-
The Nucleic Acid Laboratory, a division of the Clinical Laboratory Department, is responsible for performing real-time PCR (qPCR) assays to rapidly detect infectious diseases, genetic disorders, and more[1]. As the gold standard, qPCR has played an indispensable role in diagnosing pandemic infections, such as the coronavirus disease 2019 (COVID-19)[2].
Despite the widespread use of qPCR, its high sensitivity can result in false positives if nucleic acid contamination occurs in the laboratory. This contamination can significantly impact the accuracy of the test[3]. Leaked nucleic acid extractions or amplified nucleic acid products can create aerosols that linger in the air, eventually adhering to various surfaces, including the outer gloves of operators, door handles, and centrifuges[4]. Once nucleic acid contamination occurs in a laboratory, it can lead to a temporary suspension of work for several days or even months. Sometimes, laboratories may need to establish new facilities to continue their operations[5]. Therefore, prevention and disinfection of nucleic acid contamination are crucial for nucleic acid analysis laboratories.
Conventional methods to decontaminate nucleic acid in labs include using 75% alcohol, ultraviolet (UV) irradiation, and spraying with 5,500 mg/L chlorine-containing disinfectant. While 75% alcohol primarily sterilizes, it has limited effectiveness in deactivating DNA and RNA[6]. Chlorine-containing disinfectants denature viral proteins but require rinsing due to their corrosive nature. UV radiation inhibits DNA replication but doesn’t eliminate nucleic acid contamination[7], and it’s limited to surface decontamination. These methods have limitations, so an alternative decontamination strategy should be put forward.
Ozone is a highly reactive molecule with strong oxidizing ability. Through the reactive oxygen species (ROS) it generates, such as hydroxyl radicals, ozone can directly attack and decompose DNA or RNA[8,9]. Based on this, ozone could serve as an effective tool to eliminate nucleic acid contamination. In this study, we constructed a model to simulate nucleic acid contamination and used it to evaluate the effectiveness of ozone compared to conventional methods. We also explored the optimal concentration and working time of ozone.
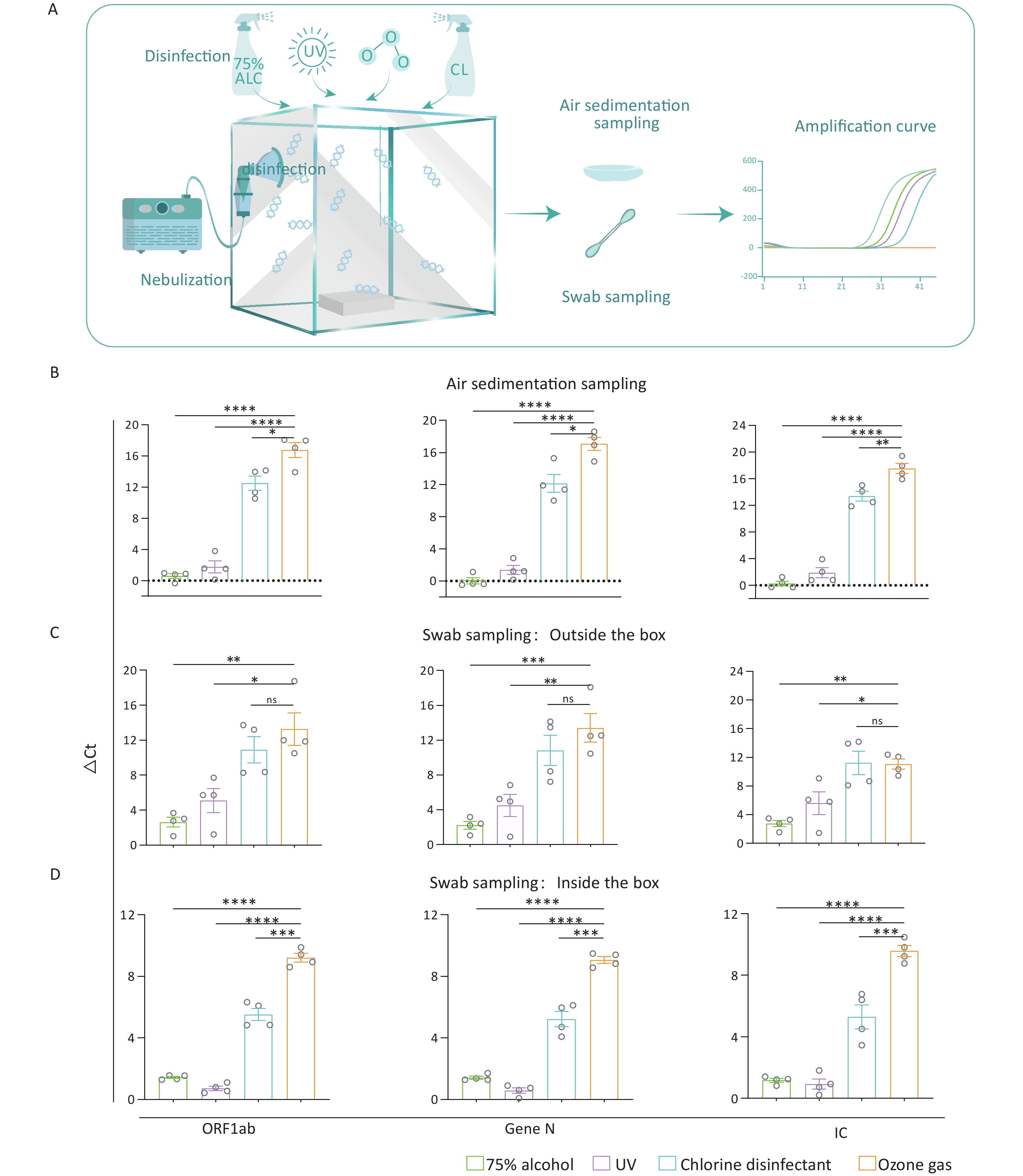
We used five sealed chambers measuring 50 cm × 50 cm × 50 cm to emulate a nucleic acid-contaminated laboratory environment. Inside each chamber, we placed a non-sealed rectangular plastic box (11 cm × 8 cm × 9 cm) to emulate common laboratory instruments. Nucleic acid fragments (ORF1ab, Gen N, IC) (BDSbiotech, Guangzhou, China) were loaded into a nebulizer. Then, the nebulizer was used to fill an air-tight acrylic chamber with the fragments through a pore with a diameter of 2 cm on the left side wall of the chamber. When the nebulization was completed, the pore was completely sealed with a rubber plug, followed by static settling for 5 min. After settling, the nucleic acid contamination model was constructed. (Figure 1A).
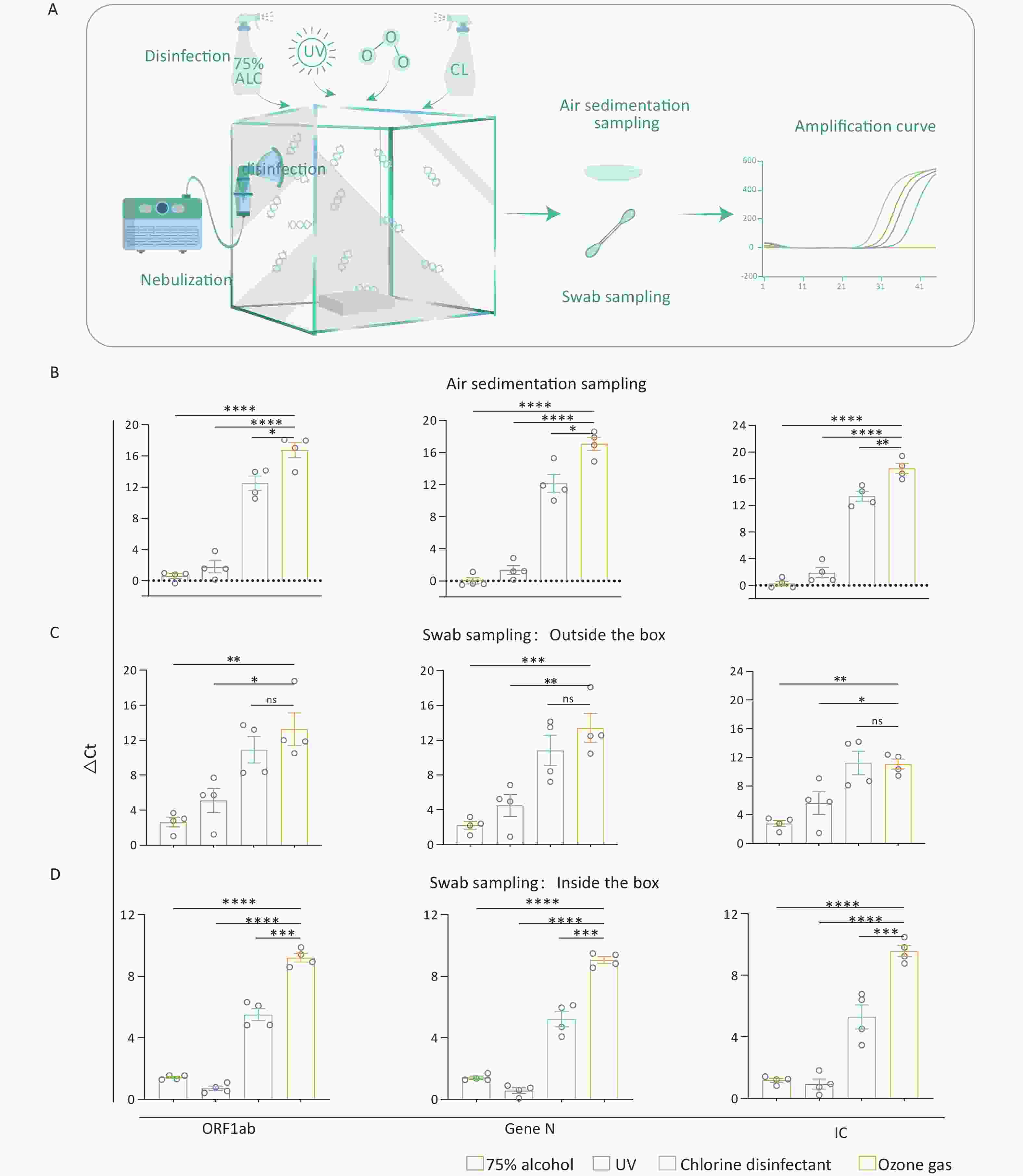
Figure 1. Effectiveness of various decontamination strategies in a nucleic acid contamination model. (A) Schematic diagram illustrating the nucleic acid contamination model, which includes the steps of nebulization, disinfection, sampling, and amplification. (B–D) Bar graph showing the ΔCt values for four nucleic acid fragments in the air, on the inner or outer surfaces of the box after four different disinfectants. All experiments were performed as independent in at least quadruplicate, and data are shown as mean ± SEM. Student’s t-test was used to compare the two groups. The difference was considered significant if P < 0.05.
Different treatments to eliminate the nucleic acid contamination were applie. In the 75% alcohol and chlorinated disinfectant groups, we used a sprayer to disperse the respective agents into the chamber via the 2 cm-diameter pore, promptly sealing it afterward. In the UV group, a 6 w UV light was installed inside the chamber’s top, and UV exposure was carried out for 2 hours to eliminate nucleic acid contamination. The ozone generator was used to provide ozone into the model, and the introduction of ozone was halted once the in-box ozone concentration achieved the 120 mg/m3 for 2 h. The control group was set by nebulizing nucleic acid fragments into the chamber as mentioned above, but without applying any disinfection treatment. The above five groups conduct experiments at the same time.
Residual nucleic acids were collected by swabbing 5 standard areas (3 cm × 3 cm) on both the inner and outer surfaces of the box. For air sampling, 40-mm-diameter Petri dishes containing 3 mL of deionized water (ddH2O) were placed at the fixed sampling point inside the chamber and were left for 2 h after decontaminating. Residual nucleic acids were then collected by harvesting the water from the Petri dish.
To detect nucleic acid fragments, we employed the Liferiver Novel Coronavirus (2019-nCoV) real-time multiplex RT-PCR kit (Liferiver Biotech, Shanghai, China). A 25 μL reaction comprised 5 μL sample, 19 μL 2019-nCoV Super Mix, and 1 μL Enzyme Mix. PCRs were conducted as per the manufacturer’s recommendations using the Real Time PCR Detection System-Gentier96E (Tian Long, China). The thermal cycling conditions were as follows: 95 °C for 3 min, followed by 45 cycles of 95 °C for 15 s, 58 °C for 30 s. Results were interpreted based on Cycle threshold (Ct) values, with Ct > 41 considered a negative detection signal. The relative reduction in nucleic acid levels post disinfection was determined by comparing the Ct values of treated samples with untreated controls. The ΔCt value represents the difference between the treated sample and the control.
Among the samples collected through air sedimentation, those treated with ozone showed the highest ΔCt values, indicating the most effective decontamination of the nucleic acid in the air among four groups (P < 0.05) (Figure 1B). In addition, PCR results indicated that while there was no significant difference in ΔCt values between ozone and chlorine-containing disinfectant for fragments on the outer surface (P > 0.05), both treatments produced higher ΔCt values than the other two methods (P < 0.05) (Figure 1C). Besides, the ozone treatment showed the highest ΔCt values of the fragments on the inner surface among the four disinfectants (P < 0.01) (Figure 1D). Consistent with the result of air sedimentation, the ozone exhibited the best effect on eliminating the outer and inner surfaces of appliances.
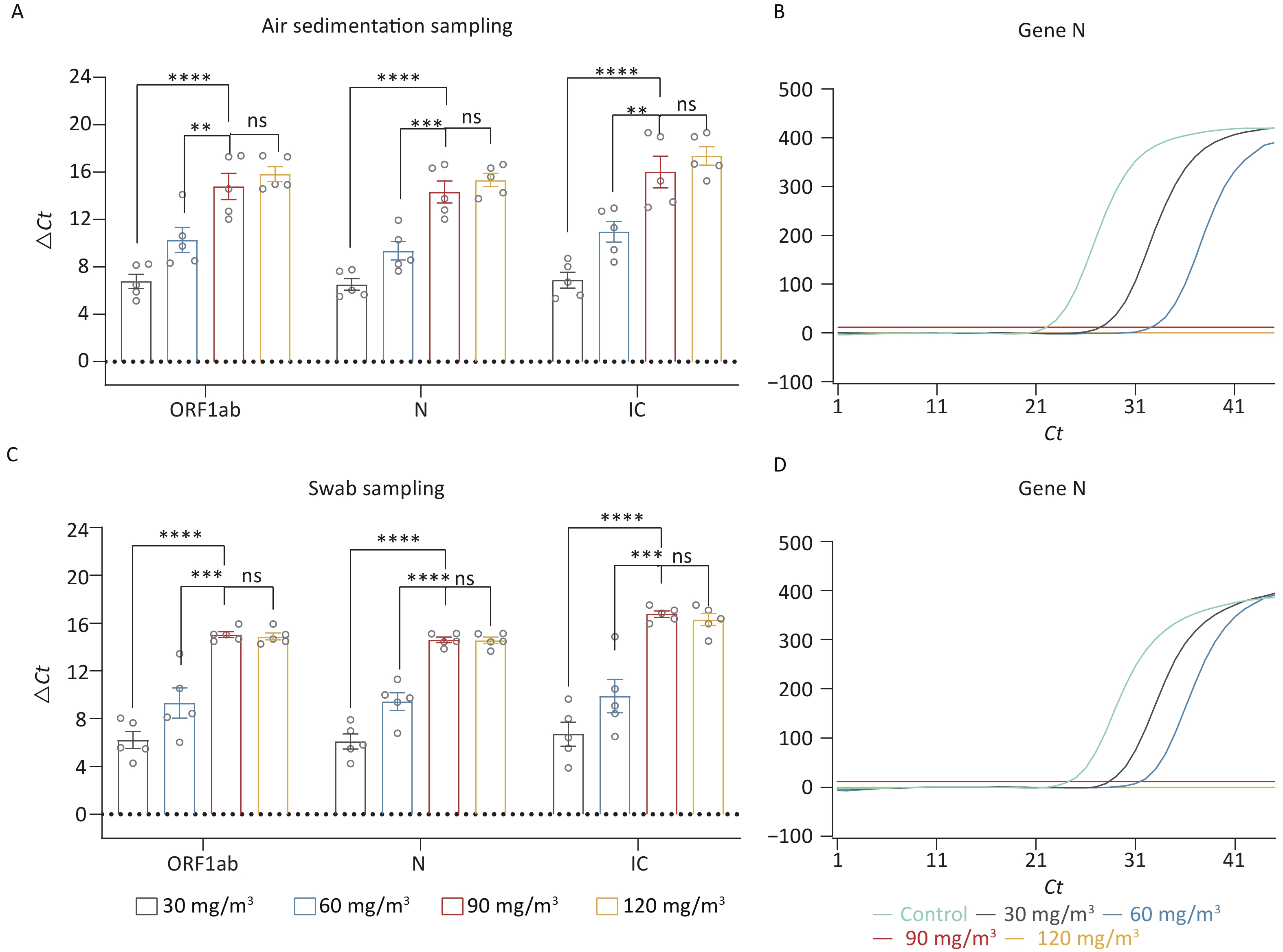
In the afore mentioned research, we preliminarily determined that ozone is an effective method for eliminating nucleic acid contamination. Therefore, we further explored the optimal working concentration of ozone. Four different concentrations of ozone (30 mg/m3, 60 mg/m3, 90 mg/m3, 120 mg/m3) were used to eliminate the nucleic acid. Figure 2A exhibited the ΔCt values of the fragments (ORF 1ab, Gene N, IC) in the air disinfected by different concentrations of ozone. Compared with the concentrations of 30 mg/m3 and 60 mg/m3, both 90 mg/m3 and 120 mg/m3 concentrations were more effective in eliminating nucleic acid contamination (P < 0.05). However, there was no significant difference in the elimination efficacy of these two concentrations (P > 0.05). One of the amplification curves of Gene N even showed no amplification peak in 90 mg/m3 and 120 mg/m3 groups (Figure 2B), which indicated that nucleic acid levels in these two groups fell beneath the detection threshold. The nucleic acid fragments collected by swab sampling showed a similar result. The concentrations of 90 mg/m3 and 120 mg/m3 were more effective in eliminating the surface nucleic acid fragments (P < 0.05) (Figure 2C), and the amplification curves for Gene N showed no peaks (Figure 2D).
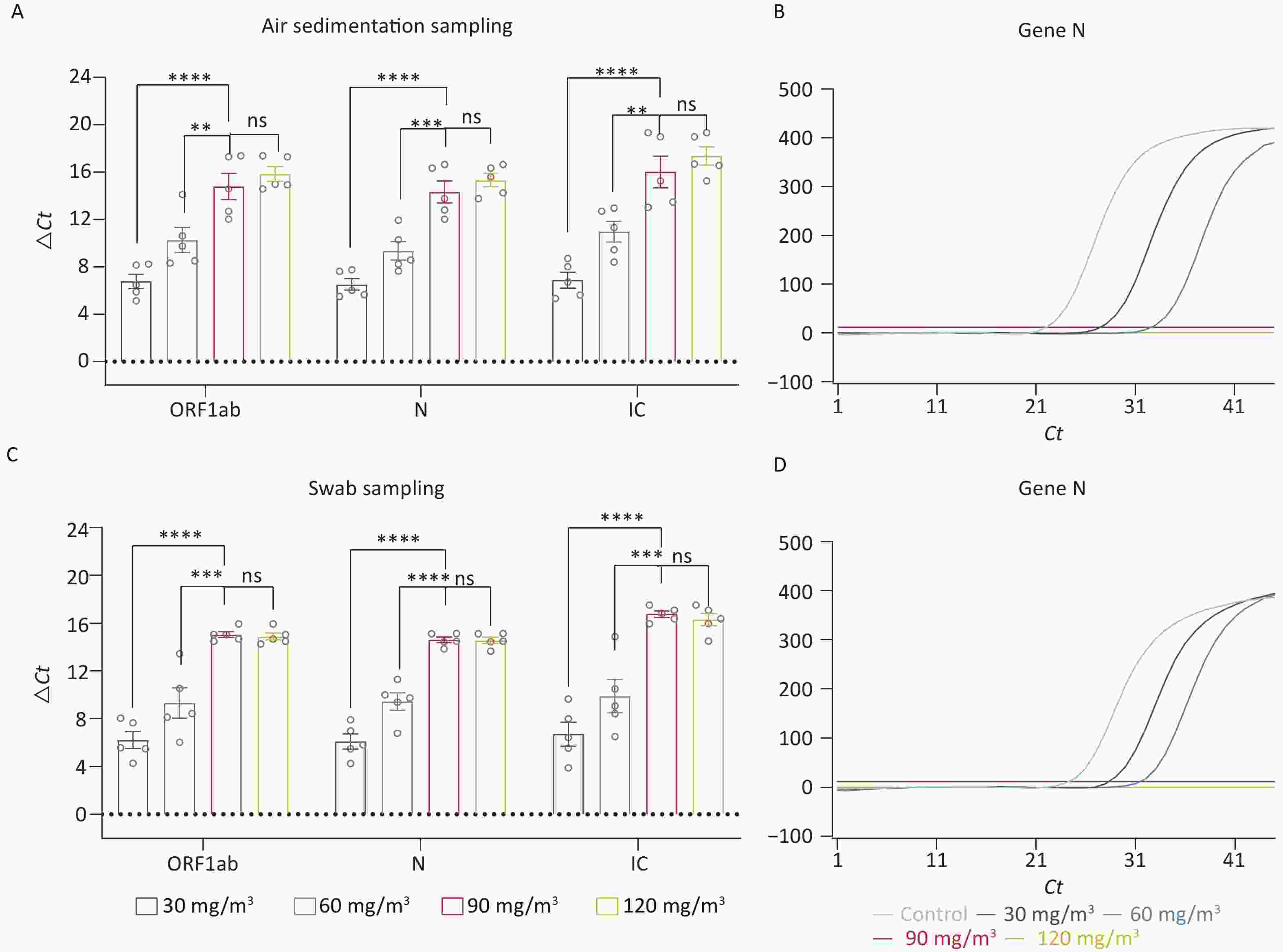
Figure 2. Investigation of the optimal working concentration of ozone for nucleic acid elimination. (A) ΔCt values of the fragments (ORF 1ab, Gene N, IC) in the air disinfected by different ozone concentrations. (B) The amplification curves of Gene N. (C) ΔCT values of the fragments on the inner and outer surfaces disinfected by different concentrations of ozone. (D) The amplification curves of Gene N. All experiments were performed as independent in at least quadruplicate, and data are shown as mean ± SEM. Student’s t-test was used to compare the two groups. The difference was considered significant if P < 0.05. ns, no significance; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Although 90 mg/m3 and 120 mg/m3 both effectively eliminated nucleic acid contamination, 90 mg/m3 is recommended as the optimal concentration based on cost-effectiveness and personnel safety considerations.
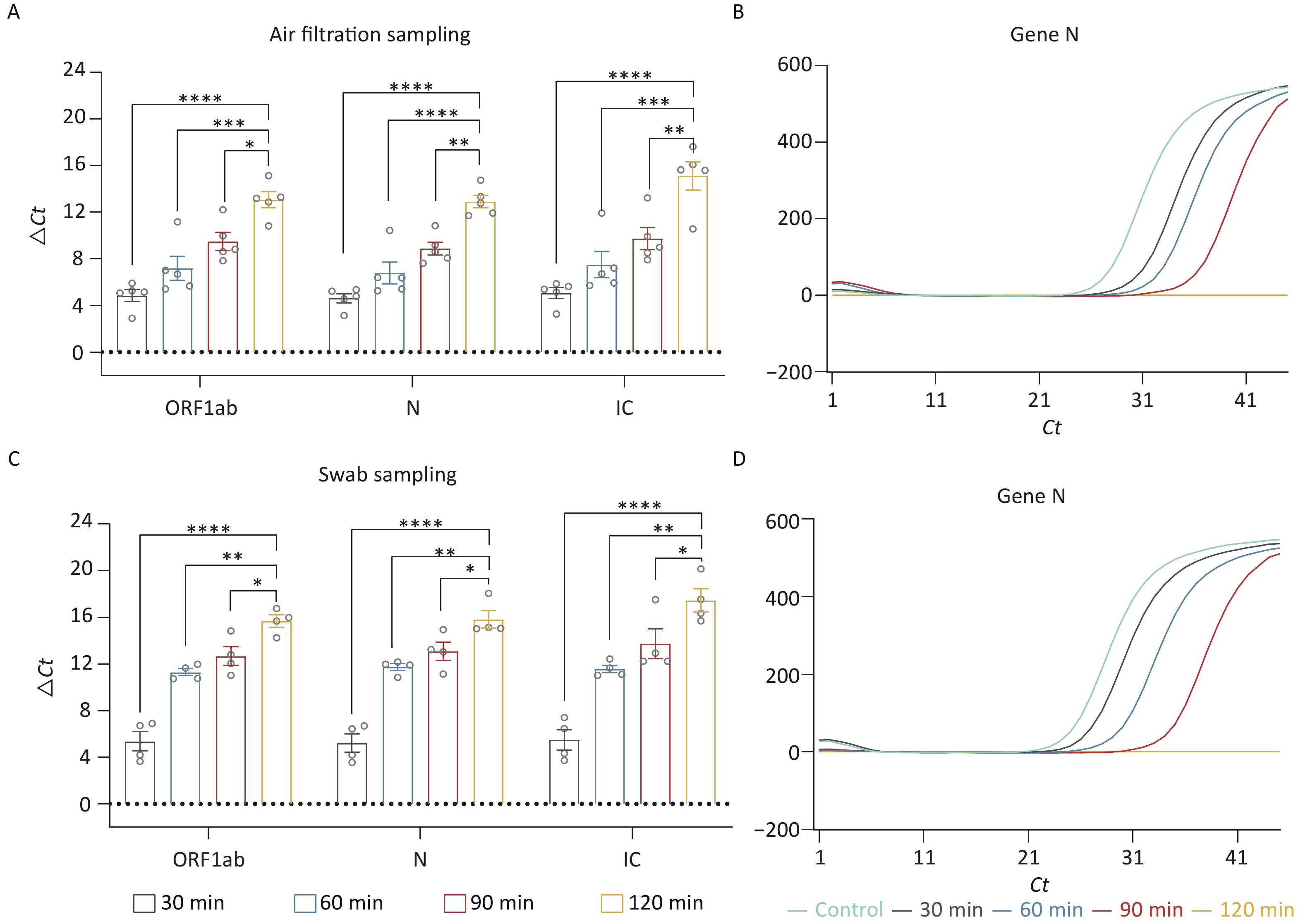
Based on the optimal ozone concentration of 90 mg/m3, we further investigated the ideal working time for ozone. We evaluated the following times: 30 min, 60 min, 90 min, and 120 min. Since determining the optimal ozone working time requires the collection of residual nucleic acids in the air within a brief timeframe, the air sedimentation sampling as mentioned above is not feasible. Therefore, membrane filtration was used to collect the nucleic acids in the air. After the disinfection process, the existing rubber plug was promptly replaced with a pre-soaked membrane having a diameter of 3 cm. A pump was then employed to capture any residual airborne nucleic acid onto the membrane. Finally, the nucleic acids adhered to the membrane were eluted using ddH2O. Through air filtering, we collected the residual fragments in the air. The ΔCt values of these fragments showed that the group of 120 min had the most effective elimination (P < 0.05) (Figure 3A). Additionally, there was no amplification peak in the N Gene amplification curve after 120 min of treatment (Figure 3B), suggesting that nucleic acid contamination in the air had been nearly eliminated. We also collected the residual fragments by swab sampling. Similar to the result of air samples, treatment for 120 min had cleared up the nucleic acid contamination on the surface of appliances (Figure 3C–D).
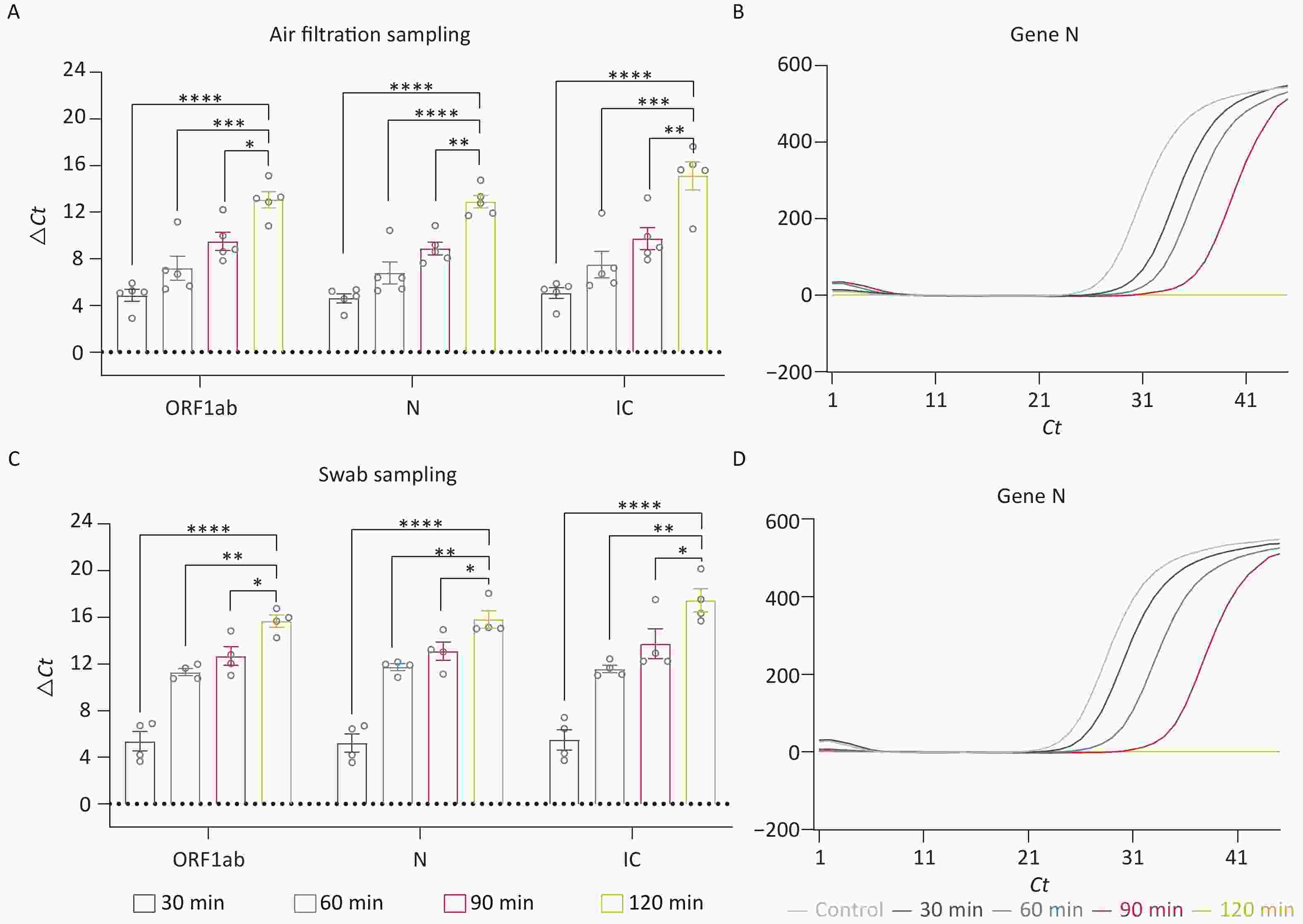
Figure 3. Exploration of optimal working time for nucleic acid decontamination. (A, C) ΔCt values of the fragments (ORF 1ab, Gene N, IC) in the air and surface disinfected by different working times of ozone. (B, D) The amplification curves of Gene N. All experiments were performed as independent in at least quadruplicate, and data are shown as mean ± SEM. Student’s t-test was used to compare the two groups. The difference was considered significant if P < 0.05.*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
In this study, we created a model to simulate nucleic acid contamination and used it to confirm the efficacy of ozone in eliminating nucleic acids. Our results also showed that ozone has advantages over other commonly used disinfectants. In addition, we explored the optimal concentration and time for decontamination of nucleic acids using ozone. Our findings provide nucleic acid analysis laboratories or other laboratories prone to nucleic acid contamination with an alternative approach to dealing with nucleic acid contamination.
Interestingly, when examining the effect of ozone on the elimination of nucleic acids from the surface of laboratory instruments (simulated by a plastic box), we found that it was comparable to chlorine-containing disinfectants in eliminating nucleic acid fragments attached to the outer surface of appliances. However, ozone was more effective than chlorine-containing disinfectants when it came to deactivating nucleic acid fragments attached to inner surfaces. This enhanced disinfection capability of ozone may be due to its gaseous nature, allowing it to permeate more effectively into the interior of equipment to eliminate nucleic acids. Notably, in cases of severe nucleic acid contamination, ozone could be chosen for its efficacy both in the air and on the surfaces of laboratory equipment.
However, due to site constraints, our nucleic acid contamination model may not be able to mimic a real laboratory fully. Room size, concentration and type of nucleic acid fragments may differ from actual laboratory contamination and may affect actual elimination. To further validate our findings and strengthen the evidence supporting the use of ozone for eliminating nucleic acid contamination, we plan to conduct validation studies with the real laboratory environment in future research. Moreover, we will further construct COMSOL models in the future to simulate different concentrations of nucleic acid contamination and different room parameters, including laboratory size and shape. This will help us optimize the working time and concentration of ozone for various scenarios.
Furthermore, because of the practical implications of this study, it’s essential to emphasize the potential hazards of ozone to humans[10]. Whenever possible, the technicians need to wait until ozone has degraded before entering the laboratory.
In conclusion, this study demonstrated the strong nucleic acid elimination ability of ozone and provides the optimal concentration and working time for nucleic acid analysis laboratories to eliminate nucleic acid contamination.
HTML
Competing Interest The authors declare no conflict of interest.
Ethics Approval and Consent to Participate The Research Ethics Committee of The Second People’s Hospital of Jiulongpo District confirmed that no ethical approval was required.
Availability of Data and Materials All data in the study can be accessed from the corresponding author (wangjing44599@163.com) after the application.
&These authors contributed equally to this work.
Reference







 Quick Links
Quick Links
 DownLoad:
DownLoad: