-
Strontium-90, one of the most important long-lived radionuclides, emits high energy β rays as fission products of 235U and 239Pu. Its chemical characteristics are very similar to those of calcium, and it accumulates mainly in the bones and teeth, and damages blood-producing cells in humans[1]. Therefore, the ability to precisely detect low level 90Sr in environmental and biological samples is essential for environmental protection and health and safety. Numerous techniques for removing strontium from radioactive wastewater have been developed[2-5]. Adsorption is regarded as an efficient technology to remove Sr2+ from aqueous solutions, concerning its good adsorption capacity, low cost, and simplicity of use.
Metal organic frameworks (MOFs) have exceptionally high surface areas and porosity, and have been used for penetration, accumulation and separation of various species, such as heavy metals, radionuclides, and other toxic chemicals from both gas and liquid phases[6-11]. Since the magnetic properties of the sorbent aid in rapid and easy separation of the new solid phase from solution, introducing additional functionality via magnetic nanoparticles can be achieved by synthesizing magnetic framework composites.In our study, facile preparation of magnetic metal organic framework (Fe3O4@UiO-66-NH2) was achieved, and the magnetic metal organic framework (MMOF) was applied as a sorbent for the extraction of Sr2+ ions from liquid. This sorbent was characterized by X-ray diffraction (XRD), vibrating sample magnetometry (VSM), fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Brunauer-Emmett-Teller analysis (BET), and elemental analysis. The magnetic properties of the sorbent allowed for rapid and easy isolation of the solid phase from a solution. Fe3O4@UiO-66-NH2 exhibited both magnetic characteristics and high porosity, thus making it an excellent sorbent for wastewater treatment.
All reagents were of analytical grade. Strontium standard [GBW(E) 080242] solution was obtained from the National Research Center for Certified Reference Materials, Beijing, China.
An ICP-MS Element 2 (Thermo Fisher Scientific, Waltham, Mass., USA) mass spectrometer was used for determination of Sr2+. SEM images of the prepared materials were captured on a S4800 electron microscope (Hitachi, Tokyo, Japan). High angle X-ray diffraction patterns were obtained on a RAPID IP diffractometer with Cu Kα radiation (Rigaku, Japan). The magnetic properties of the materials were characterized with a PPMS-9 vibrating sample magnetometer (Quantom, USA). Thermodynamic investigation was performed with a synchronous thermal analyzer under nitrogen with a heating rate of 5 ℃/min (STA449c, Netzsch). A Fourier transform infrared spectrometer (Jasco FT/IR-660 plus, Germany) was also used.
Fe3O4 nanoparticles were prepared through the conventional coprecipitation method with some modifications[12]. FeCl3·6H2O (23.6 g) and FeCl2·4H2O (8.6 g) were dissolved in 500 mL deionized water under nitrogen gas with vigorous stirring at 80 ℃. NH3·H2O (25 mL, 25 wt.%) was added to the solution. The magnetic precipitates (Fe3O4 magnetic nanoparticles) were isolated from the solvent through magnetic decantation. The precipitates were washed with deionized water three times. Then Fe3O4 nanoparticles were initially functionalized with Mercaptoacetic acid (MAA)[11]. Fe3O4 (0.10 g) was added to 20 mL of an ethanol solution of MAA (0.58 mmol/L) with shaking for 24 h. The product was collected under an external magnetic field and washed several times with deionized water and ethanol under ultrasonication, then dispersed in a mixture containing 15 mL DMF, 0.12 g ZrCl4, and 0.09 g 2-aminoterephthalic acid under oscillation for 30 min to ensure uniform dispersion. The mixture was transferred into an autoclave, sealed and maintained for 24 h at 120 ℃. After being cooled to room temperature, the precipitate was collected with a magnet and washed with N, N-dimethylformamide.
Portions of strontium standard solution (100 mg/L) and appropriate volumes of ultrapure water were mixed in 10 mL test tubes, and then 5 mg of Fe3O4@UiO-66-NH2 was added during mixing; the total volume of the suspension was then adjusted to 5.0 mL. The mixed solutions were then shaken at room temperature for 60 min to ensure complete adsorption of Sr2+ onto the sorbent. Subsequently, the magnetic sorbent with adsorbed Sr2+ was separated from the suspension with a permanent magnet. The concentration of strontium was determined through inductively coupled plasma mass spectrometry with a calibration curve prepared by dilution of the strontium standard solution with 2% (v/v) HNO3. All experiments were performed twice, and mean values were used in data analysis. Control experiments without sorbent were carried out to determine the degree of strontium removal in test tubes.
The XRD patterns of Fe3O4, UiO-66-NH2, and Fe3O4@UiO-66-NH2 are shown in Figure 1A. The diffraction peaks of the synthesized microspheres showed that the MOF crystal and Fe3O4 were well-retained, because all characteristic peaks of the magnetic microspheres were readily indexed to Fe3O4 and UiO-66-NH2. The magnetic properties of the samples are shown in Figure 1B. The magnetic hysteresis loop showed no remanence or coercivity at room temperature, thus suggesting that the particles had superparamagnetic character, such that no magnetization remained when the applied magnetic field was removed. The magnetic Fe3O4 and Fe3O4@UiO-66-NH2 particles had magnetic saturation (MS) values of approximately 60.4 and 7.5 emu/g, respectively. The lower MS value of Fe3O4@UiO-66-NH2 particles was due to the dielectric property of the UiO-66-NH2. Owing to the paramagnetic quality of the MMOF, the particles could be easily separated from aqueous solution after adsorption of Sr2+ nuclides by using an external magnetic field. To investigate the surface morphology of the Fe3O4-MAA, MOF and MMOF nanocomposite, samples were characterized by TEM and SEM. As illustrated in Figure 1C, D, E, Fe3O4 nanoparticles were functionalized with MAA, and the size of Fe3O4-MAA particles was approximately 10 nm. The original UiO-66-NH2 crystals had smooth surfaces and an average size of 100 nm. However, the surface of the MMOF tended to be rougher after immobilization with MAA-Fe3O4.
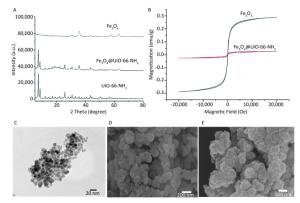
Figure 1. (A) XRD patterns of Fe3O4@UiO-66-NH2, pure UiO-66-NH2 powder, and Fe3O4 particles; (B) magnetic hysteresis of Fe3O4 nanoparticles and Fe3O4@UiO-66-NH2; (C) TEM images of Fe3O4-MAA; (D) SEM images of UiO-66-NH2; (E) SEM images of Fe3O4@UiO-66-NH2.
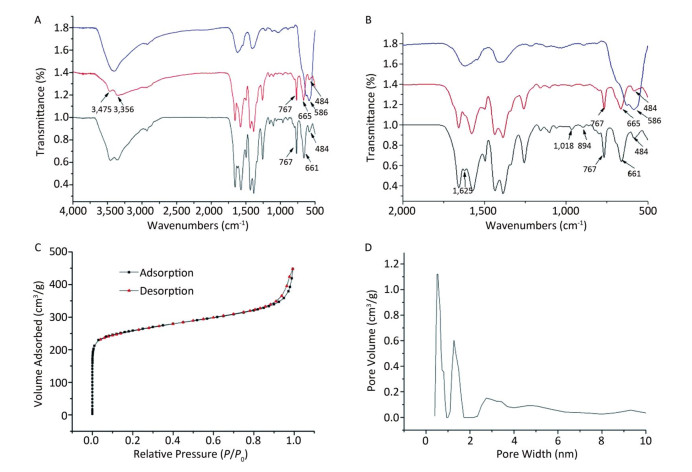
The FT-IR spectra of Fe3O4, UiO-66-NH2, and Fe3O4@ UiO-66-NH2 are presented in Figure 2A, B. In the FT-IR spectra of Fe3O4, the peaks observed at 586 cm-1 were due to Fe-O vibration. In the FT-IR spectra of UiO-66-NH2, a triplet at 767, 665, and 484 cm-1 was ascribed to Zr-O2[13]. The intensities of peaks at 3, 475 and 3, 356 cm-1 were attributed to symmetric and asymmetric stretching of primary amines[14], respectively. In the spectra of Fe3O4@UiO-66-NH2, the MMOF had an infrared absorption similar to that of UiO-66-NH2, but the corresponding absorption shifted slightly from 665 cm-1 to 661 cm-1 and increased because of the Fe-O vibration. At 1, 625 cm-1, new absorption associated with vibration of carboxylic iron was found, and the bands around 1, 018 and 894 cm-1 observed in Fe3O4@UiO-66-NH2 were attributed to C-S vibration. N2 adsorption- desorption analysis was also performed to investigate the pore structure parameters of the as-prepared MMOF composites. The results (Figure 2C) indicated the presence of micropores, such that a steep uptake at low relative pressure was followed by nearly horizontal adsorption and desorption curves. Fe3O4@UiO-66-NH2 showed hysteresis loops at high relative pressure, most probably as a result of textural porosity formed by the stacking of nanoparticles. The BET surface area and pore volume (Vt) were 878 m2 g–1 and 0.69 mL/g, respectively. The BET surface area was lower than that of pure UiO-66-NH2 (1, 112 m2/g)[15], mainly because of the heavier mass. The pore size distributions for the as-synthesized Fe3O4@UiO-66-NH2, estimated on the basis of density functional theory (DFT), were mainly 0.54 nm, 1.2 nm, and 2.7 nm, and were attributed to micropore and mesopore cages (Figure 2D).

Figure 2. (A) FTIR spectrum of Fe3O4@UiO-66-NH2, UiO-66-NH2, and Fe3O4 particles; (B) FTIR spectrum of Fe3O4@UiO-66-NH2, UiO-66-NH2, and Fe3O4 particles in detail; (C) N2 sorption-desorption isotherms of the as prepared Fe3O4@UiO-66-NH2; (D) Pore size distribution of Fe3O4@UiO-66-NH2.
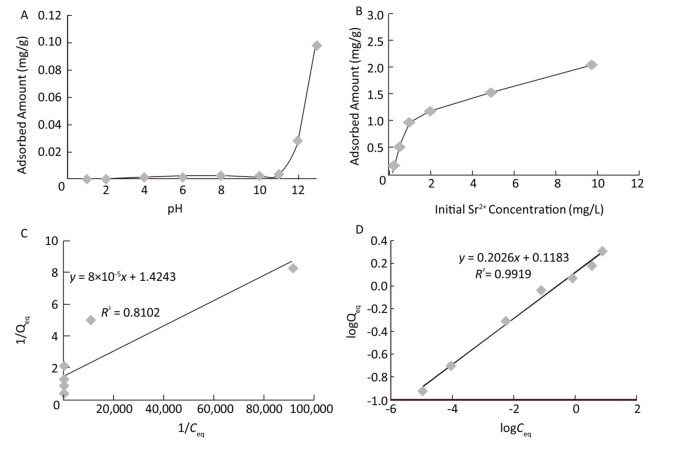
The effect of pH on sorption of strontium on the MMOF is shown in Figure 3A. The solution pH was one of the most important variables affecting adsorption characteristics, and an appropriate pH value could improve the adsorption efficiency[4]. Thus, the effect of pH on the sorption behavior of Fe3O4@UiO-66-NH2 was investigated by using 5 mL of 100 μg/L strontium solution and 5 mg magnetic adsorbent at different pH values ranging from 1.0 to 13.0. The influence of initial pH on the adsorption of strontium from water (Figure 3A) indicated that the amount of adsorption remained constant from pH 1.0 to 10.0, then increased sharply from pH 11.0 to 13.0. This observation was due to protonation of the actives sites of MMOF. In alkaline conditions, OH– reacts with H+ in solution, such that much more negative ions form, and the protonation of these active sites decreased. Therefore, the negative charge density of MMOF aggregated, and the conditions were more conducive to the adsorption of Sr2+. To demonstrate that the decrease in strontium concentration was not caused by the formation of strontium hydroxide, the same concentrations of strontium solution without addition of adsorbent at pH 9.0-13.0 were separated and detected in the same way. The results showed that the amount of strontium did not decrease. Other studies have also reported a sharp increase in adsorption capacity of Sr2+ to other adsorbents around neutral to slightly alkaline pH[4]. The effect of initial strontium concentration (ranging from 0.2 mg/L to 10 mg/L) on the adsorption capacity of the MMOF was studied at pH 13 with a contact time of 1 h (Figure 3B). The amount of strontium absorbed on the MMOF increased with increasing initial strontium concentration. In general, the flexible and highly porous structure of MOFs allowed guest species such as metal ions to diffuse into their bulk structure[16].
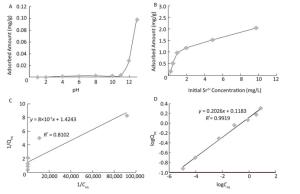
Figure 3. (A) Effect of pH on sorption of strontium on the MMOF; (B) Effect of strontium concentration on adsorption of strontium by the MMOF; (C) Langmuir isotherms for strontium at pH 13; (D) Freundlich isotherms for strontium absorbed by Fe3O4@UiO-66-NH2 at pH 13.
Adsorption isotherms were used to describe the adsorption mechanism of adsorbent to adsorbate. In the present study, Langmuir and Freundlich models were used. The Langmuir equation can be expressed by the following equation:
$$ {Q_{eq}} = \frac{{{Q_{max}}b{C_{eq}}}}{{1 + b{C_{eq}}}} $$ (1) where Qeq (expressed in mg/g) is the adsorption capacity, and Ceq (expressed in mg/L) is the equilibrium concentration of metal in solution. Qmax is the maximum adsorption capacity (mg/g), and b is a constant related to the enthalpy of adsorption (expressed in L/mg). The value of Qmax represents a practical limiting adsorption capacity when the adsorbent surface is fully covered with metal ions.
The Freundlich isotherm describes a more complicated adsorption model wherein interactions occur between adsorbed molecules. The Freundlich model is as follows:
$$ {Q_{eq}} = kC_{eq}^{1/n} $$ (2) where the constant k is related to the adsorption capacity, and the constant 1/n represents the intensity of the reaction, with a value less than one indicating favorable adsorption. The Langmuir and Freundlich isotherms were determined for strontium concentrations of 0.1-10 mg/L and are shown graphically in Figure 3C and 3D. The Freundlich model successfully fitted the data for adsorption of Sr2+ on MMOF (R2 = 0.9919). In Figure 3B, the highest of sorption capacity was 2.0 mg/g, but it was not possible to determine the maximum sorption capacity, because the concentration range of strontium was below saturation.
In the present work, we developed a simple and facile strategy for preparing MMOF. The chemical bonding between Fe3O4 and UiO-66-NH2 endows the MMOFs with high chemical stability and desirable durability. Fe3O4@UiO-66-NH2 was first used as sorbent for strontium in aqueous solution. The adsorption capacity was affected by the pH, and maximum adsorption was found at pH 13. The Freundlich model yielded a good fit for the isotherm model. Combinations of MOFs and magnetic nanoparticles of MMOF composite have obvious advantages in their adsorption and separation and have potential for applications as magnetic adsorbents for radionuclides.
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Facile Synthesis of the Magnetic Metal Organic Framework Fe3O4@UiO-66-NH2 for Separation of Strontium
doi: 10.3967/bes2018.065
the National Natural Science Foundation of China 20477058
the Chinese Ministry of Science and Technology 2014YF211000
- Received Date: 2018-01-03
- Accepted Date: 2018-04-18
-
Key words:
- Magnetic metal organic framework (MMOF) /
- Fe3O4@UiO-66-NH2 /
- Radioactivity /
- Strontium /
- Adsorbents
Abstract: A magnetic metal organic framework (MMOF) was synthesized and used to separate Sr2+ in aqueous solution. The shape and structure of prepared Fe3O4@UiO-66-NH2 were characterized, and the absorbed concentration of strontium was determined through inductively coupled plasma mass spectrometry. The results indicated that Fe3O4 and UiO-66-NH2 combined through chemical bonding. The experimental adsorption results for separation of Sr2+ in aqueous solution indicated that the adsorption of Sr2+ to Fe3O4@UiO-66-NH2 increased drastically from pH 11 to pH 13. The adsorption isotherm model indicated that the adsorption of Sr2+ conformed to the Freundlich isotherm model (R2 = 0.9919). The MMOF thus inherited the superior qualities of magnetic composites and metal organic frameworks, and can easily be separated under an external magnetic field. This MMOF thus has potential applications as a magnetic adsorbent for low level radionuclide 90Sr.
| Citation: | YIN Liang Liang, KONG Xiang Yin, ZHANG Yao, JI Yan Qin. Facile Synthesis of the Magnetic Metal Organic Framework Fe3O4@UiO-66-NH2 for Separation of Strontium[J]. Biomedical and Environmental Sciences, 2018, 31(6): 483-488. doi: 10.3967/bes2018.065 |








 Quick Links
Quick Links
 DownLoad:
DownLoad: