-
Hyperthyroidism refers to a clinical state that results from inappropriately high thyroid hormone levels in the tissues[1]. Ⅰ-131 therapy plays a critical role and provides a remarkable curative effect in targeting thyroid diseases. Thyroid cells can take up isotope Ⅰ-131, which emits not only beta rays but also gamma rays. Beta rays are used for killing thyroid cancer cells and for destroying a number of glandular tissues, which is a key in achieving treatment goals. However, gamma rays with strong ionizing radiation effects can cause radioactive contamination, which causes radiation hazard to other individuals who come into contact with patients. Radiation can induce carcinogenesis, such as leukemia and skin cancer. Therefore, more and more attention is being paid to radiation safety and protection against the radiation hazard from Ⅰ-131[2, 3].
The external dose rate or retained body activity of Ⅰ-131 in patients with hyperthyroidism have been well characterized[2]. However, the functional relations of the external dose rate and retained body activity has not been established[4-6]. Therefore, an appropriate relationship between external dose rate and retained body activity must be established for patients with hyperthyroidism, and a quantitative analysis on the effects of radiation on other individuals should be carried out.
This study was approved by our institutional review board. Data used in this study were collected from 72 patients with hyperthyroidism (14 women and 58 men) receiving Ⅰ-131 therapy at the nuclear medicine department of a hospital, and a written informed consent was obtained after providing an explanation to the patients. The patients' age ranged from 20 to 53 (mean: 44 ± 10), and they received a dose of 340-740 MBq. To reduce errors, all selected patients were first treated with Ⅰ-131 and were prescribed with a low-iodine diet for 5-7 weeks before therapy.
The external dose rates were measured at 0.5, 4, 8, 24, 48, 72, 96, 120, 144, 168, 192, and 288 h after the administration of Ⅰ-131 as well as at 0.6, 1, and 2 m in front of the patients' thyroid gland at a height of 1 m above the floor. All measurements were conducted in a room where the natural background was measured before the external dose rate was obtained. Then, the net external dose rate was calculated by removing the natural background. To reduce errors, three values were recorded for each measurement, and these values were averaged. In this experiment, a laser distance meter (Disto D2, Leica Geosystems AG, St. Gallen, Switzerland) was used to measure the distance from the patients, and a portable radiation survey meter with an NaI (Tl) scintillator detector (RadEye PRD, Thermo Fisher Scientific Inc, Waltham, MA, the USA) was utilized to detect the external dose rate.
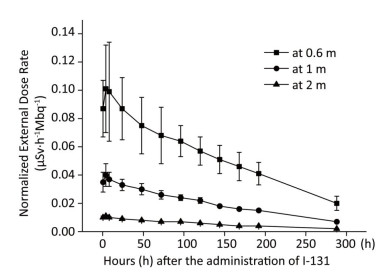
The external dose rate was normalized to administered activity to facilitate comparison. The initial external dose rate obtained at 0.5 h was considered 100% of the administered activity. Figure 1 shows the normalized external dose rate at various times and distances. Even though the external dose rates measured at different distances have a similar trend over time, a highly significant difference was observed in the degree of variability. At 0.6 m, the external dose rate was relatively high, and it significantly fluctuated. Moreover, the external dose rate at 2 m was relatively small, and it was easily affected by the natural background dose. The experimental outcomes showed a good correlation between the external dose rate at 1 m and the elapsed time after the administration of Ⅰ-131. Therefore, the results at 1 m were proposed to describe the relationship between the external dose rate and elapsed time after the administration of Ⅰ-131. The external dose rates at 0.5-288 h at 1 m were 0.035 ± 0.007, 0.040 ± 0.009, 0.037 ± 0.005, 0.033 ± 0.004, 0.300 ± 0.004, 0.026 ± 0.003, 0.024 ± 0.002, 0.022 ± 0.002, 0.018 ± 0.001, 0.016 ± 0.001, 0.015 ± 0.001, and 0.007 ± 0.001 μSv·h-1Mbq-1, respectively (with a mean half-life of 6.53 days). The function was described using Equation (1).
$$ \mathit{{N}_{1}}=-0.009+0.047{{e}^{\frac{-t}{273.4}}} $$ (1) 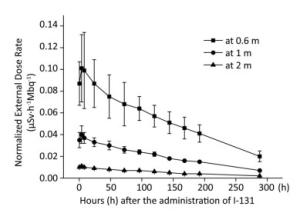
Figure 1. External dose rates at 0.6, 1, and 2 m plotted as a function of time after the administration of Ⅰ-131.
where N1 is the normalized external dose rate at 1 m and t is the time after the administration of Ⅰ-131.
The activity distribution in patients with hyperthyroidism is often assumed as a point source, and the dose at a given distance is calculated using the inverse square law[6]. However, in this study, a different law was revealed for patients receiving Ⅰ-131 therapy. Their relationship was identified using Equation (2).
$$ {{Y}_{r}}={{r}^{-1.71}}{{Y}_{1}} $$ (2) where Yr and Y1 are the external dose rates at r and 1 m in front of the patients' thyroid gland, respectively.
In fact, patients with hyperthyroidism who are treated with Ⅰ-131 are not an ideal point source. Firstly, due to the difference in organ conversation factors for Ⅰ-131, the absorbed dose in human organs is diverse, and then, it is distributed in the body non-homogeneously. According to the International Commission on Radiological Protection (ICRP)[7], the thyroid is subjected to the maximum dose of Ⅰ-131. Meanwhile, other organs also possess a small quantity of radioactive Ⅰ-131. Secondly, the volume of the thyroid cannot be ignored. Theoretically, thyroid can be approximated as a point when it is far enough away from the thyroid, but the distance (0.6, 1, and 2 m) in this experiment was relatively short. Consequently, the assumption that patients treated with Ⅰ-131 are an unshielded point source is biased. JA Siegel, et al. have indicated that the patient can be modeled as a line source, and this consideration describes the situation more accurately[6]. In future research, line source model or better models should be explored in depth.
A significant portion of the administered activity is not taken up by the thyroid gland and is rapidly excreted in the urine[8]. In relation this reason, body activity can be estimated using the excreted activity in the patient's urine. Urine collections were scheduled at 4, 8, 24, 48, 72, 96, 120, 144, 168, 192, and 288 h after the administration of Ⅰ-131. The volume of urine between each collection was recorded, and the activity in 1 mL of urine was measured using a high-purity germanium detector (GX5019, CANBERRA, the USA). To proportionally measure radioactivity, urine was stirred before each measurement.
$$ Q={{Q}_{0}}\cdot \frac{{\scriptstyle{}^{{{N}_{0}}}\!\!\diagup\!\!{}_{{{t}_{0}}}\;}-{\scriptstyle{}^{{{N}_{\text{b}}}}\!\!\diagup\!\!{}_{{{t}_{\text{b}}}}\;}}{{\scriptstyle{}^{N}\!\!\diagup\!\!{}_{t}\;}-{\scriptstyle{}^{{{N}_{\text{b}}}}\!\!\diagup\!\!{}_{{{t}_{\text{b}}}}\;}}\cdot \frac{1}{MD} $$ (3) where Q and Q0 are the activity of Ⅰ-131 in urine samples and standard samples (Bq/mL), respectively; N, N0, and Nb are the gamma ray spectrum counts of the urine sample, standard sample, and background of empty box, respectively; t, t0, and tb are the measurement time of urine sample, standard sample, and background of empty box (s), respectively; M is the urine sample volume (mL); and D is the decay correction coefficient, which was taken as 1 in this analysis.
Then, the activity in the urine was calculated based on the measured activity Q and urine volume. The retained body activity was estimated using the following Formula:
$$ {{A}_{i}}=\frac{{{A}_{j}}{{e}^{-0.693t}}}{{{T}_{p}}}-{{A}_{ij}} $$ (4) where i and j are the hours after the administration of Ⅰ-131; Ai and Aj are the retained body activities at i h and j h (i > j), respectively; Aij and t are the activity in the urine and time span between i h and j h, respectively; and Tp is the physical half-life of Ⅰ-131[7]. The retained body activity was also immediately measured using the whole body counter (ACCUSCAN2260, CANBERRA, the USA) after the external dose rate was obtained.
The metabolic model of Ⅰ-131 in the patients was obtained and described using Equations (5) and (6).
$$ {{P}_{1}}=0.069+0.867\ {{e}^{\frac{-t}{208.6}}} $$ (5) $$ {{P}_{2}}=0.078+0.840\ {{e}^{\frac{-t}{197.9}}} $$ (6) where P1 and P2 are the percentages of retained activity in the patients estimated via urine analysis and using the whole-body counter, respectively, and t is the hours after the administration of Ⅰ-131. The retained body activities in the patients decreased exponentially with an average half-life of 6.07 days via urine analysis and 5.68 days using the whole body counter.
As shown in Table 1, the retained body activity obtained using the whole body counter was smaller than that obtained via urine analysis. The difference was statistically significant (P < 0.001). This phenomenon may be attributed to several reasons. In addition to the urinary system, there are many other excretion routes, such as exhalation, perspiration, and saliva[5]. Furthermore, during urine collection, activity in the urine is constantly attenuating.
Table 1. Results Obtained Using the Two Methods of Detection (x ± s)*
Group Retained Body Activity (MBq) Male participants Female participants Urine 380.59 ± 172.32 358.44 ± 168.53 Whole body counter 375.16 ± 173.72 350.54 ± 171.12 Difference in value 5.44 ± 15.29 7.90 ± 9.81 t 4.61 21.25 P < 0.001 < 0.001 95% CI 3.11-7.76 7.18-8.64 Note.*: Paired t test. Based on the fact that the different levels of retained body activities can lead to different external dose rates at a given distance, the relationship between the external dose rate and retained body activity was explored in this study. A significant positive correlation between these two variables was observed, as shown in Figure 2. It showed a linear relationship at 1 m with a correlation coefficient R2 of 0.978 for urine analysis and 0.979 for using the whole-body counter. Therefore, the retained body activities in the patients can be estimated by directly measuring the external dose rate. Compared with the two conventional methods (urine analysis and whole body counter) utilized for calculating the retained body activity, the measurement of the external dose rate at 1 m cannot only reduce radiation dose for medical staff but also decrease costing.
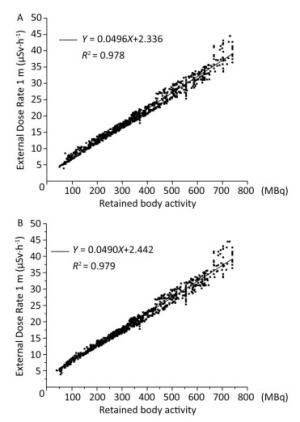
Figure 2. Retained body activity corresponding to external dose rate. (A) Monitored via urine analysis. (B) Monitored using the whole-body counter.
Patients with hyperthyroidism receiving Ⅰ-131 therapy will continually release radiation when they have activities in their bodies. Hence, medical staff who treat or care for these patients and individuals close to them may be at risk of radiation. There are two modes of exposure: external exposure and internal intake (due to contamination and environmental pathways)[2, 9]. The external exposure was detected using the thermoluminescence dosimeter that assesses the extent of radiological hazards acquired by 20 medical staff. Results showed that the absorbed annual effective radiation dose of the medical staff was lower than 1 mSv, which meets the ICRP Publication 60 with respect to annual radiation exposure limits (5 mSv).
When medical staff inject radiopharmaceuticals to patients, the drug volatilizes and is inhaled by the medical personnel. Moreover, when medical staff take care of patients, they are directly in contact with the patient's items (sheets, clothes, excreta, etc.) and may take radioactive Ⅰ-131. In the past, external irradiation dose alone was primarily monitored. However, whether the medical staff may be subjected to internal irradiation is worth assessing. To confirm the abovementioned conjecture, the whole body radioactivity of 10 medical staff who got into contact with patients with hyperthyroidism receiving Ⅰ-131 therapy were measured using the whole body counter. Result showed that none of the medical staff exceeded the limit.
This outcome may be attributed to several reasons: First, the hospital has proper radiation protection equipment (masks, lead aprons, etc.), which reduce Ⅰ-131 absorption among medical staff; Second, volatile in the air is low. Previous investigations have indicated that the internal dose due to contamination was usually less than 10% of the external dose[10]. Moreover, the physical half-life of Ⅰ-131 is relatively short, which indicates that the iodine absorbed into the body can be easily attenuated. However, evidence did not suggest that it would not cause health problems. Thus, exposure to unnecessary radiation must be reduced as much as possible.
Based on the abovementioned measurements, evaluating the retained body activity of patients by directly measuring the external dose rate is feasible, and this provides experiment-based clinical practice guidelines for reducing radiation exposure among the patients' families, nurses, and physicians.
doi: 10.3967/bes2018.124
Study of the External Dose Rate and Retained Body Activity of Patients with Hyperthyroidism Who Are Receiving Ⅰ-131 Therapy
-
-
Table 1. Results Obtained Using the Two Methods of Detection (x ± s)*
Group Retained Body Activity (MBq) Male participants Female participants Urine 380.59 ± 172.32 358.44 ± 168.53 Whole body counter 375.16 ± 173.72 350.54 ± 171.12 Difference in value 5.44 ± 15.29 7.90 ± 9.81 t 4.61 21.25 P < 0.001 < 0.001 95% CI 3.11-7.76 7.18-8.64 Note.*: Paired t test. -
[1] DS Ross, HB Burch, DS Cooper, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and other causes of Thyrotoxicosis. Thyroid, 2016; 26, 1343-421. doi: 10.1089/thy.2016.0229 [2] SY Cui, L Jiao, J Tan, et al. Estimating radiation absorbed dose of individuals nearby 131I-treated hyperthyroid patients. Health Phys, 2014; 106, 365-9. doi: 10.1097/HP.0b013e3182a1c8d5 [3] Demir M, Kabasakal L, Onsel C. Evaluation of external radiation exposure rate from radioiodine-treated hyperthyroid patients and radiation safety considerations. Nucl Med Commun, 1996; 17, 692-5. doi: 10.1097/00006231-199608000-00008 [4] Sartor O, Hoskin P, Bruland OS. Targeted radio-nuclide therapy of skeletal metastases. Cancer Treat. Rev, 2013; 39, 18-26. doi: 10.1016/j.ctrv.2012.03.006 [5] MJ O'Doherty, AG Kettele, CNEustance, et al. Radiation dose rates from adult patients receiving 131I therapy for thyrotoxicosis. Nucl Med Commun, 1993; 14, 160-8. doi: 10.1097/00006231-199303000-00003 [6] JA Siegel, CS Marcus, RB Sparks. Calculating the absorbed dose from radioactive patients:the line-source versus point-source model. J Nucl Med, 2002; 43, 1241-4. http://europepmc.org/abstract/MED/12215565 [7] International Commissioin on Radiological Protection. ICRP Publication 53. Radiation Dose to Patients from Radiopharmaceuticals. Pergamom Press: Oxford, UK, 1988. [8] SR Thomas, HR Maxon, KM Fritz, et al. A comparison of methods for assessing patient body burden following 131I therapy for thyroid cancer. Radiology, 1980; 137, 839-42. doi: 10.1148/radiology.137.3.7444070 [9] JA Siegel, CS Marcus, MG Stabin. Licensee over-reliance on conservatisms in NRC guidance regarding the release of patients treated with 131I. Health Phys, 2007; 93, 667-77. doi: 10.1097/01.HP.0000270300.34270.44 [10] AP Jacobson, PA Plato, D Toeroek. Contamination of the home environment by patients treated with iodine-131:initial results. Am J Public Health, 1978; 68, 225-30. doi: 10.2105/AJPH.68.3.225 -




 下载:
下载:



 Quick Links
Quick Links