-
Japanese encephalitis (JE) is a mosquito-borne zoonotic disease caused by the Japanese encephalitis virus (JEV)[1]. JEV is the major cause of viral encephalitis in the Asia-Pacific region[2,3]. More than three billion individuals living in 24 countries in this region are at risk of JEV infection. The estimated annual number of JE cases was 67,900[3,4]. Although most JE cases are asymptomatic, the fatality rate among patients with encephalitis is as high as 20%–30%, and approximately 30%–50% of survivors have long-term neurological sequelae[2,5]. Encephalitis primarily affects children. Most adults in endemic countries develop natural immunity after childhood infections. However, individuals of any age can be affected[6].
JEV belongs to the genus Orthoflavivirus, the family Flaviviridae, and the JEV serological complex. The JEV genome spans approximately 11,000 nucleotides and contains a single open reading frame that encodes three structural proteins (C, PrM/M, and E) and seven non-structural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5). The viral genome is flanked by 5' and 3' untranslated regions[1,7,8]. Moreover, it is divided into five genotypes (G1–5) based on the E gene and the whole genome[9,10]. G1, G3, and G5 JEVs have been isolated in China[11,12].
JE is a disease that can be prevented via vaccination. Vaccination has significantly reduced the incidence of JE and JE-related deaths in endemic areas[13]. All JE vaccines currently used are derived from G3 JEV[14], which provides protection against G1 and G3 JEV infections but does not provide adequate protection against G5 JEV infections[15-17]. In 2015, a case of JE caused by G5 JEV was reported in a South Korean patient with a history of JE vaccination[18]. In China, a G5 JEV strain was isolated from Culex tritaeniorhynchus collected in Nyingchi, Tibet[19], which confirmed the presence of G5 JEV in China. This suggested that attention should be given to the existence of this genotype in JEV cases in China.
The primary diagnostic criteria for JE are viral isolation, serological evidence, and molecular biology evidence[20,21]. However, JEV has only one serotype; therefore, current enzyme-linked immunosorbent assay (ELISA) methods cannot distinguish the viral genotype when detecting JEV infections. Furthermore, the detection of JEV nucleic acids in cerebrospinal fluid and serum specimens is complicated. Therefore, it is difficult to distinguish between the JEV genotypes in most cases. Cross-neutralization experiments have been used to distinguish flaviviral infections[22], and serological investigations have aimed to determine the JEV genotype[23,24]. In this study, we compared neutralizing antibody (nAb) titers produced by serum specimens from the same patient against G1, G3, and G5 JEV strains. A 4-fold difference in the nAb titer enabled the identification of the JEV genotype causing the infection.
Shandong, Zhejiang, and Guangdong provinces were selected for serological investigations. Cross-neutralization tests were performed on laboratory-confirmed JE cases using G1, G3, and G5 JEV strains to determine whether G5 JE cases had appeared in China. We aimed to identify the major genotypes of JEV that cause human diseases in selected coastal areas of China.
-
Serum specimens were collected from patients diagnosed with JE in the Guangdong, Zhejiang, and Shandong provinces of China from 2018 to 2020. This was a retrospective study, and epidemiological information was obtained from the JE Reporting Information Management System. Serum specimens were obtained as part of routine diagnostic testing, and the names and other identifying information of the patients were not included in the data analysis. Therefore, the Ethical Review Committee of the China Center for Disease Control and Prevention exempted this study from review by the Institutional Review Board.
-
The JEV strains used in this study were maintained at the Department of Arbovirus, National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention (IVDC, China CDC). G1 JEV is the NX1889 strain (GenBank accession no.: MT134112.1), G3 JEV is the P3 strain (GenBank accession no.: U47032.1), and G5 JEV is the XZ0934 strain (GenBank accession no.: JF915894.1). The titers of the three JEV strains were measured[16], and the viral suspensions were stored at -80 °C. BHK-21 cells were aseptically cultured in minimum essential medium (MEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (PS) at 37 °C in an atmosphere of 5% CO2.
-
The serum specimens were subjected to anti-JEV IgM antibody detection. A JEV IgM Capture ELISA kit (Shanghai B & C Enterprise Development Co., Ltd., Shanghai, China) was used to detect JEV IgM antibodies. Detection was performed according to the manufacturer’s instructions.
-
Nucleic acids were extracted from all serum specimens using a QIAamp Viral RNA Mini Kit (QIAGEN, Dusseldorf, Germany) and subjected to a reverse transcription one-step polymerase chain reaction (RT-PCR). The RNA (5 µL) was added to the reaction volume (20 µL) according to the instructions of the TaqPath-IDTM 1-Step Multiplex Master Mix (Thermo Fisher Scientific, Waltham, MA, USA). Universal primers and probes for JEV have been used to detect G1, G3, and G5 JEV[25]. Specimens with Ct values of < 35.00 were considered positive.
-
A 90% plaque reduction neutralization test (PRNT90) was used to measure the titers of nAbs against G1, G3, and G5 JEV. Dispensed BHK-21 cells were seeded in six-well plates and cultured until a monolayer was formed. Serum specimens were inactivated at 56 °C for 30 min and diluted 2-fold from 1:5 to 1:10,240. Each dilution was mixed with an equal volume of virus suspension at a titer of 200 plaque-forming units (PFU)/100 µL. After incubation for 1 h at 37 °C, the mixture was used to inoculate BHK-21 cells in six-well plates. In addition, the virus was added (100, 10, and 1 PFU) to the cells in six-well plates as a reference. All specimens and references were incubated at 37 °C for 1 h. The liquid in each well was discarded, and the cells were overlaid with methylcellulose-MEM containing 2% FBS and 1% PS, followed by incubation at 37 °C for 5 d. The methylcellulose-MEM in each well was discarded, and the cells were stained with crystal violet. Next, the number of plaques in each well was determined. The titers of nAbs were determined as the maximum dilution level that resulted in a 90% reduction in plaque number compared to the reference.
-
The value of PRNT90 ≥1:10 was defined as a positive result. The ratios of the nAb titers for the various viral strains were calculated. If one nAb titer was 4-fold different from the other two or only if this nAb was positive in the same serum specimen, we determined that the patient was infected with the JEV genotype. Statistical analyses were performed using the R software (Version 3.6.3). Proportions were compared using a χ2 test with a test level of ɑ = 0.05.
-
In this study, acute serum specimens from clinically reported JE cases were obtained for laboratory confirmation from hospitals in Shandong (92 cases), Zhejiang (192 cases), and Guangdong (77 cases) between 2018 and 2020. These specimens were stored at -80 °C and transported on dry ice to the Department of Arbovirus, National Institute for Viral Disease Control and Prevention, China CDC, for JEV serological tests and nucleic acid detection.
-
All 361 specimens were negative for JEV nucleotides, and only 70 serum specimens were positive for JEV IgM antibodies.
-
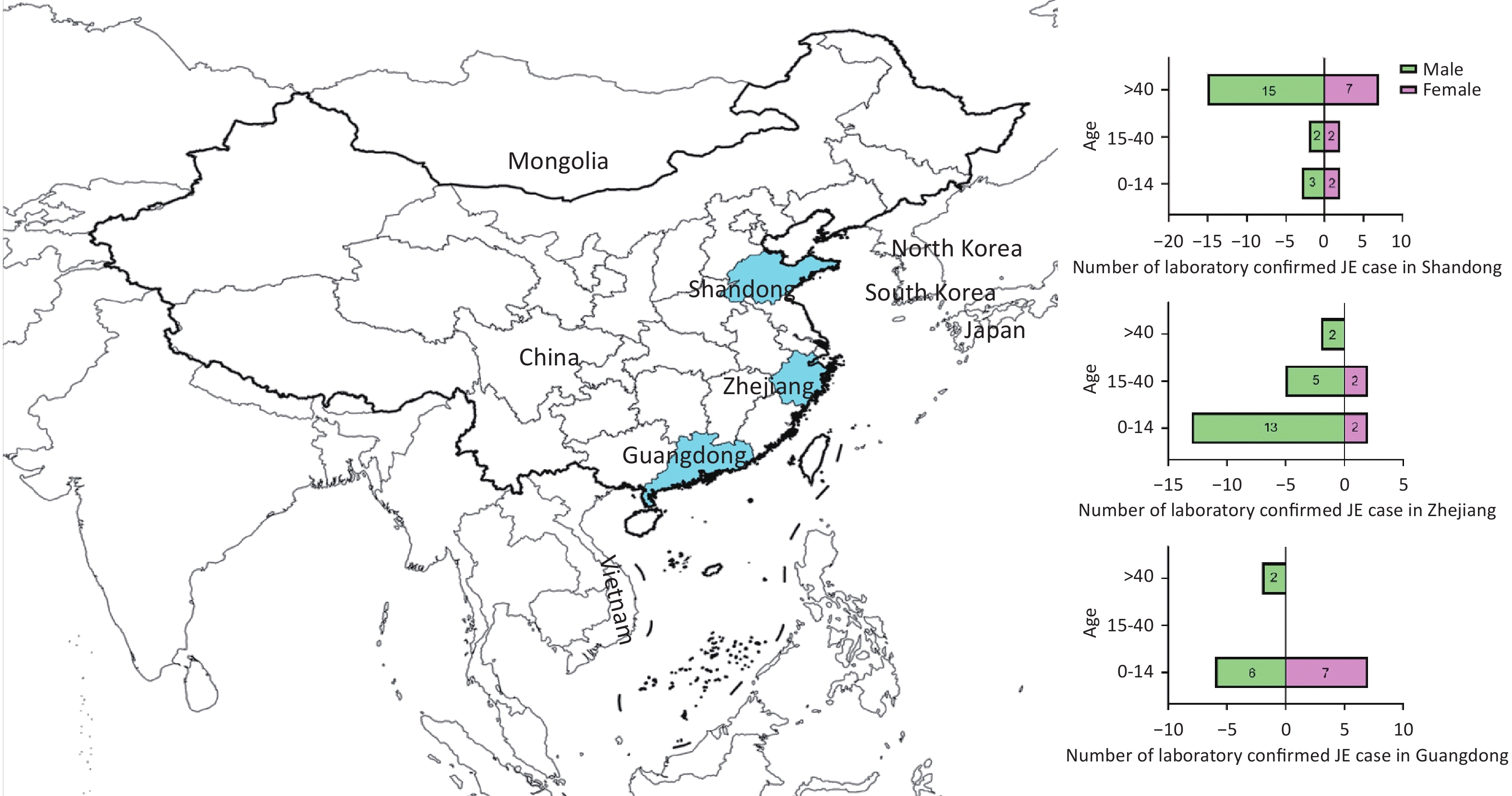
Seventy laboratory-confirmed JE cases from three coastal provinces of China between 2018 and 2020 were included. The highest number of cases was reported in 2018. The sex ratio of 48 males to 22 females was 2.18:1. Their mean age was 29.63 ± 25.17 years. Thirty-three individuals were 0–14 years old, 11 were 15–40 years old, and 26 were > 40 years old. Farmers, students, and children accounted for the largest proportion of JE cases. A considerable proportion of cases (24/70, 34.28%) were classified as severe. In addition, approximately one-third (21/70) of the patients received the JE vaccination. The remaining two-thirds (49/70) were unvaccinated against JE or had unknown vaccination status (Table 1). Individuals aged > 40 years accounted for the largest proportion of cases in Shandong province, whereas those aged 0–14 years dominated Zhejiang and Guangdong provinces (Figure 1).
Table 1. Summary of basic characteristics of the laboratory-confirmed case of JE
Year Main class Detail class Shandong (31 cases) Zhejiang (24 cases) Guangdong (15 cases) 0–14 15–40 > 40 0–14 15–40 > 40 0–14 15–40 > 40 2018-2020 Gender Male 3 2 15# 13*# 5 2 6 2 Female 2 2 7 2 2 7* Occupation Farmer 1 20 1 1 2 Student 3 8 1 8 Scattered children 2 7 5 Worker 1 4 1 Job-waiting people 1 1 Unknown 2 1 Clinic classification Mild 2 1 3 3 2 1 6 1 Moderate 2 1 5 4 3 4 Severity 1 2 10 7 1 1 2 1 Extreme Severity 2 1 1 Unclassified 2 1 JE vaccination status Vaccinated 2 10 9 Unvaccinated 2 1 6 2 1 1 4 1 Unknown 1 3 16 3 6 1 1 Note. *: indicates the G1 JEV infection case is included in this group. #: indicates the G5 JEV infection case is included in this group. JE, Japanese encephalitis. 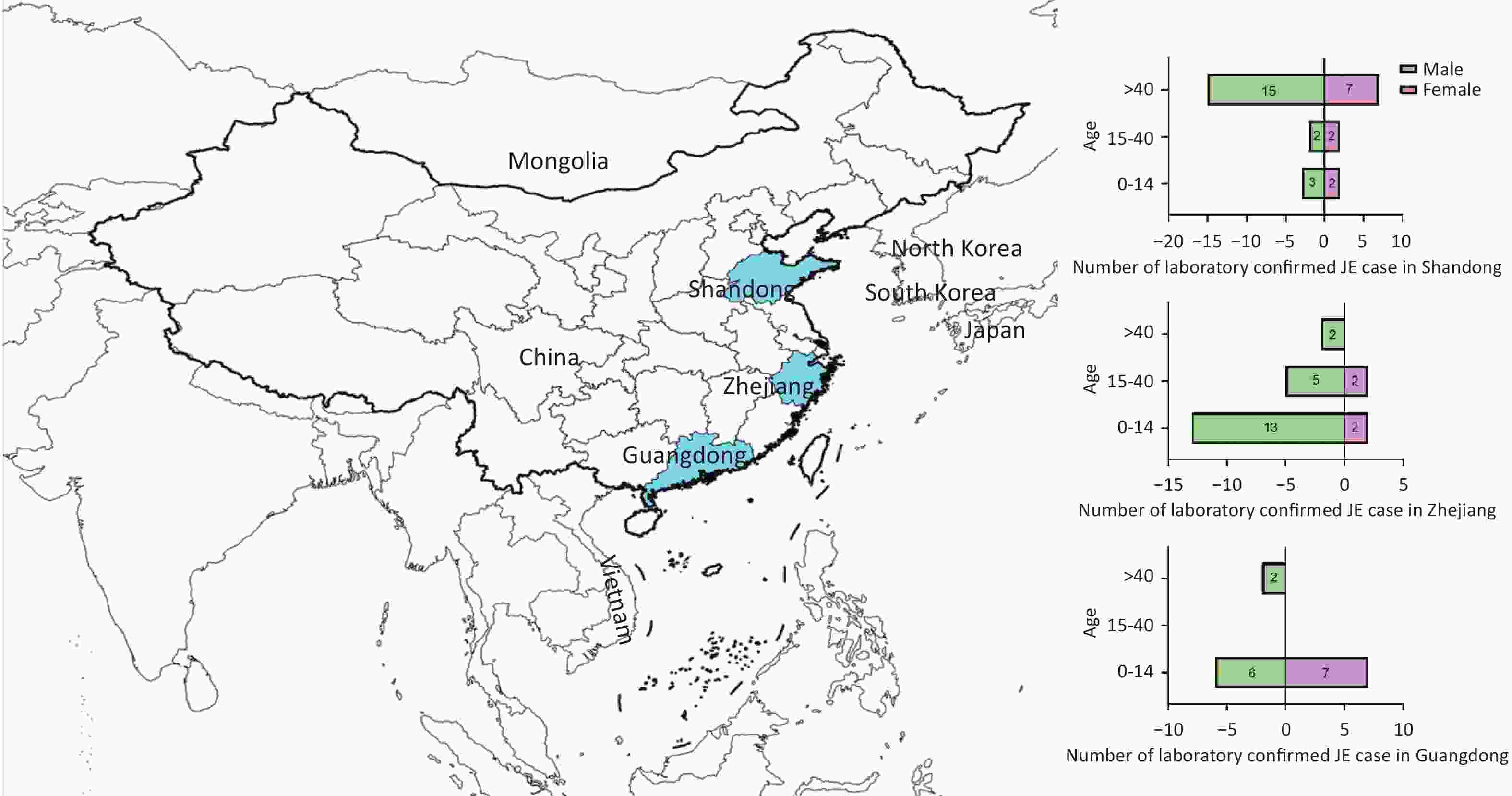
Figure 1. Geographical location of the serum specimens and demographic characteristics of the JE case for our survey. The provinces highlighted in blue from north to south are Shandong, Zhejiang, and Guangdong. The pyramid shows the number of laboratory-confirmed JE cases and the age and gender of participants from each province. A total of 70 laboratory-confirmed cases of JE are observed, with more males than females. The number of cases from Shandong, Zhejiang, and Guangdong provinces was 31, 24, and 15, respectively. The majority of cases in Shandong province were > 40 years old, whereas the majority of cases in Zhejiang province and Guangdong province were 0–14 years old.
-
Of the 36 specimens with identifiable infection genotypes, two cases of G1 (5.6%), 32 cases of G3 (88.8%), and two cases of G5 (5.6%) JEV infections were identified using the cross-neutralization test. Thirty-two patients in Shandong, Zhejiang, and Guangdong provinces were confirmed to be infected with G3 JEV. One case in Zhejiang and Guangdong provinces was G1 JEV. One case from Shandong and Zhejiang provinces was confirmed to be infected with G5 JEV (Table 2). Seven specimens tested negative for the G1, G3, and G5 JEV nAbs. The JEV genotype in the other 27 cases could not be inferred using a cross-neutralization test. Nine specimens were positive for three nAbs, 17 were positive for G1 and G3 JEV nAbs but negative for G5 JEV nAbs, and one specimen was positive for G3 and G5 JEV nAbs but negative for G1 JEV nAbs (Table 2).
Table 2. Results of PRNT90 and the information of JE cases with definite JEV infection genotype result
No. Provincea Year Age Genderb Days after onsetc Titers of nAbs Genotyped Clinic classification Vaccination
statuse(1:-) G1 G3 G5 1 ZJ 2019 14 M − 320 20 < 10 G1 Severe UK 2 GD 2019 8 F 4 10 < 10 < 10 G1 Extremely severe V 3 SD 2018 18 M 4 40 320 40 G3 Mild UK 4 SD 2018 19 F 14 10 2560 80 G3 Severe UK 5 SD 2018 53 M 5 20 320 10 G3 Extremely severe UK 6 SD 2018 57 M − 160 5120 < 10 G3 Unclassified UK 7 SD 2018 60 M 20 20 80 < 10 G3 Unclassified UK 8 SD 2018 67 M 13 10 40 < 10 G3 Moderate No 9 SD 2018 67 F 21 < 10 160 < 10 G3 Severe No 10 SD 2018 73 M 31 < 10 40 < 10 G3 Severe UK 11 SD 2018 74 M 7 < 10 40 < 10 G3 Mild No 12 SD 2019 2m M 7 < 10 20 < 10 G3 Mild No 13 SD 2019 55 F − < 10 20 < 10 G3 Unclassified UK 14 SD 2020 34 M 10 320 1280 320 G3 Moderate No 15 SD 2020 49 M 7 40 2560 < 10 G3 Severe UK 16 SD 2020 54 F 3 < 10 20 < 10 G3 Unclassified No 17 SD 2020 54 M 5 160 640 < 10 G3 Moderate UK 18 SD 2020 55 F 11 < 10 10 < 10 G3 Mild UK 19 SD 2020 60 M 0 < 10 20 < 10 G3 Unclassified UK 20 SD 2020 63 M 7 < 10 10 < 10 G3 Severe UK 21 ZJ 2018 1 M 6 10 80 10 G3 Mild V 22 ZJ 2018 10 M − 20 640 10 G3 Severe UK 23 ZJ 2018 15 M − 320 1280 10 G3 Moderate UK 24 ZJ 2019 14 M − 80 320 < 10 G3 Severe V 25 ZJ 2019 15 M 11 10 40 < 10 G3 Mild UK 26 ZJ 2019 55 M 17 40 320 < 10 G3 Unclassified No 27 ZJ 2020 1 M 16 10 160 < 10 G3 Mild V 28 ZJ 2020 30 F 11 10 40 < 10 G3 Mild No 29 ZJ 2020 31 M 7 20 320 < 10 G3 Moderate UK 30 GD 2018 3 F 18 < 10 10 < 10 G3 Unclassified No 31 GD 2018 71 M 25 < 10 10 < 10 G3 Unclassified UK 32 GD 2020 10m M 2 < 10 10 < 10 G3 Moderate V 33 GD 2020 8 M 6 40 1280 10 G3 Moderate V 34 GD 2020 66 M 4 < 10 10 < 10 G3 Mild No 35 SD 2020 58 M 8 40 160 640 G5 Severe UK 36 ZJ 2019 2 M 8 40 40 320 G5 Moderate V 37 SD 2020 14 F 7 < 10 < 10 < 10 − Mild No 38 SD 2020 61 F 5 < 10 < 10 < 10 − Moderate UK 39 SD 2020 65 M 13 < 10 < 10 < 10 − Severe UK 40 SD 2020 70 M 5 < 10 < 10 < 10 − Severe UK 41 ZJ 2020 1 M 4 < 10 < 10 < 10 − Moderate No 42 GD 2018 11 F 10 < 10 < 10 < 10 − Mild V 43 GD 2019 5 F 22 < 10 < 10 < 10 − Mild V 44 SD 2018 14 M 7 10 20 < 10 − Severe UK 45 SD 2018 32 F 27 10 20 < 10 − Severe UK 46 SD 2018 53 F 13 20 20 < 10 − Severe UK 47 SD 2018 62 F 10 20 20 < 10 − Moderate No 48 SD 2018 64 M 3 10 20 < 10 − Severe No 49 SD 2019 9 F 5 10 20 < 10 − Unclassified V 50 SD 2020 7 M 5 20 40 < 10 − Moderate V 51 ZJ 2018 10 M − 80 80 < 10 − Severe V 52 ZJ 2018 49 M 13 10 10 < 10 − Severe UK 53 ZJ 2019 14 M 5 20 20 < 10 − Mild V 54 GD 2018 1 M 1 20 40 < 10 − Moderate No 55 GD 2018 8 F 9 20 20 < 10 − Mild V 56 GD 2018 14 F 18 20 10 < 10 − Severe V 57 GD 2019 8 M 35 20 40 < 10 − Mild V 58 GD 2019 10 M 26 80 160 < 10 − Mild No 59 GD 2019 14 F − 20 10 < 10 − Severe No 60 GD 2020 3 M 1 10 20 < 10 − Moderate V 61 ZJ 2018 26 M 6 < 10 20 20 − Extremely severe UK 62 SD 2018 53 M 5 20 40 10 − Severe UK 63 ZJ 2018 1 F 8 40 80 20 − Severe No 64 ZJ 2018 6 M − 160 320 160 − Moderate V 65 ZJ 2018 11 M 8 160 320 10 − Unclassified V 66 ZJ 2018 12 M 9 10 20 10 − Severe V 67 ZJ 2018 18 M 5 20 20 20 − Unclassified UK 68 ZJ 2018 22 F 3 40 80 20 − Severe UK 69 ZJ 2019 4 F 18 10 10 10 − Severe UK 70 ZJ 2019 8 M − 160 160 20 − Unclassified V Note. aSD, Shandong Province; ZJ, Zhejiang Province; GD, Guangdong Province. bGender: M, male; F, female. cDays after onset, the days from onset at serum collection. dGenotype, the genotype of JEV that caused JE of the patient. eUK, unknown; No, unvaccinated; V, vaccinated. JE, Japanese encephalitis. -
To explore the relationship between vaccination status and the clinical classification of the 70 cases, the clinical classification of the 21 cases with a vaccination history was primarily mild (33.3%, 7/21) or moderate (38.1%, 8/21). The clinical classification of the 49 patients with no or unknown vaccination history was primarily mild (24.5%, 12/49) or severe (42.9%, 21/49). No statistically significant differences were observed between the two groups (Table 3). The incidence of symptoms such as acute onset, fever, depression, and dysfunction of consciousness was similar in the different vaccination status groups (all > 50%). The incidence of dizziness, nausea, irritability, convulsions, small and localized twitches, and recurrent or continuous twitches was similar (10%–50%). Abdominal pain, diarrhea, and circulatory failure were less common (< 10%). No statistically significant differences were observed between vaccination status and clinical symptoms (Table 4).
Table 3. Comparison of the difference in clinical staging in different vaccination status
Clinical typing Vaccination history, n (%) χ2 P value Yes (n = 21) NO/Ominous (n = 49) Mild 7 (33.3) 12 (24.5) 0.581 0.553 Moderate 8 (38.1) 11 (22.4) 1.82 0.242 Severe 4 (19.0) 21 (42.9) 3.63 0.091 Extremely severe 1 (4.8) 3 (6.1) 0.051 1 Unclassified 1 (4.8) 2 (4.1) 0.017 1 Table 4. Comparison of clinical symptoms in in different vaccination status
Clinical symptoms Vaccination history, n (%) χ2 P value Yes (n = 21) NO/Ominous (n = 49) Acute onset 14 (66.7) 46 (93.9) 2.694 0.131 Fever 16 (76.2) 41 (83.7) 0.039 1 <39 °C 7 (33.3) 21 (42.9) 0.287 0.779 39-40 °C 6 (28.6) 13 (26.5) 0.144 0.762 >40 °C 3 (14.3) 7 (14.3) 0.016 1 Headache 8 (38.1) 27 (55.1) 1.241 0.381 Dizziness 8 (38.1) 17 (34.7) 0.284 0.777 Abdominal pain 0 (0.0) 3 (6.1) 1.248 0.559 Diarrhea 2 (9.5) 4 (8.2) 0.082 1 Nausea 6 (28.6) 18 (36.7) 0.219 0.778 Vomiting 11 (52.4) 17 (34.7) 3.101 0.096 Jet vomiting 3 (14.3) 3 (6.1) 1.541 0.332 Depression 14 (66.7) 36 (73.5) 0.016 1 Irritability 1 (4.8) 11 (22.4) 2.955 0.15 Drowsiness 9 (42.9) 30 (61.2) 1.516 0.236 Dysphoria 5 (23.8) 20 (40.8) 1.466 0.251 Convulsions 3 (14.3) 11 (22.4) 0.429 0.74 Dysfunction of consciousness 11 (52.4) 27 (55.1) 0.019 1 Small localized twitches 3 (14.3) 9 (18.4) 0.082 1 Recurrent or continuous twitches 4 (19.0) 9 (18.4) 0.048 1 Respiratory failure 2 (9.5) 12 (24.5) 1.775 0.308 Cirulatory failure 0 (0.0) 3 (6.1) 1.248 0.559 -
In China, JE is the most common form of viral encephalitis [26]. Clinically reported cases of JE are usually confirmed using ELISA methods for detecting JEV IgM antibodies in specimens. However, this method cannot distinguish between genotypes of infected JEV. Furthermore, because it is difficult to detect viral nucleic acids in case specimens, the infected JEV genotype cannot be identified using molecular biology methods. In this study, the nAb titers of G1, G3, and G5 JEV strains against the same serum specimen were used to determine the infection genotypes of the JE cases. G1, G3, and G5 JEV infections were evident among JE cases in Shandong, Zhejiang, and Guangdong provinces from 2018 to 2020. G3 was the primary infection genotype among the JE cases with a definite infection genotype, and infection caused by G5 JEV was confirmed in China.
Although G5 JEV was isolated in 2009 in China, there have been no previous reports of G5 JEV infections[19]. In this study, two cases of G5 JEV infection were identified in the Zhejiang and Shandong provinces in 2019 and 2020, respectively. In contrast to the age and sex characteristics of patients infected with G5 JEV reported in Korea (27 years of age and females, respectively)[18], the patients in this study were both males (2 and 58 years of age). These findings suggest that G5 JEV can cause JE infections in all age groups. Similar to the Korean case described above, the child infected with G5 JEV in this study was vaccinated with two doses of the inactivated vaccine. These findings support the hypothesis that G3 JEV-derived JE vaccines do not provide adequate protection against G5 JEV at the individual level[16,17]. The three individual cases mentioned above show that G5 JEV has an underestimated capacity and potential to spread among humans, and there is a risk of G5 JEV transmission in China. These results suggest that it is necessary to conduct G5 JEV nAb surveillance in healthy populations to gain a more comprehensive understanding of G5 JEV infections. G4, a relatively rare JEV genotype similar to G5, caused an outbreak in Australia in 2022. Inactivated vaccines and chimeric-attenuated live vaccines used to control outbreaks in Australia are derived from G3 JEV. To date, no relevant research has evaluated the protective effects of G3 vaccines against G4 JEV infection. Further studies focusing on the protective efficiency of JE vaccines against G4 and G5 JEV are required, and a polyvalent vaccine to prevent epidemics caused by the five JEV genotypes should be developed.
JE is a disease that can be prevented by vaccination. The vaccines currently in domestic use in China are the live attenuated JE vaccine (SA-14-14-2) and the inactivated JE vaccine (P3)[27,28]. Shandong, Zhejiang, and Guangdong provinces included the JE vaccine in their childhood immunization programs beginning in 1986, 1986, and 2004, respectively[29-31]. Children aged 8 and 24 months received two free doses of the live-attenuated vaccine. This has led to a decline in JE incidences[26,32]. In 2008, China included the JE vaccine in the Expanded Program on Immunization, continuously improving JE vaccination in children and strengthening JE surveillance, making the recording and preservation of vaccination information complete and traceable[33]. In this study, among the 21 patients with a history of JE vaccination, six completed the full vaccination schedule with two doses of the live-attenuated vaccine. However, a majority (49/70) of the patients had either not been vaccinated or had unclear vaccination histories; 25.71% (18/70) had not received the JE vaccine; and 44.29% (31/70) had unclear vaccination backgrounds. Most of those who had not been vaccinated or whose vaccination status was unclear were middle-aged and elderly individuals born during an era when the JE vaccine was not widely available. Therefore, among the objectives of this study, the vaccination information of children can be queried, but the vaccination information of adults is incomplete or inaccurate, which impacts the statistical analysis. Statistical analyses incorporating vaccination status data failed to yield statistically significant results.
In patients 0–14 years of age, the numbers of cases from Shandong, Zhejiang, and Guangdong provinces were 16% (5/31), 62.5% (15/24), and 86.7% (13/15), respectively. For those aged > 40 years, the proportions were 71% (22/31) in Shandong, 8.3% (2/24) in Zhejiang, and 13.3% (2/15) in Guangdong (Figure 1). Based on the age demographics of JE cases in these provinces, Shandong in Northern China primarily encounters adult cases, whereas in Zhejiang and Guangdong in Southern China, pediatric cases are primarily observed, which is consistent with previous research[34,35]. As pediatric JE is increasingly mitigated by vaccination, adult cases have become more prominent. Studies have reported lower titers of nAbs against JEV in the sera of older populations in Northern China, resulting in outbreaks in adults[36-39]. After large-scale vaccination in these older adult age groups, the incidence and severity of individual cases decreased significantly[40]. Therefore, surveillance for adult JE should be implemented in the northern regions of China. Vaccination programs in endemic areas should maintain and strengthen regular immunization of children and supplemental vaccination of adults with unclear vaccination histories. Collectively, a dynamic “precision immunization” strategy should be employed.
Serological crossover exists between flaviviruses, and serological crossover occurs between different JEV genotypes. The detection of IgM or IgG antibodies cannot distinguish the viral genotype infection, and there are no relevant studies to specifically distinguish the crossover titer. Because we only had a single serum specimens, a cross-neutralization test of different JEV genotypes was used in this study to identify JE cases infected with different viral genotypes. Cross-neutralization tests revealed that among the JE cases with identifiable infection genotypes, 32 of 36 were infected with G3 JEV, and two of 36 were infected with G1 JEV. From 1949–2000, G3 was the predominant JEV genotype isolated and identified in China. Since 2000, both the G1 and G3 JEVs have been circulating in China. In recent years, G1 has become the dominant genotype in mosquitoes[41-44]. Concurrently, G1 and G3 JEV co-circulate in the swine population. The findings of this study indicate that the natural infection rate of G3 JEV remains high among human cases in the coastal provinces of Guangdong, Zhejiang, and Shandong. Therefore, although a shift in the JEV genotypes has occurred in mosquitoes, G3 remains the primary genotype responsible for human infections. However, the outbreaks caused by other JEV genotypes should not be ignored. In 2006, G1 JEV was first isolated from the cerebrospinal fluid of a patient with encephalitis in Guizhou province[45]. Subsequently, more G1 JEV strains were isolated from cerebrospinal fluid specimens[46], and multiple outbreaks caused by G1 JEV have been reported in Northern China. For example, during the 2018 JE outbreak in the Ningxia Hui Autonomous Region, both clinical and natural specimens tested positive for G1 JEV[37], suggesting that G1 can replace G3 JEV in humans. These findings indicate the necessity for genotype monitoring in future JE cases.
Based on the cross-neutralization test, we could not deduce the causative JEV genotype in 27 cases. The timing of the production and the peak of nAbs are related to several factors. A considerable difference was observed in the titers of specific neutralizing antibodies produced after the initial exposure and re-exposure to a pathogen. Second, the time interval between onset and serum collection affected the determination of nAb titers. Previous studies have shown that on the day of onset, JEV IgG nAbs can be detected in the sera of a small number of JE cases. By the 30th day of the disease, the positivity rate of IgG nAbs reaches 100%, and the IgG antibody level peaks[47]. In this study, the demographic and geographical patterns of the 27 cases were not observed. A follow-up investigation will increase the specimen size and continue to focus on determining the infection genotype of JE cases in other areas of the country, with the hope of drawing more comprehensive and rich conclusions.
-
In conclusion, this study confirmed the presence of G1, G3, and G5 JEV infections in China using serological methods. G3 was the primary infection genotype among the JE cases with a definite infection genotype. The data confirmed the insufficient protection conferred by the current JE vaccine at the individual level, and the transmission of G5 JEV in the population is important to consider. In addition, this study revealed that the majority of JE cases in northern China occurred in adults. To prevent and control JE, strengthened surveillance in adults and supplementary JE vaccination should be considered. Serological investigations in different populations are important to clarify the threat posed to population health.
doi: 10.3967/bes2024.078
Serological Investigation into the Infected Genotypes of Patients with Japanese Encephalitis in the Coastal Provinces of China
-
Abstract:
Objective Genotypes (G) 1, 3, and 5 of the Japanese encephalitis virus (JEV) have been isolated in China, but the dominant genotype circulating in Chinese coastal areas remains unknown. We searched for G5 JEV-infected cases and attempted to elucidate which JEV genotype was most closely related to human Japanese encephalitis (JE) in the coastal provinces of China. Methods In this study, we collected serum specimens from patients with JE in three coastal provinces of China (Guangdong, Zhejiang, and Shandong) from 2018 to 2020 and conducted JEV cross-neutralization tests against G1, 3, and 5. Results Acute serum specimens from clinically reported JE cases were obtained for laboratory confirmation from hospitals in Shandong (92 patients), Zhejiang (192 patients), and Guangdong (77 patients), China, from 2018 to 2020. Seventy of the 361 serum specimens were laboratory-confirmed to be infected with JEV. Two cases were confirmed to be infected with G1 JEV, 32 with G3 JEV, and two with G5 JEV. Conclusion G3 was the primary infection genotype among JE cases with a definite infection genotype, and the infection caused by G5 JEV was confirmed serologically in China. -
Key words:
- Japanese encephalitis virus /
- Serological investigation /
- Plaque reduction neutralization test /
- Cross-neutralization test /
- Genotype
The authors declared no conflict of interest.
&These authors contributed equally to this work.
注释:1) AUTHOR CONTRIBUTIONS: 2) CONFLICT OF INTEREST: -
Figure 1. Geographical location of the serum specimens and demographic characteristics of the JE case for our survey. The provinces highlighted in blue from north to south are Shandong, Zhejiang, and Guangdong. The pyramid shows the number of laboratory-confirmed JE cases and the age and gender of participants from each province. A total of 70 laboratory-confirmed cases of JE are observed, with more males than females. The number of cases from Shandong, Zhejiang, and Guangdong provinces was 31, 24, and 15, respectively. The majority of cases in Shandong province were > 40 years old, whereas the majority of cases in Zhejiang province and Guangdong province were 0–14 years old.
Table 1. Summary of basic characteristics of the laboratory-confirmed case of JE
Year Main class Detail class Shandong (31 cases) Zhejiang (24 cases) Guangdong (15 cases) 0–14 15–40 > 40 0–14 15–40 > 40 0–14 15–40 > 40 2018-2020 Gender Male 3 2 15# 13*# 5 2 6 2 Female 2 2 7 2 2 7* Occupation Farmer 1 20 1 1 2 Student 3 8 1 8 Scattered children 2 7 5 Worker 1 4 1 Job-waiting people 1 1 Unknown 2 1 Clinic classification Mild 2 1 3 3 2 1 6 1 Moderate 2 1 5 4 3 4 Severity 1 2 10 7 1 1 2 1 Extreme Severity 2 1 1 Unclassified 2 1 JE vaccination status Vaccinated 2 10 9 Unvaccinated 2 1 6 2 1 1 4 1 Unknown 1 3 16 3 6 1 1 Note. *: indicates the G1 JEV infection case is included in this group. #: indicates the G5 JEV infection case is included in this group. JE, Japanese encephalitis. Table 2. Results of PRNT90 and the information of JE cases with definite JEV infection genotype result
No. Provincea Year Age Genderb Days after onsetc Titers of nAbs Genotyped Clinic classification Vaccination
statuse(1:-) G1 G3 G5 1 ZJ 2019 14 M − 320 20 < 10 G1 Severe UK 2 GD 2019 8 F 4 10 < 10 < 10 G1 Extremely severe V 3 SD 2018 18 M 4 40 320 40 G3 Mild UK 4 SD 2018 19 F 14 10 2560 80 G3 Severe UK 5 SD 2018 53 M 5 20 320 10 G3 Extremely severe UK 6 SD 2018 57 M − 160 5120 < 10 G3 Unclassified UK 7 SD 2018 60 M 20 20 80 < 10 G3 Unclassified UK 8 SD 2018 67 M 13 10 40 < 10 G3 Moderate No 9 SD 2018 67 F 21 < 10 160 < 10 G3 Severe No 10 SD 2018 73 M 31 < 10 40 < 10 G3 Severe UK 11 SD 2018 74 M 7 < 10 40 < 10 G3 Mild No 12 SD 2019 2m M 7 < 10 20 < 10 G3 Mild No 13 SD 2019 55 F − < 10 20 < 10 G3 Unclassified UK 14 SD 2020 34 M 10 320 1280 320 G3 Moderate No 15 SD 2020 49 M 7 40 2560 < 10 G3 Severe UK 16 SD 2020 54 F 3 < 10 20 < 10 G3 Unclassified No 17 SD 2020 54 M 5 160 640 < 10 G3 Moderate UK 18 SD 2020 55 F 11 < 10 10 < 10 G3 Mild UK 19 SD 2020 60 M 0 < 10 20 < 10 G3 Unclassified UK 20 SD 2020 63 M 7 < 10 10 < 10 G3 Severe UK 21 ZJ 2018 1 M 6 10 80 10 G3 Mild V 22 ZJ 2018 10 M − 20 640 10 G3 Severe UK 23 ZJ 2018 15 M − 320 1280 10 G3 Moderate UK 24 ZJ 2019 14 M − 80 320 < 10 G3 Severe V 25 ZJ 2019 15 M 11 10 40 < 10 G3 Mild UK 26 ZJ 2019 55 M 17 40 320 < 10 G3 Unclassified No 27 ZJ 2020 1 M 16 10 160 < 10 G3 Mild V 28 ZJ 2020 30 F 11 10 40 < 10 G3 Mild No 29 ZJ 2020 31 M 7 20 320 < 10 G3 Moderate UK 30 GD 2018 3 F 18 < 10 10 < 10 G3 Unclassified No 31 GD 2018 71 M 25 < 10 10 < 10 G3 Unclassified UK 32 GD 2020 10m M 2 < 10 10 < 10 G3 Moderate V 33 GD 2020 8 M 6 40 1280 10 G3 Moderate V 34 GD 2020 66 M 4 < 10 10 < 10 G3 Mild No 35 SD 2020 58 M 8 40 160 640 G5 Severe UK 36 ZJ 2019 2 M 8 40 40 320 G5 Moderate V 37 SD 2020 14 F 7 < 10 < 10 < 10 − Mild No 38 SD 2020 61 F 5 < 10 < 10 < 10 − Moderate UK 39 SD 2020 65 M 13 < 10 < 10 < 10 − Severe UK 40 SD 2020 70 M 5 < 10 < 10 < 10 − Severe UK 41 ZJ 2020 1 M 4 < 10 < 10 < 10 − Moderate No 42 GD 2018 11 F 10 < 10 < 10 < 10 − Mild V 43 GD 2019 5 F 22 < 10 < 10 < 10 − Mild V 44 SD 2018 14 M 7 10 20 < 10 − Severe UK 45 SD 2018 32 F 27 10 20 < 10 − Severe UK 46 SD 2018 53 F 13 20 20 < 10 − Severe UK 47 SD 2018 62 F 10 20 20 < 10 − Moderate No 48 SD 2018 64 M 3 10 20 < 10 − Severe No 49 SD 2019 9 F 5 10 20 < 10 − Unclassified V 50 SD 2020 7 M 5 20 40 < 10 − Moderate V 51 ZJ 2018 10 M − 80 80 < 10 − Severe V 52 ZJ 2018 49 M 13 10 10 < 10 − Severe UK 53 ZJ 2019 14 M 5 20 20 < 10 − Mild V 54 GD 2018 1 M 1 20 40 < 10 − Moderate No 55 GD 2018 8 F 9 20 20 < 10 − Mild V 56 GD 2018 14 F 18 20 10 < 10 − Severe V 57 GD 2019 8 M 35 20 40 < 10 − Mild V 58 GD 2019 10 M 26 80 160 < 10 − Mild No 59 GD 2019 14 F − 20 10 < 10 − Severe No 60 GD 2020 3 M 1 10 20 < 10 − Moderate V 61 ZJ 2018 26 M 6 < 10 20 20 − Extremely severe UK 62 SD 2018 53 M 5 20 40 10 − Severe UK 63 ZJ 2018 1 F 8 40 80 20 − Severe No 64 ZJ 2018 6 M − 160 320 160 − Moderate V 65 ZJ 2018 11 M 8 160 320 10 − Unclassified V 66 ZJ 2018 12 M 9 10 20 10 − Severe V 67 ZJ 2018 18 M 5 20 20 20 − Unclassified UK 68 ZJ 2018 22 F 3 40 80 20 − Severe UK 69 ZJ 2019 4 F 18 10 10 10 − Severe UK 70 ZJ 2019 8 M − 160 160 20 − Unclassified V Note. aSD, Shandong Province; ZJ, Zhejiang Province; GD, Guangdong Province. bGender: M, male; F, female. cDays after onset, the days from onset at serum collection. dGenotype, the genotype of JEV that caused JE of the patient. eUK, unknown; No, unvaccinated; V, vaccinated. JE, Japanese encephalitis. Table 3. Comparison of the difference in clinical staging in different vaccination status
Clinical typing Vaccination history, n (%) χ2 P value Yes (n = 21) NO/Ominous (n = 49) Mild 7 (33.3) 12 (24.5) 0.581 0.553 Moderate 8 (38.1) 11 (22.4) 1.82 0.242 Severe 4 (19.0) 21 (42.9) 3.63 0.091 Extremely severe 1 (4.8) 3 (6.1) 0.051 1 Unclassified 1 (4.8) 2 (4.1) 0.017 1 Table 4. Comparison of clinical symptoms in in different vaccination status
Clinical symptoms Vaccination history, n (%) χ2 P value Yes (n = 21) NO/Ominous (n = 49) Acute onset 14 (66.7) 46 (93.9) 2.694 0.131 Fever 16 (76.2) 41 (83.7) 0.039 1 <39 °C 7 (33.3) 21 (42.9) 0.287 0.779 39-40 °C 6 (28.6) 13 (26.5) 0.144 0.762 >40 °C 3 (14.3) 7 (14.3) 0.016 1 Headache 8 (38.1) 27 (55.1) 1.241 0.381 Dizziness 8 (38.1) 17 (34.7) 0.284 0.777 Abdominal pain 0 (0.0) 3 (6.1) 1.248 0.559 Diarrhea 2 (9.5) 4 (8.2) 0.082 1 Nausea 6 (28.6) 18 (36.7) 0.219 0.778 Vomiting 11 (52.4) 17 (34.7) 3.101 0.096 Jet vomiting 3 (14.3) 3 (6.1) 1.541 0.332 Depression 14 (66.7) 36 (73.5) 0.016 1 Irritability 1 (4.8) 11 (22.4) 2.955 0.15 Drowsiness 9 (42.9) 30 (61.2) 1.516 0.236 Dysphoria 5 (23.8) 20 (40.8) 1.466 0.251 Convulsions 3 (14.3) 11 (22.4) 0.429 0.74 Dysfunction of consciousness 11 (52.4) 27 (55.1) 0.019 1 Small localized twitches 3 (14.3) 9 (18.4) 0.082 1 Recurrent or continuous twitches 4 (19.0) 9 (18.4) 0.048 1 Respiratory failure 2 (9.5) 12 (24.5) 1.775 0.308 Cirulatory failure 0 (0.0) 3 (6.1) 1.248 0.559 -
[1] Pierson TC, Lazear HM, Diamond MS. Flaviviruses. In: Howley PM, Knipe DM, editors. Fields Virology, volume 1. Lippincott Williams & Wilkins Press. 2021; 866-8. [2] Solomon T. Control of Japanese encephalitis--within our grasp? N Engl J Med, 2006; 355, 869-71. [3] Wang HY, Liang GD. Epidemiology of Japanese encephalitis: past, present, and future prospects. Ther Clin Risk Manage, 2015; 11, 435−48. [4] Campbell GL, Hills SL, Fischer M, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ, 2011; 89, 766−74. doi: 10.2471/BLT.10.085233 [5] Griffiths MJ, Turtle L, Solomon T. Japanese encephalitis virus infection. Handb Clin Neurol, 2014; 123, 561−76. [6] Halstead SB, Hills SL, Marfin AA, et al. Japanese encephalitis vaccines. In: Plotkin SA, Orenstein WA, Offit PA. Vaccine. Elsevier. 2023, 577-600. [7] Roy U. Structural and molecular analyses of functional epitopes and escape mutants in Japanese encephalitis virus envelope protein domain III. Immunol Res, 2020; 68, 81−9. doi: 10.1007/s12026-020-09130-y [8] Liu H, Zhang J, Niu YZ, et al. The 5' and 3' untranslated regions of the Japanese Encephalitis Virus (JEV): molecular genetics and higher order structures. Front Microbiol, 2021; 12, 730045. doi: 10.3389/fmicb.2021.730045 [9] Uchil PD, Satchidanandam V. Phylogenetic analysis of Japanese encephalitis virus: envelope gene based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent. Am J Trop Med Hyg, 2001; 65, 242−51. doi: 10.4269/ajtmh.2001.65.242 [10] Mohammed MAF, Galbraith SE, Radford AD, et al. Molecular phylogenetic and evolutionary analyses of Muar strain of Japanese encephalitis virus reveal it is the missing fifth genotype. Infect Genet Evol, 2011; 11, 855−62. doi: 10.1016/j.meegid.2011.01.020 [11] Liang GD, Li XL, Gao XY, et al. Arboviruses and their related infections in China: a comprehensive field and laboratory investigation over the last 3 decades. Rev Med Virol, 2018; 28, e1959. doi: 10.1002/rmv.1959 [12] Zhang WJ, Xu CX, Nie K, et al. Genotype 5 Japanese encephalitis virus—old genotype, new threat. Zoonoses, 2022; 2, 23. [13] Hu YL, Lee PI. Safety of Japanese encephalitis vaccines. Hum Vaccin Immunother, 2021; 17, 4259−64. doi: 10.1080/21645515.2021.1969852 [14] Japanese Encephalitis Vaccines: WHO position paper – February 2015. Wkly Epidemiol Rec, 2015; 90, 69-87. (查阅网上资料, 未找到作者信息, 请确认补充) [15] Fan YC, Chen JM, Chiu HC, et al. Partially neutralizing potency against emerging genotype I virus among children received formalin-inactivated Japanese encephalitis virus vaccine. PLoS Negl Trop Dis, 2012; 6, e1834. doi: 10.1371/journal.pntd.0001834 [16] Cao L, Fu SH, Gao XY, et al. Low protective efficacy of the current Japanese encephalitis vaccine against the emerging genotype 5 Japanese encephalitis virus. PLoS Negl Trop Dis, 2016; 10, e0004686. doi: 10.1371/journal.pntd.0004686 [17] Tajima S, Yagasaki K, Kotaki A, et al. In vitro growth, pathogenicity and serological characteristics of the Japanese encephalitis virus genotype V Muar strain. J Gen Virol, 2015; 96, 2661−9. doi: 10.1099/vir.0.000213 [18] Woo JH, Jeong YE, Jo JE, et al. Genetic characterization of Japanese encephalitis virus genotype 5 isolated from patient, South Korea, 2015. Emerg Infect Dis, 2020; 26, 1002−6. doi: 10.3201/eid2605.190977 [19] Li MH, Fu SH, Chen WX, et al. Genotype V Japanese encephalitis virus is emerging. PLoS Negl Trop Dis, 2011; 5, e1231. doi: 10.1371/journal.pntd.0001231 [20] World Health Organization. Manual for the laboratory diagnosis of Japanese encephalitis virus infection. 2007; 1-52. https://cdn.who.int/media/docs/default-source/immunization/vpd_surveillance/lab_networks/manual-lab-diagnosis-je.pdf?sfvrsn=e2b62a35_4. [2023-10-19]. [21] Ye XF, Wang HY, Fu SH, et al. Etiological spectrum of clinically diagnosed Japanese encephalitis cases reported in Guizhou Province, China, in 2006. J Clin Microbiol, 2010; 48, 1343−9. doi: 10.1128/JCM.01009-09 [22] Calisher CH, Karabatsos N, Dalrymple JM, et al. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol, 1989; 70, 37−43. doi: 10.1099/0022-1317-70-1-37 [23] Maeki T, Tajima S, Kyaw AK, et al. Comparison of neutralizing antibody titers against Japanese encephalitis virus genotype V strain with those against genotype I and III strains in the sera of Japanese encephalitis patients in Japan in 2016. Jpn J Infect Dis, 2018; 71, 360−4. doi: 10.7883/yoken.JJID.2018.126 [24] Nguyen TTT, Tajima S, Ikeda M, et al. Neutralization potency of sera from Vietnamese patients with Japanese encephalitis (JE) against genotypes I and V JE viruses. Jpn J Infect Dis, 2019; 72, 115−7. doi: 10.7883/yoken.JJID.2018.232 [25] Shao N, Li F, Nie K, et al. TaqMan real-time RT-PCR assay for detecting and differentiating Japanese encephalitis virus. Biomed Environ Sci, 2018; 31, 208−14. [26] Chen XJ, Wang HY, Li XL, et al. Japanese encephalitis in China in the period of 1950-2018: from discovery to control. Biomed Environ Sci, 2021; 34, 175−83. [27] Eckels KH, Yu YX, Dubois DR, et al. Japanese encephalitis virus live-attenuated vaccine, Chinese strain SA14-14-2; adaptation to primary canine kidney cell cultures and preparation of a vaccine for human use. Vaccine, 1988; 6, 513−8. doi: 10.1016/0264-410X(88)90103-X [28] Gu PW, Ding ZE. Inactivated Japanese encephalitis (JE) vaccine made from hamster kidney cell culture (a review). Jpn Encephalitis Haemorrhagic Fever Renal Syndr Bull, 1987; 2, 15−26. [29] Lin XJ, Liu GF, Zhang L, et al. Epidemiological analysis of the Japanese encephalitis in Shandong province during 1986-2010. Mod Prev Med, 2013; 40, 2389−91. (In Chinese [30] Deng X, Yan R, Tang XW, et al. An analysis on epidemiological characteristics of Japanese encephalitis in Zhejiang Province. Prev Med, 2017; 29, 994−8. (In Chinese [31] Liang J, Liu MZ, Hu P, et al. Epidemiological and clinical characteristics of Japanese encephalitis in Guangdong, 2009-2015. Mod Prev Med, 2016; 43, 3865−8. (In Chinese [32] Shi TS, Meng L, Li DH, et al. Effect of different vaccine strategies for the control of Japanese encephalitis in mainland China from 1961 to 2020: a quantitative analysis. Vaccine, 2022; 40, 6243−54. doi: 10.1016/j.vaccine.2022.09.030 [33] Gao XY, Li XL, Li MH, et al. Vaccine strategies for the control and prevention of Japanese encephalitis in Mainland China, 1951-2011. PLoS Negl Trop Dis, 2014; 8, e3015. doi: 10.1371/journal.pntd.0003015 [34] Li XL, Cui SH, Gao XY, et al. The Spatio-temporal distribution of Japanese encephalitis cases in different age groups in mainland China, 2004 - 2014. PLoS Negl Trop Dis, 2016; 10, e0004611. doi: 10.1371/journal.pntd.0004611 [35] Wu D, Chen XJ, Liu WJ, et al. Emergence of Japanese encephalitis among adults 40 years of age or older in northern China: Epidemiological and clinical characteristics. Transbound Emerg Dis, 2021; 68, 3415−23. doi: 10.1111/tbed.13945 [36] Wang LH, Fu SH, Wang HY, et al. Japanese encephalitis outbreak, Yuncheng, China, 2006. Emerg Infect Dis, 2007; 13, 1123−5. doi: 10.3201/eid1307.070010 [37] Liu WJ, Fu SH, Ma XM, et al. An outbreak of Japanese encephalitis caused by genotype Ib Japanese encephalitis virus in China, 2018: A laboratory and field investigation. PLoS Negl Trop Dis, 2020; 14, e0008312. doi: 10.1371/journal.pntd.0008312 [38] Zhang XS, Jin N, Tu AX, et al. Adults in northwest China experienced the largest outbreak of Japanese encephalitis in history 10 years after the Japanese encephalitis vaccine was included in the national immunization program: A retrospective epidemiological study. J Med Virol, 2023; 95, e28782. doi: 10.1002/jmv.28782 [39] Zhang XH, Dong MX, Yang XJ, et al. Investigation on the neutralizing antibody against Japanese encephalitis virus in healthy people in Southeast Gansu province in 2018. Chin J Exp Clin Virul, 2022; 36, 591−4. (In Chinese [40] Wang GW, Li HN, Yang XJ, et al. Guillain-barré syndrome associated with JEV infection. N Engl J Med, 2020; 383, 1188−90. doi: 10.1056/NEJMc1916977 [41] Gao XY, Liu H, Li XL, et al. Changing geographic distribution of Japanese encephalitis virus Genotypes, 1935-2017. Vector Borne Zoonotic Dis, 2019; 19, 35−44. doi: 10.1089/vbz.2018.2291 [42] Li F, Feng Y, Wang GW, et al. Tracing the spatiotemporal phylodynamics of Japanese encephalitis virus genotype I throughout Asia and the western Pacific. PLoS Negl Trop Dis, 2023; 17, e0011192. doi: 10.1371/journal.pntd.0011192 [43] Chai CX, Wang Q, Cao SJ, et al. Serological and molecular epidemiology of Japanese encephalitis virus infections in swine herds in China, 2006-2012. J Vet Sci, 2018; 19, 151−5. doi: 10.4142/jvs.2018.19.1.151 [44] Chen C, Zhao T, Jiang YT, et al. Vector mosquito ecology and Japanese encephalitis virus genotype iii strain detection from Culex tritaeniorhynchus and Pig in Huaihua, China. Vector Borne Zoonotic Dis, 2019; 19, 933−44. doi: 10.1089/vbz.2019.2453 [45] Wang LH, Fu SH, Zhang HL, et al. Identification and isolation of Genotype-I Japanese encephalitis virus from encephalitis patients. Virol J, 2010; 7, 345. doi: 10.1186/1743-422X-7-345 [46] Zhang JS, Zhao QM, Guo XF, et al. Isolation and genetic characteristics of human genotype 1 Japanese encephalitis virus, China, 2009. PLoS One, 2011; 6, e16418. doi: 10.1371/journal.pone.0016418 [47] Burke DS, Nisalak A, Ussery MA, et al. Kinetics of IgM and IgG responses to Japanese encephalitis virus in human serum and cerebrospinal fluid. J Infect Dis, 1985; 151, 1093−9. doi: 10.1093/infdis/151.6.1093 -




 下载:
下载:



 Quick Links
Quick Links