-
The cerebrospinal venous system is an essential component of the cerebral circulatory system. Unlike the arterial system, which ensures the supply of oxygen and nutrients, the venous system plays a critical role in waste clearance, cerebrospinal fluid collection, immune surveillance, and intracranial pressure regulation[1]. Cerebral venous outflow disorders (CVOD) arise from intraluminal obstructions, extrinsic compression, or venous valve incompetence, leading to increased trans-stenosis pressure gradients, impaired cerebral blood flow, and tissue hypoxia[2-4]. These pathological changes can cause intractable symptoms, including sleep disturbances, tinnitus, head noise, dizziness, and headaches[5-8]. Furthermore, chronic venous stasis and cerebral perfusion deficiency caused by CVOD may promote neurodegeneration and cognitive decline[9]. Current therapeutic approaches, including symptomatic management, endovascular stenting, and surgical procedures (like steroidectomy), offer limited benefits and lack conclusive evidence for long-term efficacy[10-12]. This underscores the urgent need for novel and effective therapies that address the underlying pathophysiology of CVOD.
Intermittent hypoxia–hyperoxia training (IHHT) is a novel non-pharmacological intervention that combines controlled periods of hypoxia and hyperoxia stimulation. This approach has shown promise in various clinical contexts by improving cerebral oxygenation, energy metabolism, and circulatory function[13-20]. The rationale for applying IHHT in CVOD is based on its potential to address the disorder’s fundamental pathophysiology. CVOD is characterized by impaired venous drainage, leading to cerebral venous congestion and tissue hypoxia. IHHT counteracts these pathological changes through multiple mechanisms. During hyperoxic intervals, IHHT rapidly elevates tissue oxygen levels, alleviating hypoxia secondary to venous outflow impairment. This may mimic normobaric oxygen therapy and potentially relieve hypoxic-related non-focal neurological symptoms[21]. IHHT also exerts therapeutic effects on CVOD through intermittent hypoxia (IH) stimulation. IH induces physiological acclimatization and enhances tolerance to hypoxic conditions, which may effectively alleviate hypoxia-related symptoms associated with CVOD[14,22]. Additionally, IH stimulates hypoxia-inducible factor-1 (HIF-1) expression, which promotes angiogenesis and improves vascular function, potentially normalizing venous outflow patterns[23]. IH also modulates autonomic function, reducing sympathetic overactivity and enhancing metabolic stability, thereby supporting hemodynamic balance[24-26]. Despite these theoretical benefits, evidence regarding the safety and efficacy of IHHT in CVOD remains limited and inconclusive. Therefore, this study aims to evaluate the safety and feasibility of IHHT in patients with CVOD, with particular focus on its potential to improve cerebral perfusion, venous outflow dynamics, and symptomatology.
-
This single-arm, self-controlled study (NCT06738706) recruited patients with CVOD from the Department of Neurology at Xuanwu Hospital, Capital Medical University. All participants provided written informed consent after receiving detailed information about the study. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki and approved by the Institutional Ethics Committee of Xuanwu Hospital, Capital Medical University (Approval No. 2023[142]). Inclusion criteria were as follows: 1) age 18–80 years; 2) diagnosis of CVOD confirmed by contrast-enhanced magnetic resonance venography, digital subtraction angiography, computed tomography venography, or ultrasound, including cerebral venous sinus stenosis (CVSS), internal jugular venous stenosis (IJVS), or internal jugular venous valve incompetence (IJVVI)[7]; 3) unexplained chronic neurological deficits or other symptoms lasting > 3 months; and 4) signed informed consent from the patient or a legally authorized representative. Exclusion criteria included: 1) life-threatening comorbidities; 2) clinical symptoms and signs attributable to other diseases; 3) intracranial hypertension; 4) moderate to severe intracranial/extracranial arterial stenosis; 5) history of ischemic/hemorrhagic stroke or prior cerebral endovascular surgery; 6) intracranial abnormalities, including tumors, abscesses, vascular malformations, or cerebral venous sinus thrombosis; 7) confirmed sleep apnea, plateau residency, traveling history of altitude > 1000 m, or relative hypoxic exposure within last 6 months; and 8) poor compliance.
-
All participants underwent 14 sessions of IHHT intervention over 2–3 weeks. During each session, patients remained seated, awake, and at rest under normobaric conditions. Each IHHT session comprised four cycles, each consisting of 20 minutes of hyperoxia (38% O2) followed by 10 minutes of hypoxia (11% O2). Both hypoxic and hyperoxic exposures were administered via a face mask connected with the plastic tubing of a hypoxicator, which blended oxygen and nitrogen extracted from ambient air to achieve the target oxygen partial pressure[27]. The intervention could be supervised by physicians or self-administered by patients.
-
Adverse events were monitored throughout the intervention. Hypoxia-related discomforts, including headache, gastrointestinal disturbance, fatigue, weakness, and dizziness, were assessed using the Lake Louise Score (LLS; 0–12 score). Physiological parameters, including peripheral oxygen saturation (SpO2), heart rate (HR), and brachial arterial blood pressure (BP), were continuously monitored during the intervention using a multiparameter monitor (HC-2010®, HealthCare NewTech Co., Ltd., Beijing, China). Regional cerebral oxygen saturation (rScO2) was noninvasively monitored via near-infrared spectroscopy (NIRS; BRS-1®, Casibrain Technology Co., Ltd., Beijing, China) with sensors placed bilaterally on the forehead. Parameters were recorded every 5 minutes from baseline until 30 minutes post-intervention of the first session. Twenty-four-hour ambulatory blood pressure was assessed at baseline and post-intervention using methods previously described[28]. Parameters, including 24-hour, daytime, and nighttime average systolic and diastolic BP, were recorded. Fasting blood samples were collected at baseline and post-intervention for biochemical and hematologic analyses using the HITACHI 7600 automated biochemical analyzer (Hitachi Ltd., Tokyo, Japan) and Sysmex XN-350 hematology analyzer (Sysmex Corporation, Kobe, Japan). All laboratory analyses were conducted at the blood biochemistry laboratory of Xuanwu Hospital, Capital Medical University.
The Patient Global Impression of Change (PGIC) scale was administered after the 14 IHHT sessions to assess patients’ perceived overall improvement. The PGIC is a numeric scale ranging from 1 (very much improved) to 7 (very much worse), with lower scores indicating greater improvement. Clinical effects of IHHT were further assessed using the Tinnitus Handicap Inventory (THI), Headache Impact Test-6 (HIT-6), and a visual analogue scale (VAS), administered before and after the intervention. Color-coded duplex sonography was performed at baseline and post-intervention by an experienced physician (over 20 years of experience, Jia LY), blinded to participants’ clinical data. The detailed method has been previously described[29]. The inner diameter (mm) and flow volume (FV, mL/min) were measured at the proximal (J1) and distal (J3) segments of the bilateral internal jugular veins. Magnetic resonance imaging using multi-post-labeling delay arterial spin labeling (ASL) was performed to evaluate changes in cerebral perfusion. Data were post-processed using CereFlow software to obtain cerebral perfusion parameters (corrected cerebral blood flow, cCBF)[30].
-
Continuous variables are expressed as mean ± standard deviation (SD) or median (interquartile range, IQR), according to their distribution. Differences between pre- and post-intervention measures were assessed using paired sample t-tests and Wilcoxon signed-rank tests, as appropriate. Categorical variables were reported as numbers (frequencies and proportions) and analyzed using chi-square or Fisher’s exact tests. Statistical significance was set at P < 0.05. Statistical analyses were conducted using SPSS statistical software (version 26; IBM Corp., Armonk, NY, USA) and GraphPad Prism (Version 10.1.0).
-
A total of 30 patients with CVOD were screened, of whom 24 met the inclusion criteria and were enrolled in the study. Four patients did not complete the intervention: three withdrew due to scheduling conflicts, and one discontinued after the first three IHHT sessions due to perceived lack of benefit. Ultimately, 20 patients (6 males, 14 females), aged 23–73 years, completed the entire training protocol. The cohort had a mean age of 49.80 ± 14.35 years, a mean weight of 62.45 ± 10.55 kg, a mean height of 164.65 ± 9.20 cm, and a mean body mass index of 22.95 ± 2.87 kg/m2 (Table 1). The most prevalent symptoms among participants were tinnitus (including tinnitus cerebri), headache, and sleep disturbances. Observed CVOD subtypes included IJVS, IJVVI, and CVSS (Table 1).
Parameters Patients (n = 20) Demographic Information Age (year) 49.80 ± 14.35 Gender (female) 14 (70%) Height (cm) 164.65 ± 9.20 Weight (kg) 62.45 ± 10.55 Body Mass Index (kg/m2) 22.95 ± 2.87 Major Symptoms, n (%) Tinnitus, Tinnitus Cerebri, or both 16 (80%) Headache 9 (45%) Sleep Disturbance 14 (70%) Cerebral Venous Outflow Disorders, n (%) Internal Jugular Vein Stenosis (IJVS) 16 (80%) Internal Jugular Vein Valve Incompetence (IJVVI) 5 (30%) Cerebral Venous Sinus Stenosis (CVSS) 3 (15%) Table 1. Demographic information and clinical characteristics
All 20 participants completed the protocol with good tolerance; no significant discomfort or adverse events were observed. Following the first intervention, 50% of patients (10/20) experienced mild-to-moderate fatigue, 10% (2/20) reported dizziness, and 10% (2/20) reported mild headaches, with a mean Lake Louise Score of 0.75 ± 0.55. Additionally, 20% of patients (4/20) noted the induction or exacerbation of head noise or tinnitus during hypoxic stimulation, which resolved spontaneously or alleviated rapidly following hyperoxic or room-air inhalation. By the seventh IHHT session, no further discomforts were reported. Throughout the IHHT conditioning period, no new-onset insomnia or worsening of sleep quality was observed, and no participants reported palpitations, dyspnea, or gastrointestinal disturbances (Supplementary Table S1).
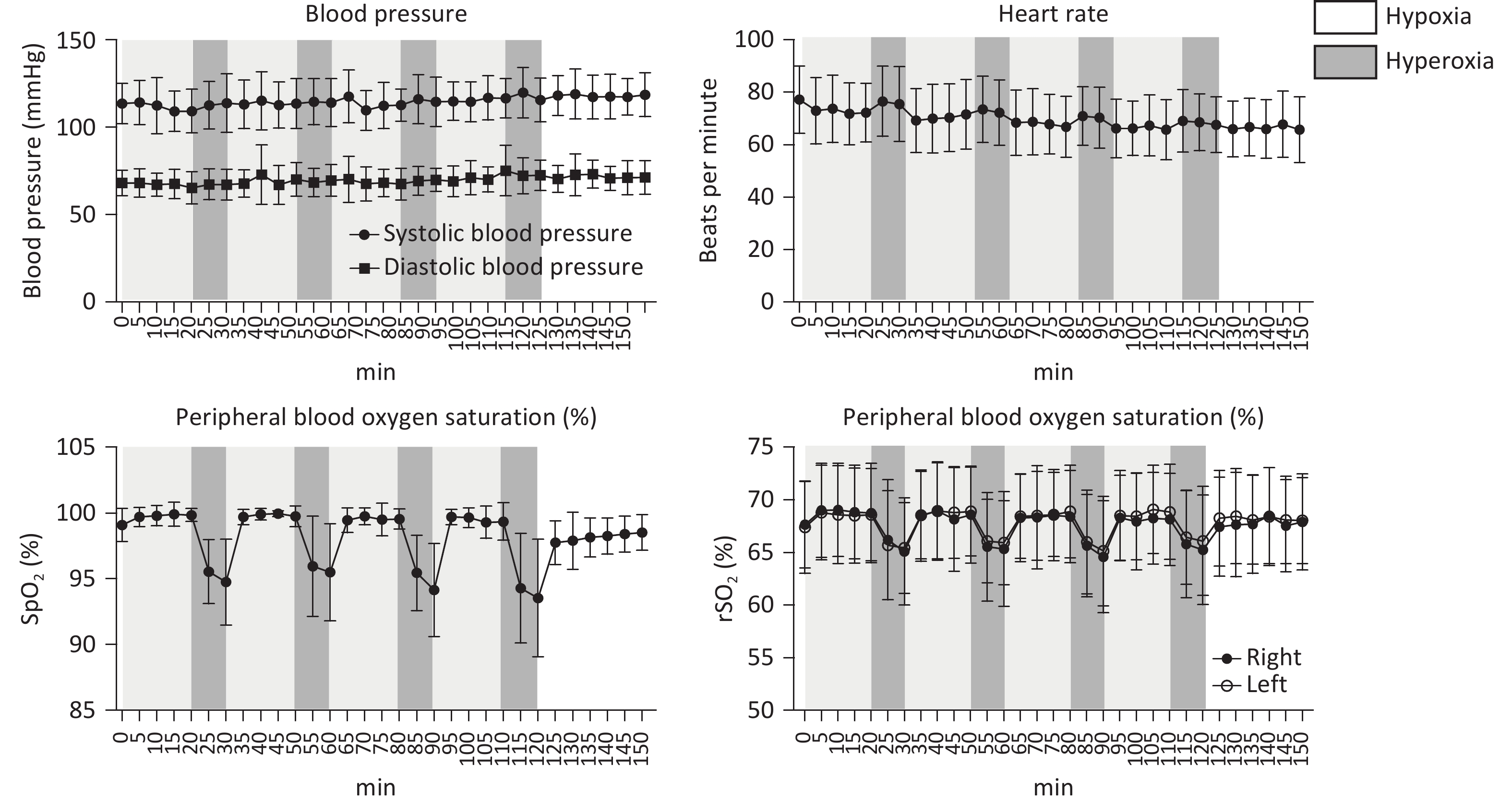
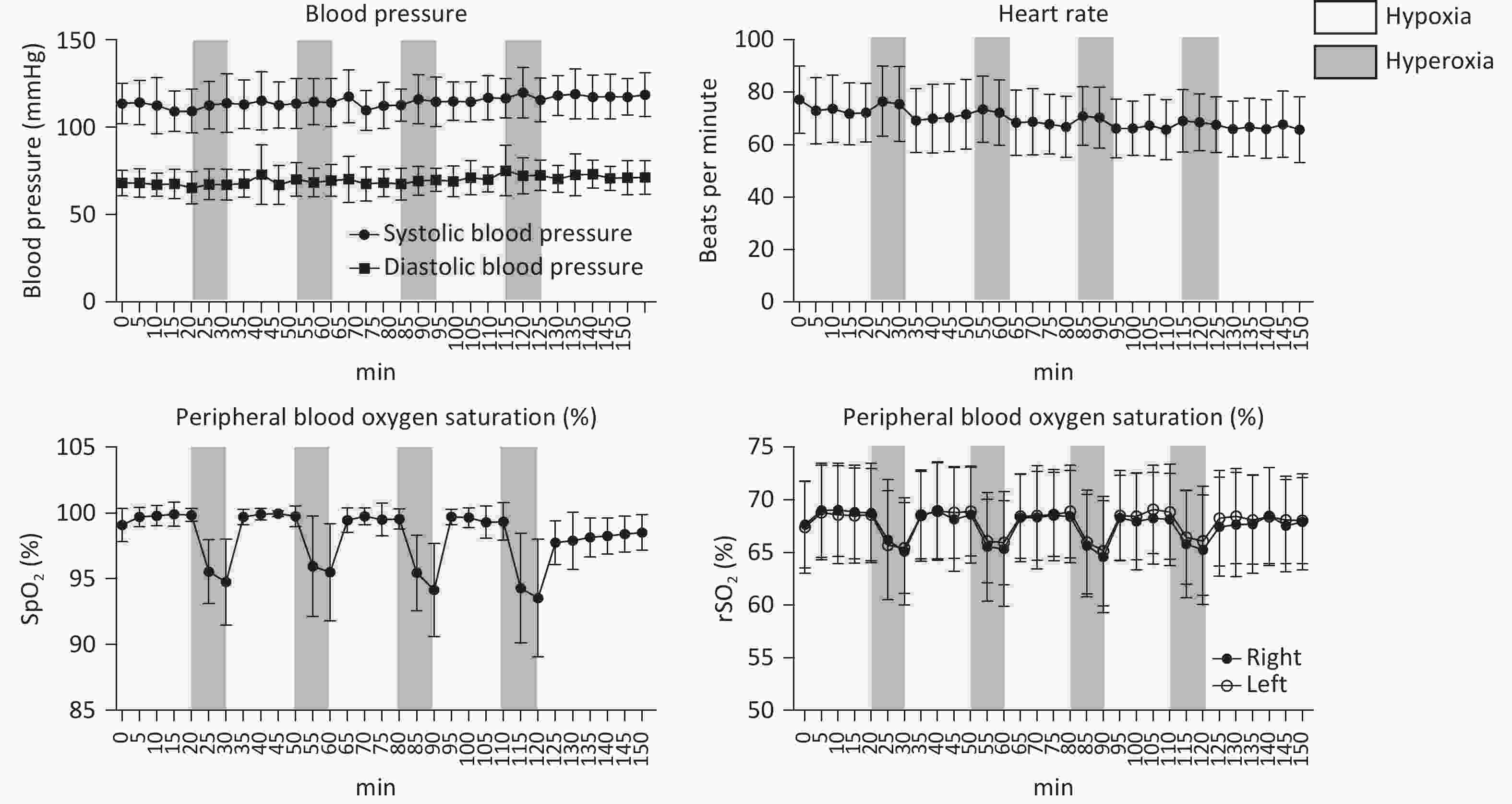
Systolic blood pressure (SBP), diastolic blood pressure (DBP), HR, SpO2, and rScO2 of all patients remained within normal physiological ranges throughout the study. During the IHHT intervention, both SpO2 and rScO2 exhibited dynamic changes in response to variations in inhaled oxygen concentration. Specifically, compared to baseline, both parameters significantly decreased during the 10-minute hypoxic stimulation phase and rapidly recovered within 5 minutes following transition to hyperoxic intervals or during the room-air resting periods after completing the intervention (Figure 1A–D).
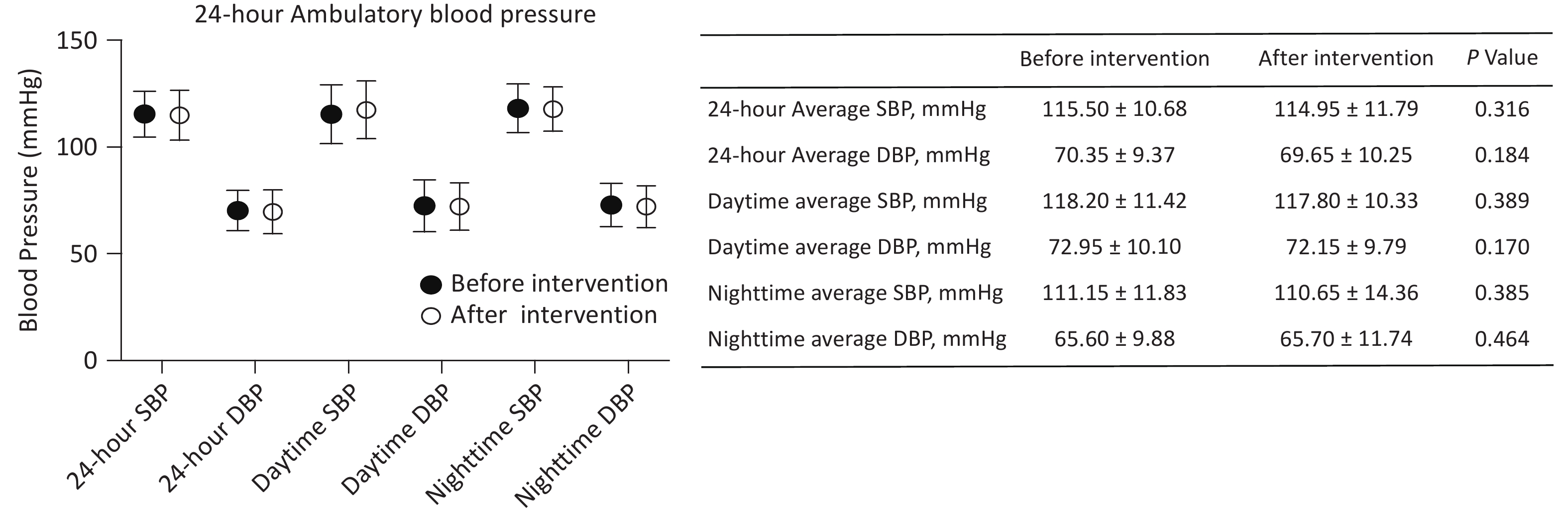
The 24-hour ambulatory blood pressure was monitored to assess the impact of the intervention (Figure 2), revealing no significant changes in 24-hour, daytime, and nighttime average SBP and DBP. The 24-hour average SBP was 115.50 ± 10.68 mmHg pre-intervention and 114.95 ± 11.79 mmHg post-intervention (P = 0.316), while the 24-hour average DBP was 70.35 ± 9.37 mmHg and 69.65 ± 10.25, respectively (P = 0.184). These findings indicate that the IHHT intervention did not significantly alter participants’ overall blood pressure profiles.
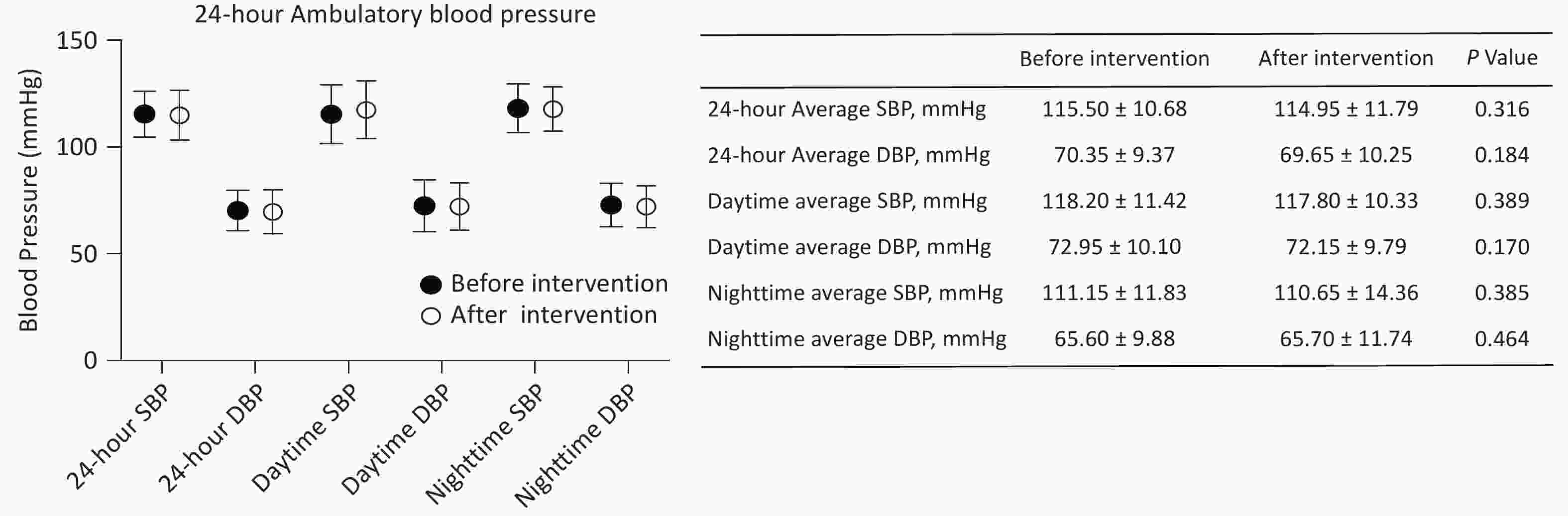
Figure 2. The pre-IHHT and post-IHHT 24-hour ambulatory blood pressure of patients with cerebral venous outflow disorders. DBP, diastolic blood pressure; SBP, systolic blood pressure.
Hematological analyses revealed no significant changes in white blood cell concentration and distribution pre- and post-intervention. Additionally, no statistically significant alterations were observed in hematopoietic function indicators (Supplementary Table S2). Serum biochemical analyses further demonstrated that liver function, kidney function, and nutritional status indices remained stable in all patients following IHHT (all P > 0.05). In terms of the comprehensive metabolic panel, although post-intervention trends suggested reductions in triglyceride, low-density lipoprotein, and total cholesterol levels, along with increased high-density lipoprotein levels, these changes did not reach statistical significance (P > 0.05). Similarly, glucose and albumin levels remained unchanged (Supplementary Table S3).
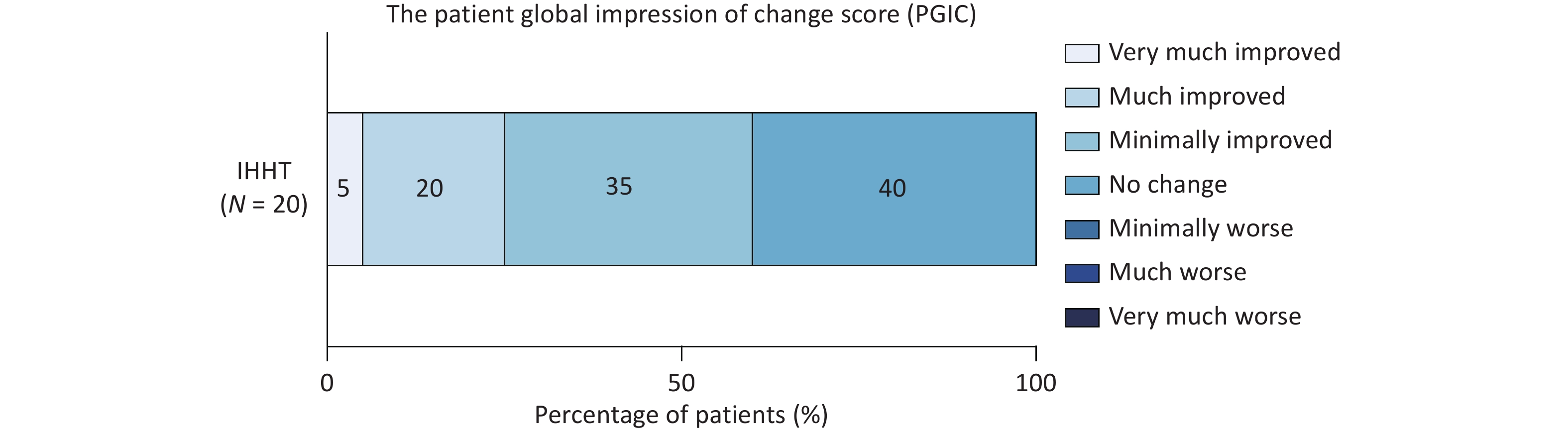
The PGIC scale was applied to assess the therapeutic efficacy of IHHT. Among the 20 patients, twelve (60%) reported symptom improvement: seven noted mild improvement, four reported moderate improvement, and one experienced remarkable improvement. The remaining eight patients reported no change after completing the 14 sessions, and no patients reported symptom deterioration following IHHT (Figure 3). For patients presenting with headache and tinnitus (including tinnitus, tinnitus cerebri, or both), additional assessments were conducted to evaluate the specific effects of IHHT on these symptoms. Compared with baseline, VAS scores indicated a significant reduction in headache severity after the 14 sessions (6.33 ± 1.22 vs. 4.89 ± 2.03, P = 0.016); however, no significant change was observed in the HIT-6 scores (59.11 ± 8.77 vs. 56.89 ± 5.51, P = 0.459). The mean THI score also decreased (pre-IHHT vs. post-IHHT: 62.44 ± 29.66 vs. 53.38 ± 26.62), although this reduction did not reach statistical significance (P = 0.057). Similarly, VAS scores for tinnitus did not differ significantly before and after IHHT (Supplementary Table S4). Patients with IJVS underwent additional ASL scanning and jugular venous ultrasound evaluations. Following 14 IHHT sessions, cCBF significantly increased in the left insula, left internal capsules, bilateral occipital lobes, and bilateral lentiform nucleus (all P < 0.05). Although slight cCBF elevations were observed in other regions, these changes did not reach statistical significance (Table 2). The flow volume at the J3 segment of the left internal jugular vein also increased significantly post-intervention (107.27 [47.50, 160.00] vs. 140.83 [55.00, 210.00] mL/min, P = 0.011). In contrast, the diameters and flow volumes of other jugular segments did not differ significantly from baseline (all P > 0.05; Table 3).
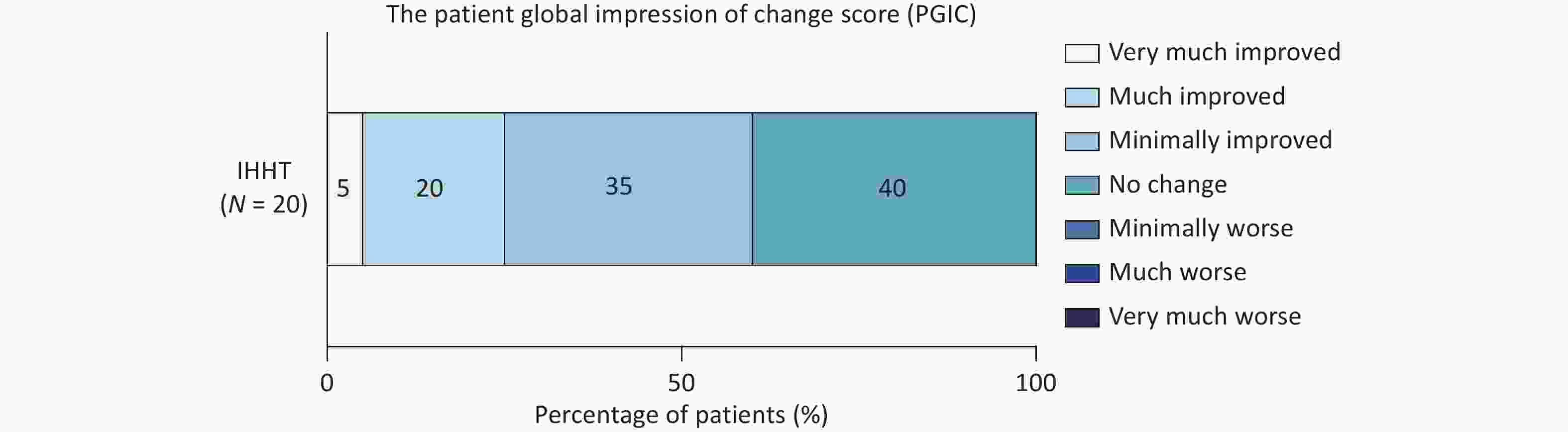
Figure 3. The Patient Global Impression of Change score distribution of patients with CVOD after IHHT. CVOD, cerebral venous outflow disorders; IHHT, intermittent hypoxia–hyperoxia training.
Brain Areas Left Right Pre-IHHT Post-IHHT P-value Pre-IHHT Post-IHHT P-value Whole brain 42.87 ± 7.43 45.61 ± 7.48 0.168 43.87 ± 9.69 46.68 ± 7.09 0.225 Cerebrum 43.55 ± 7.81 46.36 ± 7.81 0.182 44.53 ± 10.14 47.59 ± 7.43 0.203 Cerebellum 36.98 ± 6.10 38.34 ± 6.01 0.335 38.19 ± 7.41 37.73 ± 6.71 0.804 Temporal lobe 42.91 ± 4.84 46.68 ± 8.96 0.056 47.08 ± 9.11 50.19 ± 9.23 0.124 Frontal lobe 42.40 ± 10.68 44.41 ± 7.42 0.504 41.18 ± 12.63 44.15 ± 6.65 0.407 Parietal lobe 44.56 ± 10.50 46.40 ± 8.67 0.553 46.82 ± 11.54 49.33 ± 8.15 0.353 Occipital lobe 43.53 ± 7.31 48.18 ± 8.43 0.014 46.96 ± 8.74 51.22 ± 8.93 0.041 Caudate nucleus 36.83 ± 8.36 41.91 ± 11.41 0.106 39.45 ± 8.41 43.37 ± 8.96 0.157 Internal capsules 36.12 ± 4.67 41.48 ± 6.35 0.005 37.24 (35.23, 37.44) 43.14 (40.35, 47.01) 0.062 Lenticula 42.29 ± 5.45 47.49 ± 8.11 0.022 41.39 ± 5.84 46.05 ± 7.53 0.006 Insula 44.36 ± 8.12 48.36 ± 7.43 0.024 51.38 ± 8.74 54.94 ± 11.20 0.087 M1 42.88 ± 10.24 44.84 ± 8.36 0.514 43.04 (36.24, 48.79) 45.86 (41.78, 54.48) 0.248 M2 47.64 ± 6.94 51.35 ± 9.73 0.089 49.98 ± 8.37 53.41 ± 8.83 0.070 M3 44.01 ± 4.70 48.99 ± 9.53 0.053 47.09 ± 7.07 50.68 ± 8.11 0.063 M4 42.03 ± 11.30 42.99 ± 7.13 0.803 42.39 ± 10.61 44.73 ± 6.08 0.510 M5 42.67 ± 10.92 44.94 ± 8.58 0.470 43.42 ± 9.04 46.54 ± 7.49 0.239 M6 43.86 ± 9.72 46.29 ± 9.87 0.395 47.91 ± 10.31 51.38 ± 8.10 0.128 Note. cCBF, corrected cerebral blood flow; M1, anterior MCA cortex, corresponding to the frontal operculum; M2, MCA cortex lateral to the insular ribbon, corresponding to the anterior temporal lobe; M3, posterior MCA cortex corresponding to the posterior temporal lobe; M4, anterior MCA territory immediately superior to M1; M5, Lateral MCA territory immediately superior to M2; M6, posterior MCA territory immediately superior to M3; IHHT, intermittent hypoxia–hyperoxia training. Table 2. Regional perfusion status (cCBF) before and after the intervention
Timepoints Sides J1 J3 Diameter (mm) FV (mL/min) Diameter (mm) FV (mL/min) Pre-IHHT Left 8.48 ± 1.66 640.83 ± 391.33 4.10 (2.70, 5.20) 107.27(47.50, 160.00) * Right 9.98 (7.50, 12.13) 844.17 ± 362.50 5.09 ± 1.76 132.73 ± 83.20 Bilateral 18.47 ± 2.72 1485.00 ± 319.93 9.19 ± 1.73 220.00 (190.00, 280.00) Post-IHHT Left 8.54 ± 1.50 697.50 ± 505.88 4.49 (3.15, 5.70) 140.83 (55.00, 210.00) * Right 10.32 (8.50, 12.00) 863.33 ± 354.90 5.19 ± 1.78 137.27 ± 58.33 Bilateral 18.86 ± 2.60 1560.00 ± 429.55 9.68 ± 1.73 255 (230, 275) Note. FV, flow volume; IJV, internal jugular vein; J1, the level of IJV influx into the innominate vein; J3, the IJV level representing the bifurcation level of the common carotid artery; * P < 0.05. Table 3. Comparisons of the IJV flow volumes and diameters before and after the intervention
-
We conducted a single-arm pilot study of IHHT in 20 patients with CVOD. The intervention demonstrated favorable safety and feasibility, with no severe adverse events observed. Post-treatment evaluations revealed no significant changes in 24-hour ambulatory blood pressure, liver/kidney function, or complete blood cell counts. During individual IHHT sessions, vital signs remained within normal ranges, and both peripheral and cerebral oxygen saturation levels varied in accordance with hypoxic–hyperoxic cycles. Additionally, improvements were observed in symptom severity, regional cerebral perfusion, and jugular venous outflow patterns, suggesting IHHT as a potential therapeutic option for CVOD.
Previous studies have demonstrated the safety and tolerability of IHHT in various disease entities[31]. Our study further demonstrated the feasibility in patients with CVOD, as evidenced by a relatively high recruitment rate (24/30, 80%), low withdrawal rate (4/24, 16.7%), and strong adherence rate (20/24, 83.3%). Although hypoxia exposures can theoretically induce hypoxemia and relative symptoms resembling acute mountain sickness[32,33], no significant complications or severe adverse events were observed in our cohort throughout the intervention. The most frequently reported side effect was fatigue, occurring in 50% of participants (10/20). Interestingly, some patients subjectively associated this fatigue with improved sleep quality; however, in the current study, the objective assessment of sleep quality by the Pittsburgh Sleep Quality Index, which requires a one-month assessment period, was not administered due to limited follow-up duration. Other adverse events, including dizziness and headache, were also detected during the intervention, which were mild, self-limiting, and well-tolerated, consistent with findings from prior IHHT studies[27,34,35]. Notably, during initial IHHT sessions, patients with pre-existing tinnitus or head noise reported symptom induction or exacerbation during hypoxic exposures, suggesting a latent relationship between hypoxia and the pathogenesis of these symptoms. Moreover, these effects were not reported in later sessions, indicating the development of adaptive responses and improved hypoxic tolerance over time.
The literature on IH’s effects on blood pressure, blood cell counts, and systemic metabolism remains inconsistent. Prolonged, highly frequent, and severe IH protocols have been shown to elevate blood pressure, mimicking the pathophysiological effects of sleep-disordered breathing[36]. In contrast, shorter-duration and mild-to-moderate protocols have been associated with beneficial outcomes, including a discernible reduction in both SBP and DBP[26,37]. In our study, BP, HR, and oxygen saturation—sensitive indicators of physiological tolerance to hypoxia—remained within normal ranges during IHHT sessions. No significant BP elevation was observed within 30 minutes post-intervention, and 24-hour ambulatory BP parameters showed no significant changes after the 14 sessions, suggesting that patients with CVOD tolerated the oxygen saturation oscillations without hypertensive responses. Similarly, hepatic and renal function remained stable post-intervention. Regarding lipid metabolism, prior studies have reported both beneficial and detrimental effects of IH. While some preclinical and clinical studies have reported transient triglyceride increases following IH exposure[38-40] and a higher prevalence of dyslipidemia in patients with obstructive sleep apnea[41], other studies suggest that IH training may improve lipid profiles and offer protective benefits against metabolic syndrome and vascular diseases[35,42]. In our study, the 14-session mild-to-moderate IHHT protocol was associated with a declining trend in triglycerides and low-density lipoprotein cholesterol (commonly considered “bad” cholesterol), alongside an increase in high-density lipoprotein cholesterol (commonly regarded as “good” cholesterol). These divergent outcomes across studies may stem from variations in hypoxia exposure patterns, including intensity, duration, and frequency. Current evidence suggests that brief (5–10 minutes), moderate (FiO2: 9–16%), low-frequency (3–15 cycles/day) IH stimulation, interspersed with normoxia or hyperoxia, can promote cognitive and physical function, enhance quality of life, and improve metabolic condition. Overall, our findings demonstrated the physiological stability in patients with CVOD undergoing IHHT, supporting its potential as a safe and well-tolerated therapeutic intervention for cerebral vascular disease.
In addition to demonstrating safety and feasibility, the IHHT intervention exhibited potential therapeutic benefits in symptom relief and hemodynamic improvement. Following 14 IHHT sessions, 60% of patients (12/20) reported symptom amelioration based on subjective assessments using the PGIC score. Furthermore, THI and VAS scores indicated a slight attenuation of tinnitus and head noise, and HIT-6 and VAS scores revealed a decrease in chronic headache severity. We hypothesize that IHHT efficacy may be attributed to multiple mechanisms. IHHT combines intermittent hypoxic exposure (IHE) with hyperoxic intervals, leading to synchronized fluctuations in peripheral and cerebral oxygen saturation (ScO2) in response to changes in inhaled oxygen concentration (Figure 1). Previous research has linked reduced prefrontal cerebral oxygenation to increased susceptibility to altitude-related headaches[43]. The elevated ScO2 during hyperoxic phases may help alleviate cerebral ischemic–hypoxic conditions, thereby relieving hypoxia-related non-focal neurological symptoms. The hyperoxia intervals of IHHT could also act similarly to normobaric oxygen (NBO) therapy, which has shown protective effects in the CVOD population[21].
Conversely, brief cyclic hypoxemic exposures may enhance resistance to hypoxic injury and promote acclimatization[44]. IHE can stimulate the transcription of angiogenic molecules, such as vascular endothelial growth factor (VEGF)[23,45], and regulate CBF through nitric oxide-dependent vasodilation during hypoxic challenges[46]. Collectively, these mechanisms may improve cerebral perfusion and compensate for oxygen deficiency, thereby stabilizing cerebral oxygen supply[47-49]. In our study, absolute quantification of CBF using ASL revealed increased regional cerebral perfusion following IHHT intervention among patients with IJVS, particularly in deep brain structures such as the lentiform nucleus and insula. Ultrasound assessments also demonstrated discernible increases in jugular vein outflow volumes and diameters, further supporting the IHHT’s positive impact on venous outflow patterns. These hemodynamic improvements may underlie the alleviation of symptoms associated with abnormal collateral vessel networks and outflow turbulence, including tinnitus and head noise. Improved regional cerebral perfusion may also account for the broader symptomatic benefits of IHHT observed. For instance, a six-week IHHT protocol adjuvant to cycling exercise was found to be more effective than aerobic exercise alone in improving global cognitive function, physical performance, and functional mobility in geriatric patients[19]. Another potential mechanism underlying these therapeutic effects is hypoxia acclimatization, mediated by IHE-induced regulation of erythropoiesis and blood oxygen transport capacity[50,51]. However, our study detected no significant hematological changes. This discrepancy from previous findings may be due to differences in assessment timing, hypoxic protocols, or individual variability[52,53]. In our study, complete blood count measurements were obtained within a short interval (≤ 3 weeks) before and after the intervention, which may have been insufficient to detect changes in routine hematological parameters. In conclusion, our findings indicate that IHHT may alleviate hypoxia-related symptoms by improving cerebral oxygen supply and mitigate structural abnormality-related symptoms by enhancing cerebral circulation and venous outflow patterns. Future research is warranted to optimize IHHT protocols in terms of intensity, volume, and frequency, as well as to further elucidate the mechanisms underlying its therapeutic effects in patients with CVOD.
This study acknowledges certain limitations. First, as a single-arm preliminary study, the observed effects of IHHT on symptoms, cerebral perfusion, and jugular venous hemodynamics should be interpreted cautiously. Future randomized controlled trials with larger sample sizes are needed to enhance statistical power and improve the generalizability of the findings. Second, the study did not assess the long-term effects of IHHT on symptoms or hemodynamic parameters, nor did it explore other potential adaptive responses to the intervention. Third, physiological parameters were recorded only during the first IHHT session, leaving it unclear whether they changed after repeated exposure over 14 sessions. Finally, although blinded to patients’ diagnoses and clinical characteristics, the medical sonographer was aware of whether the examination was conducted pre- or post-intervention due to repeated measurements. Addressing these limitations in future studies will be essential for validating and refining IHHT as a therapeutic approach in CVOD.
-
In this exploratory pilot study, patients with CVOD demonstrated good tolerance to the 14-session IHHT intervention, which consists of four daily cycles of 10-minute moderate hypoxic stimulation alternated with 20-minute hyperoxic episodes. The intervention was associated with enhanced cerebral perfusion, improved jugular venous outflow patterns, and reduced symptom severity. These findings suggest that IHHT may represent a novel, non-pharmacological therapeutic approach for CVOD; however, further research is needed to validate its efficacy and elucidate its underlying mechanisms.
Intermittent Hypoxia–hyperoxia Training Ameliorates Symptoms and Improves Cerebral Perfusion Status in Patients with Cerebral Venous Outflow Disorders: A Pilot Study
doi: 10.3967/bes2025.118
-
Key words:
- Cerebral vascular disease /
- Intermittent hypoxia-hyperoxia training /
- Cerebral venous outflow disorders /
- Cerebral perfusion /
- Acclimatization
Abstract:
This study was approved by the Institutional Ethics Committee of Xuanwu Hospital of Capital Medical University (Approval No. [2023]142). All procedures involving human participants were performed in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
| Citation: | Milan Jia, Chenxia Zhou, Hui Li, Jing Lan, Wenbo Zhao, Lingyun Jia, Sijie Li, Changhong Ren, Chen Zhou, Lu Liu, Xunming Ji. Intermittent Hypoxia–hyperoxia Training Ameliorates Symptoms and Improves Cerebral Perfusion Status in Patients with Cerebral Venous Outflow Disorders: A Pilot Study[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.118 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: