-
Today, tuberculosis (TB) remains a global public health threat associated with significantly high rates of morbidity and mortality. The World Health Organization's (WHO) Global Tuberculosis Report 2018[1] has reported that in 2017, 10.0 million people across the world had developed TB diseases that resulted in an estimated 1.6 million deaths, and 889, 000 people developed TB in China that led to 39, 000 TB-related deaths. Therefore, rapid and accurate detection of Mycobacterium tuberculosis (MTB) is important for initiating early treatment and reducing mortality. Traditional diagnostic methods for pulmonary TB incorporate chest radiography and sputum smear microscopy; however, several cases of tuberculosis go undiagnosed because of the low sensitivity of smear microscopy[2]. Hence, the most urgent need in TB control is the availability of rapid, accurate, affordable, and easy-to-use diagnostic tests for MTB detection, particularly at peripheral and low-resource settings[3]. Numerous technologies based on nucleic acid amplification have been developed to address this gap, which include Xpert MTB/RIF (Cepheid, USA), RealAmp (real-time loop-mediated isothermal amplification, Deaou Chemical Co., Ltd, China), and CPA (cross-priming amplification, Ustar Biotechnology Co., Ltd, China). The Xpert MTB/RIF assay is an in vitro diagnostic technology involving a semi-nested, real-time fluorescent quantitative polymerase chain reaction that targets the 81-bp rifampin resistance-determining region of the rpoB gene. The Xpert MTB/RIF test automates DNA extraction, amplification, and detection inside a closed cartridge, which reduces the chances of cross-contamination. Conducting this assay is simple, which enables laboratory technicians (with no prior molecular testing experience) to perform the assay and produce accurate results[4]. The RealAmp technique uses a real-time loop-mediated isothermal amplification method to amplify the target sequence of IS6110 under isothermal conditions. The amplified products emit fluorescent signals after reacting with a nucleic acid dye. The RealAmp test consists of the following three steps: DNA extraction (50 min), isothermal amplification (30 min), and real-time detection (0.5-1 min). The speed of the reaction, the lack of requirement for a thermocycler, and the ease of use make the RealAmp test an excellent platform for the molecular detection of MTB in developing countries[5]. Using multiple cross-linked primers, the CPA method can amplify a target nucleic acid under isothermal conditions without the requirement for any specialized equipment. The amplified products are detected on a lateral flow strip housed within an enclosed and sealed plastic housing designed to prevent the leakage of amplicons. The CPA testing protocol for TB detection includes sample preparation (60 min), nucleic acid isothermal amplification (60 min), and hybridization and detection using a patented cross-contamination proof device (20 min)[6].
Previous studies have demonstrated excellent performance of Xpert MTB/RIF, RealAmp, and CPA[4-6]. However, these three assays were evaluated in different study populations, due to which their performance cannot be compared accurately. Therefore, we conducted a clinical study at three sites in Henan Province, China, to compare the sensitivity and specificity of Xpert MTB/RIF, RealAmp, and CPA with the aim of determining the sensitivity and specificity of each method. A secondary goal of our study was to explore the potential of combining these new molecular rapid diagnostic methods with more traditional diagnostic methods with the aim of developing a more efficient and accurate diagnostic algorithm for use in clinical settings.
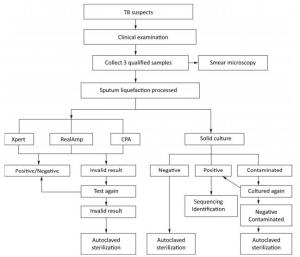
Individuals suspected to have TB who can provide three sputum samples (morning, night, and on-the-spot) of adequate volume (at least 1.5 mL) were consecutively enrolled in this study from August 2014 to August 2015. After obtaining the informed consent from all recruited individuals suspected with TB, all the three sputum samples were tested directly by Ziehl-Neelsen (ZN) microscopy. Morning sputum samples were processed with N-acetyl-L-cysteine and sodium hydroxide (NALC-NaOH), followed by centrifugation, and the resulting pellets were suspended in 3 mL of physiological saline that can be readily used for solid culture (Lowenstein–Jensen), Xpert MTB/RIF, RealAmp, and CPA tests (Figure 1).
Culture-positive samples were collected by the NTRL staff for identification as either MTB or nontuberculosis Mycobacterium (NTM). Analysis of the 16S-23S rDNA ITS sequence was performed using the forward primer 5'-GGCCTAACCCTCGGGAGGGAG -3' and the reverse primer 5'-CCCGAGGCATATCGCAG CCTC-3'[7]. Direct sequencing was performed on the ABI 3730 DNA automated sequence analyzer. Sequencing results were entered into the Basic Local Alignment Search Tool (BLAST), an international data bank (www.ncbi.nlm.nih.gov/BLAST), and compared against the sequences of reference Mycobacterium strains. Strains with no sequencing results (possibly due to contamination or identified as NTM) were excluded from the final analysis.
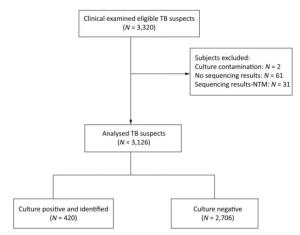
Of the 3, 220 enrolled cases, 303 (9.4%) showed smear-positive results, 2 had contaminated culture results, and 512 (15.9%) had culture-positive results (Table 1). The rates of positive results for Xpert MTB/RIF, RealAmp, and CPA tests were 17.1% (551/3, 220), 18.8% (605/3, 220), and 15.7% (504/3, 220), respectively (Table 1). The performance of the solid culture, which was used as the gold standard for this research, may have affected the evaluation of the molecular assay. The culture contamination rate was found to be very low, potentially due to harsh specimen decontamination procedures. This may have decreased the sensitivity of the solid culture that can detect only live bacteria.
Table 1. Different Test Results for 3, 220 Recruited Suspects
Items Culture Smear Xpert LAMP CPA Positive 512 (15.9%) 303 (9.4%) 551 (17.1%) 605 (18.8%) 504 (15.7%) Negative 2, 706 (84.0%) 2, 917 (90.6%) 2, 649 (82.3%) 2, 615 (81.2%) 2, 716 (84.3%) No result 2 (0.1%) 0 (0%) 20 (0.6%) 0 (0%) 0 (0%) Total 3, 220 3, 220 3, 220 3, 220 3, 220 A total of 512 culture-positive samples underwent the 16S-23S rDNA ITS sequence analysis, of which 11.9% (61/512) gave no sequencing results, and 6.1% (31/512) were identified as NTM. Finally, 420 culture-positive cases were identified as TB, 2, 706 cases were culture-negative, and 2 cases had culture contamination (Figure 2). Therefore, of the 3, 220 patients, 3, 126 were included for the analysis. Compared with solid culture, the sensitivity and specificity of the assays were as follows: 91.1% and 95.3% for Xpert MTB/RIF, 89.5% and 93.3% for RealAmp, and 88.1% and 96.9% for CPA, respectively (Table 2). The sensitivity of Xpert MTB/RIF was found to be comparable to that reported in previous studies[8, 9]. The Xpert MTB/RIF assay demonstrated the highest sensitivity in detecting MTB compared with the other two molecular methods. Due to its rapidity, simplicity, and accuracy, the Xpert MTB/RIF method has been recommended by the WHO for detecting Mycobacterium tuberculosis and rifampicin-resistant MTB globally. However, the Xpert MTB/RIF platform has one limitation, i.e., it requires sophisticated instruments and is relatively expensive, thus rendering it unfeasible to be adopted at a wide scale in low-resource settings. RealAmp and CPA, two locally developed (China) diagnostic tools, were comparable with Xpert MTB/RIF in terms of sensitivity and specificity. This local production and the nearly instrument-free amplification methods have made RealAmp and CPA attractive to the China NTP.
Table 2. The Sensitivity and Specificity of the Three Molecular Diagnostic Methods Compared with Solid Culture and Species Identification
Items Culture + 16S Sensitivity (%, 95% CI) Specificity (%, 95% CI) PPV (%, 95% CI) NPV (%, 95% CI) POS NEG Subtotal Xpert POS 380 124 504 91.1 (87.9-93.6) 95.3 (94.5-96.1) 75.4 (71.4-79.0) 98.6 (98.0-99.0) NEG 37 2, 565 2, 602 Subtotal 417 2, 689 3, 106 LAMP POS 376 181 557 89.5 (86.1-92.2) 93.3 (92.3-94.2) 67.5 (63.4-71.3) 98.3 (97.7-98.7) NEG 44 2, 525 2, 569 Subtotal 420 2, 706 3, 126 CPA POS 370 85 455 88.1 (84.5-91.0) 96.9 (96.1-97.5) 81.3 (77.4-84.7) 98.1 (97.5-98.6) NEG 50 2, 621 2, 671 Subtotal 420 2, 706 3, 126 Smear POS 249 18 267 59.3 (54.4-64.0) 99.3 (98.9-99.6) 93.3 (89.4-95.8) 94.0 (93.1-94.8) NEG 171 2, 688 2, 859 Subtotal 420 2, 706 3, 126 Note. 16S: 16S-23S rDNA internal transcribed spacer sequence analysis; CI: Confidence interval. PPV: Positive predictive value. NPV: Negative predictive value. Although ZN smear microscopy is less sensitive, it still remains the most frequently used diagnostic test for pulmonary TB in low-income countries, probably due to its rapidity and low cost. The sensitivity of smear microscopy in this study was 59.3% (Table 2), which was comparable to that reported in previous research[10]. The sensitivity of Xpert MTB/RIF-Smear assay was 92.1%, while LAMP-Smear and CPA-Smear demonstrated sensitivities of 91.7% and 90.5%, respectively, showing significant differences compared with the conventional smear-alone method (59.3%) but not with the Xpert MTB/RIF-Smear method (Table 3).
Table 3. The Sensitivity and Specificity of the Three Molecular Diagnostic Methods when Combined with Smear Microscopy
Items Culture + 16S Sensitivity (%, 95% CI) Specificity (%, 95% CI) PPV (%, 95% CI) NPV (%, 95% CI) POS NEG Subtotal Xpert-Smear POS 387 129 516 92.1 (89.0-94.5) 95.2 (94.3-96.0) 75.0 (71.0-78.6) 98.7 (98.2-99.1) NEG 33 2, 577 2, 610 Subtotal 420 2, 706 3, 126 LAMP-Smear POS 385 185 570 91.7 (88.5-94.0) 93.2 (92.1-94.1) 67.5 (63.5-71.3) 98.6 (98.1-99.0) NEG 35 2, 521 2, 556 Subtotal 420 2, 706 3, 126 CPA-Smear POS 380 90 470 90.5 (87.2-93.0) 96.7 (95.9-97.3) 80.9 (74.9-84.3) 98.5 (97.9-98.9) NEG 40 2, 616 2, 656 Subtotal 420 2, 706 3, 126 Based on these performance parameters and the simplicity of use, we recommend the application of the Xpert MTB/RIF assay for the diagnosis of TB in high-income countries, because this assay has the ability to not only diagnose Mycobacterium tuberculosis with the highest sensitivity but also detect rifampin resistance. In low-resource settings, RealAmp or CPA can be used as an alternative to Xpert MTB/RIF. The introduction and rollout of these two molecular diagnostic methods developed in China will significantly improve the speed and accuracy of diagnosis of pulmonary tuberculosis at the peripheral level and in low-resource settings in China, which could result in corresponding improvements in TB control.
-
We are grateful to Dr. LI Ya Ru (National Institute for Nutrition and Health, Chinese Center for Diseases Control and Prevention) for help with data analysis. We also thank all staff in these project laboratories who contributed their efforts to this work.
doi: 10.3967/bes2019.029
Comparison of Xpert MTB/RIF, RealAmp, and CPA Tests in Detecting Mycobacterium tuberculosis
-
-
Table 1. Different Test Results for 3, 220 Recruited Suspects
Items Culture Smear Xpert LAMP CPA Positive 512 (15.9%) 303 (9.4%) 551 (17.1%) 605 (18.8%) 504 (15.7%) Negative 2, 706 (84.0%) 2, 917 (90.6%) 2, 649 (82.3%) 2, 615 (81.2%) 2, 716 (84.3%) No result 2 (0.1%) 0 (0%) 20 (0.6%) 0 (0%) 0 (0%) Total 3, 220 3, 220 3, 220 3, 220 3, 220 Table 2. The Sensitivity and Specificity of the Three Molecular Diagnostic Methods Compared with Solid Culture and Species Identification
Items Culture + 16S Sensitivity (%, 95% CI) Specificity (%, 95% CI) PPV (%, 95% CI) NPV (%, 95% CI) POS NEG Subtotal Xpert POS 380 124 504 91.1 (87.9-93.6) 95.3 (94.5-96.1) 75.4 (71.4-79.0) 98.6 (98.0-99.0) NEG 37 2, 565 2, 602 Subtotal 417 2, 689 3, 106 LAMP POS 376 181 557 89.5 (86.1-92.2) 93.3 (92.3-94.2) 67.5 (63.4-71.3) 98.3 (97.7-98.7) NEG 44 2, 525 2, 569 Subtotal 420 2, 706 3, 126 CPA POS 370 85 455 88.1 (84.5-91.0) 96.9 (96.1-97.5) 81.3 (77.4-84.7) 98.1 (97.5-98.6) NEG 50 2, 621 2, 671 Subtotal 420 2, 706 3, 126 Smear POS 249 18 267 59.3 (54.4-64.0) 99.3 (98.9-99.6) 93.3 (89.4-95.8) 94.0 (93.1-94.8) NEG 171 2, 688 2, 859 Subtotal 420 2, 706 3, 126 Note. 16S: 16S-23S rDNA internal transcribed spacer sequence analysis; CI: Confidence interval. PPV: Positive predictive value. NPV: Negative predictive value. Table 3. The Sensitivity and Specificity of the Three Molecular Diagnostic Methods when Combined with Smear Microscopy
Items Culture + 16S Sensitivity (%, 95% CI) Specificity (%, 95% CI) PPV (%, 95% CI) NPV (%, 95% CI) POS NEG Subtotal Xpert-Smear POS 387 129 516 92.1 (89.0-94.5) 95.2 (94.3-96.0) 75.0 (71.0-78.6) 98.7 (98.2-99.1) NEG 33 2, 577 2, 610 Subtotal 420 2, 706 3, 126 LAMP-Smear POS 385 185 570 91.7 (88.5-94.0) 93.2 (92.1-94.1) 67.5 (63.5-71.3) 98.6 (98.1-99.0) NEG 35 2, 521 2, 556 Subtotal 420 2, 706 3, 126 CPA-Smear POS 380 90 470 90.5 (87.2-93.0) 96.7 (95.9-97.3) 80.9 (74.9-84.3) 98.5 (97.9-98.9) NEG 40 2, 616 2, 656 Subtotal 420 2, 706 3, 126 -
[1] World Health Organization, 2018. Global Tuberculosis Report. Geneva, Switzerland: WHO; 2018. http://www.who.int/tb/publications/global_report/en/.[2019-1-25] [2] Li Q, Zhao YL, Wang Q, et al. Evaluation of the new automatic mycob. T stainer and scanner for detecting acid-fast bacilli in China. Biomed Environ Sci, 2018; 31, 572-8. http://d.old.wanfangdata.com.cn/Periodical/bes201808002 [3] Li Q, Bao XD, Liu Y, et al. Comparison of two molecular assays for detecting smear negative pulmonary tuberculosis. Biomed Environ Sci, 2016; 29, 248-53. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=bes201604002 [4] Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med, 2010; 363, 1005-15. doi: 10.1056/NEJMoa0907847 [5] Ou X, Wang S, Dong H, et al. Multicenter evaluation of a real-time loop-mediated isothermal amplification (RealAmp) test for rapid diagnosis of Mycobacterium tuberculosis. J Microbiol Methods, 2016; 129, 39-43. doi: 10.1016/j.mimet.2016.07.008 [6] Ou X, Song Y, Zhao B, et al. A multicenter study of cross- priming amplification for tuberculosis diagnosis at peripheral level in China. Tuberculosis (Edinb), 2014; 94, 428-33. doi: 10.1016/j.tube.2014.04.006 [7] Gürtler V, Stanisich VA. New approaches to typing and identification of bacteria using the 16S-23S rDNA spacer region. Microbiology, 1996; 142, 3-16. doi: 10.1099/13500872-142-1-3 [8] Agrawal M, Bajaj A, Bhatia V, et al. Comparative study of GeneXpert with ZN stain and culture in samples of suspected pulmonary tuberculosis. J Clin Diagn Res, 2016; 10, Dc09-12. http://europepmc.org/abstract/MED/27437212 [9] Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet, 2011; 377, 1495-505. doi: 10.1016/S0140-6736(11)60438-8 [10] Moreira Ada S, Huf G, Vieira MA, et al. Liquid vs solid culture medium to evaluate proportion and time to change in management of suspects of tuberculosis: a pragmatic randomized trial in secondary and tertiary health care units in Brazil. PloS One, 2015; 10, e0127588. doi: 10.1371/journal.pone.0127588 -




 下载:
下载:



 Quick Links
Quick Links