-
Hepatitis B is an infectious disease mainly caused by hepatitis B virus (HBV). This disease places heavy economic burdens on patients, their families, and societies. An estimated total of 300,000 people die of hepatitis B-related liver cancer or cirrhosis every year, accounting for 37%–50% of all hepatitis B-related deaths worldwide[1,2]. In Asia, China is considered an intermediate-to-high hepatitis B-endemic region[3].
Since its initial inclusion in the national immunization program in 1992, the hepatitis B vaccine has led to a significant decrease in the prevalence of HBV infection in China. Particularly, the positive rate of HBV surface antigen (HBsAg) has decreased to < 1% among children younger than 10 years[4]. A recent meta-analysis revealed that the rate of HBsAg positivity in the general Chinese population (age range: 1–59 years) was 5.7%, a 20.61% decline from the rate of 7.18% recorded 10 years earlier in 2006[5]. Moreover, data from the Statutory Infectious Diseases Report indicate that the incidence of hepatitis B in China decreased from 89.00/100,000 in 2007 to 68.74/100,000 in 2016[6]. Despite these decreases, the population of HBsAg carriers continually exceeds 80 million residents due to the highly populous nature of China. Therefore, the prevention and control of hepatitis B in China will have a considerable impact on the global elimination of viral hepatitis[7-9].
Although China has made remarkable progress in controlling the spread of HBV by expanding infant immunization programs, efforts to eliminate this virus in the country have been limited, mainly due to regional imbalances in infection. According to the results of a national serosurvey conducted in 2006, the prevalence of HBsAg in Western China remained as high as 8.2%[4]. Although no national HBV serosurvey was conducted on the whole population between 2006 and 2019, a recent meta-analysis of HBV infection reported the highest rate in Western China [8.92%; 95% confidence interval (CI): 7.19%–10.64%][10]. The resistance to viral elimination in this region poses the greatest challenge to the goal of nationwide viral hepatitis eradication in 2030[11]. Consequently, Western China has become the focus of national hepatitis B prevention and treatment efforts.
Wuwei, located in the middle of Gansu province, is the eastern gate of Silk Road into Hexi Corridor and Xinjiang. This typical agricultural city in Western China is characterized by a multi-ethnic and largely rural population, poor economic conditions, underdeveloped medical and health facilities, a large number of migrant workers, and high population mobility. The incidence of hepatitis B in Wuwei increased from 571/100,000 in 2005 to 742/100,000 in 2008, which is eight times higher than the national average according to National Disease Supervision Information Management System (NDSIMS)[12].
To prevent and control the HBV spread, the Ministry of Science and Technology of China developed demonstration areas in seven locations, including Wuwei city, in 2008. In 2009, the project of hepatitis B prevention and control demonstration area in Wuwei was launched. A cross-sectional survey of the prevalence of hepatitis B in Wuwei in 2010 revealed HBV infection rates of 7.19% and 8.56% among people older than 1 year of age and among rural adults, respectively[13]. These rates were significantly higher than those reported in a nationwide hepatitis B epidemiological survey conducted in 2006[4]. In summary, HBV infection represents a serious public health issue faced by the residents of Wuwei and Western China. Since 2009, special funds have been provided to enable the implementation of a series of prevention and control measures, including vaccination, health education, and specialized training for physicians, in Western China. This study was to compare the results of three serosurveys conducted in 2010, 2013, and 2015 in the Wuwei hepatitis B prevention and control demonstration area. Specifically, this study aimed to evaluate the effects of specific hepatitis B prevention and control measures and to provide the most recent data in support of further improvements to these measures in Western China.
-
The three surveys were conducted from January 2010 to March 2010, from January 2013 to May 2013, and from September 2015 to December 2015. The research object was defined as the whole population of Wuwei, and the same serosurvey sampling method was adopted in 2010[13], 2013, and 2015. The minimum sampling unit was a village or subdistrict. Therefore, the rural population comprised subjects whose minimum residential unit was a village, whereas the urban population consisted of those whose minimum residential unit was a subdistrict.
-
All serosurveys used similar methods and questionnaires. All investigators were trained by professionals at the Air Force Medical University and designated as official investigators after passing an examination. The investigators conducted face-to-face interviews of each subject (or designated guardian) in accordance with the method of centralized and household entry investigation, with assistance from the local township health centers, village committees, and other departments. A standardized questionnaire survey method was used to collect basic information, including gender, date of birth, education level (for those older than 15 years), ethnicity, residential area, and HBV immunization history, from the respondents.
HBsAg positive was defined as HBV infection. HBV infection case records pertaining to Wuwei were obtained via the Legal Infectious Disease Surveillance System. Three serum surveys were conducted using population data (including gender and age) from the 2010 national Census of Wuwei city (see Supplementary Table S1 available in www.besjournal.com). Data corresponding to the total population of China from 2010 to 2015 were obtained from the World Bank (https://data.worldbank.org.cn/). National hepatitis B incidence data were obtained from NDSIMS.
Age (years) Liangzhou Gulang Minqin Tianzhu Male Female Male Female Male Female Male Female 0– 5,703 4,404 3,786 3,709 11,353 10,747 4,701 4,069 5– 27,786 21,787 5,760 6,238 14,840 14,046 4,802 4,162 10– 24,337 19,130 14,678 16,558 37,601 35,591 14,956 13,370 20– 68,771 62,692 18,150 18,891 34,927 33,058 12,555 12,519 30– 79,950 74,240 19,354 20,972 38,924 36,843 13,077 12,440 40– 88,510 83,790 28,461 29,618 27,451 25,983 20,050 18,926 50– 83,930 76,810 15,072 13,913 18,054 17,088 10,494 8,710 60– 80,500 77,710 13,783 16,384 12,590 11,917 6,928 7,258 70– 71,068 72,652 12,972 14,698 6,832 6,466 2,927 3,531 80– 3,317 3,627 7,379 10,492 1,974 1,868 514 606 Total 534,672 496,042 139,395 151,473 204,546 193,607 91,056 85,573 Table S1. Population of different ages and genders in four districts and counties of Wuwei in 2010
-
Sera were collected from the subjects’ blood samples (5 mL) and stored at −80 °C. Enzyme-linked immunosorbent assays (Yantai Laboratory System, Beijing, China) were used to screen the serum samples for HBsAg, anti-HBs, and anti-HBc.
-
Data were entered into EpiData 3.1 software (EpiData Association, Odense, Denmark) and analyzed using SAS 9.2 statistical software (SAS Institute, Cary, NC, USA). The complex sampling design was based on Taylor series linearization method, and the standard errors were estimated accurately. The calculated prevalence rates of HBV markers were weighted based on gender, age, ethnicity and occupation, and other selected determinants to account for differences in the selected probabilities across subdistricts, communities, and villages and between age and gender categories. The weight for each person,
$ i $ , is expressed as follows:$$ {W}_{kji}=\frac{{W}_{i}}{{W}_{k}\times{W}_{j|k}} $$ (1) where
$ i $ refers to the respondents;$ {W}_{k} $ and$ {W}_{j|k} $ represent the sampling probabilities of the county and village, respectively;$ {W}_{i} $ denotes the adjustment factor for person$ i $ (based on the age and gender composition of the 2010 Wuwei Census data). The survey data from 2013 were divided on the basis of the age groups used in the 2010 hepatitis B serosurvey in Wuwei city to facilitate direct comparisons. Both data sets were adjusted based on the age and gender composition of the population of Wuwei city based on the 2010 Census.The prevalence rates of HBV seromarkers were reported as point estimates and estimated 95% CIs. The latter ranges were compared when contrasting the prevalence rates of each seromarker, such that 95% CIs that did not overlap between groups were considered statistically significant (P < 0.05). Changing trends in the prevalence rates were calculated using the chi-square test. Multivariate unconditional logistic regression model was used to analyze the factors influencing HBV infection. With HBsAg positive as the dependent variable and multiple research factors (including gender, age, race, education level, urban and rural areas) as independent variables, a multi-factor regression analysis was conducted. The age group is divided into two groups: those over 14 years old and those under 14 years old; The ethnicity are divided into han nationality and minority nationality; Gender, age, race, urban and rural data were dichotomous variables. Education is an unordered variable, assigned a value of 1 to 4.
-
The study protocol was approved by the Ethics Committee of the Air Force Medical University. Consent to participate in the survey was obtained from all participants or their parents/guardians (for minor children). Specifically, each participant or their parent/guardian signed an informed consent form and also provided written consent on the first page of the questionnaire.
-
By 2015, the overall HBV immunization rate was 99.87%, and the rate of timely first vaccination among newborn infants was 96.71%. Moreover, 85.64%, 82.71%, and 82.16% of middle school students, primary school students, and residents in this area, respectively, reported an awareness of hepatitis B and health education, indicating that the project had yielded significant social benefits. The rate of awareness about hepatitis B prevention and treatment among community physicians who served rural populations increased from 67% before the intervention to above 81% upon its completion (Table 1).
Measures Respondents Index Free vaccination Children, adults Vaccination of 294,943 residents: 75,561 newborns, 53,111 susceptible adults, 26,649 members of high-risk and key groups (e.g., hospital medical staff and families of HBsAg-positive individuals), 1,138 rural doctors, and 137,820 people younger than 15 years. Health education and knowledge promotion Residents A total of 304 hepatitis B prevention and control slogans (10,000 m2); A total of 920,000 SMS sent monthly to all mobile phone users; Production of hepatitis B prevention and control public service advertisements for rolling broadcasts four times per day; Printing and distribution of 50,000 health education posters, 26,000 copies of hepatitis B prevention and control information manuals, 20,000 copies of hepatitis B prevention and control leaflets, two types of Health Education Prescriptions (10,000 copies), 10,000 copies of medical institution posters, 20,000 copies of publicity calendars, 8,000 publicity handbags, 10,000 publicity pens, and 105 coloring/drawing publicity pages. Physician training Clinicians, managing physicians, village and community physicians Training of 1,022 clinicians and managing physicians in infectious disease at public health departments in township (or higher-level) hospitals, and 2107 rural and community-level physicians (total = 3,129), with 100% training coverage. Table 1. HBV prevention and control measures applied in Wuwei city in 2010–2015
-
Overall, 28,044, 35,232, and 20,358 samples were analyzed in the 2010, 2013, and 2015 serosurveys, with male-to-female ratios of 0.77:1, 0.93:1, and 0.81:1, respectively. All serosurvey populations included similar distributions of age groups, ethnic groups, education levels (among those older than 15 years), and urban and rural residents. Table 2 shows the demographic characteristics of the three groups.
Category 2010 2013 2015 Frequency Proportion (%) Frequency Proportion (%) Frequency Proportion (%) Overall 28,044 100.00 35,232 100.00 20,358 100.00 Age (years) 0–9 1,649 5.88 2,532 7.19 1,076 5.28 10–19 4,844 17.27 5,204 14.77 4,251 20.88 20–59 17,535 62.53 23,312 66.17 12,157 59.72 ≥ 60 4,016 14.32 4,184 11.87 2,874 14.12 Gender Male 12,218 43.57 16,939 48.08 9,090 44.65 Female 15,826 56.43 18,293 51.92 11,268 55.35 Ethnicity Han 26,515 94.55 33,611 95.40 19,068 93.66 Uighur 263 0.94 296 0.84 159 0.78 Tibetan 990 3.53 1,207 3.43 857 4.21 Others 276 0.98 118 0.33 274 1.35 Education (subjects aged 15–59 years) Illiterate or primary school 8,125 40.51 9,981 38.08 4,335 34.17 Middle school 7,239 36.10 11,146 42.52 4,947 38.99 High school 3,397 16.94 3,521 13.43 1,671 13.17 Junior college or undergraduate degree 1,294 6.45 1,566 5.97 1,735 13.67 Urban/rural Urban 6,092 21.72 8,768 24.89 5,221 25.65 Rural 21,952 78.28 26,464 75.11 15,137 74.35 Table 2. Characteristics of the study populations in the 2010, 2013, and 2015 serosurveys
-
A serological investigation conducted in Wuwei in 2010 yielded HBsAg, anti-HBs, and anti-HBc positivity rates of 7.19% (95% CI: 6.28%–8.11%), 49.07% (95% CI: 45.50%–52.65%), and 43.89% (95% CI: 40.37%–47.41%), respectively. In 2013, the corresponding rates for HBsAg, anti-HBs, and anti-HBc positivity were 6.51% (95% CI: 6.29%–6.73%), 53.66% (95% CI: 52.42%–54.91%), and 32.87% (95% CI: 31.43%–34.30%), respectively. In 2015, the corresponding positivity rates were 5.87% (HBsAg, 95% CI: 5.41%–6.25%), 53.72% (anti-HBs, 95% CI: 50.27%–55.78%), and 28.46% (anti-HBc, 95% CI: 26.42%–30.65%). Compared with 2010, the positive rate of anti-HBs in 2015 was significantly increased, while the positive rates of HBsAg and anti-HBc were significantly decreased (P < 0.05; Table 3).
Year HBsAg (%) (95% CI) Anti-HBs (%) (95% CI) Anti-HBc (%) (95% CI) 2010 7.19 (6.28–8.11) 49.07 (45.50–52.65) 43.89 (40.37–47.71) 2013 6.51 (6.29–6.73) 53.66 (52.42–54.91) 32.87 (31.43–34.30) 2015 5.87 (5.41–6.31) 53.72 (50.27–55.78) 28.46 (26.42–30.65) Table 3. Rates of HBV serological marker seropositivity in the 2010, 2013, and 2015 survey populations
-
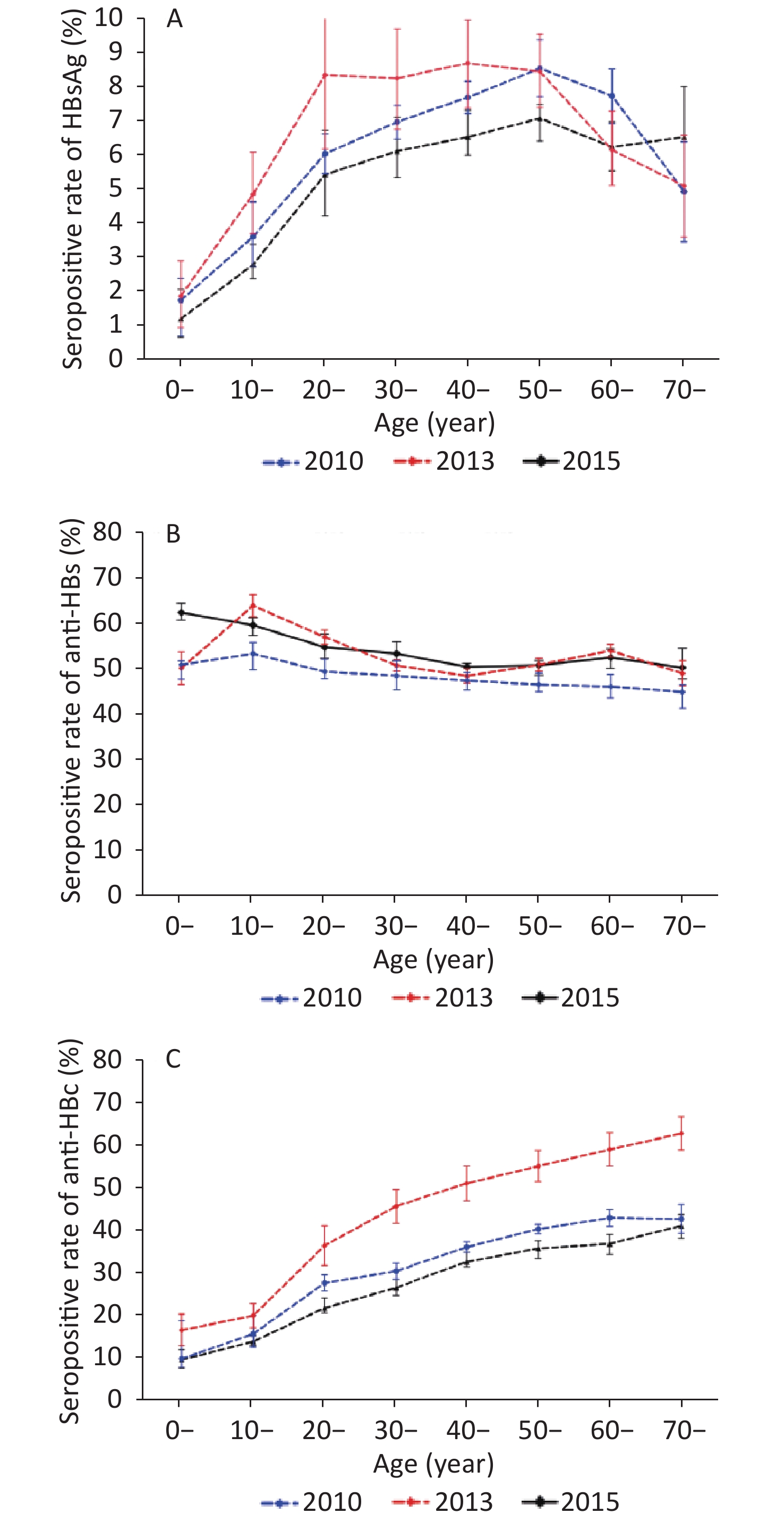
The rates of HBsAg, anti-HBs, and anti-HBc positivity differed significantly between age groups (P < 0.05). In 2010, the rate of HBsAg seropositivity increased with age, peaking at 8.72% in the age group of 40–49 years, and declined. In 2013, the rate of HBsAg seropositivity increased with age, peaking at 8.57% in the age group of 50–59 years and decreasing to 7.76% and 4.96% in those aged 60–69 and ≥ 70 years, respectively. In 2015, the rate of HBsAg seropositivity increased with age, peaking at 7.47% in the 60–69-year-old age group and decreasing to 6.42% in those aged in ≥ 70 years. Despite these fluctuations, all rates of HBsAg positivity among adults aged 20–69 years remained higher than 5.46%. Moreover, the HBsAg seropositive rate of children aged 0–9 years was over 1% in 2010 (1.91%), 2013 (1.78%), and 2015 (1.24%). Compared with the data of 2010, except for the 10–19-year-old age group, the serum positive rate of HBsAg in other age groups was not statistically significant in 2015 (Figure 1A).
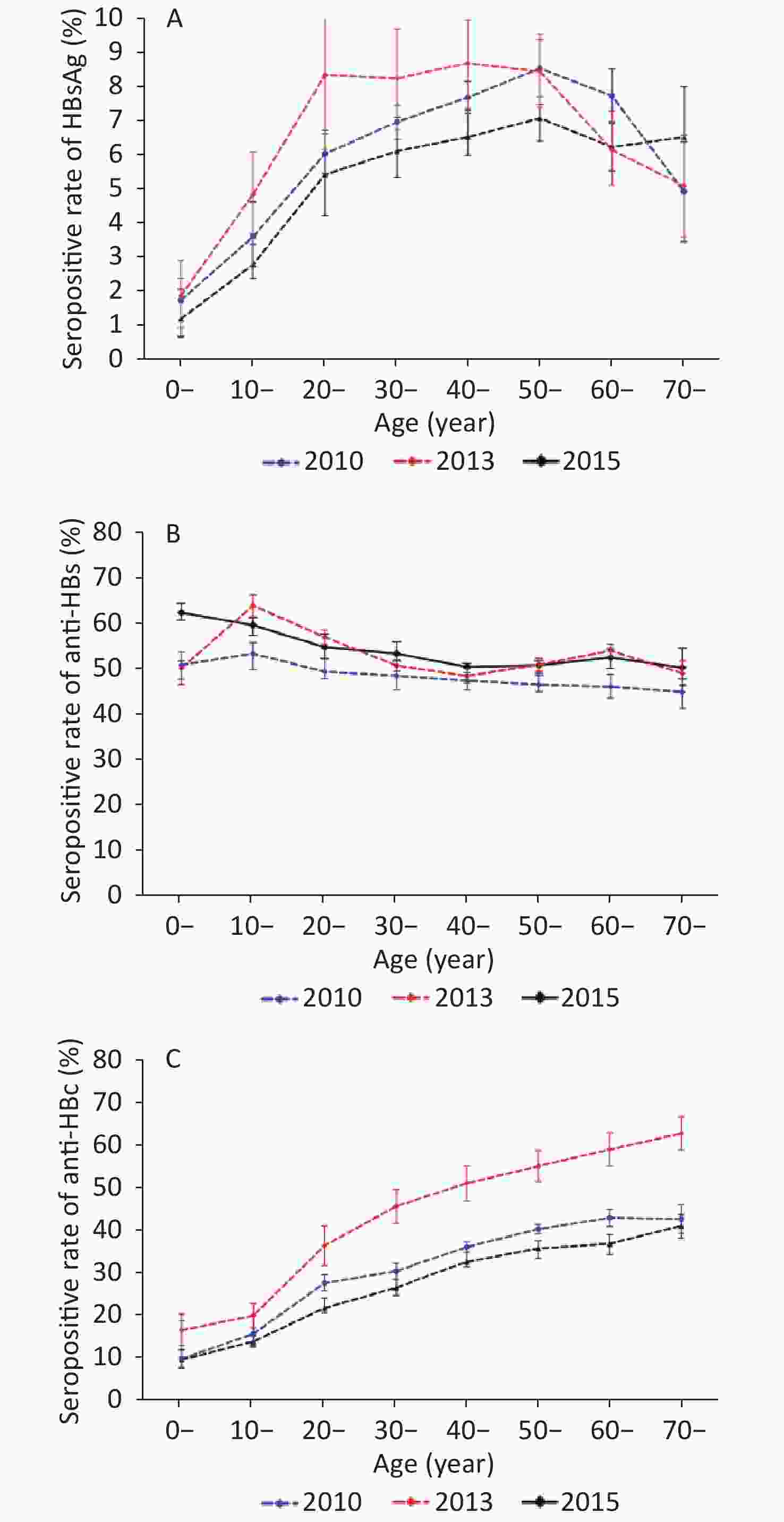
Figure 1. Rates of HBV seromarker seropositivity stratified by age group in 2010, 2013, and 2015 survey populations. Bars indicate the 95% CI. (A) Comparison of HBsAg seropositivity. (B) Comparison of anti-HBs seropositivity. (C) Comparison of anti-HBc seropositivity.
The results demonstrated a decrease in the rate of anti-HBs seropositivity with age. In 2010, the highest rate of anti-HBs positivity (53.26%) was observed among people aged 10–19 years, followed by that among adolescents (50.76%) aged 0–9 years. The positive rate of anti-HBs in other age groups was between 40% and 50%. In 2013, the highest rate of anti-HBs positivity (63.92%) was observed among people aged 10–19 years, followed by that in adults aged 20–29 years (56.93%). All other age groups had anti-HBs positivity rate of approximately 50%. In 2015, the positive rate of anti-HBs was over 50% in all age groups, with the highest value recorded in the population aged 0–9 years (62.37%) followed by the 10–19-year-old group (59.56%). Compared with the data of 2010, except for the 0–9 and 40–49-year-old age groups, the positive rate of anti-HBs in other age groups was significantly higher in 2013 and 2015 (P < 0.05) (Figure 1B). Furthermore, we analyzed the positive rate of anti-HBs at 1–5 years old in 2013 and 2015, and found that the anti-HBs positive rate in children aged 1–5 years were 70.00%, 71.79%, 60.53%, 43.46%, and 42.98%. The rate was significantly the highest among children aged 2 years and decreased thereafter (χ2= 26.27, P < 0.01) (see Supplementary Table S2 available in www.besjournal.com).
Age (years) Investigation (n) Anti-HBs χ2 P Positive Positive rate (%) 1 10 7 70.00 26.27 < 0.01 2 39 28 71.79 3 152 92 60.53 4 283 123 43.46 5 356 153 42.98 Table S2. Total anti-HBs positive rate of 1–5-year-old children in 2013 and 2015 surveys
The three serosurveys showed that the rate of anti-HBc seropositivity also increased with age. Compared with the data of 2010, the rates of anti-HBc seropositivity decreased in subjects aged 10 years or older (P < 0.05). However, the positive rate of anti-HBc in adults over 20 years old remained at a high level, especially in the age group over 40 years, i.e., above 30% (Figure 1C).
-
In an analysis stratified by gender, male subjects had higher rates of HBsAg positivity than female subjects in the three serosurveys, but the difference was not statistically significant (P > 0.05). The prevalence of anti-HBc decreased, whereas the positive rate of anti-HBs increased in 2010–2015 (P < 0.05). In particular, the prevalence rates of HBsAg and anti-HBc decreased in 2015 compared with 2010 among males and females, but a statistically significant decrease was identified only for HBsAg prevalence in males (Table 3).
In an analysis stratified by ethnicity, the participants of Han ethnicity had higher rates of HBsAg positivity than those from Tibetan, Uighur, and other ethnic groups in the three serosurveys. However, the participants of Uighur ethnicity had the highest rate of anti-HBs positivity. The rates of anti-HBc positivity were highest in the Han group and lowest in the Uighur group in 2013 and 2015. In the three serosurveys, the rates of anti-HBc positivity decreased significantly in the Han and Uighur groups, whereas the rates of anti-HBs positivity increased significantly (P < 0.05; Table 4).
Variables Sample tested (n) HBsAg (%) (95% CI) Anti-HBs (%) (95% CI) Anti-HBc (%) (95% CI) 2010 2013 2015 2010 2013 2015 2010 2013 2015 2010 2013 2015 Sex Male 15,826 16,939 9,354 7.78
(6.51–9.03)7.23
(6.12–7.59)5.91
(5.27–6.43)a24.42 (22.40–26.34) 52.78 (52.01–54.40) 53.82
(51.56–56.57)b43.8
(39.98–47.79)32.96
(31.25–34.67)28.32
(26.03–29.87)cFemale 12,218 18,293 11,448 6.81
(5.92–7.59)6.27
(5.43–6.88)5.82
(5.28–6.13)22.81 (20.49–25.23) 54.13 (52.61–55.66) 52.91
(50.68–54.61)b43.9
(40.42–47.40)32.78
(31.46–34.09)26.17
(24.46–27.07)cEthnicity Han 26,515 33,611 19,068 7.31
(6.29–8.22)6.63
(6.40–6.85)5.91
(5.44–6.42)23.52 (21.62–25.49) 54.53 (53.33–55.74) 53.47
(51.27–54.69)b44.2
(40.58–47.82)33.19
(31.78–34.60)28.21
(26.65–29.93)cUighur 263 296 159 4.68
(3.82–5.61)3.38
(2.71–4.05)3.76
(0.78–6.77)20.68 (19.03–22.42) 56.08 (49.29–62.87) 60.65
(53.34–67.68)b43.1
(41.09–45.02)15.65
(12.20–19.11)19.64
(13.27–25.72)cTibetan 990 1,207 857 5.78
(4.50–7.09)3.78
(2.25–5.30)5.15
(3.75–6.74)24.91 (16.58–33.28) 33.21 (29.50–36.92) 53.36
(51.50–56.18)b24.5
(20.88–28.21)24.46
(20.62–28.06)26.13
(23.32–29.21)Others 276 118 274 5.21
(2.89–7.51)5.47
(2.42–8.64)5.37
(2.76–8.18)19.06 (6.82–31.29) 37.97 (18.62–57.33) 50.11
(49.18–59.03)b31.5
(24.21–38.89)23.58
(20.31–26.85)22.36
(18.32–28.40)Education (15–59 years) Illiterate and
primary school8,125 9,981 4,335 8.28
(6.81–9.78)7.12
(6.78–7.54)6.78
(6.43–7.36)16.54 (14.72–18.40) 47.29 (45.32–49.04) 50.34 (49.01–52.03)b 43.21 (38.89–47.58) 37.55 (35.42–38.46) 36.24 (34.36–39.23) Middle school 7,239 11,146 4,947 8.79
(7.26–10.28)7.68
(7.21–8.32)7.14
(6.49–7.84)21.46 (19.51–23.46) 53.27 (50.24–55.36) 52.16 (51.57–54.35)b 45.38 (41.56–49.28) 35.42 (33.46–37.58) 29.27 (27.44–31.12)c High school 3,397 3,521 1,671 8.21
(7.18–9.16)6.65
(6.36–7.24)5.41
(4.41–6.94)a26.21 (22.78–29.68) 59.89 (57.64–62.56) 57.43 (55.02–59.76)b 43.78 (39.68–47.89) 26.86 (24.66–28.87) 26.49 (24.44–29.73)c Junior college or
undergraduate
degree1,294 1,566 1,735 5.79
(4.31–7.20)3.83
(3.56–4.48)3.17
(2.26–4.15)a34.23 (30.21–38.18) 74.33 (69.62–76.32) 72.08 (70.05–74.27)b 42.32 (39.45–45.12) 20.24 (18.67–23.24) 22.75 (20.04–23.97) Urban/rural Urban 6,092 8,768 5,221 6.12
(5.32–7.01)5.04
(4.68–5.54)4.83
(4.28–5.45)24.02 (19.68–28.42) 57.14 (54.76–62.51) 55.18 (53.27–57.96)b 47.50 (45.71–49.28) 28.48 (26.34–32.42) 20.35 (18.72–21.83)c Rural 21,952 26,464 15,137 7.72
(6.51–8.87)6.60
(6.15–7.05)6.04
(5.65–6.41)a23.31 (21.53–25.02) 52.79 (51.88–53.70) 49.68 (48.33–51.93)b 42.28 (37.68–46.79) 34.29 (33.44–35.14) 30.26 (28.15–31.65)c Note. CI, confidence interval; HBsAg, hepatitis B surface antigen; anti-HBs, antibody against hepatitis B surface antigen; anti-HBc, antibody against hepatitis B core antigen.
aP < 0.05 for the comparison of the seropositive rate of HBsAg between 2010 and 2015.
bP < 0.05 for the comparison of the seropositive rate of anti-HBs between 2010 and 2015.
cP < 0.05 for the comparison of the seropositive rate of anti-HBc between 2010 and 2015.Table 4. Rates of HBV serological marker positivity stratified by sex, ethnicity, education, and residential area in 2010, 2013, and 2015
In an analysis stratified by education level, the results of the three surveys revealed that the rate of HBsAg positivity decreased as the education level increased, with the highest and lowest rates observed in the groups with junior middle school education and junior college or higher education, respectively. Moreover, with the rise in educational level, the rates of anti-HBs and anti-HBc positivity increased and decreased, respectively. The results obtained in both surveys were generally similar, although the rates of anti-HBc and anti-HBs positivity significantly decreased and increased, respectively, from 2010 to 2015 (Table 4).
In an analysis stratified by area of residence, the three surveys demonstrated lower rates of HBsAg and anti-HBc positivity and higher rates of anti-HBs positivity in urban areas than in rural areas (P > 0.05). Compared with 2010, the rates of HBsAg and anti-HBc positivity decreased in urban and rural areas in 2013 and in 2015, although only the latter was significant (P < 0.05). Moreover, the rates of anti-HBs positivity increased significantly over time in urban and rural areas (P < 0.05; Table 4).
We also analyzed the factors that may affect HBV infection, such as sex, age, ethnicity, urban and rural areas, education, etc. The results showed that age, urban and rural areas, and education were the main factors influencing HBV infection. Among the factors influencing HBV infection, the OR value estimates of male and female in 2010, 2013, and 2015 were 1.32, 1.26, and 1.22, which indicated that after excluding other factors, the probability of HBV infection in male was higher than that in female; however, the difference was not statistically significant (P > 0.05). In addition, the OR values of the age groups above 14 and below 14 were 2.31, 2.18, and 2.43, respectively, indicating that people in higher age groups were more likely to be infected with HBV (P < 0.05). The possibility of HBV infection in ethnic minority group was slightly higher than that in the Han nationality group, but the difference was not statistically significant. The probability of HBV infection in rural population was significantly higher than that in urban population (P < 0.05) (See Supplementary Table S3 available in www.besjournal.com).
Variables 2010 2013 2015 B SE OR (95% CI) P B SE OR (95% CI) P B SE OR (95% CI) P Sex Female 1.00 1.00 1.00 Male 0.78 0.18 1.32 (1.21–1.44) > 0.05 0.68 0.24 1.26 (1.01–1.44) > 0.05 0.88 0.16 1.22 (1.09–1.35) > 0.05 Age ≤ 14 1.00 1.00 1.00 > 14 1.88 0.04 2.31 (1.92–2.68) < 0.05 1.68 0.06 2.18 (1.86–2.38) < 0.05 1.48 0.05 2.43 (1.92-2.81) < 0.05 Ethnicity Han 1.00 1.00 1.00 Minorities 0.42 0.28 1.04 (1.02-1.14) > 0.05 0.52 0.12 1.12 (1.06–1.27) > 0.05 0.36 0.06 1.08 (1.02–1.15) > 0.05 Education (15–59 years) Illiterate and primary school 1.00 1.00 1.00 Middle school 0.59 0.04 1.02 (0.92–1.08) > 0.05 0.47 0.06 0.92 (0.82–1.04) > 0.05 0.46 0.04 0.94 (0.84–1.01) > 0.05 High school 0.68 0.06 1.04 (0.84–1.12) > 0.05 0.48 0.12 0.98 (0.89–1.12) > 0.05 0.88 0.11 0.89 (0.74–0.96) > 0.05 Junior college or undergraduate degree 0.76 0.12 0.64 (0.52–0.96) < 0.05 0.86 0.22 0.74 (0.56–0.94) < 0.05 0.56 0.12 0.63 (0.55–0.87) < 0.05 Urban/rural Urban 1.00 1.00 1.00 Rural 0.93 0.04 1.28 (1.14–1.36) > 0.05 1.02 0.03 1.24 (1.16–1.35) > 0.05 1.01 0.05 1.31 (1.14–1.42) > 0.05 Note. B: regression coefficient; SE: standard error; OR: odds ratio Table S3. Multiple factor weighted logistic regression analysis of HBV infection in general population of Wuwei
-
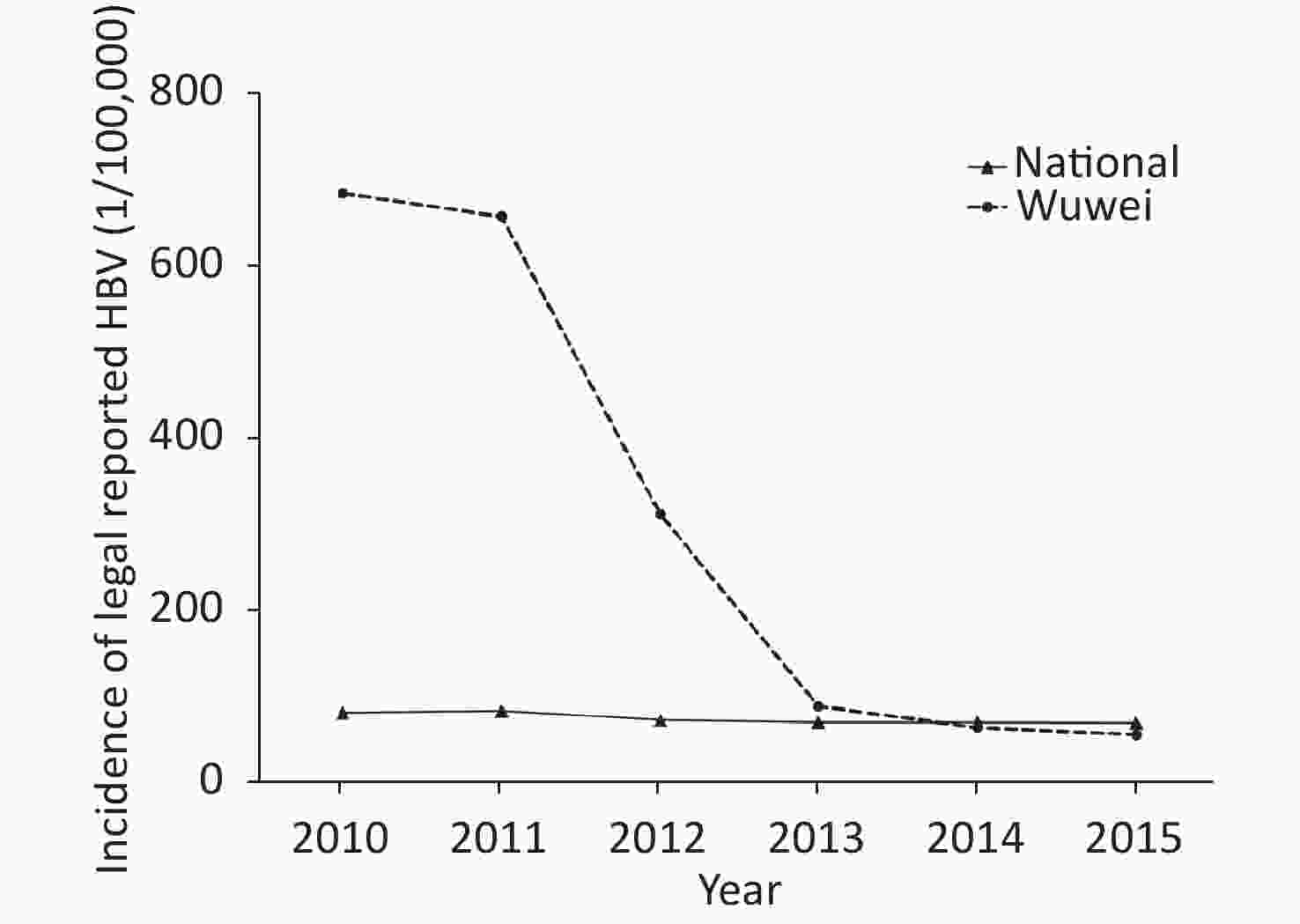
The incidence rates of legally reported HBV in Wuwei from 2010 to 2015 were 686.53/100,000, 659.37/100,000, 311.17/100,000, 87.16/100,000, 62.20/100,000, and 53.72/100,000, respectively. The average annual reported incidence of HBV exhibited a declining trend (P < 0.05) with an average decrease of 310.03/100,000. Nationwide, the statutory reported incidences of hepatitis B infection in China from 2010 to 2015 were 79.67/100,000, 81.73/100,000, 80.88/100,000, 71.29/100,000, 68.59/100,000, and 68.13/100,000, respectively. Compared with the overall statutory incidence of hepatitis B in China, Wuwei reported significantly higher incidence rates from 2010 to 2015 (P < 0.05; Figure 2).
-
Since its designation as a hepatitis B prevention and control demonstration area in 2009, Wuwei has implemented a series of prevention and control measures, including vaccination, health education, and physician training, with support from special funds and strong cooperation from local governmental departments. In this study, we investigated the prevalence of HBV serum markers in three representative populations in 2010, 2013, and 2015 in Wuwei. In general, the effects of the vaccine-based prevention and control measures implemented in 2010 became evident 5 years later. The HBsAg-positive rate in 2015 was 5.87% compared with the 7.19% recorded in 2010. The rate of anti-HBs positivity increased from 23.49% in 2010 to 53.72% in 2015 and was significantly higher in male and female participants, those of Han and Hui ethnicity, and urban and rural residents (all P < 0.05). Meanwhile, the incidence of statutory reports of HBV infection in Wuwei city decreased with each passing year from 2010 to 2015. By contrast, the rates of anti-HBc in these groups decreased significantly from 2010 to 2015 (all P < 0.05).
Despite the above-listed improvements, 1.24% of children younger than 10 years in Wuwei were HBsAg-positive in 2015. This rate was significantly higher than that reported for the same age group (less than 1%) in a 2014 Chinese national serological survey[14], suggesting that measures intended to block the transmission of HBV from mother-to-child had not been fully achieved in Wuwei. A previous study investigated the risk factors for mother-to-child transmission of Hepatitis B among 221 HBsAg positive mothers who had delivered 247 infants in the obstetrics and gynecology departments of three Grade B hospitals in Wuwei city from 2008 to 2010 [15]. The results showed that less than a third of premature and low-weight infants in Wuwei city had received the first dose of hepatitis B vaccine in time. The delayed injection of the first dose of HBV vaccine after premature birth was a possible risk factor for HBV mother-to-child transmission. Routinely, pregnant women with a high HBV load are administered with a blocking treatment that combines immunoglobulin with a HBV vaccine. However, this treatment fails to prevent HBV immunization in certain cases, which constitutes an important cause of vertical HBV transmission from mother-to-child in China[16-18]. The treatment of high viral load pregnant women infected with HBV with nucleotide analogs can reduce the level of HBV DNA in pregnant women, thus reducing the risk of mother-to-child transmission[19]. The high rate of infection observed among infants and young children in the 2015 survey may be attributable mainly to the fact that high-risk pregnant women in Wuwei had not received antiviral treatment during the survey period. Moreover, although the vaccination rate of hepatitis B vaccine for children under 5 years old in the study area was over 99%, the positive rate of anti-HBs was about 50%, which gradually decreased with the increase in age. Hence, the antibody titer of children should be monitored.
Compared with the results of the 2010 serosurvey, the rate of anti-HBs positivity increased significantly in 2013 and 2015. However, the prevalence of HBsAg in rural areas was higher than that reported by a national serological survey conducted during the same period[20]. This finding shows that the comprehensive prevention and control measures based on vaccination have achieved initial results in rural areas, but these findings need further confirmation. The three serosurveys also revealed that the rate of HBsAg positivity decreased, and that the rate of anti-HBs positivity increased as the education level increased. Given the generally lower level of educational attainment in the rural Chinese population, this factor may contribute to the lack of knowledge about hepatitis B prevention and immune awareness and consequently, to the high incidence of hepatitis B in this area. Therefore, strategies that target the rural villages of Western China should include a free vaccination policy to reduce the disease burden and the distribution of stronger propaganda intended to enhance the knowledge about hepatitis B prevention and treatment by community physicians serving village households. Furthermore, the positive rates of HBsAg and anti-HBc in 20–69-year-old adults remained high. Up to 20%–30% of adults with chronic HBV infection are estimated to develop cirrhosis or liver cancer[21]. The burden of hepatitis B-related diseases in Western China remains serious. At present, the main antiviral drugs for hepatitis B patients include nucleoside (acid) analogs and interferon. Hepatitis B patients treated with nucleoside analogs often need long-term use of drugs to inhibit HBV DNA replication in vivo and reduce the risk of cirrhosis and liver cancer[22, 23]. New nuclear nucleoside drugs, and the development of sequential or joint treatment, such as Peg interferon, offer a new hope of ‘functional cure’ to hepatitis B patients[24, 25]. However, given the economic backwardness of the region, the economic burden of antiviral treatment on patients cannot be ignored.
To eliminate hepatitis B by 2030 as proposed by the World Health Organization, we recommend the following improved strategies for hepatitis B prevention and control in Western China based on our findings: (1) implementation of antiviral drug intervention among HBsAg-positive pregnant women to block vertical HBV transmission; (2) strengthened monitoring of antibody titer in children in this region and implementation of a more comprehensive vaccination strategy; (3) strengthened publicity and education initiatives targeting the rural population, with rural physicians serving as mediators; (4) implementation of free adult vaccination strategies to reduce the economic burden associated with hepatitis B and related diseases. We also propose to establish and implement a comprehensive strategy for hepatitis B prevention and treatment that will integrate prevention, screening, diagnosis, and treatment throughout the human lifespan.
In summary, although vaccine-based prevention and control measures have achieved certain results in Wuwei area, the positive rate of HBsAg in children and rural population is still high, and the burden of hepatitis B remains heavy. We should further strengthen the prevention and control of rural population and children and the management of the infected to reduce the patient burden.
-
The authors would like to thank the health workers of the Health Bureaus and the Centers for Disease Control and Prevention in Wuwei, Liangzhou district, Gulang county, Minqin county, and the Tianzhu Autonomous County for their collaboration in this study.
-
The authors declare that they have no conflict of interest.
-
Experiments conceived and designed by: YAN Yong Ping, GUO Zhi Wen, and SHAO Zhong Jun; Experiments carried out by: LIU Nan, JI Zhao Hua, GAO Jie, and LIU Yi Wen; Data analysis by: CHEN Hui, PU Zhong Shu, JI Zhao Hua, and YAN Yong Ping; Paper written by: CHEN Hui, JI Zhao Hua, and YAN Yong Ping.
Assessment on the Effects of Hepatitis B Prevention and Control Measures in Western China: A Comparison of Three Population-based Serosurveys
doi: 10.3967/bes2020.098
- Received Date: 2020-01-25
- Accepted Date: 2020-07-22
-
Key words:
- Hepatitis B /
- Prevention and control measures /
- Serosurvey /
- Western China
Abstract:
| Citation: | CHEN Hui, LIU Nan, JI Zhao Hua, PU Zhong Shu, GUO Zhi Wen, GAO Jie, SHAO Zhong Jun, LIU Yi Wen, YAN Yong Ping. Assessment on the Effects of Hepatitis B Prevention and Control Measures in Western China: A Comparison of Three Population-based Serosurveys[J]. Biomedical and Environmental Sciences, 2020, 33(10): 735-744. doi: 10.3967/bes2020.098 |














 Quick Links
Quick Links
 DownLoad:
DownLoad:
