-
Bisphenol A (BPA) is a prevalent industrial organic synthetic material primarily utilized in the production of plastic bottles and inner coating of food cans[1,2]. Prior animal studies have demonstrated that BPA disrupts the endocrine system’s homeostasis, induces reproductive toxicity, triggers oxidative imbalance, and elicits diverse adverse effects on human health[3-5]. Additionally, BPA exposure has been linked to disturbances in gut microbial communities that can cause various local and systemic diseases, such as metabolic syndrome, type II diabetes, and obesity[6]. Since 2005, numerous countries have implemented policy ban or restrict BPA[7]. Consequently, certain BPA analogs or derivatives, such as bisphenol S (BPS) and bisphenol F (BPF), which are functionally or structurally similar to BPA, have been adopted as substitutes[8-10]. With their unrestricted use, these substances have been detected in food, environmental samples, and even in human blood, and urine[11]. The reported detection rate of BPF in body lotions, shampoos, soaps, and other personal care products is 2.6%–13.4%[12]. Numerous studies have reported that BPF exhibits toxic effects similar to those of BPA, including reproductive toxicity and endocrine disorders[13,14]. Thus, the safety of these BPA substitutes has become the subject of widespread public and government concern.
The gut microbiota refers to the microbial community residing in the gut, which plays a pivotal role in maintaining individual health and the origin and progression of diseases[15,16]. The composition of the microbiota community varies based on host genetics, diet, exogenous exposure, and location in the body, exerting a significant impact on the metabolic and immune systems[17,18]. Due to its sensitivity and specificity, the gut microbiota has emerged as a key area of interest for evaluating the toxic effects and mechanisms of environmental pollutants in recent years. Various environmental pollutants, such as microplastics, nanoparticles, and heavy metals, can alter the structure, composition, quantity, and metabolism of microbiota[19].
Bisphenols can bioaccumulate in the gut[20]. Previous studies have reported that high doses of BPA induce dysbiosis of gut microbiota and metabolic disturbances[21-23]. Feng et al. reported that dietary intake of BPA induced hepatic steatosis, which was closely related to dysbiosis of gut microbiota, elevated endotoxin levels, and increased liver inflammation[24]. Exposure to tetrabromobisphenol and BPS early in life has been shown to produce an overall trend of immunosuppression, manifested as an increase in anti-inflammatory secondary bile acids and propionic acid in the stool[25]. Bisphenol exposure resulted in concentration-dependent growth inhibition, alterations in membrane lipid composition, changes in short-chain fatty acid production, and shifts in the functional spectrum[26]. Limited knowledge exists regarding the potential impact of BPF, a substitute for BPA, on gut microbiota. A recent study demonstrated that exposure to BPF disrupts the gut microbial community in zebrafish[21]. This finding raises significant concerns, as alterations in the composition and abundance of specific bacterial groups have been associated with various diseases and conditions, including inflammatory bowel disease, irritable bowel syndrome, and colorectal cancer[27,28]. Moreover, elevated levels of bacterial groups that may pose a risk to human health, such as Pseudomonadota, are associated with gut dysbiosis and the development of gut-related pathologies. In contrast, reduced levels of beneficial bacterial groups, such as Bacteroidota and Bacillota, have been associated with obesity, type 2 diabetes, and other chronic diseases[27,29]. Consequently, further research is necessary to investigate the effects of BPF on gut microbiota in mammals.
The purpose of the present study was to evaluate the effects of BPA and its alternative, BPF, on the colonic fecal community and structure of mice using 16S rRNA sequencing. Additionally, the study aimed to compare the differences in the structure and abundance of bacterial flora between BPA- and BPF-exposed mice. These findings serve as a foundation for future research on the intestinal toxicity of BPs and contribute to an overall understanding of the adverse effects of BPA and its alternatives in vertebrates, including humans.
-
Six-to-eight-week-old C57BL/6 male mice (18–20 g) obtained from Charles River (Beijing, China) were reared in a specific pathogen-free laboratory for this study. After 1 week of adaptive feeding, 25 mice were randomly divided into five treatment groups: control, 50 μg/(kg∙day) BPA, 50 μg/(kg∙day) BPF, 5 mg/(kg∙day) BPA, and 5 mg/(kg∙day) BPF. Mice that received the same chemicals were housed together to prevent intergroup interference. The exposure doses for BPA and BPF (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) were set based on the United States Environmental Protection Agency’s recommendation of the no observed adverse effect level (NOAEL) for BPA [5 mg/(kg∙day)] and a reference dose of 50 μg/kg/day calculated from the NOAEL.
The BPA or BPF was first dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA). Ten microliters of the 2 mg/mL drug solution were dissolved in 100 mL of sterile drinking water (approximately 5 mL/d/20 g mouse). The DMSO concentration in drinking water was 0.001% (v/v) in all groups, including the control group. This test was repeated three times, and all protocols were approved by the Fifth Affiliated Hospital of Guangxi Medical University (No. 2022-090-01). All study procedures followed the guidelines for the care and use of animals of the Ethics Committee of the Fifth Affiliated Hospital of Guangxi Medical University.
-
After 14 days of exposure, the mice were euthanized in a clean biosafety cabinet. Stool samples were taken aseptically from the colon, placed in sterile DNA/RNase-free 1.5 mL Eppendorf tubes, and stored at −80 °C. Total bacterial DNA was extracted from the colonic stool samples using the AllPrep PowerFecal DNA/RNA Kit (Cat. No. 80244; Qiagen, Hilden, Germany) following the manufacturer’s instructions. Subsequently, 16S rRNA gene sequencing was performed as previously described[30]. Briefly, the 16S V3 + V4 variable regions of the qualified DNA samples were subjected to polymerase chain reaction (PCR) amplification, library preparation, and library quality inspection. The TAG sequences were used to distinguish the samples. The HiSeq 2500 high-throughput sequencing platform (Illumina, San Diego, CA, USA) was used to sequence the libraries.
-
Based on the overlapping relationship of paired-end reads obtained by HiSeq sequencing, the double-ended sequences were spliced into target region sequences using FLASH software[31]. The chimera sequences were detected and filtered using search 8.0 in QIIME software (http://qiime.org/). The final optimized sequence was generated by comparing the filtered sequence with a reference database and removing the chimeric sequence. The UCLUST algorithm in QIIME was used for operational taxonomic unit (OTU) cluster analysis[32]. The OTUs of each sample were annotated by taxonomy based on the Silva reference database (https://www.arb-silva.de/documentation/release-128/). Species differences and structural analyses were based on taxonomic information.
For comparison with equal variance, the Dunnett’s test and one-way analysis of variance (ANOVA) were conducted. Otherwise, the Wilcoxon test was used to compare means. Alpha diversity and principal coordinate analysis (PCoAs) for the microbial community structure were performed using QIIME and R software (The R Foundation for Statistical Computing, Vienna, Austria). Pairwise comparisons of bacterial taxa were conducted using a two-sided White’s nonparametric t-test in STAMP (v2.1.3; https://beikolab.cs.dal.ca/software/STAMP). Signaling pathways were predicted using phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of 16S rRNA sequencing data. Differences with a P < 0.05 were considered statistically significant.
-
The 16S rRNA sequencing revealed that most of the PCR products in the fecal samples of each group had an average length of 400 bp, which is consistent with the size of the V3 + V4 variable regions. A total of 1,236 OTUs were identified using Illumina high-throughput sequencing, representing nine phyla and 81 genera.
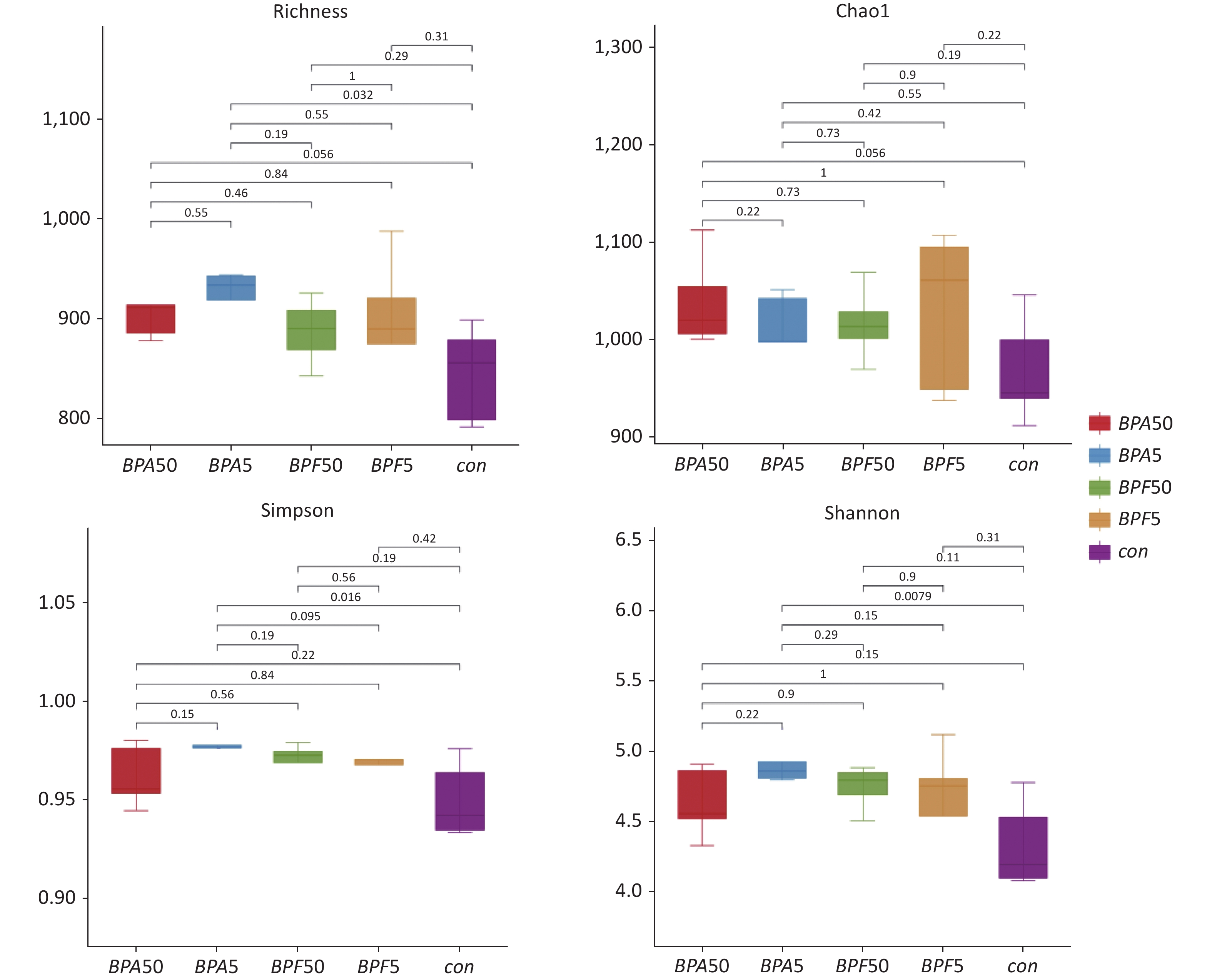
The key indices of bacterial alpha diversity (richness, Chao1, Simpson, and Shannon indices) of the different groups were analyzed to evaluate the complexity of microbial species diversity. Figure 1 displays, the species richness, Shannon, and Simpson indices of the group receiving 5 mg/(kg∙day) BPA group was notably higher than those of the DMSO control group (P < 0.05). However, the Chao1 index showed a slight increase without significance (P > 0.05). Notably, after two weeks of 50 μg/(kg∙day) BPA or BPF treatment, there was no substantial difference in the alpha diversity metrics. Community richness showed no difference between the two groups, and there was no difference in community diversity between the BPA and BPF treatments.
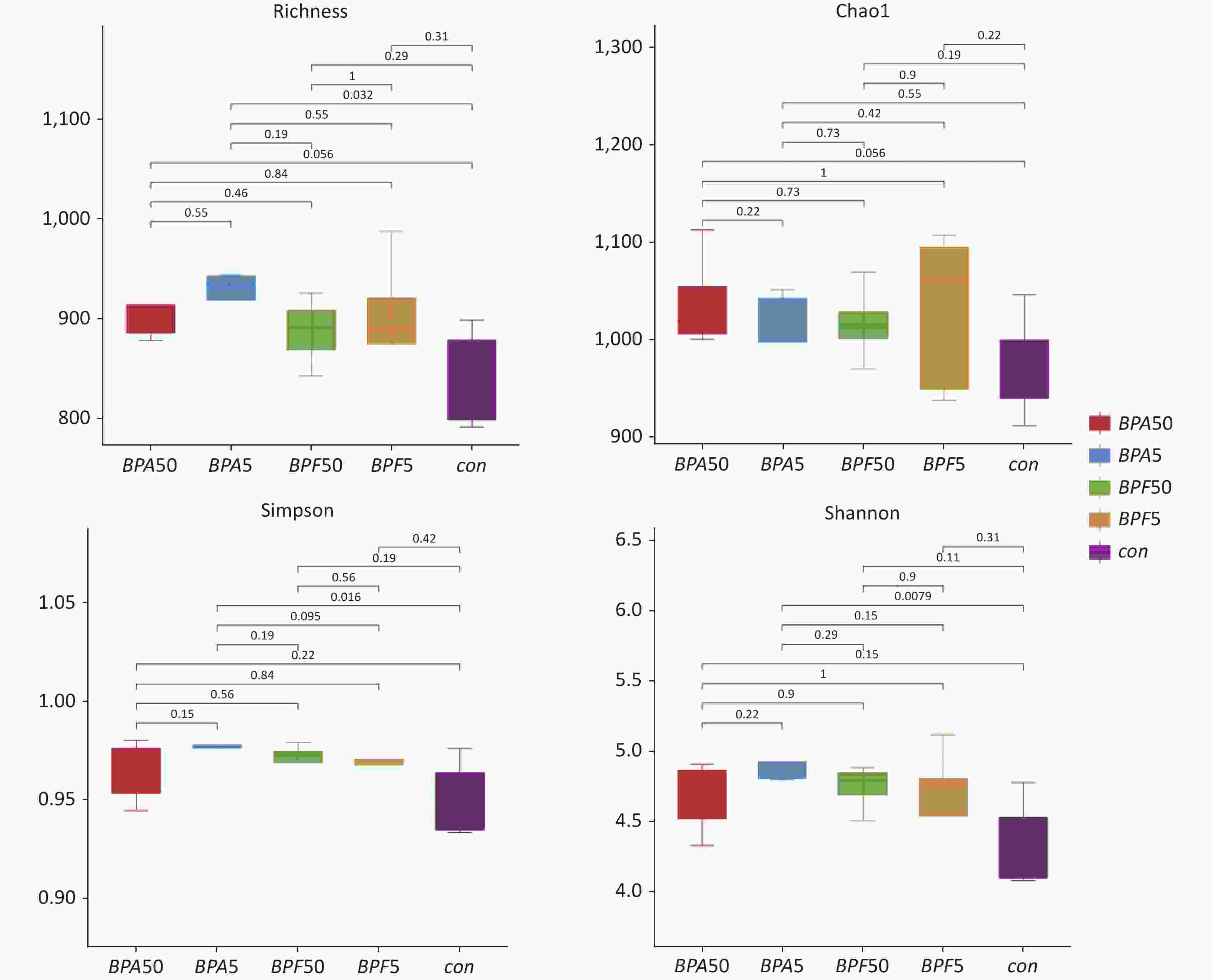
Figure 1. Alpha diversity metrics for BPA and BPF exposure. The study utilized 16S rRNA gene sequencing on 14-day-old mice exposed to BPA and BPF. The figure represents species richness, Shannon diversity, Simpson’s, and Chao1 indices. The experiment consisted of three biological replicates, each with five mice. BPA refers to bisphenol A, while BPF stands for bisphenol F.
-
The differences in bacterial composition between the treated animals and controls were analyzed using PCoA based on the OTU tables of the bacterial communities. Compared to the DMSO controls, exposure to different concentrations of BPA resulted in differences in beta diversity (Figure 2). In particular, PCoA clearly indicated that the confidence ellipses of the bacterial communities in the 50 μg/(kg∙day) BPA and 50 μg/(kg∙day) BPF treatments deviated significantly from those of the control group. In contrast, the confidence ellipse of the bacterial communities in the high-dose BPF treatment group covered that of the control group during the 14 days of exposure, indicating a similarity between the bacterial communities in the 5 mg/(kg∙day) BPF and control groups.
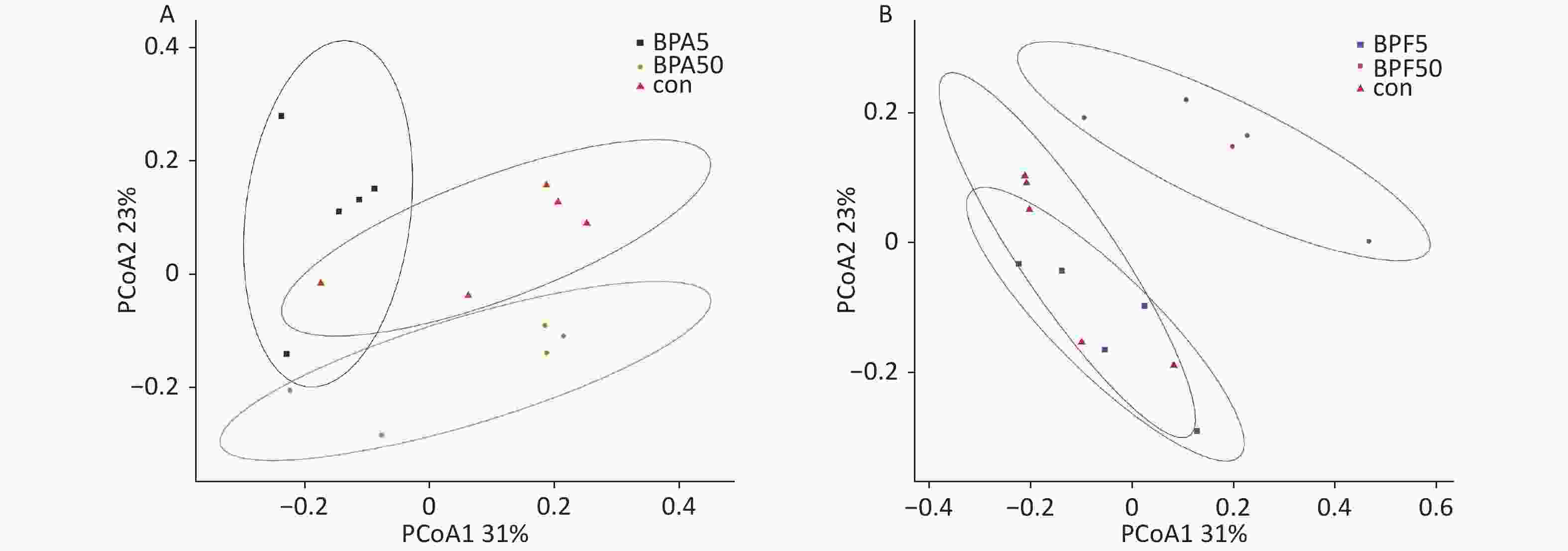
Figure 2. PCoA profile of pairwise community dissimilarity indices (Bray–Curtis), calculated from the OTU table of the bacterial communities on samples after BPA and BPF exposure, is shown here. Ovals indicate the 95% confidence intervals for each sample type. (A) PCoA profile after BPA exposure, (B) PCoA profile after BPF exposure. PCoA, principal coordinate analysis; OTU-operational taxonomic unit; BPA, bisphenol A; BPF, bisphenol F.
Based on the OTU classification results, we analyzed the abundance of specific species at the phylum level. Among the different groups, most of the species belonged to Bacteroidota, Bacillota, Pseudomonadota, Verrucomicrobia, Actinomycetota, Saccharibacteria, Deferribacteres, and Cyanobacteria. The relative abundance of Bacillota and Bacteroidota accounted for > 98% within the groups (Supplementary Figure S1, available in www.besjournal.com) and exhibited only minor changes among the BPA and DMSO control groups. An increase in the abundance of Bacillota and a decrease in the relative abundance of Bacteroidota were observed in both BPF groups. However, the difference was not significant (P > 0.05).
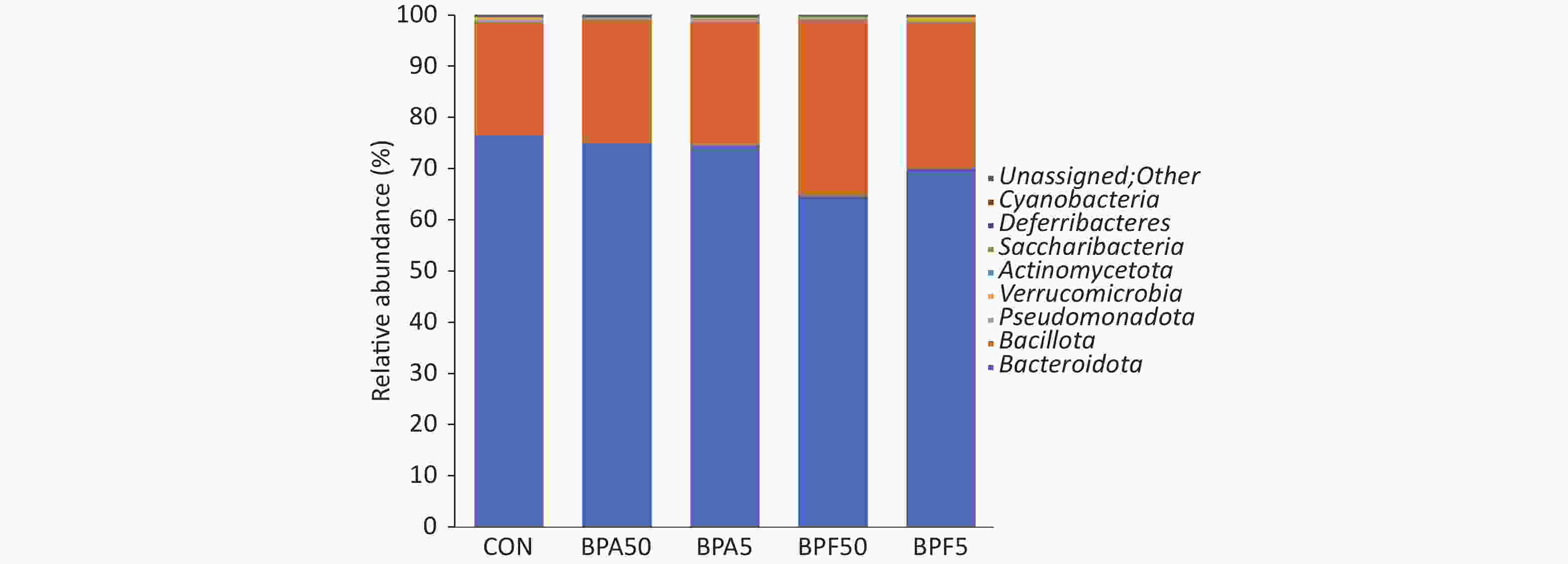
Figure S1. Sample species composition analysis at the phylum level. Three biological replicates, each with five mice per replicate. Con, control; BPA, bisphenol A; BPF, bisphenol F.
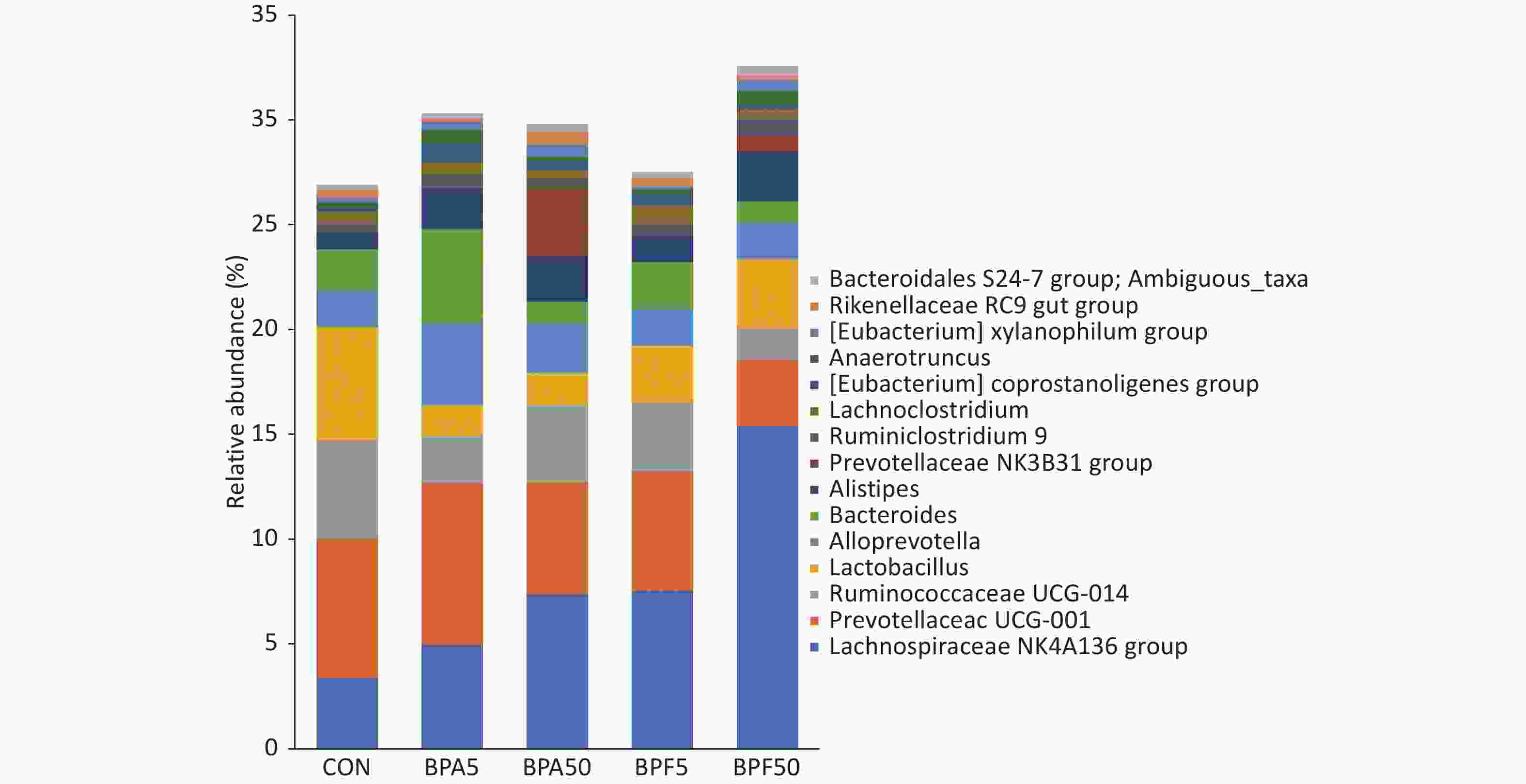
As illustrated in Figure 3, the 15 most abundant genera (in order of relative abundances) were Lachnospiraceae NK4A136, Prevotellaceae UCG-001, Ruminococcaceae UCG-014, Lactobacillaceae family, Alloprevotella, Bacteroides, Alistipes, Prevotellaceae NK3B31, Ruminiclostridium 9, Lachnoclostridium, Eubacterium coprostanoligenes, Anaerotruncus, Eubacterium xylanophilum, Rikenellaceae RC9 gut, and Bacteroidales S24-7. There were differences observed in the proportions of most bacterial genera among the treatment groups. Relative to that in the DMSO controls, the abundance of Lachnospiraceae NK4A136 increased in all treatment groups. This increase was higher in the low-concentration BPA and BPF groups than in the high-concentration BPA and BPF groups. The abundance of Prevotellaceae UCG-011 in the high-dose BPA treatment group increased, whereas it decreased in the other treatment groups, particularly in the 50 μg/(kg∙day) BPF group. The abundance of Ruminococcaceae UCG-014 and Lactobacillaceae decreased in all treatment groups, particularly in the 50 μg/(kg∙day) BPF group.
-
To identify the specifically altered bacteria at the genus level, a differential analysis of bacterial abundance among the treated groups and controls was performed using White’s nonparametric t-test. The results are depicted in Figure 4.
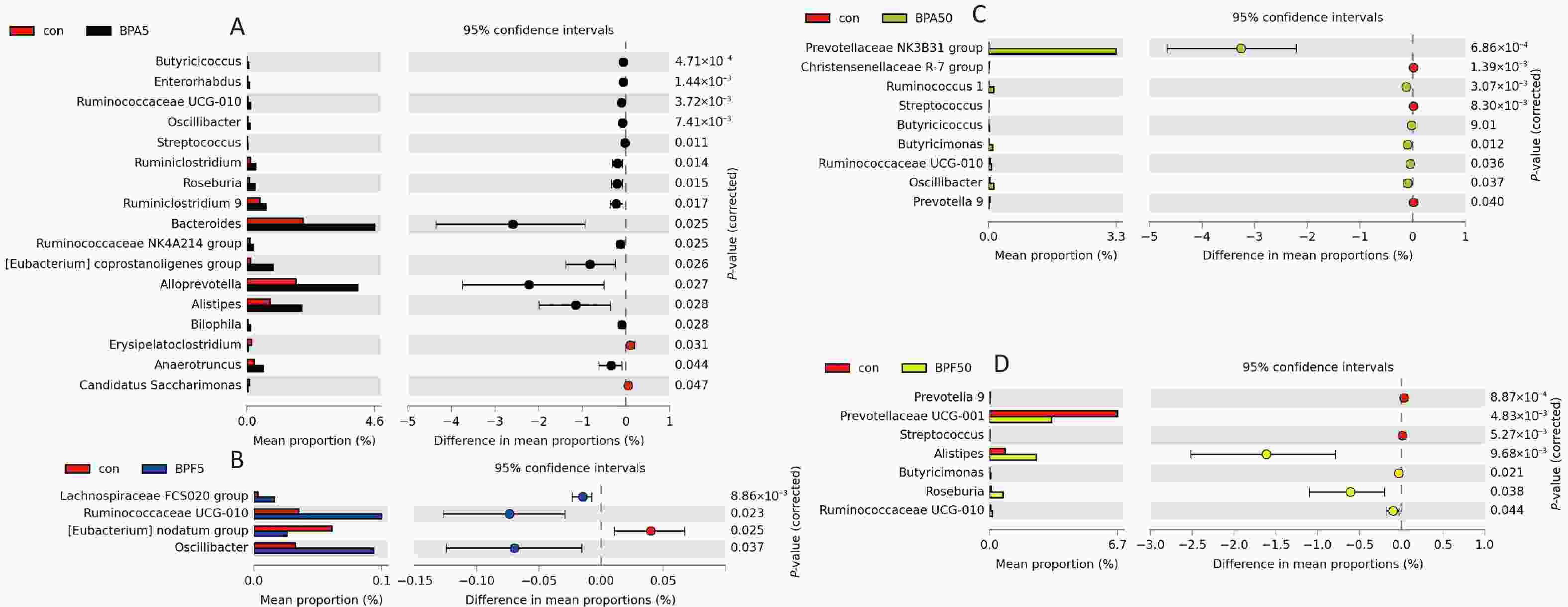
Figure 4. Extended error bar plots showing significant changes in genus level taxa after exposure to (A) 5 mg/(kg∙day) BPA, (B) 5 mg/(kg∙day) BPF, (C) 50 μg/(kg∙day) BPA, or (D) 50 μg/(kg∙day) BPF. White’s nonparametric t-test was used to identify significance (P < 0.05) for taxa classified at the genus level. Three biological replicates each with five mice per replicate were included. BPA, bisphenol A; BPF, bisphenol F.
Compared with those in the control group, 17 and 9 bacterial taxa were significantly changed in the 5 mg/(kg∙day) and 50 μg/(kg∙day) BPA-treated groups, respectively (P < 0.05, Figure 4A, C). Specifically, Butyricicoccus, Enterorhabdus, Ruminococcaceae UCG-010, Oscillibacter, Streptococcus, Ruminiclostridium, Roseburia, Ruminiclostridium 9, Bacteroides, Ruminococcaceae NK4A214 group, Eubacterium coprostanoligenes group, Alloprevotella, Alistipes, Bilophila, and Anaerotruncus were predominant in the 5 mg/(kg∙day) BPA group. Meanwhile, the abundance of Prevotellaceae NK3B31, Ruminococcus 1, Butyricicoccus, Butyricimonas, Ruminococcaceae UCG-010, and Oscillibacter significantly increased in the 50 μg/(kg∙day) BPA-treated group (P < 0.05). However, there were fewer significant changes in the bacterial taxa in the 5 mg/(kg∙day) and 50 μg/(kg∙day) BPF-treated groups (four and seven, respectively) than in the BPA-treated group (P < 0.05; Figure 4B, D). As depicted in Figure 4C, Lachnospiraceae FCS020, Ruminococcaceae UCG-010, and Oscillibacter were considerably enriched in the 5 mg/(kg∙day) BPF group. Correspondingly, Ruminococcaceae UCG-010, Alistipes, Butyricimonas, Roseburia, and Prevotellaceae UCG-001E were more abundant in the 50 μg/(kg∙day) BPF group than in the DMSO control group. However, the opposite effect was observed for Prevotella 9 and Streptococcus spp. (Figure 4D).
The number of bacterial genera predominantly enriched in the control group was greater than that in all the chemically treated groups. Additionally, a similar shift in microbial differences was observed among the treatment groups. The relative abundance of Ruminococcaceae UCG-010 was significantly downregulated in both BPA and BPF groups (P < 0.05). Except for the low-dose BPF group, the relative abundance of Oscillibacter was also significantly downregulated in the treatment groups (P < 0.05). Furthermore, Prevotella 9 and Streptococcus were significantly decreased in the low-dose BPA (Figure 4B) and BPF (Figure 4D) groups. Conversely, the abundance of Streptococcus markedly increased in the 5 mg/(kg∙day) BPA group.
Analysis of the genus level variation between the control and treatment groups revealed that Ruminococcaceae UCG-010, Butyricimonas, Ruminococcus 1, Roseburia, Lachnospiraceae FCS020, Enterorhabdus, Alistipes, Desulfovibrio, Oscillibacter, Eubacterium coprostanoligenes, and Prevotellaceae NK3B31 were more abundant in the treatment groups than in the control group (Figure 5A). Notably, no specific increase in genus abundance was observed in the control group.
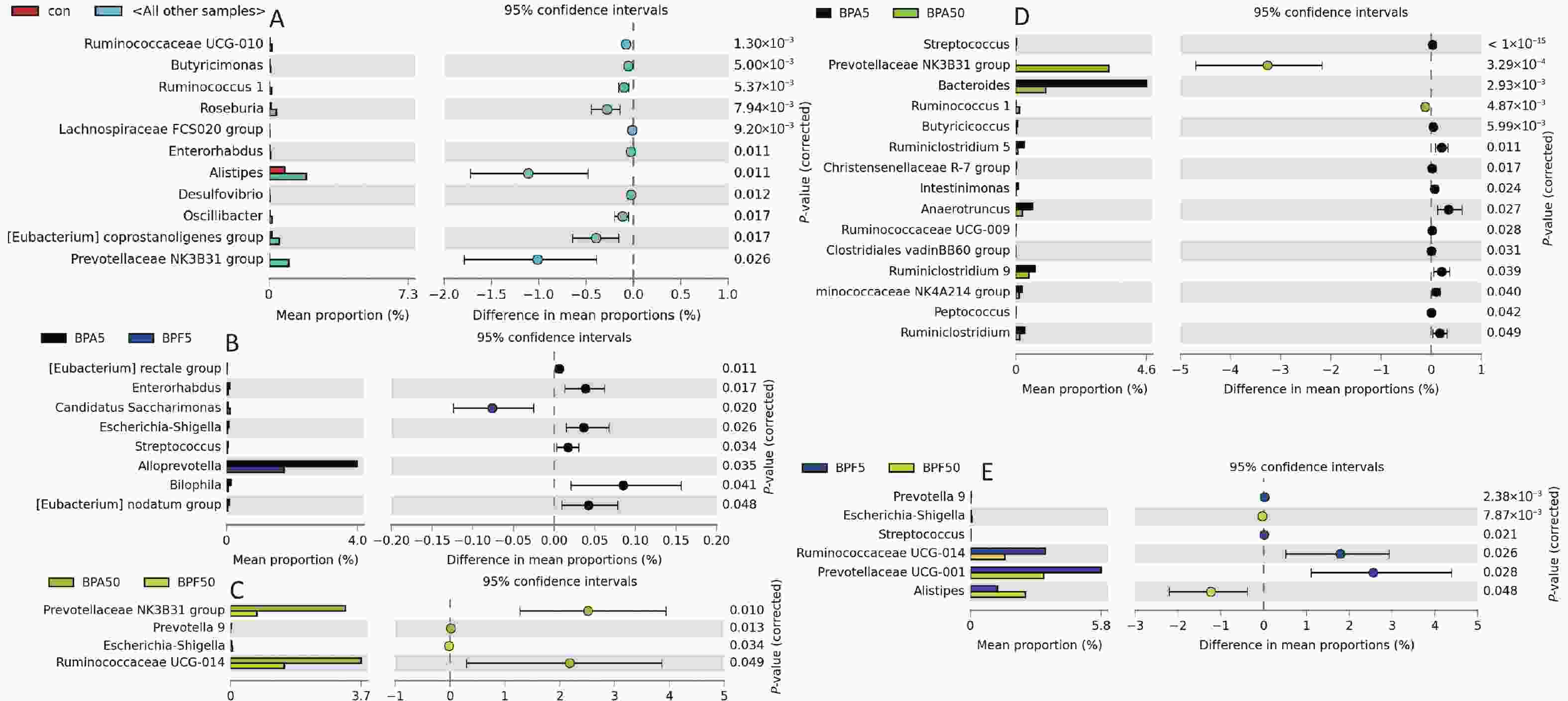
Figure 5. Bar plot of differential bacterial communities between (A) the control and all other treatment groups, (B) 5 mg/(kg∙day) BPA and 5 mg/(kg∙day) BPF group, (C) 50 μg/(kg∙day) BPA and 50 μg/(kg∙day) BPF group, (D) 5 mg/(kg∙day) BPA and 50 μg/(kg∙day) BPA group, (E) 5 mg/(kg∙day) BPF and 50 μg/(kg∙day) BPF groups, which was performed using the White’s nonparametric t-test (P < 0.05). Three biological replicates, each with five mice per replicate, were included. BPA, bisphenol A; BPF, bisphenol F.
When compared across the same exposure concentrations, six genera (Eubacterium rectale group, Candidatus Saccharimonas, Escherichia-Shigella, Streptococcus, Alloprevotella, Bilophila, and Eubacterium nodatum group) in the 5 mg/(kg∙day) BPA group and three genera (Prevotellaceae NK3B31, Prevotella 9, and Ruminococcaceae UCG-014) in the 50 μg/(kg∙day) BPA group were significantly upregulated (P < 0.05). Correspondingly, Enterorhabdus and Escherichia-Shigella were significantly downregulated in both these groups (Figure 5B, C).
Subsequently, we analyzed and identified the predominant differential flora among the different treatment groups. A total of 15 genera were significantly different between the BPA groups, but only six genera were altered in the BPF groups (P < 0.05, Figure 5D, E). As shown in Figure 5D, with increasing BPA exposure concentrations, Streptococcus, Bacteroides, Butyricicoccus, Ruminiclostridium 5, Christensenellaceae R-7, Intestinimonas, Anaerotruncus, Ruminococcaceae UCG-009, Clostridiales vadin BB60, Ruminiclostridium 9, Ruminococcaceae NK4A214, Peptococcus, and Ruminiclostridium significantly increased, whereas Prevotellaceae NK3B31 and Ruminococcus 1 were downregulated (P < 0.05). For the high-concentration BPF treatment, Prevotella 9, Streptococcus, Ruminococcaceae UCG-014, and Prevotellaceae UCG-001 were upregulated, whereas Alistipes and Escherichia-Shigella were significantly downregulated (P < 0.05; Figure 5E).
-
A phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) analysis was conducted to predict the functions of the gut microbial community based on the 16S rRNA gene sequencing data. After mapping the KEGG Orthology categories to the KEGG pathways and removing those with low effect size (< 0.01), two pathways differed significantly between the high-dose BPA group and the DMSO control group: 13 pathways for the 50 μg/(kg∙day) BPA group and four pathways for the 50 g/(kg∙day) BPF group (P < 0.05, Figure 6). No differences in microbiological functions were observed between the high-dose BPF and control groups. Furthermore, the KEGG categories indicated that functions related to amino acid metabolism (phenylalanine metabolism) and infectious diseases (Salmonella infection) were significantly enriched in male mice exposed to 5 mg/(kg∙day) BPA (P < 0.05). The microbiological functions changed considerably in the 50 μg/(kg∙day) BPA group and were associated with various processes, including carbohydrate metabolism (pentose and glucuronate interconversions, ascorbate and aldarate metabolism, and pyruvate metabolism), glycan biosynthesis and metabolism (peptidoglycan biosynthesis and degradation proteins), metabolism (amino acid metabolism and energy metabolism), replication and repair (chromosome and associated proteins, mismatch repair pathway, DNA replication, and DNA replication proteins), transport, and catabolism (prokaryotic defense system, ribosome biogenesis, and RNA polymerase). In contrast, the microbiological function was less affected in the mice after exposure to 50 μg/(kg∙day) BPF, which showed clear differences from 50 μg/(kg∙day) BPA, and it was primarily related to carbohydrate metabolism (pentose and glucuronate interconversions), enzyme families (peptidases), glycan biosynthesis and metabolism (peptidoglycan biosynthesis), and metabolism of other amino acids (cyanoamino acid metabolism) (Figure 6C).
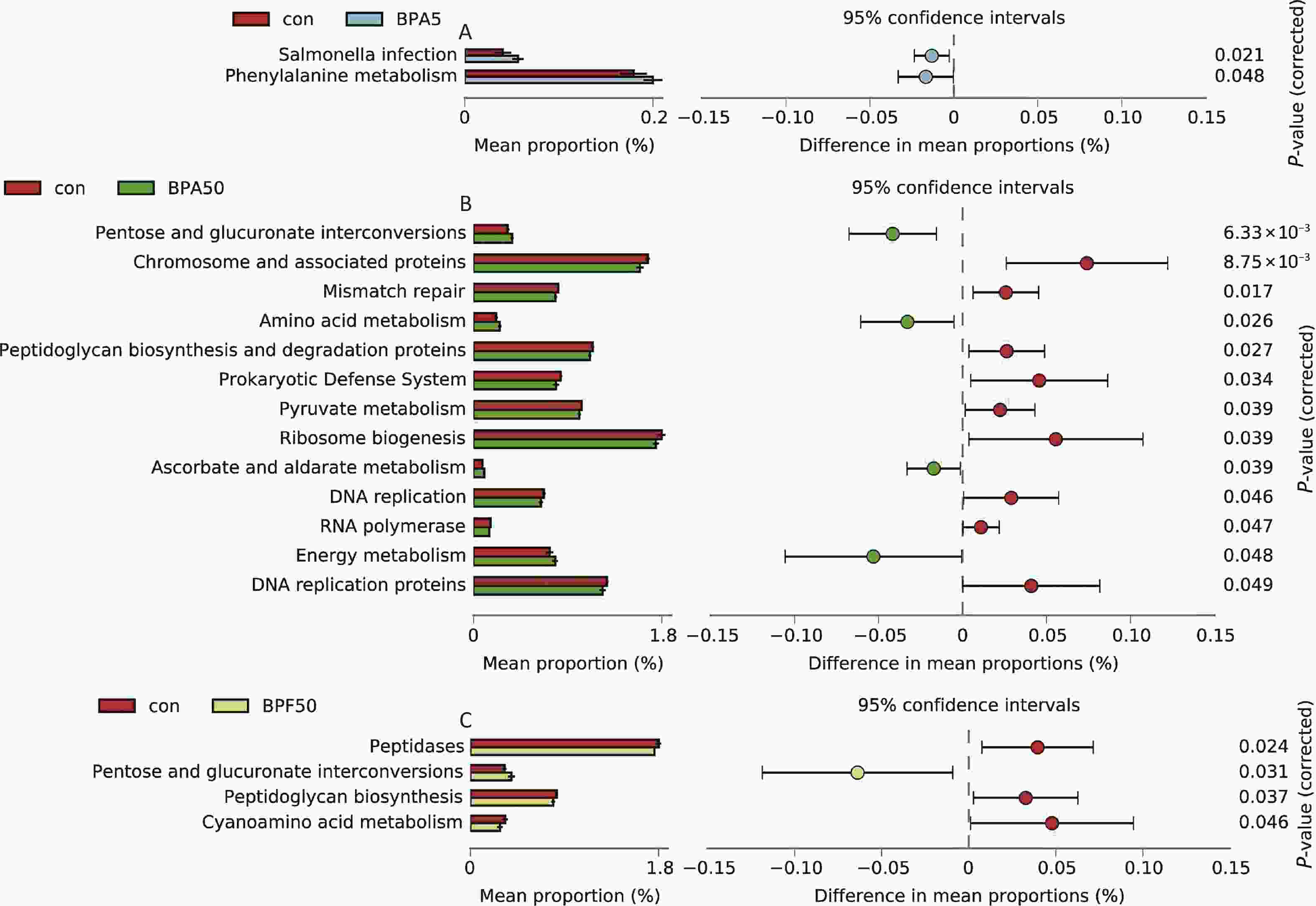
Figure 6. Predictive functional profiling was analyzed using PICRUSt and KEGG categories between (A) the control and 5 mg/(kg∙day) BPA group, (B) the control and 50 μg/(kg∙day) BPA group, (C) the control and 50 μg/(kg∙day) BPF group. Three biological replicates, each with five mice per replicate, were included. con, control; BPA, bisphenol A; BPF, bisphenol F; PICRUSt, phylogenetic investigation of communities by reconstruction of unobserved states; KEGG, Kyoto Encyclopedia of Genes and Genomes.
-
In recent years, 16S rRNA gene sequencing technology has been commonly used to study the intestinal microflora due to its advantages of high sensitivity, strong specificity, high throughput, low cost, and rapid operation[33,34]. Gut microbiota are increasingly recognized to play an important role in the maintenance of host health by regulating various physiological activities such as immune responses, energy metabolism, and neural signaling[35]. Alterations in the intestinal flora may cause physiological dysfunction in the host animal and lead to the occurrence of various diseases. In the current study, we evaluated the effects of BPA and its alternative, BPF, on the gut microbiota of male mice after 14 days of exposure. We found that BPA and BPF altered the microbial community structure, increased the diversity of species, regulated the relative abundance of different microbiota, and exerted different toxic effects.
A decrease in gut microbiota diversity has been associated with several human intestinal disorders[36]. Based on alpha diversity analysis, we observed a significant increase in the number of observed species and higher Shannon and Simpson indices in male mice following two weeks of exposure to 5 mg/(kg∙day) BPA (P < 0.05). However, the observed species did not increase substantially at 50 μg/(kg∙day) BPA, suggesting that this dosage may not be sufficient to have a significant effect on gut microbial diversity (P > 0.05). No significant differences were detected between the BPF and control groups or between the BPA and BPF treatments (P > 0.05). Feng et al. reported that the species diversity of the gut microbiota was reduced in male CD-1 mice treated with 50 μg/(kg∙day) BPA for 24 weeks[24]. These differences in diversity may be attributed to variations in mouse strains and exposure times among the studies.
The results of the difference analysis at the genus level demonstrated that the variation in bacterial numbers was greater in the BPA group than that in the BPF group at the same exposure concentration. Moreover, 15 bacterial species were not changed by BPF treatment but significantly decreased or increased under BPA (P < 0.05). These microbial differences may be attributed to the variation in the types and concentrations of the relevant exposed chemicals. Here, we have demonstrated for the first time that BPF, the most commonly detected BPA alternative in the environment, induces gut microbiota disorders in mammals and emphasizes the potential negative impact of BPF on the intestines. Similarly, Catron et al. demonstrated that exposure to BPF led to a significant dose-dependent disturbance in the microbial community structure in zebrafish[21]. Notably, BPF treatment did not alter the abundance of Butyricicoccus, Enterorhabdus, Ruminiclostridium, Ruminiclostridium 9, Bacteroides, Ruminococcaceae NK4A214, Eubacterium coprostanoligenes, Alloprevotella, Bilophila, Prevotellaceae NK3B31, Ruminococcus 1, or Anaerotruncus, whereas BPA treatment significantly increased their abundances (P < 0.05). The abundance of Erysipelatoclostridium and Candidatus Saccharimonas significantly decreased in the BPA groups (P < 0.05); however, BPF treatment did not affect these bacteria. Furthermore, BPF treatment increased the abundance of Prevotellaceae UCG-001E and Lachnospiraceae FCS020. However, BPA treatment did not alter these bacteria. Although Streptococcus was significantly increased in the 5 mg/(kg∙day) BPA group, it was significantly decreased in the 50 g/(kg∙day) BPA and BPF groups (P < 0.05). Similar to the results reported in a previous study, the relative abundances of Ruminococcus 1, Oscillibacter, Christensenellaceae R-7, Ruminococcaceae NK4A214, Ruminococcaceae UCG-010, and Ruminiclostridium 9 were significantly altered in BPA-exposed male mice[37]. In addition, the change in Christensenellaceae R-7 group and Ruminiclostridium 9 could lead to functional profile disorder and diminished levels of single-chain fatty acids[37].
Disruption of the microbiota may cause adverse effects, and its correlation with infection or disease severity warrants further investigation. Previous research has reported that the Bacillota/Bacteroidota ratio may be a relevant marker of intestinal microbiota dysregulation in obese patients[38]. Ni et al. observed that the Bacillota/Bacteroidota ratio increased in male C57BL/6 mice exposed to BPA for 22 weeks, which might be associated with memory function and learning ability[37]. In our study, an increasing trend in the Bacillota/Bacteroidota ratio was observed in both BPF groups, although the difference was not significant (P > 0.05). We found that Bacteroides were significantly upregulated in the 5 mg/(kg∙day) BPA group, which is consistent with the results of previous studies. For example, pre-copulation exposure to BPA during lactation in mice results in a sex-dependent increase in Bacteroides species[39]. Xu et al. also reported that high-dose BPA exposure increased Bacteroides in males after 122 days of chronic treatment but decreased the abundance after 16 days of sub-acute BPA exposure[40]. Similarly, in our study, Prevotella 9 levels were significantly decreased following exposure to 50 g/(kg∙day) of BPA or BPF. Diamante et al. reported that Prevotella levels were low or not observed in the samples of a BPA-treated female offspring[41]. Additionally, previous research on different doses of BPA revealed changes in the relative abundances of Ruminococcaceae and Lachnospiraceae[40]. In our current study, we found a significant increase in the abundance of Ruminococcaceae UCG-010 and Lachnospiraceae FCS020 following BPA or BPF exposure (P < 0.05).
PICRUSt analysis revealed that compared to the 5 mg/(kg∙day) BPF treatment, the other treatments significantly enriched many KEGG pathways, indicating that the microbes in our study responded to both BPA and BPF (P < 0.05). These findings suggest that mice exposed to different BPA analogs exhibit different metabolic functions in their gut microbiota, which could potentially have varying effects on the physiological functions of mice and produce different biological toxicities. However, exposure to BPA and BPF did not result in dose-dependent changes in the relative abundance of the predicted functions. The enriched functional profiles in response to exposure to both BPA and BPF showed similar changes in structural and predicted metabolic functions. Glycan biosynthesis and metabolism, carbohydrate metabolism, and amino acid metabolism level 3 KEGG functions were significantly enriched (P < 0.05). These pathways play critical roles in maintaining host health and are involved in many essential biological processes. Glycans are important for cell signaling and immune functions, whereas carbohydrate metabolism provides the energy required to maintain normal cellular processes[42]. Amino acid metabolism is crucial for protein synthesis, the regulation of neurotransmitters and hormones, and energy production[43]. The enrichment of these metabolic pathways indicates their significance in supporting vital physiological and cellular functions, which are necessary for overall host health, development, and homeostasis. Previous research by Yao et al. demonstrated alterations in neuroactive metabolites within the tryptophan and dopamine pathways in both the brain and serum after exposure to BPA, suggesting that the disruption of these metabolic pathways may be involved in BPA-induced neuropsychiatric disorders[44]. Previous studies have reported that exposure to BPA and BPF can lead to dose-dependent changes in the relative abundance of predicted functions; however, these changes in functions were independent of the BPS concentration[21]. These inconsistencies in the findings could be attributed to the differences in species exposed, duration of exposure, sex of the subjects, endogenous hormones, and developmental phases.
Changes in gut microbiome community richness and diversity may affect overall host health. The changes in bacterial groups observed in this study, specifically the elevated levels of Ruminococcaceae UCG-010 and Oscillibacter, and decreased levels of Prevotella 9 and Streptococcus, can also have health implications. Ruminococcaceae UCG-010 and Oscillibacter have been linked to inflammation, metabolic disorders, and an altered gut-brain axis, whereas Prevotella 9 and Streptococcus have been linked to anti-inflammatory effects and gut health[45-49]. These microbial changes induced by BPA increase oxidative stress and reduce the diversity of the gut microbiota, including a decrease in protective bacteria such as short-chain fatty acid producers[23]. An imbalance in the abundance of bacterial groups involved in carbohydrate metabolism and immune system regulation can lead to various metabolic and inflammatory disorders. Decreased diversity can result in the loss of important microbial functions and dysbiosis, which have been linked to various diseases, including metabolic, autoimmune, and mental health disorders. Elevated levels of bacteria, such as Staphylococcus, can lead to foodborne illnesses, gastrointestinal infections, skin infections, and other health issues in humans[49].
Since this study was conducted in mice, the findings may not directly translate to human health. Secondly, the study focused on a limited number of bacterial groups and did not investigate the functional outcomes associated with the changes in these bacterial communities. Additionally, the study cannot establish a causal relationship between BPA or BPF exposure and changes in the gut microbiome. However, these findings can be applied to future research or policies related to the safety of BPA and its substitutes by demonstrating the need for improved regulations and restrictions on the use of these chemicals in consumer products. Another limitation of the current study was that only male mice were used for ease of standardization, to reduce the potential variability in the results, and to focus on a specific population. However, the inclusion of both male and female animals would have enabled investigators to better understand the potential differences in the gut microbial composition between the sexes and reveal the potential sex-specific effects of BPA and BPF exposure. Previous animal studies have shown that exposure to BPA can alter gut microbiota in a sex-specific manner. Male mice exposed to BPA exhibited increased levels of Prevotellaceae, Akkermansia, and Methanobrevibacter in their gut microbiome. However, female mice did not show any changes in the relative abundance of Prevotellaceae[23]. Furthermore, due to the group housing of the mice and the use of water to deliver BPA and BPF, it was challenging to control the exact dosage of the compound to which each animal was exposed, which may have led to some variability in the results. Another limitation was the absence of baseline stool samples collected prior to exposure to BPA and BPF. Collecting samples from the same animal before and after exposure would have controlled for individual differences in the gut microbial composition and minimized the influence of other environmental factors on the microbial community.
Despite these limitations, the study results provide valuable insights into the potential health impacts of these chemicals and highlight the need for further controlled studies to better understand their effects on human health. Future research should address these limitations to minimize the experimental variability and increase the accuracy of the findings.
In summary, the results reveal disruptions in gut microbial structure and function in mice exposed to BPA or BPF, characterized by elevated levels of Ruminococcaceae UCG-010 and Oscillibacter and decreased levels of Prevotella 9 and Streptococcus. Additionally, the pathways related to carbohydrate and amino acid metabolism were enriched. Further studies with longer exposure times, a wider range of doses, and intestinal indices would help to elucidate the correlation between BPA analog-induced gut microbiota changes and infection or disease severity. Furthermore, metagenomic sequencing, proteomics, metabolomics, and other technologies would provide a comprehensive perspective on studying the toxicity of BPA analogs from the view point of gut microbiota.
-
In summary, this study has revealed marked alterations in the gut microbiome community richness and diversity, species composition, and function in mice exposed to BPA or BPF. These changes were characterized by elevated levels of Ruminococcaceae UCG-010 and Oscillibacter and decreased levels of Prevotella 9 and Streptococcus. In addition, the pathways associated with carbohydrate and amino acid metabolism were significantly enriched. These findings demonstrate that mice exposed to different BP analogs have distinct gut bacterial community richness, composition, and related metabolic pathways. Given the fundamental role of gut bacteria in maintaining intestinal homeostasis, our study highlights the intestinal toxicity of BPs in vertebrates.
-
The authors are responsible for all aspects of the work, including ensuring that any issues related to the accuracy or integrity of any part of the work have been properly investigated and resolved. All study protocols were approved by the Fifth Affiliated Hospital of Guangxi Medical University (no. 2022-090-01). All procedures followed the guidelines of the Ethics Committee of the Fifth Affiliated Hospital of Guangxi Medical University for Animal Care and Use.
doi: 10.3967/bes2024.003
Effects of Bisphenol A and Its Substitute, Bisphenol F, on the Gut Microbiota in Mice
-
Abstract:
Objective The aim of this study was to assess the impact of bisphenol A (BPA) and its substitute, bisphenol F (BPF), on the colonic fecal community structure and function of mice. Methods We exposed 6–8-week-old male C57BL/6 mice to 5 mg/(kg∙day) and 50 μg/(kg∙day) of BPA or BPF for 14 days. Fecal samples from the colon were analyzed using 16S rRNA sequencing. Results Gut microbiome community richness and diversity, species composition, and function were significantly altered in mice exposed to BPA or BPF. This change was characterized by elevated levels of Ruminococcaceae UCG-010 and Oscillibacter and decreased levels of Prevotella 9 and Streptococcus. Additionally, pathways related to carbohydrate and amino acid metabolism showed substantial enrichment. Conclusion Mice exposed to different BP analogs exhibited distinct gut bacterial community richness, composition, and related metabolic pathways. Considering the essential role of gut bacteria in maintaining intestinal homeostasis, our study highlights the intestinal toxicity of BPs in vertebrates. -
Key words:
- Bisphenol A (BPA) /
- Bisphenol F (BPF) /
- 16S rRNA /
- Microbiota
The authors declare that they have no competing interests.
&These authors contributed equally to this work.
注释:1) AUTHOR CONTRIBUTIONS: 2) COMPETING INTERESTS: -
Figure 1. Alpha diversity metrics for BPA and BPF exposure. The study utilized 16S rRNA gene sequencing on 14-day-old mice exposed to BPA and BPF. The figure represents species richness, Shannon diversity, Simpson’s, and Chao1 indices. The experiment consisted of three biological replicates, each with five mice. BPA refers to bisphenol A, while BPF stands for bisphenol F.
Figure 2. PCoA profile of pairwise community dissimilarity indices (Bray–Curtis), calculated from the OTU table of the bacterial communities on samples after BPA and BPF exposure, is shown here. Ovals indicate the 95% confidence intervals for each sample type. (A) PCoA profile after BPA exposure, (B) PCoA profile after BPF exposure. PCoA, principal coordinate analysis; OTU-operational taxonomic unit; BPA, bisphenol A; BPF, bisphenol F.
Figure 4. Extended error bar plots showing significant changes in genus level taxa after exposure to (A) 5 mg/(kg∙day) BPA, (B) 5 mg/(kg∙day) BPF, (C) 50 μg/(kg∙day) BPA, or (D) 50 μg/(kg∙day) BPF. White’s nonparametric t-test was used to identify significance (P < 0.05) for taxa classified at the genus level. Three biological replicates each with five mice per replicate were included. BPA, bisphenol A; BPF, bisphenol F.
Figure 5. Bar plot of differential bacterial communities between (A) the control and all other treatment groups, (B) 5 mg/(kg∙day) BPA and 5 mg/(kg∙day) BPF group, (C) 50 μg/(kg∙day) BPA and 50 μg/(kg∙day) BPF group, (D) 5 mg/(kg∙day) BPA and 50 μg/(kg∙day) BPA group, (E) 5 mg/(kg∙day) BPF and 50 μg/(kg∙day) BPF groups, which was performed using the White’s nonparametric t-test (P < 0.05). Three biological replicates, each with five mice per replicate, were included. BPA, bisphenol A; BPF, bisphenol F.
Figure 6. Predictive functional profiling was analyzed using PICRUSt and KEGG categories between (A) the control and 5 mg/(kg∙day) BPA group, (B) the control and 50 μg/(kg∙day) BPA group, (C) the control and 50 μg/(kg∙day) BPF group. Three biological replicates, each with five mice per replicate, were included. con, control; BPA, bisphenol A; BPF, bisphenol F; PICRUSt, phylogenetic investigation of communities by reconstruction of unobserved states; KEGG, Kyoto Encyclopedia of Genes and Genomes.
-
[1] González N, Cunha SC, Ferreira R, et al. Concentrations of nine bisphenol analogues in food purchased from Catalonia (Spain): comparison of canned and non-canned foodstuffs. Food Chem Toxicol, 2020; 136, 110992. doi: 10.1016/j.fct.2019.110992 [2] Wang CC, He JY, Xu TF, et al. Bisphenol A (BPA), BPS and BPB-induced oxidative stress and apoptosis mediated by mitochondria in human neuroblastoma cell lines. Ecotoxicol Environ Saf, 2021; 207, 111299. doi: 10.1016/j.ecoenv.2020.111299 [3] Bousoumah R, Leso V, Iavicoli I, et al. Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: a systematic review. Sci Total Environ, 2021; 783, 146905. doi: 10.1016/j.scitotenv.2021.146905 [4] Sun YX, Wang XY, Zhou YY, et al. Protective effect of metformin on BPA-induced liver toxicity in rats through upregulation of cystathionine β synthase and cystathionine γ lyase expression. Sci Total Environ, 2021; 750, 141685. doi: 10.1016/j.scitotenv.2020.141685 [5] Wu MD, Wang SY, Weng QY, et al. Prenatal and postnatal exposure to bisphenol A and asthma: a systemic review and meta-analysis. J Thorac Dis, 2021; 13, 1684−96. doi: 10.21037/jtd-20-1550 [6] Charitos IA, Topi S, Gagliano-Candela R, et al. The toxic effects of endocrine disrupting chemicals (EDCs) on gut microbiota: bisphenol A (BPA) a review. Endocr Metab Immune Disord Drug Targets, 2022; 22, 716−27. doi: 10.2174/1871530322666220325114045 [7] den Braver-Sewradj SP, van Spronsen R, Hessel EVS. Substitution of bisphenol A: a review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances. Crit Rev Toxicol, 2020; 50, 128−47. doi: 10.1080/10408444.2019.1701986 [8] Gill S, Kumara VMR. Comparative neurodevelopment effects of bisphenol a and bisphenol F on rat fetal neural stem cell models. Cells, 2021; 10, 793. doi: 10.3390/cells10040793 [9] Harnett KG, Chin A, Schuh SM. BPA and BPA alternatives BPS, BPAF, and TMBPF, induce cytotoxicity and apoptosis in rat and human stem cells. Ecotoxicol Environ Saf, 2021; 216, 112210. doi: 10.1016/j.ecoenv.2021.112210 [10] Fouyet S, Olivier E, Leproux P, et al. Bisphenol A, bisphenol F, and bisphenol S: the bad and the ugly. Where is the good? Life (Basel), 2021; 11, 314. [11] Cui FP, Yang P, Liu C, et al. Urinary bisphenol A and its alternatives among pregnant women: predictors and risk assessment. Sci Total Environ, 2021; 784, 147184. doi: 10.1016/j.scitotenv.2021.147184 [12] Liao CY, Kannan K. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch Environ Contam Toxicol, 2014; 67, 50−9. doi: 10.1007/s00244-014-0016-8 [13] Nevoral J, Havránková J, Kolinko Y, et al. Exposure to alternative bisphenols BPS and BPF through breast milk: noxious heritage effect during nursing associated with idiopathic infertility. Toxicol Appl Pharmacol, 2021; 413, 115409. doi: 10.1016/j.taap.2021.115409 [14] Liu XX, Wang ZC, Liu FJ. Chronic exposure of BPA impairs male germ cell proliferation and induces lower sperm quality in male mice. Chemosphere, 2021; 262, 127880. doi: 10.1016/j.chemosphere.2020.127880 [15] Christovich A, Luo XM. Gut microbiota, leaky gut, and autoimmune diseases. Front Immunol, 2022; 13, 946248. doi: 10.3389/fimmu.2022.946248 [16] Qian XY, Liu AK, Liang C, et al. Analysis of gut microbiota in patients with acute myocardial infarction by 16S rRNA sequencing. Ann Transl Med, 2022; 10, 1340. doi: 10.21037/atm-22-5671 [17] Huang W, Zhu L, Zhao C, et al. Integration of proteomics and metabolomics reveals promotion of proliferation by exposure of bisphenol S in human breast epithelial MCF-10A cells. Sci Total Environ, 2020; 712, 136453. doi: 10.1016/j.scitotenv.2019.136453 [18] Shreiner A, Huffnagle GB, Noverr MC. The "microflora hypothesis" of allergic disease. In: Huffnagle GB, Noverr MC. GI Microbiota and Regulation of the Immune System. Springer. 2008, 113-34. [19] Khan J, Abdul Rahman A, Islam M, et al. Effect of bisphenol A on the intestinal barrier: evidences from animal studies. Int Med J, 2019; 26, 1−6. [20] Wang YH, Wang BB, Wang QQ, et al. Intestinal toxicity and microbial community disorder induced by bisphenol F and bisphenol S in zebrafish. Chemosphere, 2021; 280, 130711. doi: 10.1016/j.chemosphere.2021.130711 [21] Catron TR, Keely SP, Brinkman NE, et al. Host developmental toxicity of BPA and BPA alternatives is inversely related to microbiota disruption in zebrafish. Toxicol Sci, 2019; 167, 468−83. doi: 10.1093/toxsci/kfy261 [22] Gálvez-Ontiveros Y, Páez S, Monteagudo C, et al. Endocrine disruptors in food: impact on gut microbiota and metabolic diseases. Nutrients, 2020; 12, 1158. doi: 10.3390/nu12041158 [23] Chiu K, Warner G, Nowak RA, et al. The impact of environmental chemicals on the gut microbiome. Toxicol Sci, 2020; 176, 253−84. doi: 10.1093/toxsci/kfaa065 [24] Feng D, Zhang HM, Jiang X, et al. Bisphenol A exposure induces gut microbiota dysbiosis and consequent activation of gut-liver axis leading to hepatic steatosis in CD-1 mice. Environ Pollut, 2020; 265, 114880. doi: 10.1016/j.envpol.2020.114880 [25] Gomez MV, Dutta M, Suvorov A, et al. Early life exposure to environmental contaminants (BDE-47, TBBPA, and BPS) produced persistent alterations in fecal microbiome in adult male mice. Toxicol Sci, 2021; 179, 14−30. doi: 10.1093/toxsci/kfaa161 [26] Riesbeck S, Petruschke H, Rolle-Kampczyk U, et al. Adaptation and resistance: how Bacteroides thetaiotaomicron copes with the bisphenol A substitute bisphenol F. Microorganisms, 2022; 10, 1610. doi: 10.3390/microorganisms10081610 [27] Wang J, Jia HJ. Metagenome-wide association studies: fine-mining the microbiome. Nat Rev Microbiol, 2016; 14, 508−22. doi: 10.1038/nrmicro.2016.83 [28] Puljiz Z, Kumric M, Vrdoljak J, et al. Obesity, gut microbiota, and metabolome: from pathophysiology to nutritional interventions. Nutrients, 2023; 15, 2236. doi: 10.3390/nu15102236 [29] Sommer F, Anderson JM, Bharti R, et al. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol, 2017; 15, 630−8. doi: 10.1038/nrmicro.2017.58 [30] Phelps D, Brinkman NE, Keely SP, et al. Microbial colonization is required for normal neurobehavioral development in zebrafish. Sci Rep, 2017; 7, 11244. doi: 10.1038/s41598-017-10517-5 [31] Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics, 2011; 27, 2957−63. doi: 10.1093/bioinformatics/btr507 [32] Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods, 2010; 7, 335−6. doi: 10.1038/nmeth.f.303 [33] Weinroth MD, Belk AD, Dean C, et al. Considerations and best practices in animal science 16S ribosomal RNA gene sequencing microbiome studies. J Anim Sci, 2022; 100, skab346. doi: 10.1093/jas/skab346 [34] Zhou H, Zhao X, Sun L, et al. Gut microbiota profile in patients with type 1 diabetes based on 16S rRNA gene sequencing: a systematic review. Dis Markers, 2020; 2020, 3936247. [35] Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol, 2021; 19, 55−71. doi: 10.1038/s41579-020-0433-9 [36] Mosca A, Leclerc M, Hugot JP. Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol, 2016; 7, 455. [37] Ni YH, Hu LT, Yang S, et al. Bisphenol A impairs cognitive function and 5-HT metabolism in adult male mice by modulating the microbiota-gut-brain axis. Chemosphere, 2021; 282, 130952. doi: 10.1016/j.chemosphere.2021.130952 [38] Magne F, Gotteland M, Gauthier L, et al. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients, 2020; 12, 1474. [39] McDonough CM, Xu HS, Guo TL. Toxicity of bisphenol analogues on the reproductive, nervous, and immune systems, and their relationships to gut microbiome and metabolism: insights from a multi-species comparison. Crit Rev Toxicol, 2021; 51, 283−300. doi: 10.1080/10408444.2021.1908224 [40] Xu J, Huang GN, Nagy T, et al. Sex-dependent effects of bisphenol A on type 1 diabetes development in non-obese diabetic (NOD) mice. Arch Toxicol, 2019; 93, 997−1008. doi: 10.1007/s00204-018-2379-5 [41] Diamante G, Cely I, Zamora Z, et al. Systems toxicogenomics of prenatal low-dose BPA exposure on liver metabolic pathways, gut microbiota, and metabolic health in mice. Environ Int, 2021; 146, 106260. doi: 10.1016/j.envint.2020.106260 [42] Zheng S, Piao C, Liu Y, et al. Glycan biosynthesis ability of gut microbiota increased in primary hypertension patients taking antihypertension medications and potentially promoted by macrophage-adenosine monophosphate-activated protein kinase. Front Microbiol, 2021; 12, 719599. doi: 10.3389/fmicb.2021.719599 [43] Mardinoglu A, Shoaie S, Bergentall M, et al. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol, 2015; 11, 834. doi: 10.15252/msb.20156487 [44] Yao JX, Wang J, Wu LL, et al. Perinatal exposure to bisphenol A causes a disturbance of neurotransmitter metabolic pathways in female mouse offspring: A focus on the tryptophan and dopamine pathways. Chemosphere, 2020; 254, 126715. doi: 10.1016/j.chemosphere.2020.126715 [45] Sidebottom AM, Chang EB. IBD microbial metabolome: the good, bad, and unknown. Trends Endocrinol Metab, 2020; 31, 807−9. doi: 10.1016/j.tem.2020.05.001 [46] Lakshmanan AP, Al Zaidan S, Bangarusamy DK, et al. Increased relative abundance of Ruminoccocus is associated with reduced cardiovascular risk in an obese population. Frontiers in Nutrition, 2022; 9, 849005. doi: 10.3389/fnut.2022.849005 [47] Yang JP, Li YA, Wen ZQ, et al. Oscillospira-a candidate for the next-generation probiotics. Gut Microbes, 2021; 13, 1987783. doi: 10.1080/19490976.2021.1987783 [48] Stoll ML. Genetics, Prevotella, and the pathogenesis of rheumatoid arthritis. Lancet Rheumatol, 2020; 2, e375−6. doi: 10.1016/S2665-9913(20)30090-4 [49] Lannes-Costa PS, de Oliveira JSS, da Silva Santos G, et al. A current review of pathogenicity determinants of Streptococcus sp. J Appl Microbiol, 2021; 131, 1600−20. doi: 10.1111/jam.15090 -
 23100+Supplementary Materials.pdf
23100+Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links