-
Psoriasis is a chronic immune-mediated papulosquamous skin disease, affecting over 125 million patients worldwide[1-3]. Its most common and frequent form, psoriasis vulgaris (PV), constitutes over 90% of cases and arises from a combination of complex genetic susceptibilities and environmental triggers[4]. Psoriatic arthritis (PsA) is a complex inflammatory condition of the peripheral and axial skeleton, which complicates skin psoriasis in up to 30% of patients[5,6], and the global prevalence has been continuously increasing over the past few decades worldwide[7-11], thus posing an increasingly unsustainable global health burden for individuals and society.
Preventing the onset and managing the adverse health outcomes associated with psoriasis requires an understanding of its modifiable risk factors[12]. The higher prevalence of autoimmune diseases in females than in males suggests that reproductive or female factors or gonadal hormones may play a critical role in these conditions[13]. While pregnancy and menopause have been shown to modulate the natural course of psoriasis in women, suggesting a female hormone-induced regulation of skin inflammation[14-16], the prospective studies investigating the association between modifiable reproductive factors and the incidence of psoriasis are limited and have yielded controversial findings. Evidence from the Nurses’ Health Study cohorts, did not support an association between psoriasis risk and parous, age at first or last birth[17]. In another study, compared with control women without psoriasis, those with psoriasis had a lower age at first delivery[18]. These complex outcomes are not surprising as conventional epidemiological studies depend on environmental information and results are likely to be affected by errors in measurement, unexpected confounding variables, and issues with reverse causality.
To the best of our knowledge, the association and mechanisms involved in the relationship between reproductive factors and psoriasis have not been fully elucidated, and there are no studies of causal intermediates or mediators. Psoriasis is intimately associated with potential confounding factors such as adiposity[19], educational attainment, and tobacco and alcohol consumption. The extent to which these established risk factors explains the total effect of reproductive factors on psoriasis has not been investigated. Large-scale genome-wide association studies (GWAS) have highlighted the genetic regulations in reproductive factors, including age at menarche (AMA), age at menopause (AMP), age at first birth (AFB), age at last live birth (ALB), number of live births (NEB), age at first sexual intercourse (AFS), and the lifetime number of sexual partners (NSP). These have leveraged data from millions of women of European ancestry, providing a valuable opportunity to employ Mendelian randomization (MR) to test causal inference by using the genetic variants (single nucleotide polymorphisms, SNPs) as instrumental variables (IVs); this approach is less susceptible to the effects of confounders and reverse causation compared to conventional observational study methodologies[20]. Therefore, in this study, we aimed to conduct the first large two-sample and two-step MR analysis of major reproductive factors on the risk of psoriasis (including overall psoriasis, PV, and PsA). We investigated whether population-level changes to potential mediators, such as BMI, are important for reducing the effect of psoriasis risk, and quantified their mediated proportion of the total causal effects, which helped us understand the role of hormonal reproductive factors (puberty, fertility, and motherhood) on psoriasis.
-
The study was conducted per the Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization (STROBE-MR) Statement (shown in the supplementary materials). The summary-level data used in this study are publicly available and validated by the IEU openGWAS database (https://gwas.mrcieu.ac.uk/), and thus no additional ethical approval was required.
Three core assumptions were strictly adhered to in this study[21]: 1) Relevance assumption: The selected SNPs are strongly associated with the reproductive traits (exposure); 2) Independence Assumption: The SNPs influence psoriasis risk (outcome) only through the reproductive traits; 3) Exclusion restriction assumption: The SNPs are not associated with any confounders that might affect the exposure-outcome relationship. A flowchart of the analysis process and the verification of the three core assumptions conducted in this study is shown in Figure 1.

Figure 1. Analysis process and verification of key MR assumptions flowchart. We utilize different colors to represent the relationship between the analysis methods and the three core assumptions of MR.
Seven different factors were identified, including age at menarche (AMA 243,944 individuals), age at menopause (AMP 143,819 individuals), age at first birth (AFB 542,901 individuals), age at last live birth (ALB 170,248 individuals), number of live births (NEB 250,782 individuals), age at first sexual intercourse (AFS 397,338 individuals), and lifetime number of sexual partners (NSP 378,882 individuals). A meta-analysis of GWAS results from 36 cohorts was conducted for AFS and AFB in individuals of European ancestry[22]. In UK Biobank, the summarized reproductive factor data (AMA, AMP, ALB, NEB, and NSP) were derived from questionnaire responses at the baseline assessment; further details can be obtained from the UK Biobank database (http://www.ukbiobank.ac.uk/)[23].
Psoriasis data (PSO 216,752 individuals including 4510 cases and 212,242 controls), psoriatic arthritis (PsA 213,879 individuals including 1,637 cases and 212,242 controls), and psoriasis vulgaris (PV 215,044 individuals including 2802 cases and 212,242 controls) were obtained from the FinnGen Biobank Analysis Consortium database (Release 5 version, available at: https://finngen.gitbook.io/documentation/)[24] with the diagnosis made according to the ICD-10 (International Classification of Diseases) criteria.
Considering the complex clinical and genetic backgrounds of the psoriasis cases, four potential confounders that were previously shown to be associated with the exposure and outcome were merged to construct an multivariable MR (MVMR) and a two-step mediation MR model to adjust the mediation effect; these included the body mass index (BMI 99,998 individuals), years of education (EDU 766,345 individuals), tobacco consumption (SMO 249,752 individuals), and alcohol consumption level (ACL 462,346 individuals). EDU[25] and ACL[26] were obtained from two GWAS studies, while BMI and SMO were from within the family GWAS consortium and the MRC-IEU consortium, respectively (available at https://gwas.mrcieu.ac.uk/).
To prevent the influence of sample overlap, we obtained all mediators from databases distinct from those used to source the outcome. Strongly associated SNPs were filtered out using a stringent genome-wide significance threshold of P < 5 × 10-8 to ensure the relevance assumption. They were then clumped to remove linkage disequilibrium (LD) with a window size of 10,000 kb and an r2 threshold of 0.001. No proxy SNPs were used in our study to avoid potential bias due to differences in LD structure between proxy and origin populations. Additionally, we matched the Phenome-Wide Association Studies (PheWAS) database to avoid any underlying links between the SNPs and confounding factors under a threshold of P < 5 × 10-6, together with the MVMR and mediation MR methods, to ensure the independence assumption. All included participants were of European ancestry to minimize the influence of a mixed population effect. Details of all the datasets were presented in Supplementary Table S1.
-
This study was conducted using R software (v4.1.3) with the R packages two-Sample MR (v0.5.6)[27], MRPRESSO (version 1.0), MVMR (v0.3.0)[28], and MendelianRandomization (v0.5.1). The following six methods were used in the UVMR: The inverse-variance weighted (IVW) method[29,30], aggregates causality estimates from individual IVs, assuming potential invalidity of some genetic instruments. MR-Egger regression analysis[31,32] evaluates horizontal pleiotropy across IVs and provides an adjusted, robust causal estimate that is independent of IV validity. The MR pleiotropy residual sum and outlier (MR-PRESSO)[33] method detects and corrects for outliers that contribute to significant pleiotropy and heterogeneity, thus refining the causal effect estimate. The weighted-median method[34] ensures consistent, valid inferences even when a majority of the IVs may be invalid. Bayesian weighted Mendelian randomization (BWMR)[35] obtains reliable causal inferences by correcting for pleiotropy violations and polygenic weak effect uncertainties within a Bayesian weighting framework. MR-Robust Adjusted Profile Score (MRAPS)[36] increases statistical power and offers robust estimates when weak instrumental bias and horizontal pleiotropy are significant.
By default, IVW results are preferred[37], but we turn to MR-Egger analysis when significant pleiotropy is detected by the MR-Egger pleiotropy test. If the MR-PRESSO global test identifies significant outliers, we prioritize results corrected by MR-PRESSO. Since six methods were employed, a Bonferroni correction was applied to minimize the risk of Type I errors due to multiple testing, setting the threshold for significance in the UVMR at a corrected P-value of < 0.0083 (0.05/6).
To supplement UVMR and jointly detect the causal effects of multiple risk factors, MVMR[38,39] and two-step mediation MR analysis[40] were used. First, all four mediators were adjusted together to obtain a merged corrected MVMR model. In the second step, these mediators were individually adjusted to obtain separate corrected MVMR models. The total effect (beta0) represents the causal effect of the exposure on the outcome and was generated using the IVW method in the UVMR. The step 1 effect (beta1) represents the causal effect of the exposure on the mediator, measured using the IVW method. Step 2 effect (beta2) represents the causal effect of the mediator on the outcome, derived from the separate corrected MVMR models. The exposure-to-outcome association was considered fully mediated by a given mediator if beta0 was non-significant while both beta1 and beta2 were significant. If beta0, beta1, and beta2 were all significant, the exposure-to-outcome association was considered partly mediated by the factor. Mediators were considered to not affect the exposure-to-outcome association if beta0 was significant while either beta1 and/or beta2 were non-significant. For any significant mediation effect identified, the direct effect, indirect effect, and proportion mediated were subsequently calculated[19]. A P-value of < 0.05 was used to determine statistical significance in the MVMR and mediation MR analyses.
Our estimates are reported per one SD unit increase, and the effect size is presented as odds ratios (OR) with their corresponding 95% confidence intervals (CI).
-
The F-statistic was used to quantify the instrument strength and verify the relevance assumption in the UVMR[41] and calculated using the formula: $ F = \left( {\dfrac{{n - k - 1}}{k}} \right)\left( {\dfrac{{{R^2}}}{{1 - {R^2}}}} \right) $. The n and k refer to the sample size and number of IVs, respectively, while the R2 refers to the proportion of phenotypic variance explained by all SNPs generated using the MR Steiger method and was used to measure heritability[42].
The conditional F-statistic was used to measure the instrument strength in the MVMR; this tested whether the SNP strongly predicted each exposure conditional on the other exposures. An F or conditional F < 10 indicates a significantly high risk of weak instrument bias. Heterogeneity due to the invalidity of IVs was measured by Cochran’s Q-statistic. A P value < 0.05 was considered to indicate significant heterogeneity[43]; a random-effects IVW model was adopted if a correction could not be made by using the MR-PRESSO method. The MR-Egger and MR-PRESSO methods were used to test the violation of the exclusion restriction assumption caused by directional pleiotropy. If significant pleiotropy was identified by the intercept of the MR-Egger or the distorted test of MR-PRESSO, their adjusted results were adopted. Otherwise, IVW results were prioritized. In addition, the Causal Analysis Using Summary Effect Estimates (CAUSE) method was also used to correct for both correlated and uncorrelated horizontal pleiotropy in the UVMR, to further ensure the exclusion restriction assumption. This approach corrects the sample overlap by utilizing the full genome-wide summary results of both exposure and outcome rather than the genome-wide significant loci only and improves the statistical power. Those associations not paralleling the results of CAUSE were likely to have a false-positive error[44]. P < 0.05 was used to determine the statistical significance of the CAUSE.
Statistical power was evaluated by utilizing the binary-outcome model from the mRnd tools (available at: https://shiny.cnsgenomics.com/mRnd/). A power lower than 80% was considered insufficient and the results from the MR. RAPS method were then preferred. A leave-one-out analysis was performed to detect unstable SNPs that singly showed a disproportionately large contribution to the results under the Bonferroni corrected threshold. Such SNPs were excluded and the results were reassessed[32].
-
A less stringent threshold of P < 5 x 10-6 was set for the ALB because only 4 SNPs were identified under the threshold of P < 5 x 10-8 that failed to meet the minimum requirements of at least 10 eligible IVs[45,46]. After the screening, 192 IVs for AMA, 112 for AMP, 62 for AFB, 65 for ALB, 11 for NEB, 175 for AFS, and 60 for NSP were identified (Supplementary Tables S2–S8). The Wald ratio estimate results of individual SNPs are shown in Supplementary Table S9–S29 and the details of the leave-one-out tests are shown in Supplementary Figures S1–S7. Notably, only in the case of AFB-PV were two outlying SNPs identified(rs1 1249939 and rs1702877), and these two outliers were subsequently excluded from further analysis.
The details of the sensitivity analysis are shown in Supplementary Table S30. Significant heterogeneity was found between the AMA, AMP, AFB, and AFS and all of the outcomes, but these were eliminated by the MR-PRESSO distorted outliers correction. Evident pleiotropy was only found in the AFB-PV (intercept-0.046; P = 0.017) and remained significant (intercept-0.040; P = 0.036) even after excluding thetwo SNPs identified by the leave-one-out test . No evident heterogeneity or pleiotropy across all mediators was found in the MVMR (Supplementary Table S31). A low risk of weak instrumental bias was found for all exposures according to the F-statistics with a range from 26.11 to 104.55 in the UVMR. However, AFB (conditional F = 2.26), ALB (1.30), NEB (2.38), and NSP (5.64) all showed a low weak instrumental strength in the MVMR. Except for AMA (5.14%), AMP (6.84%), and AFS (1.99%), the variables AFB (0.44%), ALB (0.96%), NEB (0.17%), and NSP (0.60%) all showed a relatively low heritability which may stem from an insufficient number of eligible IVs. AFB (13% to 48%), ALB (34% to 51%), and NEB (6% to 79%) showed a relatively low statistical power for all outcomes, which may arise from insufficient sample size and low heritability. The scatter plots are shown in Supplementary Figures S8–S14.
-
In the UVMR analysis, only AFS demonstrated a significant protective effect on PSO (ORIVW: 0.54; 95% CI: 0.41 to 0.72; P = 0.000). This finding was consistent with the results obtained using the CAUSE method (ORCAUSE: 0.71; 95% CI: 0.63 to 0.80; P = 0.002). No disproportionate individual IVs were detected in the leave-one-out tests (Figure 2).
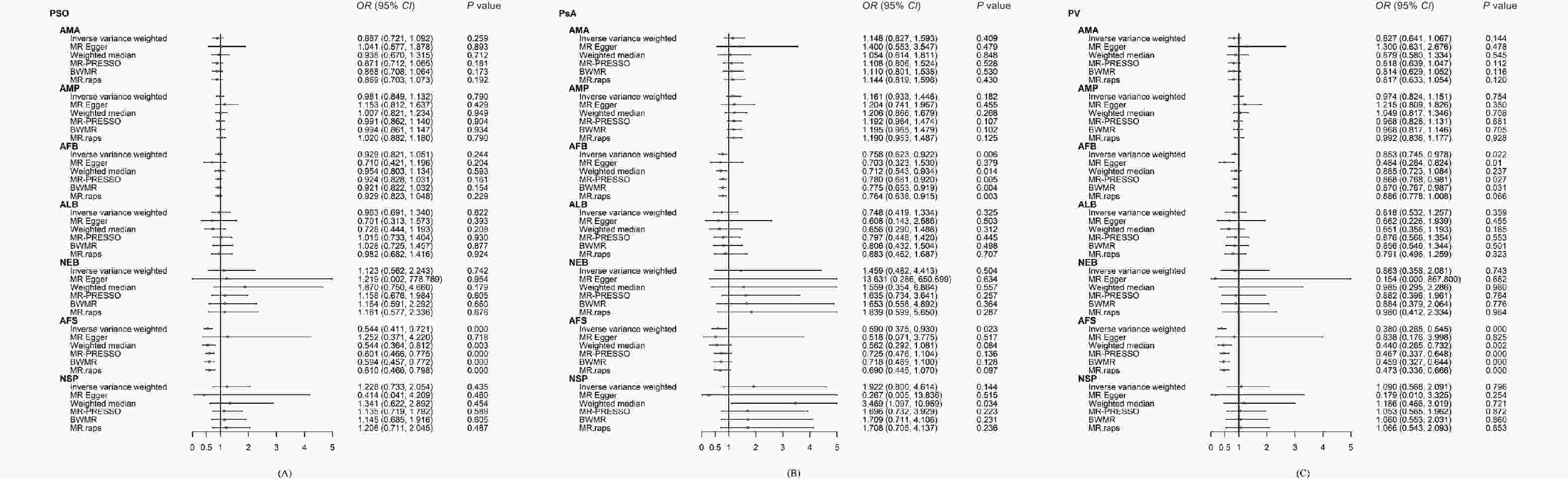
Figure 2. Forest plot of the univariable Mendelian randomisation analyses exploring associations between the women’s reproductive factors on psoriasis and its sub-types using different Mendelian randomization statistical models.
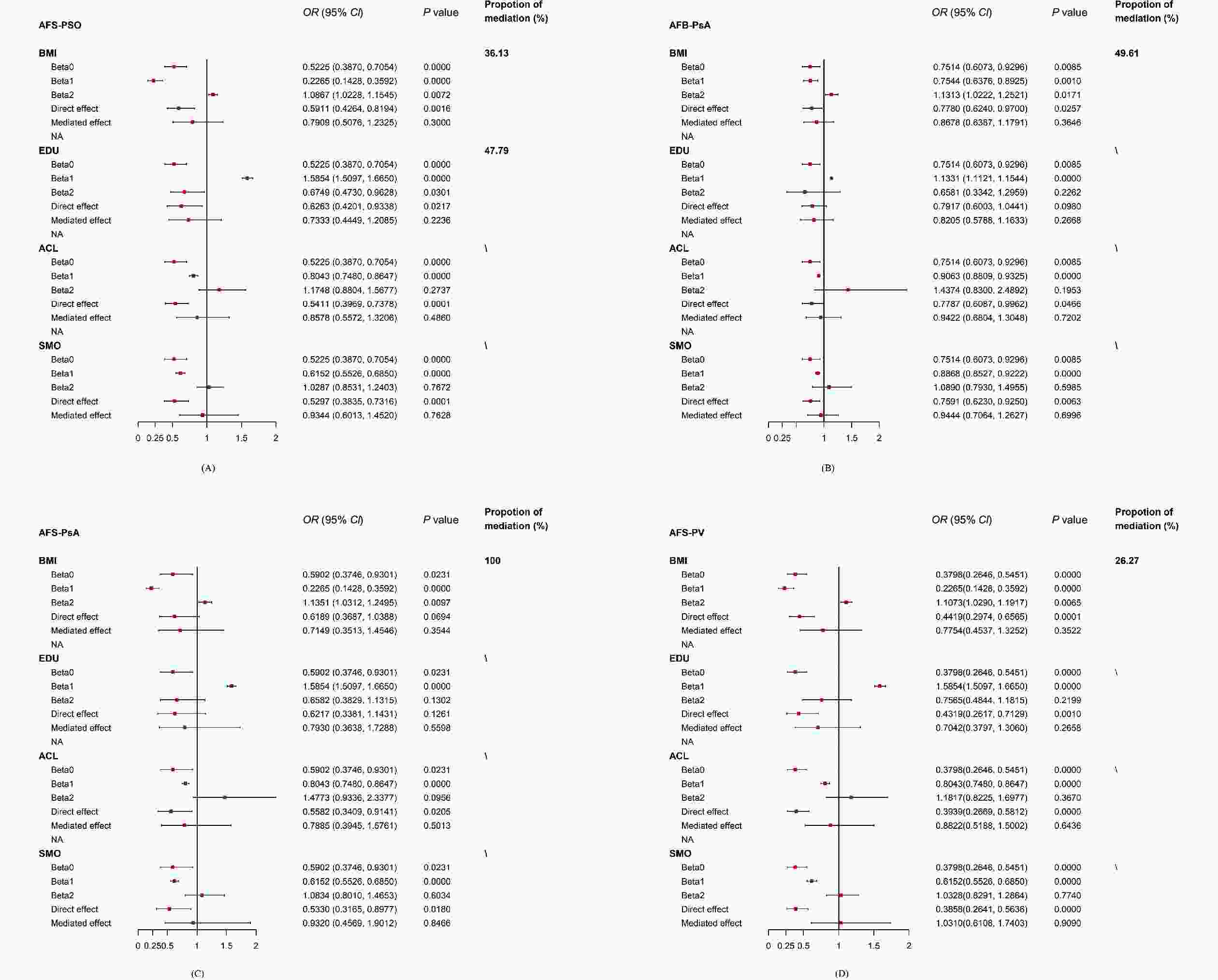
There was no significant causal association between AFS and PSO (ORMerged: 0.82; 95% CI: 0.53 to 1.26; P = 0.355) after the merged correction (Supplementary Table S31). Then, the four mediators were adjusted one by one to construct four separate MVMR models. The causal effect of AFS on PSO remained significant after adjusting ACL (ORseparate: 0.54; 95% CI: 0.40 to 0.73; P < 0.001) or SMO (ORseparate: 0.56; 95% CI: 0.40 to 0.77 P < 0.001); it was no longer significant after correcting BMI (ORseparate: 0.66; 95% CI: 0.48 to 1.12; P = 0.113) or EDU (ORseparate: 0.71; 95% CI: 0.48 to 1.06; P = 0.096) (Supplementary Table S32). A further two-step MR analysis was performed for BMI and EDU, showing that the association between AFS and PSO was partially mediated by BMI (Pbeta0 < 0.001; Pbeta1 < 0.001; Pbeta2 = 0.007) and EDU (Pbeta0 = 0.000; Pbeta1 < 0.001; Pbeta2 = 0.030). However, the indirect effect of both BMI (ORindirect effect: 0.79; 95% CI: 0.51 to 1.23; P = 0.300; the proportion of mediation: 36.13%) and EDU (ORindirect effect: 0.73; 95% CI: 0.44 to 1.21; P = 0.224; the proportion of mediation: 47.79%) were non-significant, which also suggests that direct effects dominated the causal relationship between AFS and PSO (Figure 3 and Supplementary Table S33).
-
AFB showed a significant negative association with PsA (ORMR.RAPS: 0.76; 95% CI: 0.64 to 0.92; P = 0.003) in the UVMR; this finding was consistent with the results of the CAUSE method (ORCAUSE: 0.87; 95% CI: 0.82 to 0.92; P = 0.038). AFS also had a negative effect on PsA that proved to be not significant after the Bonferroni correction (ORIVW: 0.59; 95% CI: 0.37 to 0.93; P = 0.023). No disproportional individual IV was detected in the leave-one-out tests (Figure 2).
In the merged MVMR model, there was no significant association between AFB and PsA (ORMerged: 0.92; 95% CI: 0.70 to 1.22; P = 0.571). The association between AFB and PsA remained significant in the follow-up separately corrected MVMR models of ACL (ORseparate: 0.76; 95% CI: 0.62 to 0.93; P = 0.006) and SMO (ORseparate: 0.80; 95% CI: 0.65 to 0.97; P = 0.023), but was not significant in the separate MVMR models of BMI (ORseparate: 0.87; 95% CI: 0.69 to 1.08; P = 0.201) and EDU (ORseparate: 0.92; 95% CI: 0.68 to 1.23; P = 0.558). (Supplementary Table S34). However, the further two-step analysis only revealed a partial mediating effect of BMI (Pbeta0 = 0.009; Pbeta1 = 0.001; Pbeta2 = 0.017) on the association between AFB and PsA, while the EDU (Pbeta0 = 0.009; Pbeta1 = 0.000; Pbeta2 = 0.226) did not mediate the AFB-PsA relationship. The indirect effect of BMI was also non-significant (ORindirect effect: 0.87; 95% CI: 0.64 to 1.18; P = 0.365; proportion of mediation: 49.61%), while the direct effect of AFB (ORdirect effect: 0.78; 95% CI: 0.62 to 0.97; P = 0.026) was predominant. Notably, the two-step analysis showed a fully mediated effect of BMI (Pbeta0 = 0.023; Pbeta1 = 0.000; Pbeta2 = 0.010) on AFS-PsA (ORdirect effect: 0.62; 95% CI: 0.37 to 1.04; P = 0.069), which was consistent with our findings from the UVMR and the merged MVMR (Figure 3 and Supplementary Table S35).
-
Two outliers (rs11249939 and rs1702877) were identified in the AFB-PV relationship and were removed by the leave-one-out test. AFS had a significant negative effect on PV (ORIVW: 0.38; 95% CI: 0.27 to 0.55; P < 0.001), which was also supported by the CAUSE method (ORCAUSE: 0.60; 95% CI: 0.55 to 0.65; P < 0.001). AFB showed a negative relationship with PV but this was nonsignificant following the Bonferroni correction (OREgger: 0.48; 95% CI: 0.28 to 0.82; P = 0.011) (Figure 2).
The association of AFS and PV became nonsignificant in the merged MVMR model (ORMerged: 0.64; 95% CI: 0.38 to 1.08; P = 0.092). In contrast with PSO and PsA, there remained a significant causal correlation between AFS and PV in the separately corrected MVMR models of BMI (ORseparate: 0.49; 95% CI: 0.33 to 0.73; P < 0.001), EDU (ORseparate: 0.54 95% CI: 0.33 to 0.89; P = 0.016), ACL (ORseparate: 0.39; 95% CI: 0.27 to 0.57; P < 0.001), and SMO (ORseparate: 0.37; 95% CI: 0.25 to 0.54; P = 0.000) (Supplementary Table S36). A partial but nonsignificant mediating effect was only found for BMI on AFS-PV (Pbeta0 < 0.001; Pbeta1 < 0.001; Pbeta2 = 0.007; ORindirect effect: 0.78; 95% CI: 0.45 to 1.33; P = 0.352; proportion of mediation: 26.27%), while the direct effect of AFS (ORdirect effect: 0.44; 95% CI: 0.30 to 0.66; P < 0.001) was statistically significant (Figure 3 and Supplementary Table S37).
The detailes of CAUSE analysis of the UVMR analysis of the women’s reproductive factors and psoriasis and its sub-types was shown in the Supplementary Figure S15. The Supplementary Figures S16–S18 presented the forest plots of the MVMR analyses exploring genetically determined the women’s reproductive factors and risk of overall psoriasis, PsA and PV after the adjustment for specific confounding traits.
-
In this study, we found that reproductive factors, especially AFS and AFB, were dominant risk factors for psoriasis; there were mediating effects by educational attainment and BMI. Genetically predicted early AFS led to an increased risk of overall psoriasis; 36.13% of this effect was mediated through BMI and 47.79% through educational attainment. The direct negative casual association between AFB-PsA was dominant, with 49.61% proportion of the mediation due to BMI. The mediating effect was also found for BMI on the AFS-PV relationship, which accounted for 26.27% of the proportion. Consistent null associations were identified via sensitivity and multivariable MR analyses, demonstrating the robustness of our findings.
This is the first application of an MR mediation analysis to study the mediators of the relationship between reproductive factors and psoriasis risk. In this study, we corroborate the genetic correlation between reproductive factors shown in previous studies, while showing additional correlations that were not previously investigated. For example, one study showed that women with psoriasis were younger at the time of first delivery and had greater mean durations between the first and last birth and a greater mean interpregnancy interval[18]. Another study investigated reproductive factors related to sexual history among heterosexual women, finding no significant differences in the age of the first sexual encounter between those with and without psoriasis (weighted difference –0.54 years, 95% CI –1.27 to 0.19)[47]. Several factors could underlie these discrepancies.
Firstly, reproductive factors are complex and heterogeneous traits influenced by both genetic and environmental factors. Genetics alone cannot fully capture the phenotypic variance of these traits. For instance, AFS is considered a human behavioral trait influenced by genetics, psychosocial, cultural, and financial factors. Therefore, traditional observational designs’ results are susceptible to the influence of complex confounding variables that are challenging to capture and model accurately. To address this issue, we utilized MVMR to control for adiposity, education, smoking, and alcohol intake effects. The negative results obtained corroborated our findings that AMA, AMP, ALB, NEB, and NSP were not significantly associated with psoriasis. It is also likely that the true causal effects of these reproductive factors on psoriasis were modest; as such, our study may have been underpowered to identify them. Notably, the association between AFS and PV became nonsignificant in the merged MVMR model. This may stem from an interaction between all mediators in the merged MVMR model; the effects of any single mediator could not eliminate the causal association between AFS and PV in the separate MVMR models.
The biological underlying association between hormonal factors and psoriasis development is not yet fully understood. Sex hormones have been shown to have an impact on the immune system and their interaction with environmental and genetic factors may partly explain the higher prevalence of psoriasis in women. Estrogen, in particular, is a complex modulator of the immune system with both stimulatory and inhibitory effects. For instance, estrogen levels during periovulatory to pregnancy periods may stimulate B cells and Th2 response, while also supporting the survival of auto-reactive T and B cell clones. Moreover, estrogens can suppress cell-mediated responses like Th17 cell differentiation[48-50]. Despite these findings, further research is needed to determine the exact relationship between modifiable reproductive factors and the incidence of psoriasis, as prospective studies on this topic remain limited and their findings are controversial.
Regarding the relationship of early AFS and AFB with increased risk of psoriasis, and educational attainment and BMI played a mediating role in the association, the following are some possible underlying mechanistic analyses: Firstly, mothers who give birth before the age of 20 tend to have a lower socio-economic status. Early motherhood often results in a heavier burden of childbearing, premature childbearing age, and health-related behaviors. Early childbearing may have an impact on a woman’s lifestyle and health behaviors[51,52]. For example, women who have children early may have less time and resources for higher education, which can lead to poor health literacy and reduced ability to prevent and manage chronic diseases such as psoriasis[53,54]. Secondly, lower educational attainment is often associated with poorer health cognition and behavior. People with less education may lack adequate health knowledge to effectively manage and prevent chronic diseases[55,56]. This may affect psoriasis risk in several ways: on the one hand, people with higher levels of education are generally better equipped to understand health and adopt healthy behaviors, such as eating a reasonable diet, quitting smoking, limiting alcohol, and maintaining a healthy weight[57]. For another, those with higher levels of education are generally better able to use medical resources, have regular medical check-ups, and detect and treat diseases early[58,59].
Our results revealed that BMI may also played an important role in the association between early reproductive age and psoriasis risk. Women who have children early may be more likely to be overweight or obese for a variety of reasons, such as unbalanced nutrition, lack of exercise, psychological stress, and so on. Women who become pregnant at an early age may experience greater difficulty returning to their pre-pregnancy weight[60]. Obesity can lead to chronic low-grade inflammation in the body, and psoriasis is an inflammatory disease. Obesity may induce or aggravate psoriasis by increasing the level of systemic inflammation[61,62]. High BMI is associated with metabolic syndromes (such as high blood pressure, diabetes, and dyslipidemia), and as such, a tendency for these metabolic disorders may progress to increase the risk of psoriasis[63].
In addition, those with younger AFB and AFS were also more likely to have had unintended pregnancies and may face more psychological and socioeconomic stresses that may increase the risk of psoriasis through a variety of mechanisms. Long-term psychological stress can affect immune system function through neuroendocrine pathways, inducing or aggravating psoriasis[64,65]. Besides, early childbearing can lead to increased financial burdens and weak social support systems, which can also increase psychological stress and affect health[66,67] .
The association between early reproductive age and increased risk of psoriasis is the result of a combination of factors. Educational attainment and BMI play mediating roles, and these factors might work together through influencing health behaviors, medical resource utilization, inflammation levels, and psychological stress. For the potential avenues for further investigation, progressive research can test these hypotheses through longitudinal data and multivariate analysis, and gain insight into their specific mechanisms to develop more effective prevention and intervention strategies.
To the best of our knowledge, no MR has been conducted to investigate the association between reproductive factors and psoriasis prior to this study. Our research possesses several notable strengths. We incorporated seven different but complementary reproductive traits. In the context of mediation, MR provided further robustness to non-differential measurement error in the mediator. However, we recognize some limitations. First, the available GWAS databases provide summary statistics that were limited to individuals of European descent. Second, MVMR is a powerful tool for investigating the causal relationships between multiple factors and an outcome of interest and has the potential to generate important insights into the underlying mechanisms of complex diseases, however, it has some unresolved limitations. As with any observational study, the results of a multivariable MR should be interpreted with caution, as residual confounding or unmeasured variables may still exist. For instance, we detected an abnormal result of the MVMR for AFS and PV. One potential explanation for such results is that the different mediators are related to each other in a way that is not fully captured by the MVMR model and this residual correlation is causing confounding or masking the causal effect of an individual mediator. Another possibility is that the effects of the different mediators interact with each other in complex ways, such that the net effect of multiple mediators is different from the effects of each mediator alone. In addition, there is a potential weak instrumental bias in the causal estimates generated by the MVMR, and such analyses can potentially be affected by some indeterminate pleiotropy via pathways that are not captured by the included variables. Larger and higher-quality datasets are needed to mitigate such potential weak instrumental bias and verify our findings from the MVMR. Besides that, despite using multiple methods to verify and mitigate these biases, genetic confounding could still be a potential problem. Given the complex etiology of psoriasis and the limitations of currently available datasets, it is not feasible to account for all potential mediators to eliminate the bias entirely. Therefore, the interpretation of these results should be approached with caution. Finally, the statistical power was suboptimal (< 80%), due to the relatively low overall incidence of psoriasis in the FinnGen population, and thus we urge caution in interpreting the negative results of our analysis.
-
In conclusion, the genetic predisposition to AFS was inversely associated with the risk of overall psoriasis and PV, with considerable mediation by BMI and educational attainment. Early AFB may lead to a higher risk of PsA, while the AFS-PsA association was fully mediated by BMI. Greater efforts to reduce adiposity and improve access to education appear to be clinically relevant. In addition, further research is needed to identify other environmental risk factors that act as potentially modifiable mediators of psoriasis. Modifying these factors may help substantially reduce the burden of psoriasis risk that is attributable to reproductive factors.
doi: 10.3967/bes2024.122
The Impact of Reproductive Traits on Psoriasis Risk is Mediated by Education Attainment and Body Mass Index: A Mendelian Randomization Study
-
Abstract:
Objective To explore the causality between reproductive traits and risk of psoriasis by using a large Mendelian randomization (MR) study. Methods A two-sample MR study was performed using summarized statistics from the genome-wide association studies (GWAS) conducted in reproductive traits, as well as GWAS data on overall psoriasis, psoriatic arthritis (PsA), and psoriasis vulgaris (PV). Besides univariable MR (UVMR), multivariable MR and two-step MR was used to calculate the independent effects and quantify the proportion mediated by education or body mass index (BMI). Results Genetically predicted early age at first sexual intercourse (AFS) led to an increased risk of overall psoriasis [odds ratio (OR)UVMR: 0.54]; 36.13% of this effect was mediated through BMI and 47.79% through educational attainment. The direct negative casual association between age at first birth (AFB)-PsA was dominant (ORUVMR: 0.76), with 49.61% proportion of the mediation due to BMI. The mediating effect was found for BMI on the AFS-PV relationship, which accounted for 26.27% of the proportion. AFS was inversely associated with the risk of overall psoriasis and PV, with considerable mediation by BMI and educational attainment. Conclusion Early AFB may cause a higher risk of PsA, while the AFS-PsA association was fully mediated by BMI. -
Key words:
- Age at first sexual intercourse /
- Age at first birth /
- Psoriasis /
- Psoriatic arthritis /
- Psoriasis vulgaris /
- Genetic epidemiology /
- Mendelian randomization
The authors declare that they do not have any competing interests.
Ethics approval was not needed as this study did collect any primary data from human participants or animals.
注释:1) Authors’ Contributions: 2) Competing Interests: 3) Ethics: -
Figure 1. Analysis process and verification of key MR assumptions flowchart. We utilize different colors to represent the relationship between the analysis methods and the three core assumptions of MR.
Orange indicates the relevance assumption and the methods used to verify it; green represents the independence assumption and the methods used to verify it; blue denotes the exclusion restriction assumption and the methods used for its verification. AMA, age at menarche; AMP, age at menopause; AFB, age at first birth; ALB, age at last live birth; NEB, number of live births; AFS, age at first sexual intercourse; NSP, lifetime number of sexual partners; PSO, psoriasis; PsA, psoriatic arthritis; PV, psoriasis vulgaris; BMI, body mass index; EDU, years of education; SMO, tobacco consumption; ACL, alcohol consumption level; MVMR, multivariable Mendelian randomization; UVMR, univariable Mendelian randomization; SNPs, single nucleotide polymorphisms; CAUSE, Causal Analysis Using Summary Effect Estimates; IVs, instrumental variables; IVW, inverse-variance weighted; MR-PRESSO, MR pleiotropy residual sum and outlier; BWMR, Bayesian weighted Mendelian randomization; MRAPS, MR-Robust Adjusted Profile Score.
Figure 2. Forest plot of the univariable Mendelian randomisation analyses exploring associations between the women’s reproductive factors on psoriasis and its sub-types using different Mendelian randomization statistical models.
(A) reproductive factors on PSO; (B) reproductive factors on PsA; (C) reproductive factors PV. OR, odds ratio; CIs, confidence intervals; AMA, age at menarche; AMP, age at menopause; AFB, age at first birth; ALB, age at last live birth; NEB, number of live births; AFS, age at first sexual intercourse; NSP, lifetime number of sexual partners; PSO, psoriasis; PsA, psoriatic arthritis; PV, psoriasis vulgaris; IVW, inverse-variance weighted; MR-PRESSO, MR pleiotropy residual sum and outlier; BWMR, Bayesian weighted Mendelian randomization; MRAPS, MR-Robust Adjusted Profile Score.
Figure 3. The forest plot of all the positive mediation MR analyses.
Causal estimates given as odds ratio (OR) and 95% confidence intervals (CIs) for the effect of (A) AFS-PSO, (B) AFB-PsA, (C) AFS-PsA, and (D) AFS-PV. AFS, age at first sexual intercourse; AFB, age at first birth; PSO, psoriasis; PsA, psoriatic arthritis; PV, psoriasis vulgaris. BMI, body mass index; EDU, years of education; ACL, alcohol consumption; SMO, cigarette consumption.
-
[1] Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet, 2007; 370, 263−71. doi: 10.1016/S0140-6736(07)61128-3 [2] Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature, 2007; 445, 866−73. doi: 10.1038/nature05663 [3] Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med, 2009; 361, 496−509. doi: 10.1056/NEJMra0804595 [4] Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet, 2021; 397, 1301−15. doi: 10.1016/S0140-6736(20)32549-6 [5] Fitzgerald O, Ogdie A, Chandran V, et al. Psoriatic arthritis. Nat Rev Dis Primers, 2021; 7, 59. doi: 10.1038/s41572-021-00293-y [6] Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet, 2018; 391, 2273−84. doi: 10.1016/S0140-6736(18)30830-4 [7] Cantarutti A, Donà D, Visentin F, et al. Epidemiology of frequently occurring skin diseases in Italian Children from 2006 to 2012: a retrospective, population-based study. Pediatr Dermatol, 2015; 32, 668−78. doi: 10.1111/pde.12568 [8] Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol, 2013; 133, 377−85. doi: 10.1038/jid.2012.339 [9] Springate DA, Parisi R, Kontopantelis E, et al. Incidence, prevalence and mortality of patients with psoriasis: a U. K. population-based cohort study. Br J Dermatol, 2017; 176, 650−8. doi: 10.1111/bjd.15021 [10] Tollefson MM, Crowson CS, McEvoy MT, et al. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol, 2010; 62, 979−87. doi: 10.1016/j.jaad.2009.07.029 [11] Vena GA, Altomare G, Ayala F, et al. Incidence of psoriasis and association with comorbidities in Italy: a 5-year observational study from a national primary care database. Eur J Dermatol, 2010; 20, 593−8. [12] Kanda N, Watanabe S. Regulatory roles of sex hormones in cutaneous biology and immunology. J Dermatol Sci, 2005; 38, 1−7. doi: 10.1016/j.jdermsci.2004.10.011 [13] Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun, 2012; 38, J282−91. doi: 10.1016/j.jaut.2011.11.013 [14] Murase JE, Chan KK, Garite TJ, et al. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol, 2005; 141, 601−6. [15] Raychaudhuri SP, Navare T, Gross J, et al. Clinical course of psoriasis during pregnancy. Int J Dermatol, 2003; 42, 518−20. doi: 10.1046/j.1365-4362.2003.01760.x [16] Swanbeck G, Inerot A, Martinsson T, et al. A population genetic study of psoriasis. Br J Dermatol, 1994; 131, 32−9. doi: 10.1111/j.1365-2133.1994.tb08454.x [17] Wu SW, Cho E, Li WQ, et al. Hormonal factors and risk of psoriasis in women: a cohort study. Acta Derm Venereol, 2016; 96, 927−31. doi: 10.2340/00015555-2312 [18] Lambe M, Bergstrom AV, Johansson ALV, et al. Reproductive patterns and maternal and pregnancy outcomes in women with psoriasis-A population-based study. J Am Acad Dermatol, 2020; 82, 1109−16. doi: 10.1016/j.jaad.2019.05.099 [19] Burgess S, Thompson DJ, Rees JMB, et al. Dissecting causal pathways using mendelian randomization with summarized genetic data: application to age at menarche and risk of breast cancer. Genetics, 2017; 207, 481−7. doi: 10.1534/genetics.117.300191 [20] Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ, 2018; 362, k601. [21] Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA, 2021; 326, 1614−21. doi: 10.1001/jama.2021.18236 [22] Mills MC, Tropf FC, Brazel DM, et al. Identification of 371 genetic variants for age at first sex and birth linked to externalising behaviour. Nat Hum Behav, 2021; 5, 1717−30. doi: 10.1038/s41562-021-01135-3 [23] Ruth Mitchell EB, Elsworth BL, Mitchell R, et al. Mrc ieu uk biobank gwas pipeline version 2. University of Bristol. 2019. [24] Kurki MI, Karjalainen J, Palta P, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature, 2023; 613, 508−18. doi: 10.1038/s41586-022-05473-8 [25] Lee JJ, Wedow R, Okbay A, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet, 2018; 50, 1112−21. doi: 10.1038/s41588-018-0147-3 [26] Liu MZ, Jiang Y, Wedow R, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet, 2019; 51, 237−44. doi: 10.1038/s41588-018-0307-5 [27] Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife, 2018; 7, e34408. doi: 10.7554/eLife.34408 [28] Sanderson E, Spiller W, Bowden J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat Med, 2021; 40, 5434−52. doi: 10.1002/sim.9133 [29] Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol, 2013; 37, 658−65. doi: 10.1002/gepi.21758 [30] Staley JR, Burgess S. Semiparametric methods for estimation of a nonlinear exposure-outcome relationship using instrumental variables with application to Mendelian randomization. Genet Epidemiol, 2017; 41, 341−52. doi: 10.1002/gepi.22041 [31] Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol, 2015; 44, 512−25. doi: 10.1093/ije/dyv080 [32] Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol, 2017; 32, 377−89. doi: 10.1007/s10654-017-0255-x [33] Verbanck M, Chen CY, Neale B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet, 2018; 50, 693−8. doi: 10.1038/s41588-018-0099-7 [34] Bowden J, Davey Smith G, Haycock PC, et al. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted Median estimator. Genet Epidemiol, 2016; 40, 304−14. doi: 10.1002/gepi.21965 [35] Zhao J, Ming JS, Hu XH, et al. Bayesian weighted Mendelian randomization for causal inference based on summary statistics. Bioinformatics, 2020; 36, 1501−8. doi: 10.1093/bioinformatics/btz749 [36] Zhao QY, Wang JS, Hemani G, et al. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann Statist, 2020; 48, 1742−69. [37] Slob EAW, Burgess S. A comparison of robust Mendelian randomization methods using summary data. Genet Epidemiol, 2020; 44(4), 313-29. [38] Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol, 2015; 181, 251−60. doi: 10.1093/aje/kwu283 [39] Rees JMB, Wood AM, Burgess S. Extending the MR-Egger method for multivariable Mendelian randomization to correct for both measured and unmeasured pleiotropy. Stat Med, 2017; 36, 4705−18. doi: 10.1002/sim.7492 [40] Sanderson E. Multivariable Mendelian randomization and mediation. Cold Spring Harb Perspect Med, 2021; 11, a038984. doi: 10.1101/cshperspect.a038984 [41] Haycock PC, Burgess S, Wade KH, et al. Best (but oft-forgotten) practices: the design, analysis, and interpretation of Mendelian randomization studies. Am J Clin Nutr, 2016; 103(4), 965-78. [42] Zhu HH, Zhou X. Statistical methods for SNP heritability estimation and partition: a review. Comput Struct Biotechnol J, 2020; 18, 1557−68. doi: 10.1016/j.csbj.2020.06.011 [43] Greco M FD, Minelli C, Sheehan NA, et al. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med, 2015; 34, 2926−40. doi: 10.1002/sim.6522 [44] Morrison J, Knoblauch N, Marcus JH, et al. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat Genet, 2020; 52, 740−7. doi: 10.1038/s41588-020-0631-4 [45] Savage JE, Jansen PR, Stringer S, et al. Genome-wide association meta-analysis in 269, 867 individuals identifies new genetic and functional links to intelligence. Nat Genet, 2018; 50, 912−9. doi: 10.1038/s41588-018-0152-6 [46] Gao X, Meng LX, Ma KL, et al. The bidirectional causal relationships of insomnia with five major psychiatric disorders: a Mendelian randomization study. Eur Psychiatry, 2019; 60, 79−85. doi: 10.1016/j.eurpsy.2019.05.004 [47] Armstrong AW, Follansbee MR, Harskamp CT, et al. Psoriasis and sexual behavior in U. S. women: an epidemiologic analysis using the National Health and Nutrition Examination Survey (NHANES). J Sex Med, 2013; 10, 326−32. doi: 10.1111/jsm.12003 [48] Hughes GC, Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat Rev Rheumatol, 2014; 10, 740−51. doi: 10.1038/nrrheum.2014.144 [49] Straub RH. The complex role of estrogens in inflammation. Endocr Rev, 2007; 28, 521−74. doi: 10.1210/er.2007-0001 [50] Sthoeger ZM, Chiorazzi N, Lahita RG. Regulation of the immune response by sex hormones. I. In vitro effects of estradiol and testosterone on pokeweed mitogen-induced human B cell differentiation. J Immunol, 1988; 141, 91−8. doi: 10.4049/jimmunol.141.1.91 [51] Tiruneh FN, Tenagashaw MW, Asres DT, et al. Associations of early marriage and early childbearing with anemia among adolescent girls in Ethiopia: a multilevel analysis of nationwide survey. Arch Public Health, 2021; 79, 91. doi: 10.1186/s13690-021-00610-7 [52] Scott S, Nguyen PH, Neupane S, et al. Early marriage and early childbearing in South Asia: trends, inequalities, and drivers from 2005 to 2018. Ann NY Acad Sci, 2021; 1491, 60−73. doi: 10.1111/nyas.14531 [53] Elfenbein DS, Felice ME. Adolescent pregnancy. Pediatr Clin North Am, 2003; 50, 781−800. doi: 10.1016/S0031-3955(03)00069-5 [54] Pirkle CM, De Albuquerque Sousa ACP, Alvarado B, et al. Early maternal age at first birth is associated with chronic diseases and poor physical performance in older age: cross-sectional analysis from the international mobility in aging study. BMC Public Health, 2014; 14, 293. doi: 10.1186/1471-2458-14-293 [55] Tan ST, Quek RYC, Haldane V, et al. The social determinants of chronic disease management: perspectives of elderly patients with hypertension from low socio-economic background in Singapore. Int J Equity Health, 2019; 18, 1. doi: 10.1186/s12939-018-0897-7 [56] Braverman-Bronstein A, Hessel P, González-Uribe C, et al. Association of education level with diabetes prevalence in Latin American cities and its modification by city social environment. J Epidemiol Community Health, 2021; 75, 874−80. doi: 10.1136/jech-2020-216116 [57] Grundy E, Kravdal Ø. Reproductive history and mortality in late middle age among Norwegian men and women. Am J Epidemiol, 2008; 167, 271−9. [58] Xu JX, Du SH, Dong XQ. Associations of education level with survival outcomes and treatment receipt in patients with gastric adenocarcinoma. Front Public Health, 2022; 10, 868416. doi: 10.3389/fpubh.2022.868416 [59] Sudhakar S, Aebi ME, Burant CJ, et al. Health literacy and education level correlates of participation and outcome in a remotely delivered epilepsy self-management program. Epilepsy Behav, 2020; 107, 107026. doi: 10.1016/j.yebeh.2020.107026 [60] Kac G, Benicio MH, Velásquez-Meléndez G, et al. Nine months postpartum weight retention predictors for Brazilian women. Public Health Nutr, 2004; 7, 621−8. doi: 10.1079/PHN2003579 [61] Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol, 2020; 182, 840-8. [62] Lynch M, Ahern T, Sweeney CM, et al. Adipokines, psoriasis, systemic inflammation, and endothelial dysfunction. Int J Dermatol, 2017; 56, 1103−18. doi: 10.1111/ijd.13699 [63] Hao Y, Zhu YJ, Zou S, et al. Metabolic syndrome and psoriasis: mechanisms and future directions. Front Immunol, 2021; 12, 711060. doi: 10.3389/fimmu.2021.711060 [64] MAREK-Jozefowicz L, Czajkowski R, Borkowska A, et al. The brain-skin axis in psoriasis-psychological, psychiatric, hormonal, and dermatological aspects. Int J Mol Sci, 2022; 23, 669. doi: 10.3390/ijms23020669 [65] Woźniak E, Owczarczyk-Saczonek A, Placek W. Psychological stress, mast cells, and psoriasis-is there any relationship? Int J Mol Sci, 2021; 22, 13252. [66] Davis EM, Stange KC, Horwitz RI. Childbearing, stress and obesity disparities in women: a public health perspective. Matern Child Health J, 2012; 16, 109−18. doi: 10.1007/s10995-010-0712-6 [67] Mah BL, Brown A, Eades S, et al. Psychological distress, stressful life events and social disadvantage in pregnant indigenous australian women residing in rural and remote NSW: a longitudinal cohort study. J Racial Ethn Health Disparities, 2022; 9, 2197−207. doi: 10.1007/s40615-021-01159-5 -
 24195+Supplementary Materials.pdf
24195+Supplementary Materials.pdf

-




 下载:
下载:






 Quick Links
Quick Links