)-κB signaling. Activated NF-κB stimulates the transcription of target genes, including the proinflammatory cytokines interleukin (IL)-1β and tumor necrosis factor (TNF)-α[9]. TLR4 and NF-κB signaling can induce an inflammatory response in pathological states including central nervous system injury and various diseases[10,11]; indeed, TLR2, TLR3, TLR4, TLR7, TLR8, and TLR9 were found to be upregulated in patients with ISSHL relative to normal controls[12].
HBOT has been shown to attenuate inflammation in many diseases[13,14]. In this study, we observed the changes of inflammatory cytokines TLR4 and NF-κB expression in peripheral blood of ISSHL patients after HBOT, and explored the molecular mechanism of HBOT on improving the hearing level of ISSHL patients.
-
A total of 120 participants with unilateral ISSHL seeking treatment at Beijing Chaoyang Hospital Affiliated of Capital Medical University from January 2014 to January 2017 were recruited for the study. Inclusion criteria were as follows: age between 17 and 75 years, and elapsed time between onset of hearing loss and beginning of therapy not exceeding 4 weeks. SSHL patients for whom the etiology was known were excluded, including those whose hearing loss was caused by retrocochlear lesions (as detected by acoustic brainstem response and magnetic resonance imaging) and infectious or autoimmune diseases (as detected by laboratory examinations); the remaining patients were diagnosed as ISSHL. Exclusion criteria also included acute inflammation, infection, bilateral SSHL, previous self or family history of SSHL, diabetes mellitus, systemic hypertension, chronic bronco-pulmonary obstructive syndrome, emphysema, sinusitis, seizure syndrome, pregnancy, claustrophobia in a hyperbaric environment, and an inability to meet the study requirements. Additionally, 20 healthy volunteers without hearing loss were recruited as the control group. The Ethics Committee and Institutional Review Board of Human Studies of Beijing Chaoyang Hospital approved this study, and the protocol conformed to approved guidelines. Informed consent was obtained from all patients enrolled in the study.
-
The HBOT and medicine groups (n = 60 each) were classified according to whether or not the patients elected to receive HBOT. The medicine group received 1 mg/(kg·d) oral prednisone (Xianju Pharmaceutical Co. LTD, Zhejiang, China) for 5 d followed by ginaton (Ji Sheng Chemical Pharmaceutical Co. LTD, Taiwan, China) for 14 d. The HBOT group also received prednisone and ginaton concurrently with HBOT. The HBOT session consisted of 30 min compression in air, exposure to 2.0 atm absolute pressure for 60 min, and decompression with oxygen for 30 min. Patients breathed 100% oxygen through a mask that was checked for leaks and underwent one HBOT session per day for 15 d in a multi-place hyperbaric chamber (Huaxin Oxygen Co. LTD, Weifang, China).
-
All patients underwent pure-tone audiogram tests (Conera; Interacoustics, Madsen, Denmark) immediately before the beginning of treatment (pre-treatment) and at the end of the last HBOT session (post-treatment). Masking was used when required. A qualified audiologist performed the testing and was blinded to treatment group assignment. The pure-tone average (PTA) was calculated from the bone conduction results at six frequencies (0.25, 0.5, 1, 2, 4, and 8 kHz). In accordance with American Speech and Hearing Association guidelines, hearing loss was defined as mild (20–39 dB), moderate (40–54 dB), moderate to severe (55–69 dB), severe (70–89 dB), and profound (> 90 dB).
Hearing improvement at each frequency was evaluated based on the change in hearing threshold from pre- to post-treatment[15]; outcomes were classified as follows: complete recovery, PTA ≤ 25 dB or identical to the contralateral unaffected ear; marked improvement, PTA improvement > 30 dB; slight improvement, PTA improvement between 10 and 30 dB; and non-recovery, PTA improvement < 10 dB. Effective treatment was defined as complete recovery, or marked or slight improvement.
-
In the present study, TLR4, NF-κB, and TNF-α levels in the peripheral blood of subjects were measured by enzyme-linked immunosorbent assay using commercial kits (Cusabio, Delaware, USA) according to the manufacturer’s instructions. Results are expressed as µg/mL of blood.
-
SPSS 20.0 for Windows (IBM, Armonk, NY, USA) was used for data analysis. The chi-square test was used to compare patient characteristics and the effect of treatment on hearing loss. Hearing thresholds were compared with the t-test, and differences in TLR4, NF-κB, and TNF-α levels were evaluated by one-way analysis of variance and the t-test. P < 0.05 was considered statistically significant. All statistical tests were two-sided.
-
A total of 140 subjects including 60 ISSHL patients who received only medicine treatment (medicine group), sixty who received medicine and HBOT (HBO group), and 20 healthy subjects (control group) were evaluated in this study. The clinical characteristics of the study population are shown in Table 1. There were no significant differences in sex, age, degree of hearing loss, interval between onset of hearing loss and initial treatment, smoking, tinnitus, or vertigo that could affect hearing outcomes between the medicine and HBO groups (P > 0.05).
Table 1. Clinical parameters of study subjects
Parameter Medicine group HBO group Control group Pa Sex, female/male, n 39/21 40/20 12/8 1.000 Age, years 0.556 17–45 17 21 9 46–75 43 39 11 Degree of hearing loss 0.774 Mild 19 21 − Moderate 13 9 − Moderate to severe 13 11 − Severe 7 7 − Profound 8 12 − Visit time, d 0.660 0–7 15 10 − 8–14 16 20 − 15–21 17 19 − 22–28 12 11 − Smoking, yes/no, n 8/52 12/48 4/16 0.463 Tinnitus, yes/no, n 42/18 37/23 − 0.336 Vertigo, yes/no, n 36/24 18/42 − 0.130 Note. aHBO vs. Medicine group. HBO, Hyperbaric oxygen. The HBO group underwent 15 consecutive HBOT sessions without any serious side effects, and the hearing threshold was evaluated after treatment. Treatment efficacy was higher in the HBO group (86.67%) than in the medicine group (66.67%, P < 0.05), indicating that HBOT improved the hearing threshold of ISSHL patients (Table 2).
Table 2. Hearing improvement in ISSHL patients
Group Patient number Complete recovery Marked improvement Slight improvement No recovery Effective treatment (%) Medicine 60 6 13 21 20 66.67 HBO 60 12 18 22 8 86.67a Note. aP < 0.05, HBO vs. medicine group. HBO, hyperbaric oxygen. ISSHL, sudden sensorineural hearing loss. Frequency averages before and after treatment are shown in Figure 1. There were not differences in PTA values between the medicine and HBO groups before treatment (P > 0.05); however, a decrease in the values was observed after treatment in both groups (P < 0.01). The change in hearing level was greater in the HBO group than in the medicine group at all frequencies (P < 0.01).
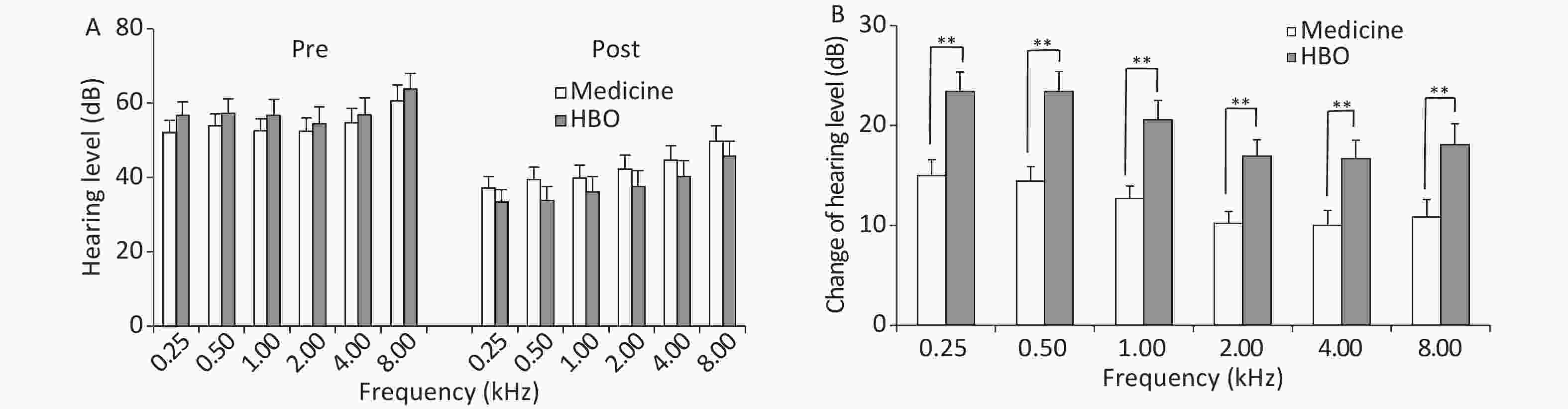
Figure 1. Hearing threshold average frequencies (A) and change in hearing threshold average frequencies (B) in the medicine and HBO groups. Values are expressed as mean ± standard error. **P < 0.01, HBO vs. medicine group. Pre, pre-treatment; Post, post-treatment; HBO, hyperbaric oxygen.
TLR4, NF-κB, and TNF-α levels in peripheral blood before treatment did not differ between the medicine and HBO groups, but were higher in ISSHL patients than in healthy control subjects (P < 0.01, Table 3). TLR4, NF-κB, and TNF-α levels in the medicine and HBO groups were lower after treatment (P < 0.05 and P < 0.01), and the levels differed significantly between the medicine and HBO groups (P < 0.05 and P < 0.01; Figure 2). These results demonstrated that HBOT lowered the expression of inflammatory cytokines TLR4, NF-κB, and TNF-α in ISSHL patients.
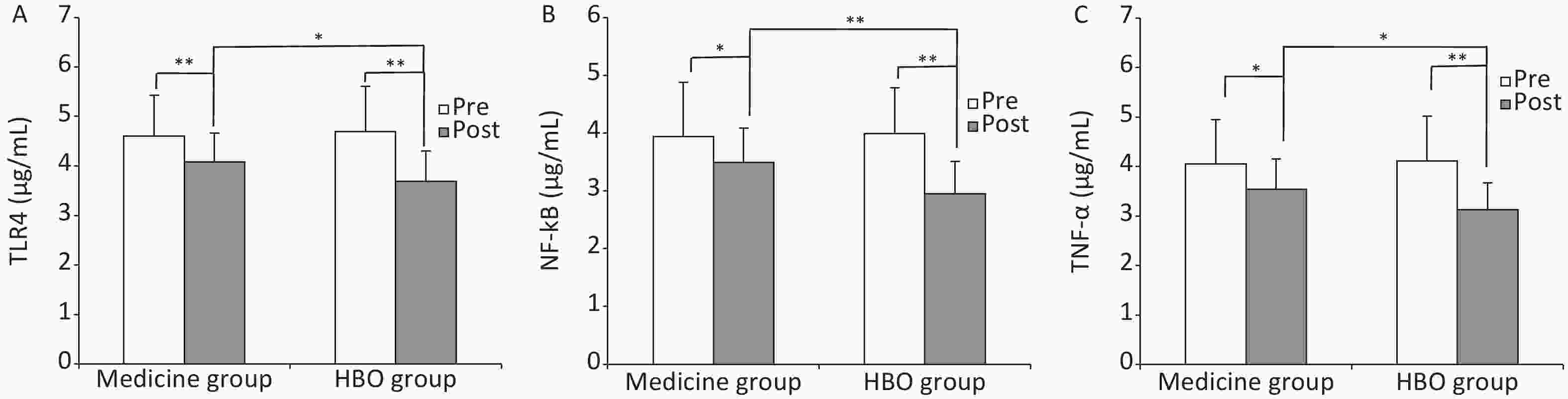
Figure 2. Expression levels of TLR4 (A), NF-κB (B), and TNF-α (C) in the medicine and HBO groups. Values are expressed as mean ± standard deviation. *P < 0.05, **P < 0.01. Pre, pre-treatment; Post, post-treatment; HBO, hyperbaric oxygen.
Table 3. Expression levels of inflammation-related factors before treatment (μg/mL)a
Parameter Medicine group HBO group Control group TLR4 4.60 ± 0.83b 4.69 ± 0.92c 1.86 ± 0.49 NF-κB 3.94 ± 0.94b 3.98 ± 0.79c 1.31 ± 0.36 TNF-a 4.05 ± 0.90b 4.11 ± 0.91c 1.16 ± 0.31 Note. aData are shown as mean ± SD. control group. bP < 0.01, Medicine vs. Control group. cP < 0.01, HBO vs. control group. HBO, hyperbaric oxygen. In addition, in ISSHL patients, the percent decrease (the posttreatment divided by the pretreatment) in TLR4, NF-κB, and TNF-α expression after treatment was greater for those who received medicine as compared to HBOT (TLR4: 0.90 ± 0.14 vs. 0.80 ± 0.13, P = 0.023; NF-κB: 0.92 ± 0.19 vs. 0.76 ± 0.17, P = 0.006; TNF-α: 0.90 ± 0.18 vs. 0.79 ± 0.17, P = 0.035). Pearson correlation analysis showed a significant negative correlation between the percent decrease of TLR4, NF-κB, and TNF-α expression after HBOT and a relative hearing gain in HBO group; in other words, greater hearing improvement was significantly associated with a larger reduction in the expression of TLR4, NF-κB, and TNF-α after HBOT (TLR4: r = –0.341, P = 0.008; NF-κB: r = –0.310, P = 0.016; TNF-α: r = –0.286, P = 0.027; Figure 3).
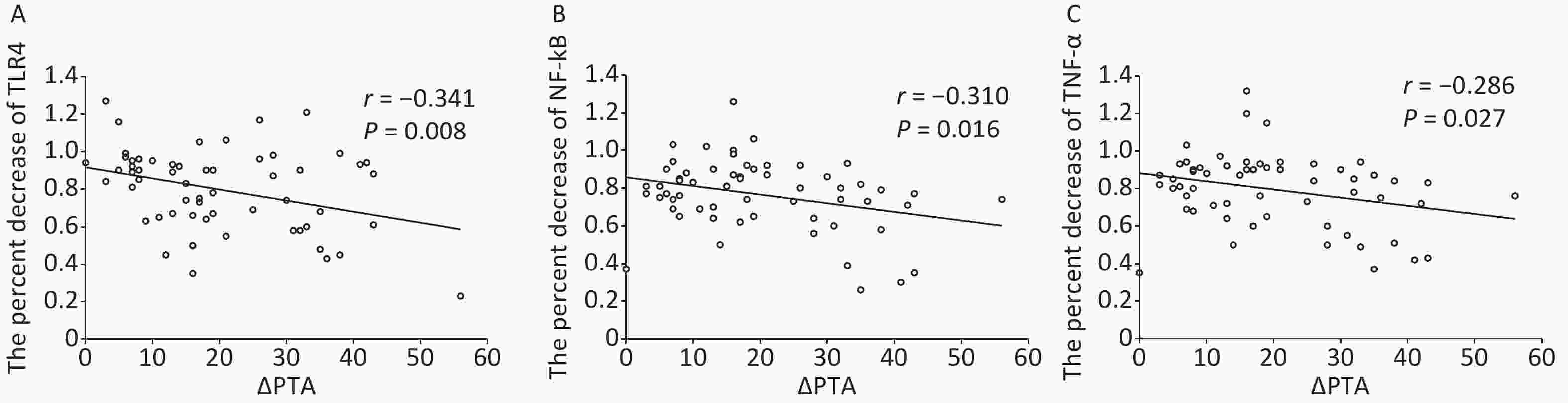
Figure 3. Association of TLR4 (A), NF-κB (B), and TNF-α (C) decline with hearing outcome. Pearson analysis showing the negative correlation between the percent decrease (the posttreatment divided by the pretreatment) of TLR4, NF-κB, and TNF-α expression after hyperbaric oxygen therapy and the relative hearing gain in HBO group (ΔPTA).
-
The results of the present study demonstrated the favorable effects of HBOT as therapy for sudden deafness. HBOT enhanced hearing threshold recovery and reduced inflammation in ISSHL patients by decreasing TLR4, NF-κB, and TNF-α expression in peripheral blood.
HBOT is used in ISSHL to increase partial oxygen pressure and improve blood profile and microcirculation. During HBOT, blood with a higher concentration of oxygen is transported through the bloodstream to the inner ear, thereby increasing microcirculation and hearing level[1, 16]. HBO combined with prednisone increased hearing gain in SSHL patients compared to prednisone treatment alone[17]. In a study examining the effects of oral steroid treatment without or with hyperbaric oxygen, the combined treatment was found to have higher efficacy (86.88% vs. 63.00%)[18]. The results of our study are consistent with these earlier reports: a significant improvement in hearing threshold was observed in the HBO as compared to the medicine group (86.67% vs. 66.67%).
Recent evidence has implicated inflammation in the development of ISSHL, as determined based on the number of white blood cells (WBCs) and their subtypes; IL-6, C-reactive protein, and TNF-α levels; and neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios[19]. In this study, we found that the level of the inflammatory factor TNF-α was elevated in the peripheral blood of ISSHL patients. TLRs recognize pathogen-associated molecular patterns of microorganisms and are thus key mediators of the innate immune response[20]. Cochlear damage induced by hypoxia stimulates the release of endogenous ligands, which are collectively referred to as danger-/damage-associated molecular patterns that are recognized by TLRs[21], which can elicit an inflammatory response in ISSHL. Activated TLRs bind to the adaptor molecule myeloid differentiation primary response 88, which associates with IL-1 receptor-associated kinase 4, leading to the sequential activation of tumor necrosis factor-associated factor 6 and inhibitor of κB complex. This leads to the degradation of inhibitor of NF-κB and activation of NF-κB, which regulates target genes including the proinflammatory cytokines TNF-α and IL-1β[22]. TLR4 expression was increased in peripheral blood leukocytes of ISSHL patients[12]. We determined that TLR4 as well as NF-κB levels were higher in peripheral blood of ISSHL patients relative to normal subjects, which indicates that the inflammatory response was activated in ISSHL.
HBOT has been shown to attenuate inflammation in various diseases[13,14]. In a model of multiple organ failure, HBOT suppressed zymosan-induced expression of TLR2 and TLR4, NF-κB activation, and cytokine production; these effects were associated with reduced injury to the lungs, liver, and intestine[23]. Exposure to HBO following trauma protected against secondary brain injury by inhibiting TLR4/NF-κB-mediated inflammation[24]. In addition, HBOT decreased the neutrophil-to-lymphocyte ratio and neutrophil and WBC counts in ISSHL patients, which alleviated inflammation and improved hearing levels[25]. In our study, HBOT combined with medicine treatment significantly decreased the expression of TLR4, NF-κB, and TNF-α compared to medicine alone. One possible mechanism underlying this effect may be that the increase of partial oxygen pressure in the cochlea after HBOT decreased production of endogenous damage-associated molecular patterns, and inhibited the activation of TLR4, NF-κB and release of TNF-α. The other possible mechanism may be that HBOT reduced TLR4 mRNA through a direct repression of gene transcription, further the activation of NF-κB and release of TNF-α[23]. We also conducted Pearson correlation analysis between the percent decrease of TLR4, NF-κB, and TNF-α expression after HBOT and a relative hearing gain in HBO group, which showed a significant negative correlation between them. So we identified that the lower levels of TLR4, NF-κB and TNF-α contribute to the improvement in hearing levels induced by HBOT. To our knowledge, this is the first report of the effects of HBOT on TLR- and NF-κB signaling-mediated inflammation in ISSHL. However, TLR4, NF-κB, and TNF-α expression was not restored to the baseline level after treatment, possibly because the ISSHL patients had not completed their treatment course. Further studies are needed to determine how many HBOT sessions are required for ISSHL patients and the precise mechanism by which HBOT influences TLR4 and NF-κB expression.
In conclusion, HBOT decreased the expression of inflammation-related cytokines TLR4, NF-κB, and TNF-α in peripheral blood, leading to an improvement in hearing levels in ISSHL patients. These results provide insight into the molecular mechanism for the therapeutic effects of HBOT on hearing loss. In order to evaluate the efficacy of HBOT compared to other treatment approaches, additional studies with larger cohorts and double-blind randomization are necessary.
-
LIU Xue Hua and YANG Jing were involved in the conception and design of work, data acquisition and interpretation, manuscript drafting and revision. LIANG Fang, JIA Xing Yuan, ZHAO Lin, and ZHOU Yan were involved in the data acquisition and analysis, manuscript revision. All authors read and approved the final manuscript.
doi: 10.3967/bes2020.045
Hyperbaric Oxygen Treatment Improves Hearing Level via Attenuating TLR4/NF-κB Mediated Inflammation in Sudden Sensorineural Hearing Loss Patients
-
Abstract:
Objective Hyperbaric oxygen treatment (HBOT) has demonstrated efficacy in improving hearing levels of patients with idiopathic sudden sensorineural hearing loss (ISSHL); however, the underlying mechanisms are not well understood. HBOT alleviates the inflammatory response, which is mediated by Toll-like receptor (TLR) 4 and nuclear factor (NF)-κB. In this study we investigated whether HBOT attenuates inflammation in ISHHL patients via alteration of TLR4 and NF-κB expression. Methods ISHHL patients (n = 120) and healthy control subjects (n = 20) were enrolled in this study. Patients were randomly divided into medicine group treated with medicine only (n = 60) and HBO group receiving both HBOT and medicine (n = 60). Audiometric testing was performed pre- and post-treatment. TLR4, NF-кB, and TNF-α expression in peripheral blood of ISSHL patients and healthy control subjects was assessed by ELISA before and after treatment. Results TLR4, NF-κB, and TNF-α levels were upregulated in ISSHL patients relative to healthy control subjects; the levels were decreased following treatment and were lower in the HBO group than that in the medicine group post-treatment (P < 0.05 and P < 0.01). Conclusion HBOT alleviates hearing loss in ISSHL patients by suppressing the inflammatory response induced by TLR4 and NF-κB signaling. -
Figure 3. Association of TLR4 (A), NF-κB (B), and TNF-α (C) decline with hearing outcome. Pearson analysis showing the negative correlation between the percent decrease (the posttreatment divided by the pretreatment) of TLR4, NF-κB, and TNF-α expression after hyperbaric oxygen therapy and the relative hearing gain in HBO group (ΔPTA).
Table 1. Clinical parameters of study subjects
Parameter Medicine group HBO group Control group Pa Sex, female/male, n 39/21 40/20 12/8 1.000 Age, years 0.556 17–45 17 21 9 46–75 43 39 11 Degree of hearing loss 0.774 Mild 19 21 − Moderate 13 9 − Moderate to severe 13 11 − Severe 7 7 − Profound 8 12 − Visit time, d 0.660 0–7 15 10 − 8–14 16 20 − 15–21 17 19 − 22–28 12 11 − Smoking, yes/no, n 8/52 12/48 4/16 0.463 Tinnitus, yes/no, n 42/18 37/23 − 0.336 Vertigo, yes/no, n 36/24 18/42 − 0.130 Note. aHBO vs. Medicine group. HBO, Hyperbaric oxygen. Table 2. Hearing improvement in ISSHL patients
Group Patient number Complete recovery Marked improvement Slight improvement No recovery Effective treatment (%) Medicine 60 6 13 21 20 66.67 HBO 60 12 18 22 8 86.67a Note. aP < 0.05, HBO vs. medicine group. HBO, hyperbaric oxygen. ISSHL, sudden sensorineural hearing loss. Table 3. Expression levels of inflammation-related factors before treatment (μg/mL)a
Parameter Medicine group HBO group Control group TLR4 4.60 ± 0.83b 4.69 ± 0.92c 1.86 ± 0.49 NF-κB 3.94 ± 0.94b 3.98 ± 0.79c 1.31 ± 0.36 TNF-a 4.05 ± 0.90b 4.11 ± 0.91c 1.16 ± 0.31 Note. aData are shown as mean ± SD. control group. bP < 0.01, Medicine vs. Control group. cP < 0.01, HBO vs. control group. HBO, hyperbaric oxygen. -
[1] Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg, 2012; 146(3 Suppl), S1−S35. [2] Purushothaman G, Purushothaman PK, Simham S, et al. A retrospective study of the clinical characteristics and post-treatment hearing outcome in idiopathic sudden sensorineural hearing loss. Audiol Res, 2017; 7, 168. [3] Eftekharian A, Amizadeh M. Pulse steroid therapy in idiopathic sudden sensorineural hearing loss: a randomized controlled clinical trial. Laryngoscope, 2016; 126, 150−5. doi: 10.1002/lary.25244 [4] Ajduk J, Ries M, Trotic R, et al. Hyperbaric oxygen therapy as salvage therapy for sudden sensorineural hearing loss. J Int Adv Otol, 2017; 13, 61−4. doi: 10.5152/iao.2017.3185 [5] Chi TH, Chiang MC, Chen RF, et al. Does the addition of hyperbaric oxygen therapy to conventional treatment modalities influence the outcome of soldiers with idiopathic sudden sensorineural hearing loss? J R Army Med Corps, 2018; 164, 69−71. doi: 10.1136/jramc-2017-000872 [6] Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother, 2015; 15, 523−31. doi: 10.1586/14737175.2015.1035712 [7] Quaranta N, De Ceglie V, D’Elia A. Endothelia dysfunction in idiopathic sudden sensorineural hearing loss: a review. Audiol Res, 2016; 6, 151. [8] Liquan C, Gaohua Z, Zhanhui Z, et al. Neutrophil-to-lymphocyte ratio predicts diagnosis and prognosis of idiopathic sudden sensorineural hearing loss: a systematic review and meta-analysis. Medicine (Baltimore), 2018; 97, e12492. doi: 10.1097/MD.0000000000012492 [9] Rider D, Furusho H, Xu S, et al. Elevated CD14 (cluster of differentiation 14) and toll-like receptor (TLR) 4 signaling deteriorate periapical inflammation in TLR2 deficient mice. Anat Rec (Hoboken), 2016; 299, 1281−92. doi: 10.1002/ar.23383 [10] Li X, Su L, Zhang X, et al. Ulinastatin downregulates TLR4 and NF-kB expression and protects mouse brains against ischemia/reperfusion injury. Neurol Res, 2017; 39, 367−73. doi: 10.1080/01616412.2017.1286541 [11] Lai JL, Liu YH, Liu C, et al. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation, 2017; 40, 1−12. doi: 10.1007/s10753-016-0447-7 [12] Yang CH, Hwang CF, Yang MY, et al. Expression of toll-like receptor genes in leukocytes of patients with sudden sensorineural hearing loss. Laryngoscope, 2015; 125, E382−7. doi: 10.1002/lary.25241 [13] Zhang Y, Yang Y, Tang H, et al. Hyperbaric oxygen therapy ameliorates local brain metabolism, brain edema and inflammatory response in a blast-induced traumatic brain injury model in rabbits. Neurochem Res, 2014; 39, 950−60. doi: 10.1007/s11064-014-1292-4 [14] Wu ZS, Lo JJ, Wu SH, et al. Early hyperbaric oxygen treatment attenuates burn-induced neuroinflammation by inhibiting the galectin-3-dependent toll-like receptor-4 pathway in a rat model. Int J Mol Sci, 2018; 19, E2195. doi: 10.3390/ijms19082195 [15] Furuhashi A, Matsuda K, Asahi K, et al. Sudden deafness: long term follow-up and recurrence. Clin Otolaryngol, 2002; 27, 458−63. doi: 10.1046/j.1365-2273.2002.00612.x [16] Attanasio G, Covelli E, Cagnoni L, et al. Does the addition of a second daily session of hyperbaric oxygen therapy to intratympanic steroid influence the outcomes of sudden hearing loss? Acta Otorhinolaryngol Ital, 2015; 35, 272−76. [17] Ersoy Callioglu E, Tuzuner A, Demirci S, et al. Comparison of simultaneous systemic steroid and hyperbaric oxygen treatment versus only steroid in idiopathic sudden sensorineural hearing loss. Int J Clin Exp Med, 2015; 8, 9876−82. [18] Alimoglu Y, Inci E, Edizer DT, et al. Comparison of oral steroid, intratympanic steroid, hyperbaric oxygen and oral steroid + hyperbaric oxygen treatments in idiopathic sudden sensorineural hearing loss cases. Eur Arch Otorhinolaryngol, 2011; 268, 1735−41. doi: 10.1007/s00405-011-1563-5 [19] Nash SD, Cruickshanks KJ, Zhan W, et al. Long-term assessment of systemic inflammation and the cumulative incidence of age-related hearing impairment in the epidemiology of hearing loss study. J Gerontol A Biol Sci Med Sci, 2014; 69, 207−14. [20] Kaisho T, Akira S. Critical roles of toll-like receptors in host defense. Crit Rev Immunol, 2000; 20, 393−405. [21] Krysko DV, Agostinis P, Krysko O, et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol, 2011; 32, 157−64. doi: 10.1016/j.it.2011.01.005 [22] Chen X, Yu G, Fan S, et al. Sargassum fusiforme polysaccharide activates nuclear factor kappa-B (NF-κB) and induces cytokine production via Toll-like receptors. Carbohyd Polym, 2014; 105, 113−20. doi: 10.1016/j.carbpol.2014.01.056 [23] Rinaldi B, Cuzzocrea S, Donniacuo M, et al. Hyperbaric oxygen therapy reduces the toll-like receptor signaling pathway in multiple organ failures. Intensive Care Med, 2011; 37, 1110−9. doi: 10.1007/s00134-011-2241-1 [24] Meng XE, Zhang Y, Li N, et al. Hyperbaric oxygen alleviates secondary brain injury after trauma through inhibition of tlr4/nf-κb signaling pathway. Med Sci Monit, 2016; 22, 284−8. doi: 10.12659/MSM.894148 [25] Li H, Zhao D, Diao M, et al. Hyperbaric oxygen treatments attenuate the neutrophil-to-lymphocyte ratio in patients with idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg, 2015; 153, 606−12. doi: 10.1177/0194599815589072 -




 下载:
下载:



 Quick Links
Quick Links