-
The remodeling processes that occur in bone tissue allow for proper functioning and can lead to the inclusion of additional elements, including toxic ones, in the remodeled bone. This condition in turn affects the metabolic processes in the bond, which may result in disturbances to the bone–joint system, as manifested by changes in bone tissue and other organs[1]. Manganese (Mn) is an essential bone component. This element is involved in the synthesis of proteins underlying connective tissue regeneration. Aluminum (Al) is incorporated into the bone matrix, and it deposits on trabecular bone surfaces and on periosteal and endosteal surfaces. Experimental research suggests that Al poisoning causes bone diseases, osteodystrophy, osteomalacia, and osteoporosis. Bone is the main tin storage organ in chronic exposures, and the biological half-life of tin in bone is approximately 100 d. Tin in combination with vitamin D affects the efficient metabolism of bone tissue. Titanium (Ti) is a trace element that shows no toxicity in the human body at high concentrations. Given its neutrality and properties, Ti is used in endoprosthetics and dentistry (dental implants). An increase in the concentration of Ti in the blood was observed in people with knee endoprosthetics due to the abrasion of the prosthesis. Barium (Ba) is a physiological antagonist of potassium and accumulates in the skeleton. Lithium (Li) interferes with metabolic processes and hormonal balance[2].
This manuscript aimed to determine the contents of aluminum, tin, titanium, barium, manganese, and lithium in the tissues of the knee joint. A correlation analysis was performed between the studied elements, and the existing relationships in the considered groups were shown. In this population, age groups were separated, and smoking was considered a source of exposure to heavy metals.
The study was conducted with the approval by the Bioethics Committee of the Medical University of Silesia No.2/2013 on 18 June 2013. The study material was obtained during a total knee endoprosthesoplasty at the Dr. Janusz Daab Hospital of Trauma Surgery in Piekary Śląskie. The study samples included tissues of tibial, femur, and meniscus. The study population included 50 patients. Table 1 presents a detailed description of the cohort.
Parameters Whole
population
(n = 50)Females
(n = 36)Males
(n = 14)Age (years) AM ± SD 67.46 ± 7.11 67.22 ± 7.09 68.07 ± 7.20 Range 54–78 54–78 56–78 Body weight (kg) AM ± SD 83.54 ± 14.56 81.45 ± 14.19 88.58 ± 14.56 Range 54–115 54–115 66–108 Height (cm) AM ± SD 164.37 ± 9.32 160.24 ± 6.14 174.33 ± 8.11 Range 149–189 149–173 165–189 Smokers (n, %) Nonsmokers 20 (40) 19 (38) 1 (2) Smokers 21 (42) 10 (20) 11 (22) Smokers in the past 9 (18) 5 (10) 4 (8) Place of live (n, %) Village 11 (22) 7 (14) 4 (8) Town 39 (78) 29 (58) 10 (20) Knee (n, %) Left 24 (48) 18 (36) 6 (12) Right 26 (52) 18 (36) 8 (16) Beginning pain (years, %) < 5 16 (32) 11 (22) 5 (10) < 10 21 (42) 15 (30) 6 (12) > 10 13 (26) 10 (20) 3 (9) Earlier knee endoprosthesis (n, %) Yes 13 (26) 10 (20) 3 (6) No 37 (74) 26 (52) 11 (22) Degenerative changes in the other knee (n, %) Yes 33 (66) 23 (46) 10 (20) No 17 (34) 13 (26) 4 (8) Contact with
chemicals in the
workplace (factory
polyvinyl chloride,
zinc smelter) (n, %)3 (6) 1 (2) 2 (4) Note. AM. arithmetic mean; SD. standard deviation Table 1. Information about the study population
The samples collected were materials rejected during knee arthroplasty. The samples were then prepared, segregated, and frozen at −22 °C. The samples were thawed before mineralization. Mineralization was performed using a microwave mineralizer Magnum II (Ertec, Poland) using 4 cm3 spectrally pure nitric acid [HNO3 (V); Supra pure]. After mineralization, the samples were transferred to 25 cm3 flasks and diluted with redistilled water (Millipore, SAS). The sampling procedure was described in our earlier work[1].
The contents of Al, Sn, Ti, Ba, Mn, and Li in samples were determined via plasma emission spectrometry technique (inductively coupled plasma–optical emission spectrometry) using a PerkinElmer Optima 5300DV spectrometer (horizontal plasma, Echelle-type diffraction net, a simultaneous semiconductor detector). This method was described in our previous work[1]. The limits of detection were from 0.001 mg/L to 0.01 mg/L.
The statistical analysis was performed using Statistica Pl. 13.1 software (StatSoft, Cracow, Poland).
Al (median 40.13 µg/g) and Ba (37.77 µg/g) accounted for the highest contents in the knee tissues, whereas Ti (0.42 µg/g), Mn (0.17 µg/g), tin (0.15 µg/g), and Li (0.035 µg/g) had notably lower contents. The highest variability was observed with Mn (93%) and the lowest with Ti (55%).
The highest concentration of these elements was recorded in the femur (Al, Ti, Ba, and Li) and the lowest in the meniscus (Al, Sn, Ti, Ba, and Li). Statistically significant differences were observed in Mn (P < 0.05), tin (P ≤ 0.05), and Ti (P < 0.05) (analysis of variance and Kruskal–Wallis rank test) in the femur, tibia, and meniscus.
Women had more Al, tin, and Ba than men. Men had higher contents of Mn, Ti, and Li. However, the differences between men and women were not statistically significant. In the femur, the contents of Al, tin, Ba, and Li were higher in samples obtained from women than those from men. The contents of Mn and tin in the tibia were higher in men compared with those in women. In the meniscus, the contents of all elements were lower in women than in men (Table 2).
Gender Tissue types Elements AM ± SD Med Range CV Women (n = 36) Tibial Mn 0.22 ± 0.21 0.14 0.07–1.03 93 Al 54.16 ± 32.08 54.95 6.27–118.58 59 Sn 0.22 ± 0.17 0.17 0.05–0.92 81 Ti 0.47 ± 0.28 0.43 0.10–1.60 60 Ba 45.56 ± 37.88 55.15 0.16–114.83 83 Li 0.041 ± 0.018 0.038 0.008–0.086 43 Femur Mn 0.23 ± 0.24 0.15 0.07–1.13 104 Al 66.91 ± 29.59 68.42 13.35–117.76 44 Sn 0.21 ± 0.11 0.19 0.05–0.48 53 Ti 0.53 ± 0.32 0.43 0.21–1.64 60 Ba 59.92 ± 32.54 69.17 1.30–114.86 54 Li 0.043 ± 0.016 0.040 0.015–0.087 38 Menicus Mn 0.27 ± 0.25 0.20 0.08–1.29 92 Al 28.81 ± 14.75 33.88 3.19–56.58 51 Sn 0.14 ± 0.05 0.14 0.06–0.33 37 Ti 0.38 ± 0.16 0.35 0.15–1.03 43 Ba 22.72 ± 18.34 32.21 0.13–53.73 81 Li 0.014 ± 0.014 0.011 0.003–0.075 103 Men (n = 14) Tibial Mn 0.21 ± 0.18 0.15 0.07-0.77 88 Al 57.81 ± 34.56 52.32 16.89–116.94 60 Sn 0.18 ± 0.08 0.20 0.02–0.28 45 Ti 0.51 ± 0.19 0.46 0.24–0.98 37 Ba 49.37 ± 43.54 52.07 2.26–115.79 88 Li 0.043 ± 0.024 0.043 0.008–0.101 56 Femur Mn 0.29 ± 0.30 0.19 0.08–1.21 105 Al 51.42 ± 40.74 24.96 7.63–115.08 79 Sn 0.16 ± 0.11 0.14 0.03–0.37 67 Ti 0.46 ± 0.20 0.47 0.07–0.83 44 Ba 40.36 ± 47.84 3.07 0.22–115.38 119 Li 0.040 ± 0.020 0.035 0.004–0.091 51 Menicus Mn 0.28 ± 0.20 0.23 0.11–0.89 70 Al 37.63 ± 15.77 37.03 3.69–72.71 42 Sn 0.15 ± 0.07 0.12 0.08–0.27 45 Ti 0.42 ± 0.23 0.41 0.14–1.04 55 Ba 27.47 ± 24.24 32.13 0.10–75.82 88 Li 0.024 ± 0.025 0.012 0.006–0.078 102 Note. AM. arithmetic mean; SD. standard deviation; Med. median; CV. coefficient of variation; Mn, manganese; Al, aluminum; Sn,tin; Ti,titanium; Ba,barium; Li,lithium
Table 2. Statistical characteristics for concentration of Mn, Al, Sn, Ti, Ba and Li in tissues of the knee joint (µg/g)
Spearman’s correlation analysis showed statistically significant correlations of Al with Ti and Ba and Li with Al, Sn, Ti, and Ba (P < 0.05). The highest number of correlations between elements in individual parts of the knee occurred in the femur and the meniscus. Upon comparing the correlations between men and women, the greatest number of correlations was found in the femur: Al with Ti, Ba, and Li; Ba with Ti and Li.
Age can affect the occurrence of elements and promotes deposition in tissues including hard tissues. The amounts of Mn, Al, and Ba increased with age. The concentration of the elements was presented as a median. For manganese, individual types of tissues showed a statistical significance in the group 61–70 years. For Al and Ti, significant differences were observed in the group up to 60 years and over 70 years. Tin showed no differences between age groups except in the subjects over 70 years. The greatest effect of age was observed in the case of Li—all three age groups showed statistically significant differences. The oldest age group (over 70 years) presented the lowest P-value (Figure 1).
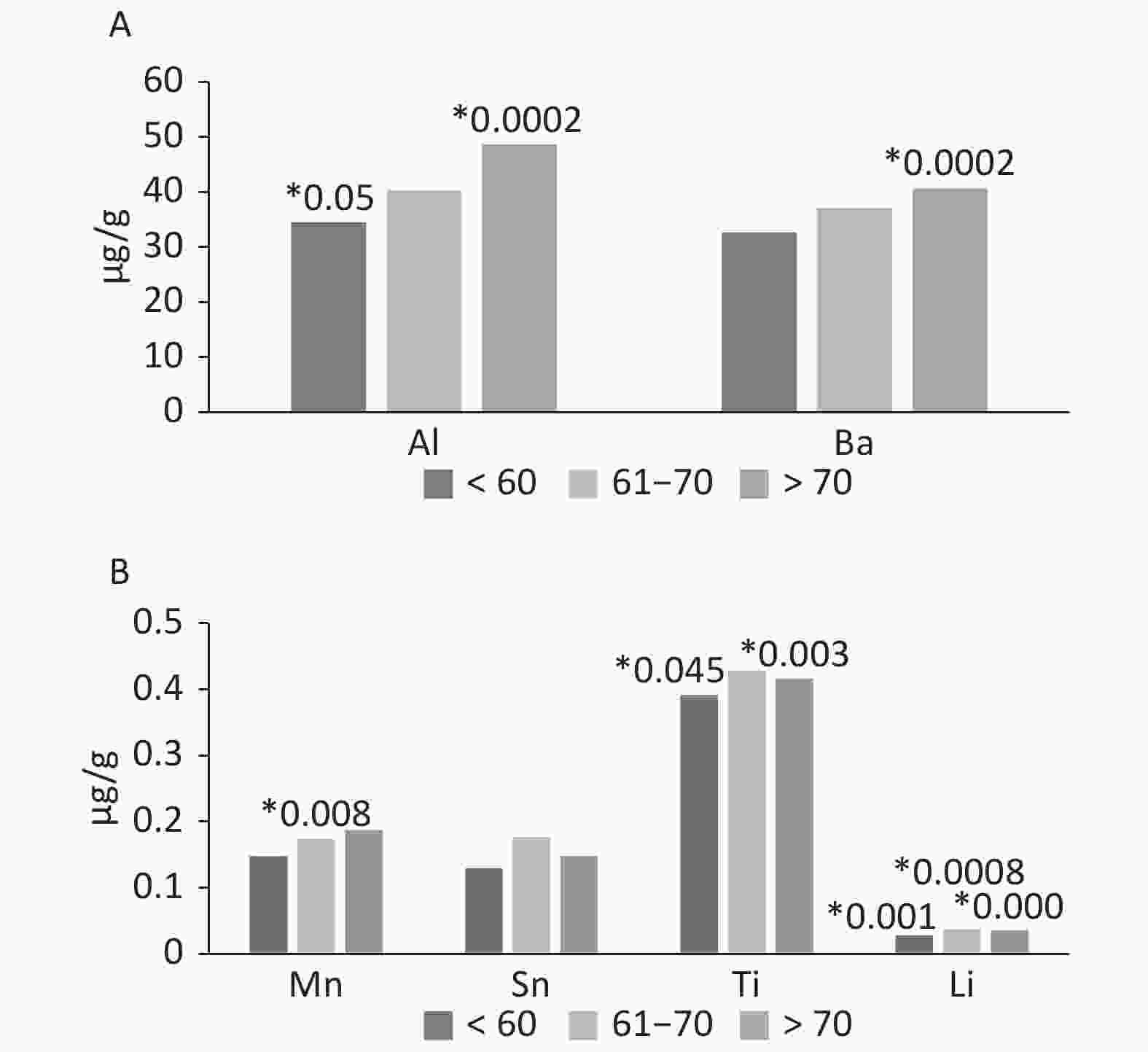
Figure 1. The occurrence of Al, Ba, Mn, Sn, Ti and Li in tissues of the knee joint, according by age (µg/g). *The level of significate in ANOVA Kruskala-Wallisa Test by ranks.
Skeletal cells work continuously to maintain remodeling, and thus, they are in a constant state of dynamic balance in terms of composition and structure; they also react to external mechanical forces. Upon considering this type of sample, we aimed to identify the differences in elemental contents. Bone and connective tissues are characterized by different structures and elemental accumulations. The connective tissue lacks hydroxyapatite. Hence, it cannot accumulate elements, especially 2-valence ones. Symptoms of reactions to metal implants occur in various forms, such as pain and dermatitis. Implant allergies are rare and not a well-recognized problem.
We compared the contents of elements in the bone (femur and tibia) and connective tissues (meniscus). Most elements were observed in higher levels in the bone tissue compared with the connective tissue. Al and Ba were 50% higher, and tin and Ti were 70% higher in the bone than in the connective tissue. For Li, the difference in the content between the bone and connective tissues was 27%. Only Mn was higher in the connective tissue (by about 40%). This element was also characterized by the highest coefficient of variation, which indicates a high migration capacity in the environment and the human body. The presented results are cognitive in nature and require more research.
Vuorinen et al.[3] reported that bones from excavations from the 16th to 17th centuries in Finland had Mn levels of 1.12 µg/g, which is slightly lower than the value observed in men (0.88 µg/g). The average Mn content in our own study was lower and amounted to 0.25 µg/g. These differences can be caused by the penetration of Mn from the soil into the tissues in which they remained for many years. Hisanaga et al.[4] studied metals in excavated ribs, where the average Mn content was 850 µg/g. This high value may indicate migration of this element from soil to tissues.
Our results for individual elements are similar to those of Kuo et al.[5]. They studied the content of metals in the ribs of residents of industrialized Taiwan, China. The content of Mn in their studies was 0.7 µg/g, and that of Al was 52 µg/g; 0.25 µg Mn/g and 50 µg Al/g were recorded. Helliwell et al.[6] determined the contents of elements in the femur of patients with fractures, degenerative changes, and a control group obtained during autopsy. The results suggest no major differences in the elemental composition of patients with fractures compared with the control group. The bone that is adjacent to the joints with degenerative disease is usually less mineralized than the control bone and the bone from patients with fractures. These changes may reflect the rapid bone rotation near the arthritic joint. Helliwell et al.[6] determined the content of aluminum at 101 µg/g, barium at 21 µg/g, and tin at 4 µg/g. The aluminum and tin levels were higher than those obtained in these tests and lower than that of barium.
The concentrations of many elements in the ribs were used as an indicator of long-term exposure in a study of 35 elderly Japanese people by Yoshinaga et al.[7]. These contents were expressed as median as follows: Li (0.05 µg/g), Mn (0.13 µg/g), Sn (0.79 µg/g), and Ba (1.30 µg/g) dry matter. These results are similar to their own research for lithium and manganese.
Garcia et al.[8] studied the contents of many elements in tissues from the inhabitants of Tarragona (Spain). The content of manganese in the bones was 0.17 µg/g wet weight, and no statistically significant differences were observed between men and women and between smokers and non-smokers. The high level of Mn in the connective tissue was probably due to the specificity of this tissue and its location. The obtained value is similar to the content obtained here. The highest content of Mn in the liver was 1.44 µg/g. The content of tin was the highest in bones at 0.47 µg/g and the lowest in the liver at 0.16 µg/g. A different element content compared with the knee joint tissues was characterized by the synovial fluid originating from this joint, in which the Ba and Li contents were 1.9 and 5.7 µg/kg, respectively[9].
Lanocha-Arendarczyk et al.[10] measured metals in the knee tissues of Polish residents. The Mn content was 0.31 mg/kg dw and slightly higher in men (0.34 mg/kg dw) than in women (0.30 mg/kg dw). However, these values were not statistically significant. A similar Mn content and a series of changes occurred in our own research: the Mn content averaged 0.25 µg/g in men, 0.026 and 0.24 µg/g in women.
The highest levels of these elements were detected in the femur, and the lowest was observed in the meniscus. Statistically significant differences were found for Mn, tin, and Ti. No statistically significant differences were observed between women and men. Age contributed to the increased content of Mn, Al, and Ba. With age, an increase in the content of these elements occurred in the knee joint tissues. The Mn content approximated the value determined in the same tissue originating in the same country. Mn also had the highest coefficient of variation, which indicates its high migration capacity in the environment and in the human body. Of the studied elements, Mn was the most frequently determined in reference to bibliographic data. Our results can be used for further study and analysis of changes occurring in the human body.
The authors declare no conflict of interest.
Concentration of Selected Elements in the Tissues of the Knee Joint
doi: 10.3967/bes2020.109
- Received Date: 2020-05-05
- Accepted Date: 2020-07-22
| Citation: | BABUŚKA-ROCZNIAK Magdalena, BRODZIAK-DOPIERAŁA Barbara, MITKO Krzysztof, CIPORA Elżbieta, ROCZNIAK Wojciech. Concentration of Selected Elements in the Tissues of the Knee Joint[J]. Biomedical and Environmental Sciences, 2020, 33(10): 807-811. doi: 10.3967/bes2020.109 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: