HTML
-
Incomplete peripheral facial paralysis is a common sequela after acoustic neuroma resection. Peripheral facial paralysis with hypoplasia, commissural distortion, mimetic convulsion, facial muscle impairment, or even blindness, seriously affect patients' physiological and psychological status as well as daily quality of life and social interactions[1]. With the progress within departments of neurosurgery and in intraoperative electrophysiological monitoring technology, in the case of acoustic neuroma surgery, more and more facial nerves are being preserved anatomically, and the incidence of complete facial paralysis has obviously decreased. Most of the patients showed incomplete facial paralysis after operation[2]. Although there are a variety of options for repair and rehabilitation of hand paralysis, the recovery of incomplete facial paralysis after acoustic neuroma surgery is not satisfactory.
The facial paralysis after acoustic neuroma surgery is often caused by the injury to the facial nerve and the brainstem, and the injured nerve cannot be directly anastomosed to the ends of the facial nerve or broken end grafts. Other motor nerves are commonly used in clinical nerve transposition surgery, such as the hypoglossal nerve, the accessory nerve, the masseteric nerve, and the lateral nerve, and facial nerve anastomosis is conducted so that paralyzed facial muscles regain nerve control to diminish facial paralysis[3-7]. End-to-end anastomosis of the hypoglossal facial nerve is the most common method for the treatment of facial paralysis after acoustic neuroma surgery[3]. However, hemi-atrophy of the tongue muscle is a weakness of this approach, and some patients experience dysfunctions in mastication, pronunciation and swallowing. Recently, to avoid the atrophy of the tongue muscle and to preserve the function of the tongue muscle, modified hypoglossal nerve and facial nerve end-to-end anastomosis was used to treat facial paralysis via guidance or transplantation of the nerve and end-to-end anastomosis between the hypoglossal nerve and the facial nerve[7]. The prognosis of facial paralysis obtained by the above neural repair methods is similar[8]. Most patients attain only H-B grade Ⅲ or grade Ⅳ, and only a small number of patients reach grade Ⅱ and still cannot be cured. The treatment method is completely targeted to the hypoglossal nerve instead of the facial muscles innervated by the facial nerve. The drawback of this method is the lack of spontaneous regeneration of the facial nerve. This method is suitable for patients with complete facial paralysis or even a preserved facial nerve anatomy, but long-term observation indicates that facial nerve function shows no signs of recovery. Therefore, this method is not suitable for the preservation of the facial nerve anatomy of incomplete paralysis patients.
For incomplete paralysis patients, the timing of recovery is controversial[9, 10]. For patients with incomplete facial paralysis with an intact and preserved facial nerve, it is necessary to measure their recovery ability and explore the advantages and disadvantages of delayed repair[11]. Facial expression muscles in cases of incomplete paralysis exhibit denervation or disuse atrophy. Electrical stimulation therapy and Chinese medicine treatment have a stimulatory effect on nerve growth but cannot replace the operation as an effective means of treatment. Long-term-delayed repair does not alter the reduction of muscle fibers, resulting in persistent facial paralysis on the affected side. The anatomic preservation of the posterior nerve in acoustic neuroma surgery is continuing to improve, and postoperative paralysis is in most cases incomplete facial paralysis; however, the result of facial nerve graft repair is not satisfactory. Clinically, rehabilitation measures are often used to promote recovery from incomplete paralysis. However, the results are not satisfactory, and this approach may miss the optimal time of nerve repair. Therefore, identifying the best time to perform nerve repair surgery without damaging the residual facial nerve is the current research direction.
The present study aimed to establish an animal model of incomplete facial paralysis at different time points after nerve grafting for facial paralysis, through hypoglossal nerve and facial nerve anastomosis. Electrophysiological examination, retrograde tracing and histological detection methods were used. The feasibility of double innervation of the facial nerve and the hypoglossal nerve in paralyzed facial muscles and the best time for performing nerve repair surgery were examined. The results as followed can provide a reliable basis for the clinical treatment of incomplete facial paralysis.
-
Thirty Sprague Dawley rats (3 months old, weighing 180-200 g) provided by Beijing Vital River Laboratory Animal Technology Co., Ltd., were used in this study. The rats were housed in Specific Pathogen Free (SPF) cages at an artificial cycle of 12 h of light and 12 h of darkness. The experiments were approved by the local animal care committee.
Thirty rats were randomly divided into the following 5 groups (n = 6 each): injury without facial nerve (FN) reconstruction (Control), immediate hemi hypoglossal nerve and facial nerve (hemiHN-FN) neurorrhaphy (IMM Neurrph), 2-week-delayed hemiHN-FN neurorrhaphy (2-WK Delayed Neurrph), 4-week-delayed hemiHN-FN neurorrhaphy (4-WK Delayed Neurrph), or 8-week-delayed hemiHN-FN neurorrhaphy (8-WK Delayed Neurrph). The bulldog clamp remains inside the rats to clamp the FN trunk continuously. Animals in the immediate and delayed neurorrhaphy groups underwent FN repair 3 months after the FN injury.
-
The FN trunk injury and hemiHN-FN neurorrhaphy were performed by an experimenting surgeon using an operating microscope. All rats underwent surgery following the intra-peritoneal injection of sodium pentobarbital (54 mg/kg). The right facial nerve trunk was completely exposed using a bulldog clamp, and the FN trunk was clamped continuously so that the injured FN axons could spontaneously regenerate, replicating the pressure of a tumor on the facial nerve to create an incomplete facial paralysis model.
A pre-degenerated nerve autograft (PNG) was prepared 1 week before the FN reconstruction by sectioning the same rat's sural nerve for pre-degeneration. We used the distal segment of the injured sural nerve in the same rat for transplantation to avoid the problem of immune rejection. Rats underwent the same general anesthesia as described above. The right sural nerve was exposed by sectioning the right thigh skin and dissecting the septum between the semimem- branosus and the gracilis. The sural nerve fibers were separated using microforceps for 1 minute of compression, but the epineurium was maintained. Then, 5-0 nylon were used to ligate the distal nerve. The surgical wound was closed with 4-0 nylon sutures, and the rats were returned to their cages.
For hemiHN-FN neurorrhaphy, the right hypoglossal nerve (HN) trunk was exposed and hemi-sectioned. The right FN was exposed, and a 5-mm epineurial window at the distal site of FN injury was created using microscissors. Next, section the right thigh skin and dissecting the septum between the semimembranosus and the gracilis, a 10-mm-long pre-degenerated sural nerve that had been prepared one week previously was harvested. The proximal end of the PNG was associated with the HN, and the other side of the PNG was joined to the FN, in which 10-0 absorbable suture for neurorrhaphy. Neither the HN nor the FN trunk was not cut off, so this procedure is referred to as 'side'-to-side neurorrhaphy. The surgical wound was closed with 4-0 nylon sutures, and the rats were returned to their cages. The rats' recovery was monitored for 3 months following the FN reconstruction. They were then evaluated using facial symmetry, electrophysiological and histological assessment methods (Figure 1).
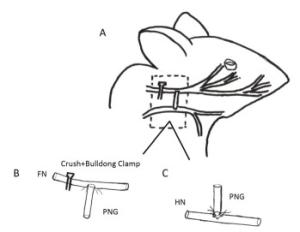
Figure 1. Schematic drawings showing the surgical procedures for facial nerve (FN) injury and repair in the rat. (A) A crush- and bulldog clamp-induced injury was caused at the beginning of the FN. A hypoglossal-facial 'side'-to-side neurorrhaphy with a pre-degenerated nerve graft (PNG) was then performed after the injury using 10-0 nylon microsutures. (B) The anastomosis between the FN and the PNG. A window was unilaterally created on the FN trunk, which was anastomosed with the distal site of the PNG in an end-to-side fashion. (C) The anastomosis between the PNG and the hypoglossal nerve (HN). The HN was hemi-sectioned, and the proximal site of the PNG was anastomosed with the sectioned site of the HN in an end-to-'side' fashion.
-
Each rat was photographed before injury, 1 week after injury and 3 months after neurorrhaphy. The extent of facial paralysis was evaluated by the nose deviation index. The tip of the nose deviates from the index, that is, the angle of the line between the two eyes and the extension of the center line. The greater the angle, the better the recovery is. An angle of approximately 90 degrees indicates complete recovery.
-
Muscle action potentials (MAPs) were recorded from the right whisker pad muscle while the PNG or the FN trunk was electrically stimulated using a Neuro-MEP-Micro electromyogram (Neurosoft, Russia) to evaluate the functional reinnervation of the FN. Recordings were conducted at 3 months after FN reconstruction. Stimulation (0.2 ms, 1 mA) was delivered through two electrodes (0.2-mm diameter) placed on the PNG or the FN distal to the neurorrhaphy site. MAPs were recorded by two electrodes (0.2-mm diameter) inserted into the right whisker pad muscle at the proximal and distal borders, thus covering the whole pad muscles. The MAP amplitude or peak area is proportional to the axon count in conducting nerves and was measured from the recorded signals.
-
The neural tracer CTB-Alexa 555 (Molecular Probes, Eugene, Oregon, USA) was used as a retrograde label of axon regrowth from the facial or hypoglossal nucleus to the paralyzed facial muscles, either via the initial FN or via the reconstructed PNG pathway. Under general anesthesia, 10 μL 1% CTB- Alexa 555 solution was injected at multiple points into the right whisker muscle site with a Hamilton syringe before each rat was electrophysiologically monitored. Then, rats were returned to their cages, where they recovered consciousness. Five days later, the rats were sacrificed using an overdose of anesthesia and then transcardially perfused with 200 mL of isotonic saline for systemic perfusion followed by 200 mL of 4% paraformaldehyde (PFA) (pH 7.2) solution. The brainstem was harvested and post-fixed in the same PFA fixative solution for 3 h. The samples were then successively cryo-preserved in 20%, 30%, and 40% sucrose at 4 ℃. All tissues were embedded in optimal cutting temperature (OCT) medium and then cross-sectioned at 30-μm thickness using a frozen slicer (Leica CM1950, Germany). The slices were placed onto clean gelatin-coated slides and observed under a fluorescence microscope (Nikon Diaphot, Japan).
-
The PNG and FN tissues were removed and fixed in 3.6% glutaraldehyde solution for 3 h. The samples were washed three times with phosphate-buffered saline (PBS, 0.1 mol/L, pH 7.0) for 10 minutes, followed by post-fixation with osmium tetroxide and embedding in Epon. Semi-thin (0.35 μm) cross-sections were obtained using an ultramicrotome (Reichert Ultracut S Wild M3z, Leica Microsystems) and stained with thionin or uranyl acetate and lead citrate. The semi-thin sections were examined under an optic microscope. The number of regenerated axons was counted by researchers who were blinded to the study, using aperio imagesope (Leica).
-
All data were presented as the means ± standard error of the mean (SEM). GraphPad Prism 6.0 (USA) was used for statistical analysis. Measurements of alpha angle, MAP amplitude, MAP area under the curve, CTB-555-labeled motor neuron count, and regenerated axon count were recorded. Three months after repair treatment (between columns), one-way ANOVA was conducted. Bonferroni's post hoc multiple comparisons tests were performed for between-group analyses. P values ≤ 0.05 were considered statistically significant (***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05).
Animals
Surgical Procedures
Facial Symmetry
Electrophysiological Examination
Retrograde Labeling
Quantitative Axon Measurements
Statistical Analysis
-
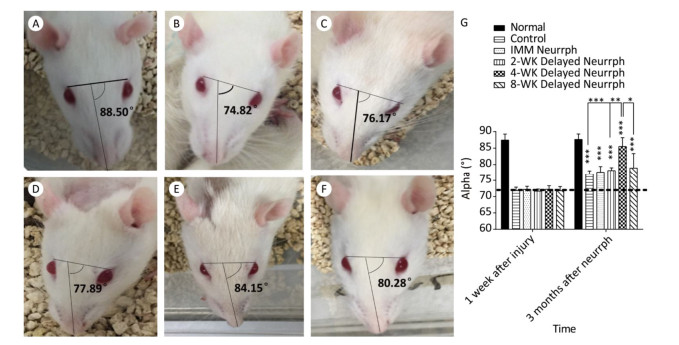
Changes in facial symmetry after FN injury and treatment were evaluated by measuring the α angle. The α angle is 90° on average in normal rats before FN injury, and it decreased to approximately 71.9° after 1 week in all injury groups. At 3 months post-surgery, increases in the α angle were observed in all the treated and non-treated groups (P < 0.001). In particular, the 4-WK Delayed Neurrph group had a mean α angle of 85.49° ± 2.68°; this group showed a significant difference from the non-repaired Control group, with a mean α angle of 76.97° ± 1.08° (P < 0.001); the IMM Neurrph group, with an alpha angle of 77.63° ± 1.77°; the 2-WK Delayed Neurrph group, with a mean α angle of 78.15° ± 0.86° (P < 0.01); and the 8-WK Delayed Neurrph group, with a mean α angle of 78.95° ± 4.39° (P < 0.05) (Figure 2).
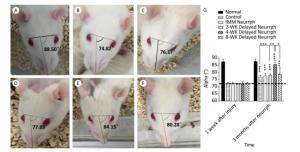
Figure 2. Assessment of the alpha angle to indicate facial symmetry. The alpha angle was defined as the acute angle between the line extending from the fold on the bridge of the nose and the line linking the two outer canthi. (A) The alpha angle of a normal rat was 88.50°. (B) The alpha angle of a control group rat was 74.82°. (C) The alpha angle of an immediate HemiHN-FN neurorrhaphy group rat was 76.17°. (D) The alpha angle of a 2-WK Delayed Neurrph group rat was 77.89°. (E) The alpha angle of a 4-WK Delayed Neurrph group rat was 84.15°. (F) The alpha angle of an 8-WK Delayed Neurrph group rat was 80.28°. (G) Measurements of the alpha angle 1 week after facial nerve injury and 3 months after neurorrphy. The results are presented as the means ± SEM. The differences between groups 3 months after repair treatment were significant based on one-way ANOVA: F (5, 25) = 13.39. Bonferroni's post hoc test for multiple comparisons was performed for comparison between groups. ***P < 0.001, compared to groups with mean values at or below the dotted line or as specifically indicated, as well as for the alpha angle in the Control group vs. that of the 4-WK Delayed Neurrph group. **P < 0.01, the alpha angle in the 2-WK Delayed Neurrph group vs. that in the 4-WK Delayed Neurrph group. *P < 0.05, the alpha angle in the 8-WK Delayed Neurrph group vs. that in the 4-WK Delayed Neurrph group.
-
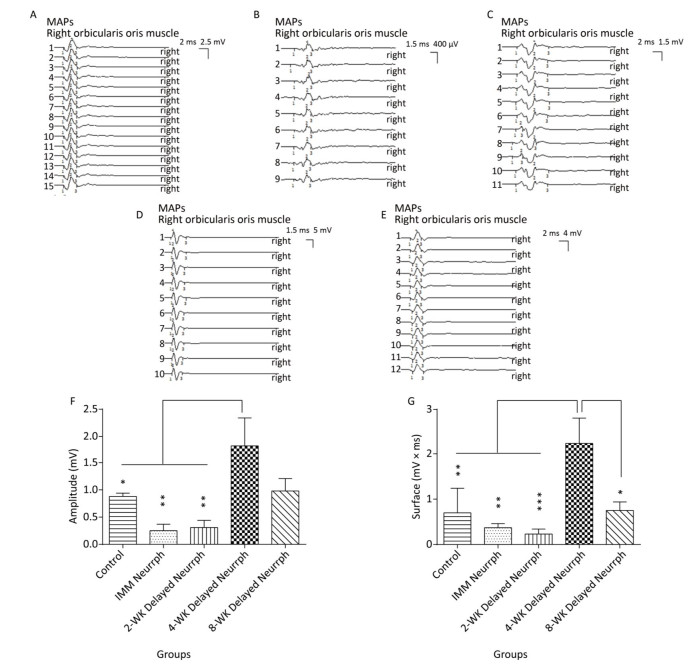
To assess functional target innervation in both the initial FN and the reconstructed PNG nerve pathways, MAPs were recorded in the whisker pad. The MAP amplitude and area under the curve were measured to evaluate the conduction effectiveness. The four hemiHN-FN neurorrhaphy groups were found to exhibit muscle action potentials in the right whisker pad in response to stimulation of the PNGs. MAPs were recorded in the 4-WK Delayed Neurrph group (1.62 ± 0.55 mV for the amplitude and 2.81 ± 0.52 mVmsec for the area under the curve), which showed a significant difference compared with the IMM Neurrph group (0.23 ± 0.08 mV for the amplitude and 0.54 ± 0.28 mVmsec for the area under the curve) and the 2-WK Delayed Neurrph group (0.25 ± 0.07 mV for the amplitude and 0.58 ± 0.31 mVmsec for the area under the curve) (P < 0.01). As for the 8-WK Delayed Neurrph group, The MAP amplitude (0.80 ± 0.30 mV) and area under the curve (1.62 ± 1.10 mVmsec) were somewhat reduced compared with those for the 4-WK Delayed Neurrph group, indicating spontaneous reinnervation in the intervening period (Figure 3).
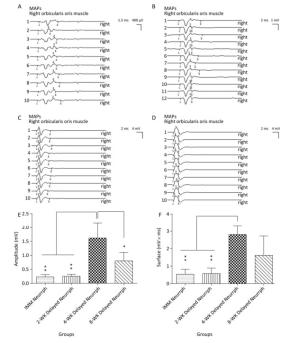
Figure 3. The evaluation of muscle action potentials (MAPs) recorded from the paralyzed whisker pad muscle in response to electro-stimulation of the PNG at 3 months after FN neurorrhaphy. The MAP amplitude and area under the curve were measured to reveal the quantity of conductive axons and their capability of synchronous conduction. (A) The MAP amplitude and area under the curve of the IMM Neurrph group. (B) The MAP amplitude and area under the curve of the 2-WK Delayed Neurrph group. (C) The MAP amplitude and area under the curve of the 4-WK Delayed Neurrph group. (D) The MAP amplitude and area under the curve of the 8-WK Delayed Neurrph group. (E) The MAP amplitude of each group is presented as the mean ± SEM. The differences between groups at 3 months after repair treatment were significant based on one-way ANOVA: F (3, 14) = 3.210. Bonferroni's post hoc test for multiple comparisons was performed for comparisons between groups. *P < 0.05, the MAP amplitude in the 8-WK Delayed Neurrph group vs. that in the 4-WK Delayed Neurrph group. **P < 0.01, the MAP amplitude in the IMM Neurrph and 2-WK Delayed Neurrph groups vs. that in the 4-WK Delayed Neurrph group. (F) The MAP area under the curve of each group at different time points is presented as the mean ± SEM. The difference between repair treatment groups was not significant based on one-way ANOVA: F (3, 13) = 2.077. Bonferroni's post hoc test for multiple comparisons was performed for comparisons between groups. **P < 0.01, the MAP areas under the curve of the IMM Neurrph and 2-WK Delayed Neurrph groups vs. that of the 4-WK Delayed Neurrph group.
Similarly, MAPs in response to FN stimulation were recorded from the whisker pad muscle of the four repaired groups and the non-repaired group. Significant differences in MAP amplitude were observed for the 4-WK Delayed Neurrph group (1.81 ± 0.53 mV) compared to the IMM Neurrph group (0.25 ± 0.12 mV) (P < 0.01), the 2-WK Delayed Neurrph group (0.31 ± 0.13 mV) (P < 0.01) and the Control group (0.88 ± 0.06 mV) (P < 0.05). There was no significant difference between the 4-WK Delayed Neurrph group and the 8-WK Delayed Neurrph group (0.75 ± 0.19 mV) (P > 0.05). The MAP area under the curve was significantly higher in the 4-WK Delayed Neurrph group (2.25 ± 0.56 mVmsec) than in the IMM Neurrph group (0.37 ± 0.09 mVmsec) (P < 0.01), the 2-WK Delayed Neurrph group (0.23 ± 0.11 mVmsec) (P < 0.001), the 8-WK Delayed Neurrph group (0.75 ± 0.19 mVmsec) (P < 0.05) and the Control group (0.70 ± 0.54 mVmsec) (P < 0.01) (Figure 4).
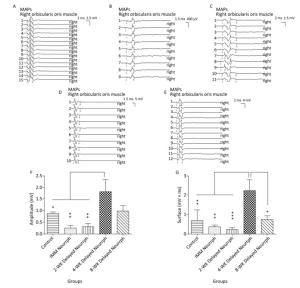
Figure 4. The evaluation of muscle action potentials (MAPs) recorded from the paralyzed whisker pad muscle in response to electro-stimulation of the FN trunk at 3 months after FN neurorrhaphy. (A) The MAP amplitude and area under the curve in the Control group. (B) The MAP amplitude and area under the curve in the IMM Neurrph group. (C) The MAP amplitude and area under the curve in the 2-WK Delayed Neurrph group. (D) The MAP amplitude and area under the curve in the 4-WK Delayed Neurrph group. (E) The MAP amplitude and area under the curve in the 8-WK Delayed Neurrph group. (F) The MAP amplitude of each group is presented as the mean ± SEM. The differences between groups 3 months after repair treatment were significant based on one-way ANOVA: F (4, 14) = 15.59. Bonferroni's post hoc test for multiple comparisons was performed for comparisons between groups. **P < 0.01, MAP amplitude in the IMM Neurrph and 2-WK Delayed Neurrph groups vs. that in the 4-WK Delayed Neurrph group. *P < 0.05, MAP amplitude in the Control group vs. that in the 4-WK Delayed Neurrph group. (G) The MAP area under the curve of each group at the different time points is presented as the mean ± SEM. The differences between groups at 3 months after repair treatment were significant based on one-way ANOVA: F (4, 14) = 12.45. Bonferroni's post hoc test for multiple comparisons was performed for comparisons between groups. ***P < 0.001, MAP area under the curve in the 2-WK Delayed Neurrph group vs. that in the 4-WK Delayed Neurrph group. **P < 0.01, MAP area under the curve in the Control and IMM Neurrph groups vs. that in the 4-WK Delayed Neurrph group. *P < 0.05, MAP area under the curve in the 4-WK Delayed Neurrph group vs. that in the 8-WK Delayed Neurrph group.
-
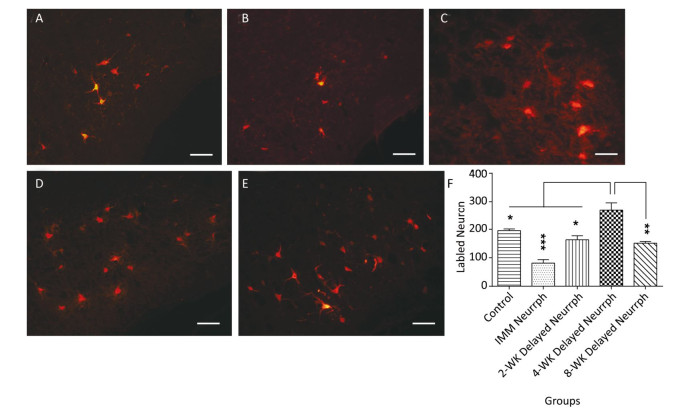
Retrograde labeling was performed by injecting CTB-Alexa 555 into the right whisker muscle 3 months after nerve neurorrhaphy. In contrast to the 4-WK Delayed Neurrph group (267 ± 29 CTB-Alexa 555-labeled neurons), 195 ± 6 (P < 0.05) retrograde-labeled neurons were found in the right facial nuclei of non-repaired rats, which indicates that there was spontaneous reinnervation. In the facial nucleus after hemiHN-FN neurorrhaphy, the number of retrograde-labeled neurons was 81 ± 12 in IMM Neurrph group (P < 0.001), 163 ± 14 in the 2-WK Delayed Neurrph group (P < 0.05) and 151 ± 6 in the 8-WK Delayed Neurrph group (P < 0.01) (n = 4). Significantly greater numbers of CTB-Alexa 555-labeled neurons were found in the 4-WK Delayed Neurrph group than in the IMM Neurrph, 2-WK Delayed Neurrph and 4-WK Delayed Neurrph groups, indicating that more axon innervation occurred when the repair was delayed by 4 weeks.
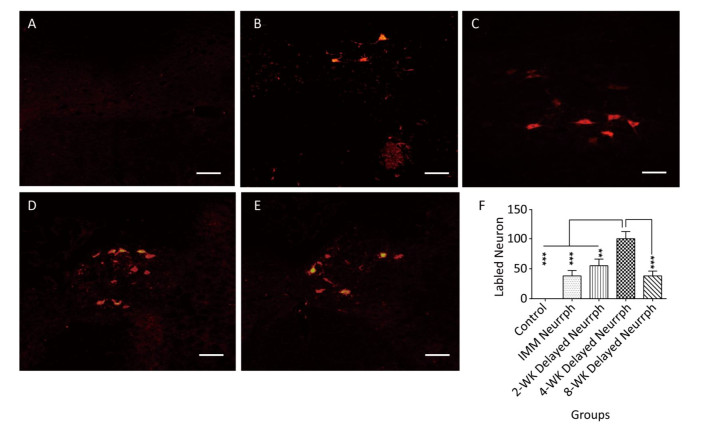
In the hypoglossal nucleus after repair, the number of CTB-Alexa 555-labeled neurons was 38 ± 9 in the IMM Neurrph group (P < 0.001), 55 ± 11 in the 2-WK Delayed Neurrph group (P < 0.01) and 38 ± 8 in the 8-WK Delayed Neurrph group (P < 0.001) (n = 4). The 4-WK Delayed Neurrph group had 100 ± 12 retrograde-labeled neurons, which was obviously different from that in the other groups. This result showed that the facial muscles were dominated by a large amount of hypoglossal fibers (Figures 5-6).
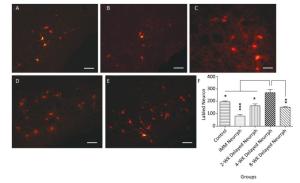
Figure 5. Observation and quantification of retrogradely labeled motor neurons in the facial nucleus 5 days after multipoint injection of Alexa CTB-555 into the paralyzed whisker pad muscle. (A) The CTB-555-labeled motor neurons (in red) in the facial nucleus of the control group 3 months after nerve neurorrhaphy. (B) The CTB-555-labeled motor neurons in the IMM Neurrph group. (C) The CTB-555-labeled motor neurons in the 2-WK Delayed Neurrph group. (D) The CTB-555-labeled motor neurons in the 4-WK Delayed Neurrph group. (E) The CTB-555-labeled motor neurons in the 8-WK Delayed Neurrph group. Bar = 50 µm. (F) The CTB-555-labeled neurons in the facial nucleus were quantified for each group at 3 months after the FN injury, and the counts are presented as the means ± SEM. The difference between groups 3 months after repair treatment were significant based on one-way ANOVA: F (4, 15) = 66.08. Bonferroni's post hoc test for multiple comparisons was performed for comparisons between groups. ***P < 0.001, the number of labeled neurons in the IMM Neurrph group vs. that in the 4-WK Delayed Neurrph group. **P < 0.01, the number of labeled neurons in the 4-WK Delayed Neurrph group vs. that in the 8-WK Delayed Neurrph group. *P < 0.05, the number of labeled neurons in the Control and 2-WK Delayed Neurrph groups vs. that in the 4-WK Delayed Neurrph group.
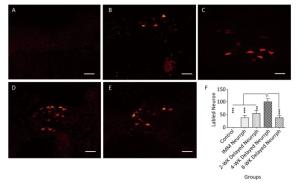
Figure 6. Observation and quantification of retrogradely labeled motor neurons in the hypoglossal nucleus 5 days after multipoint injection of Alexa CTB-555 into the paralyzed whisker pad muscle. (A) The CTB-555-labeled motor neurons (in red) were not detected in the hypoglossal nucleus of the control group after 3 months of nerve neurorrhaphy. (B) The CTB-555-labeled motor neurons in the IMM Neurrph group. (C) The CTB-555-labeled motor neurons in the 2-WK Delayed Neurrph group. (D) The CTB-555-labeled motor neurons in the 4-WK-Delayed Neurrph group. (E) The CTB-555-labeled motor neurons in the 8-WK Delayed Neurrph group. Bar = 50 µm. (F) Quantification of labeled neurons in the hypoglossal nucleus of each group. The results are presented as the means ± SEM, and one-way ANOVA: F (3, 13) = 28.67 revealed significant differences between groups. Bonferroni's post hoc test for multiple comparisons was performed for comparisons between groups. ***P < 0.001, the number of labeled neurons in the IMM Neurrph group and the 8-WK Delayed Neurrph group vs. that in the 4-WK Delayed Neurrph group. **P < 0.01, the number of labeled neurons in the 2-WK Delayed Neurrph group vs. that in the 4-WK Delayed Neurrph group.
-
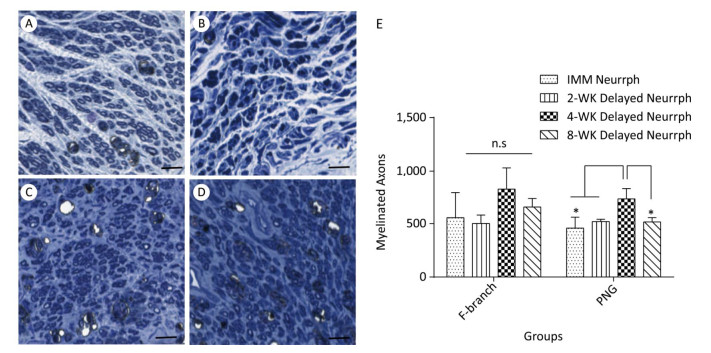
At the FN level, there were numerous regenerated axons. However, there were no differences in regenerated axons for the 4-WK Delayed Neurrph group 823 ± 207 compared with the IMM Neurrph 556 ± 235, 2-WK Delayed Neurrph 502 ± 79 and 8-WK Delayed Neurrph groups 655 ± 81 (n = 4), suggesting an approximately equal amount of axon regrowth from the distal facial nerve. However, at the PNG level, the 4-WK Delayed Neurrph group 732 ± 96 had a significant difference in the number of regenerated axons compared with the IMM Neurrph group 458 ± 102, the 2-WK Delayed Neurrph group 519 ± 21 and the 8-WK Delayed Neurrph group 516 ± 41 (P < 0.05), indicating that more axon regeneration occurred when the repair was delayed by 4 weeks (Figure 7).
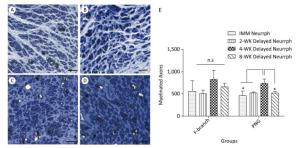
Figure 7. Representative photomicrographs of myelinated axons on thionin-stained sections at a level distal to the neurorrhaphy site of the FN and the intermediate part of the PNG 3 months after nerve neurorrhaphy. (A) Myelinated axons in the PNG of a rat subjected to immediate HemiHN-FN neurorrhaphy. (B) Myelinated axons in the PNG of a rat subjected to a 2-WK Delayed Neurrph. (C) Myelinated axons in the PNG of a rat subjected to a 4-WK Delayed Neurrph. (D) Myelinated axons in the PNG of a rat subjected to 8-WK Delayed Neurrph. Scale bars: 200 μm. (E) Myelinated axons were quantified at a level distal to the neurorrhaphy site of the FN for each group. The differences between groups 3 months after repair treatment were not significant based on one-way ANOVA: F (3, 11) = 1.946. Myelinated axons were quantified in the PNG. The differences between groups 3 months after repair treatment were significant based on one-way ANOVA: F (3, 8) = 5.332. n.s, no significant difference. *P < 0.05, myelinated axon count in the IMM Neurrph group, the 2-WK Delayed Neurrph group and the 8-WK Delayed Neurrph group vs. that in the 4-WK Delayed Neurrph group.
Recovery of Facial Symmetry
Electrophysiological Examination
Retrograde Labeling
Axon Regeneration in the Reconstructed Nerve Pathway
-
The injured facial nerve can be regenerated and repaired, which has been acknowledged in basic research and clinical settings. A large amount of clinical data show that the hypoglossal nerve center is superior to other cranial nerves in controlling facial muscle and restoring facial function. Currently, HN-FN end-to-end neurorrhaphy, either directly or via a nerve graft, is the favored procedure because of the functional similarity between the HN and the FN[12]. For example, the cortical representations of the tongue and facial muscle are close to one another[13, 14]. However, speech problems, swallowing dysfunction and atrophy of the tongue muscle are major complications after the operation that seriously affect the quality of life of these patients.
There are several main factors that determine the extent of nerve regeneration after nerve injury: the amount and route of nerve axon regeneration, the rate of nerve regeneration, the retention of target tissue structure and the re-formation of synaptic connections. In other words, in order to attain a good repair result, a sufficient amount of nerve fiber regeneration is needed within an appropriate period of time, that is, before the dominant tissue structure qualitatively changes, enabling proper matching of the target tissue for the formation of synaptic connections. Therefore, ideal facial nerve repair should be a type of treatment that does not affect the basis of facial nerve repair and that promotes recovery of facial nerve function.
Is immediate repair or delayed repair more appropriate after peripheral nerve injury? In experimental studies, Bignotti[15] showed electrophysiological and morphological changes with early suture or delayed suture of the sciatic nerve in rats. The nerve conduction velocity results showed that early suture was more effective. However, Guntinas[10] found that when the facial nerve of rats was taken as the object of observation, delayed repair was better than immediate restoration. Chen[13] showed in a study of hypoglossal-facial nerve anastomosis guinea pigs that early surgical nerve regeneration led to better outcomes because with an earlier anastomosis time, the extent of Schwann cell-mediated myelin degradation is smaller and there is less replacement by fibrous tissue, thus facilitating axonal growth and myelination. Nevertheless, Barrs[16] observed no significant difference between immediate and delayed repair in experiments of facial nerve injury in piglets. Most of the previous studies on nerve damage repair conducted complete injury, while the timing of incomplete repair has been less studied. However, facial nerve injury results in atrophy of certain facial muscles innervated by the facial nerve, nerve fibrosis, degeneration of nuclei in the pons and cerebral motor cortex, and other changes. Therefore, the repair operation should be performed before neurodegeneration occurs.
The optimal timing for nerve anastomosis resulting in maximal facial reanimation has not been precisely determined for patients and is usually influenced by personal clinical experience[17, 18]. It has been suggested that a delay in FN reconstruction may decrease functional recovery, especially when repair is delayed for more than 1 year. However, Kunihiro[3] reported that HN-FN repair can be postponed for as long as 2 years with only a minimal effect on the recovery of facial movement. Recently, some researchers have obtained similar results through animal experiments. Appropriately delayed repair is beneficial to facial nerve repair. Guntinas-Lichius[10] showed that delayed FN repair in rats within a defined denervation period of 14-112 days can result in accelerated and enhanced muscle reinnervation. Spector[19] showed that the number of regenerated nerve fibers after the delayed repair of the facial nerve was greater than that after early facial nerve repair. A study of peroneal nerve injury by other researchers suggested that the nerve repair had no adverse effect on nerve regeneration in one month, but the efficacy of nerve regeneration would be impacted by a longer delay[20].
In this study, sural nerve grafts were pre-degenerated, the axonal degradation and a large number of Schwann cell proliferation, so that the motor nerve and sensory nerve axons do not affect neural remodeling. It is necessary to hemi-section the hypoglossal nerve because the number and extension of the axonal regeneration determine the recovery of the nerve function[21]. In order to protect the remaining facial nerve axons and/or potential spontaneous regeneration ability, it is also necessary to only open the window to the facial nerve. This kind of PNG bridging for the hemiHN-FN neurorrhaphy can make the damaged facial nerve double repaired by the hypoglossal nerve and the facial nerve itself[22].
Our study generated results from facial symmetry assessments, electrophysiological examination, retrograde labeling and measurement of axon regeneration in the reconstructed nerve pathway. The optimal surgical time of the side-to-side anastomosis of the hypoglossal nerve and facial nerve was 4 weeks after the facial nerve injury, based on the data 3 months after repair. The control group without anastomosis, in some indexes, had better outcomes than the immediate repair group, the 2-week-delayed repair group, and the 8-week-delayed repair group. One possible reason for this result may be facial nerve regeneration and repair after injury. In addition, in a sense, incomplete injury is a kind of pre-degeneration that promotes the removal of myelin debris produced via Wallerian degeneration, and the nerve regeneration is guaranteed after transplantation. After 2-3 weeks of Wallerian degeneration, most myelin has been cleared, and the greatest effect on nerve regeneration is attained[23-26]. Some delay prior to repair is appropriate in order to minimize the factors hindering nerve regeneration, such as myelin degradation products and low levels of nerve growth inhibitory factor, and maximize the factors promoting nerve regeneration, such as peak levels of neurotrophic factor and an ideal for microenvironment nerve regeneration. Under these circumstances, the time for regenerating axons to reach the target tissue to promote nerve regeneration will be shortened.
It also takes a certain time to make the axon from the hypoglossal nerve into the graft, and then to the regeneration of the facial nerve, which depends on the length of the graft and the extension speed of the axons. The regeneration rate of axons is 1-4 mm/d, and the initial stage of axon regeneration is almost 2 weeks. If the length of the grafts is 10 mm, this time is 3 weeks[27, 28]. Moreover, the facial nerve of all the rats receiving anastomosis likewise underwent a secondary injury, as some nerve fibers were cut off and the formation of fibrous scars reduced the regeneration ability. Additionally, time was needed for the two nerve endpoints to heal. This also explains why the recovery index of some rats in the anastomosis groups was lower than those in the control and non-repair groups.
The findings of this study can provide a more reliable basis for the clinical treatment of incomplete paralysis, leading to diminished or even cured incomplete paralysis, pain relief, and reduced personal, social, psychological, and economic burdens for many patients. Therefore, the key to treating incomplete facial paralysis is determining how to preserve the function of the residual facial nerve to the utmost extent and finding the best time for performing the nerve repair operation.
-
The most effective treatment for peripheral facial paralysis is 'side'-to-side neurorrhaphy of the hypoglossal nerve and the facial nerve 4 weeks after incomplete facial nerve injury. That is, in this study, we determined that the optimal surgical timing is a 4-week-delayed repair for incomplete injury.
-
No conflict of interest to declare.
-
LDZ, LS, and WH conceived and designed the experiments; ZSD, FJ, and LJH performed the experiments and data cleaning; WBB, WH, and LJH analyzed the data and prepared the manuscript.
Special fund for scientific research on health development in the capital 2014-2-1073
the Basic-Clinical scientific research cooperation fund of Capital Medical University 14JL49
the National Natural Science Foundation of China 31440051








 Quick Links
Quick Links
 DownLoad:
DownLoad: