-
Prior to vaccinations which started in 1963, 7–8 million children died annually of measles worldwide[1]. Global transmission of the disease is declining because of mass vaccination efforts by governments aided by the World Health Organization (WHO), the United Nations Children’s Fund and other organizations. In 2016, it was estimated that fewer than 100,000 people died from measles for the first time in recorded history. Nevertheless, measles remains the leading cause of vaccine-preventable child mortality[2]. Several countries have eliminated measles or have made significant progress toward achieving goals for measles elimination[3]. However, measles still remains endemic in China. Following the implementation of China's 2006–2012 Action Plan for Measles Elimination, there was a drop in reported measles cases in 2012. However, there was a spike in the number of cases which started in 2013, and continued with 52,628 reported measles cases in 2014[4]. In Tianjin, there was a significant measles outbreak in 2014[5]. A total of 2,703 measles cases were reported. Morbidity was recorded at 18.36 per 100,000, the highest morbidity rate recorded in the past 30 years in Tianjin. The majority of the cases occurred in patients aged ≥ 20 years.
Based on the Hu191 strain, the live-attenuated measles virus (MV) vaccine has played a significant role in controlling measles in China[6]. The vaccine was licensed in 1965, and made available free of charge through the Expanded Program on Immunization (EPI) in 1978[7]. In fact, the Tianjin municipality began supplying the measles containing vaccine (MCV) through the EPI as early as 1973. According to the original program in 1986, one dose of MCV administered to infants at 8 months of age, and the second dose was administered at aged 7 years[8]. Eventually in 2005, the program modified its dosage to two doses at 8 months of age and between 18 and 24 months of age. Despite high population immunity following routine immunization and administration of two supplementary rounds of MCV in Tianjin[9], there is a question why measles cases continue to occur in vaccinated individuals in recent years?
In populations with high vaccination coverage, the number of susceptible individuals who are vaccinated will increase with time and will make up a larger proportion of measles cases. Therefore, vaccine failures in the measles elimination period should be a point of focus. Understanding the role of vaccine failures in measles epidemics is important for the evaluation of measles control programs in developing countries. Vaccine failures may be classified as primary and secondary failures. Primary vaccine failures (PVF) are defined by failure of seroconversion. Secondary vaccine failures (SVF) are characterized by reduced vaccination-induced immunity after seroconversion. Several risk factors may influence vaccine failures, such as vaccine properties, delivery and handling issues, administration, host and environmental factors. Therefore, it was difficult to isolate primary failures from secondary failures, as no feasible techniques had been described for this purpose[10].
A simple and reliable method for avidity testing was developed by utilizing ELISA and a mild protein-denaturing agent, and was introduced into clinical practice in 1984[11]. The first study using this technique revealed was conducted in the measles outbreak of 1998/1999 in Finland. It revealed a high secondary response rate among teenagers who had been vaccinated at a proper age, at a time when natural measles boosters were rare in Finland[12]. It showed that children vaccinated around their first birthday had an SVF rate of 36%, while those vaccinated before the first birthday had an SVF of 50%. Vaccination schedules differ considerably from country to country. Therefore, vaccine failures rates also differ in countries.
Measles IgG avidity testing has become increasingly applicable in vaccine research[13, 14], and the assay has been used to distinguish primary from secondary immune response. Virus-specific high avidity antibodies are generally associated with pre-existing memory B cells, whereas low avidity antibodies are indicative of primary immune response[12]. Therefore, measles IgG avidity assay can be used to evaluate the success of measles vaccination and identify the type of vaccine failure without knowledge of prior antibody status[15].
In this study, we used the measles IgG avidity assay to detect serum from confirmed measles cases in Tianjin, China. Tianjin, one of four municipalities in China, is located approximately 110 km southeast of Beijing in the northern part of the country. It is one of the most populous municipalities in China and serves as an important center for trade and economics. This study distinguished secondary failures with high avidity from primary failures with low avidity IgG antibodies. Furthermore, the clinical severity of measles cases across various vaccination modes and schedules, as well as the clinical and serology features of high avidity and low avidity cases was analyzed. We were particularly interested in discovering whether waning immunity in adults and time since last vaccination were associated with secondary measles vaccine failures.
-
Data on measles cases was extracted from the China Information System for Disease Control and Prevention (CISDCP), a web-based communicable disease surveillance system that allows for reporting of communicable disease information from local Centers for Disease Control and Prevention (CDC), hospitals, and other health agencies. Health care providers in China are required to report cases of 39 infectious diseases, including measles, to a public health authority under the provision of the Law of the People’s Republic of China on Prevention and Treatment of Infectious Diseases. China’s central public health authority consists of a network of CDCs, headed by China’s national CDC. Province-level CDCs, including the Tianjin CDC, have jurisdiction over their own area. Approximately 3,000 smaller CDCs at the district and local levels report to provincial CDCs. When a measles diagnosis is made, health care providers report case information to their local CDC via the CISDCP web portal. Once case-based information has been entered, all CDC levels (local, district, provincial, and national) within the appropriate jurisdiction have access to the case information and provide coordinated investigative follow-up. In the routine examination of a suspected measles case in China, a serum specimen is collected, sent to a local CDC measles network laboratory and tested for measles-specific IgM in a commercial enzyme-linked immunosorbent assay (ELISA)[4].
-
This study described measles cases reported from 2013 through 2015. In line with China’s national measles surveillance guideline[16], a suspected measles case was defined by the presence of fever, rash, and either cough, coryza, or conjunctivitis. A laboratory confirmed case was defined by serological (positive serologic test for measles IgM antibody, or a fourfold rise in measles IgG by standard serologic assay) or virological [identification of the MV RNA by reverse-transcription polymerase chain reaction (RT-PCR), or isolation of the MV from a clinical specimen] evidence of acute measles infection. In order to improve accuracy while distinguishing between PVF and SVF in this study, a confirmed measles case was described as one that had been laboratory confirmed or that met the clinical definition and was epidemiologically linked to a laboratory confirmed case.
-
Serum specimens were collected for serological testing from CDCs in all districts of Tianjin. A measles networks laboratory was established for every district CDC to analyze specimens of suspected measles cases for definite diagnosis. In this study, the acute phase serum specimens of 284 confirmed measles from 2013 to 2015 were collected by the Tianjin CDC, and the specimens were delivered to the National Institute for Viral Disease Control and Prevention of the China CDC for measles IgM, IgG, and avidity testing. This study was part of the measles surveillance public health response program and not classified as a research study, therefore ethical approval was not required.
-
Quantitative results from measles-specific IgM and IgG antibody testing were obtained by using commercial ELISA kits (Virion/Serion, Wurzburg, Germany), as previously described[17]. Threshold levels for IgM antibody test results were as follows: > 15 U/mL was considered positive, 10–15 U/mL considered equivocal, and < 10 U/mL was considered negative. Threshold levels for IgG antibody were as follows: > 200 mIU/mL was considered positive, 150–200 mIU/mL was equivocal, and < 150 mIU/mL was considered negative. Avidity testing for measles-specific IgG was performed in serum samples by using a commercial enzyme immunoassay (EUROIMMUN, Lübeck, Germany). Over the past 15 years, enzyme immunoassay (EIA) based methods employing protein-denaturing agents such as urea have been used successfully years to distinguish between high avidity and low avidity IgG antibodies, both in diagnosis and research[10]. A considerable reduction of the EIA extinction value by urea treatment confirms the presence of low avidity antibodies in a patient’s serum. For an objective interpretation, the relative avidity index (RAI) was calculated and expressed as a percentage using extinction values with and without urea treatment. The upper limit of the range of low avidity antibodies (cut-off value) recommended by EUROIMMUN was 40% RAI. Values below the indicated cut-off were to be considered as an indication of the presence of low avidity antibodies, values between 40% and 60% RAI were considered equivocal, and values above 60% RAI indicated the presence of high avidity antibodies.
-
Descriptive analysis was performed. Results were reported as frequencies and proportions for categorical variables, while continuous variables were reported as median values and ranges. Analysis of all demographic characteristics for all measles cases was performed. Pearson’s chi-squared test (χ2) was used to compare proportions and rates, while the Student’s t-test was used to compare the geometric mean concentration (GMC) of measles IgM and IgG antibodies. All statistical analyses were performed using the SPSS software (version 24.0; SPSS, Inc., Chicago, IL). A two-tailed P-value was obtained, a P < 0.05 was classified as statistically significant.
-
The acute phase serum samples from 284 confirmed measles cases, including 280 laboratories confirmed cases and 4 epidemiologically linked confirmed cases, were collected from 2013 to 2015. Of the total number of cases, 278 (97.89%) were collected in 2014. The age range for patients with confirmed measles cases in this study was between 0 and 58 years, with 262 (92.25%) cases in patients aged ≥ 20 years. Measles IgG avidity testing showed high avidity measles IgG antibodies in 172 (60.56%) cases, indicating a secondary immune response to measles (Table 1), while 80 (28.17%) cases showed low avidity measles IgG antibodies, indicating a primary immune response to a primary measles infection. High avidity was detected in only 21.43% of cases in patients aged < 1 year. The proportion of high avidity cases increased with age, being significantly higher in 70.07% of cases in patients aged 30–39 years (χ 2= 17.27, P = 0.002). Low avidity was detected at a significantly higher rate of 57.14% in patients aged < 1 year (χ2 = 12.26, P = 0.016).
Table 1. Measles IgG avidity testing results by age group
Age groups (years) Cases No. (%) of MCV doses No. (%) Avidity testing classifications 0 1 ≥ 2 Unknown High avidity Equivocal Low avidity < 1 14 8 (57.14) 6 (42.86) 0 (0) 0 (0) 3 (21.43) 3 (21.43) 8 (57.14) 1–19 8 2 (25.00) 2 (25.00) 4 (50.00) 0 (0) 4 (50.00) 1 (12.50) 3 (37.50) 20–29 58 8 (13.79) 6 (10.34) 3 (5.17) 41 (70.69) 29 (50.00) 7 (12.07) 22 (37.93) 30–39 137 28 (20.44) 27 (19.71) 0 (0) 82 (59.85) 96 (70.07) 12 (8.76) 29 (21.17) ≥ 40 67 18 (26.87) 4 (5.97) 0 (0) 45 (67.16) 40 (59.70) 9 (13.43) 18 (26.87) Total 284 64 (22.54) 45 (15.85) 7 (2.46) 168 (59.15) 172 (60.56) 32 (11.27) 80 (28.17) Overall, 64 (22.54%) patients had not been vaccinated, 52 (18.31%) had received at least a dose of MCV. The vaccination status for 168 patients (59.15%) was unknown, all of whom were aged ≥ 20 years (Table 1). Of the 52 measles cases with a vaccination history, 41 (78.85%) demonstrated high avidity, indicating SVF. This is a significantly higher proportion of high avidity cases than was observed among unvaccinated patients (χ2 = 10.23, P = 0.001) and patients with an unknown vaccination status (χ2 = 6.81, P = 0.009). Low avidity was demonstrated in 9 (17.31%) cases, indicating PVF. While a significantly higher proportion (39.06%) of those who had not been vaccinated showed low avidity (χ2 = 6.84, P = 0.033), only one patient who had received ≥ 2 doses of MCV demonstrated low avidity (Table 2).
Table 2. Measles IgG avidity testing results by MCV vaccination status
No. of
MCV dosesCases No. (%) Avidity testing classifications High avidity Equivocal Low avidity 0 64 32 (50.00) 7 (10.94) 25 (39.06) 1 45 36 (80.00) 1 (2.22) 8 (17.78) ≥ 2 7 5 (71.43) 1 (14.29) 1 (14.29) Unknown 168 99 (58.93) 23 (13.69) 46 (27.38) Total 284 172 (60.56) 32 (11.27) 80 (28.17) -
All measles cases in this study presented with fever and rash. Cough was present in 61.54% of vaccinated patients, 76.56% of non-vaccinated patients, and in 80.95% of patients with unknown vaccination status. There were no significant differences in the presentation of coryza, conjunctivitis, and Koplik spots among three groups of patients, as classified by vaccination status (Table 3). In the 52 vaccinated patients, there was no significant difference in severity of clinical symptoms between high avidity and low avidity measles cases. Similarly, there were no significant differences in the presentation of cough, coryza, conjunctivitis and Koplik spots between high avidity and low avidity cases overall (Table 4). Regardless of vaccination status, clinical severity was significantly lower in high avidity measles cases than in low avidity measles cases (P < 0.001).
Table 3. Clinical symptoms in measles cases classified by vaccination status
Symptoms Cases Vaccinated patients Unvaccinated patients Patients with unknown status Chi-square P No. % No. % No. % Fever 284 52 100.00 64 100.00 168 100.00 − − Rash 284 52 100.00 64 100.00 168 100.00 − − Cough 217 32 61.54 49 76.56 136 80.95 8.30 0.016 Coryza 132 17 32.69 32 50.00 83 49.40 4.87 0.088 Conjunctivitis 144 21 40.38 34 53.13 89 52.98 2.27 0.258 Koplik spots 115 17 32.69 28 43.75 70 41.67 1.69 0.429 Table 4. Clinical symptoms in high avidity and low avidity measles cases
Symptoms Vaccinated patients All cases High avidity Low avidity Chi-square P High avidity Low avidity Chi-square P No. % No. % No. % No. % Cough 22 53.66 8 88.89 2.49 0.115 111 64.53 76 95.00 26.48 < 0.001 Coryza 10 24.39 5 55.56 2.09 0.148 57 33.14 54 67.50 26.16 < 0.001 Conjunctivitis 14 34.15 5 55.56 0.67 0.413 67 38.95 54 67.50 17.83 < 0.001 Koplik spots 10 24.39 5 55.56 2.09 0.148 52 30.23 44 55.00 14.20 < 0.001 -
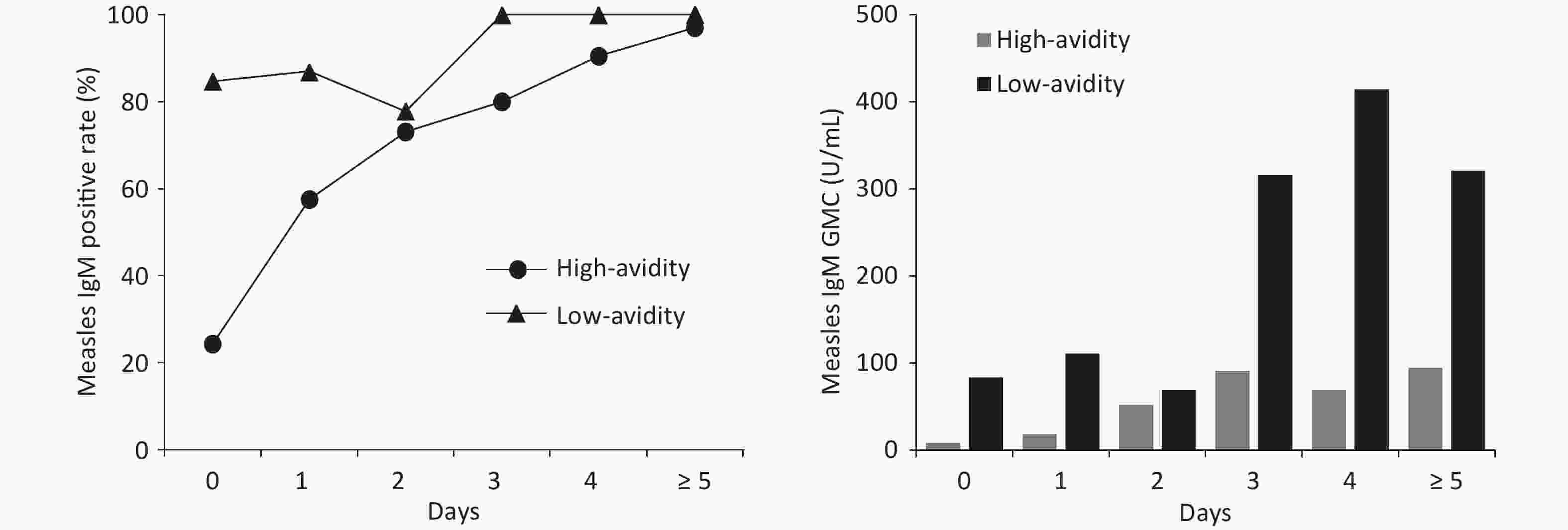
Of the 284 measles cases, the serum collection period was 0–24 days after rash onset. The median was 2 days and interval of quartiles (IQR) was 1–4 days. A positive measles IgM result was obtained in 76.06% (216) of cases. The positive measles IgM rate for high avidity and low avidity measles cases were 66.28% and 91.25%, respectively. The rate was significantly lower in high avidity measles cases (χ2 = 17.79, P < 0.001). When serum samples were collected on 0 day after rash onset, positive IgM rate only was 24.32% in high avidity measles cases, compared with 83.33% in low avidity measles cases. Similarly, GMC of measles IgM was significantly lower (33.73 U/mL) in high avidity cases than in low avidity cases (166.07 U/mL) (t = −6.99, P < 0.001). Positive IgM rate and GMC remained lower in high avidity cases several days after rash onset (Figure 1).
-
In 2005, the Regional Committee for the WHO Western Pacific Region (WPR) established a goal for measles elimination by 2012. To achieve this, all 37 WPR countries including China implemented the recommended strategies in the WPR Plan of Action for Measles Elimination[18]. Although that goal was unrealized, the WPR is on track to eliminate measles by 2020. However, 3,258 measles cases were reported in Tianjin from 2013 to 2015. In the 2014 Tianjin outbreak, 2,036 cases (75.32%) occurred in patients aged ≥ 20 years, the highest percentage in China. Following Tianjin was Beijing at 71.36% and Shanghai at 69.18%. In addition, the Chinese national average was only 42.78%[19]. A study investigating the population profile for measles susceptibility between 2011 and 2015 was conducted in Tianjin in collaboration with the University of Michigan. In this study, 2818 people were enrolled from 120 villages and communities, and it showed that the negative measles IgG rate was over 10% in participants aged between 20 and 39 years in Tianjin. Furthermore, immunity to measles was lower than the herd immunity threshold[20]. As a result, the majority of measles cases found in our study were in patients aged ≥ 20 years.
As shown by a measles IgG avidity assay, more than 70% of cases in patients aged 30–39 years demonstrated high avidity, significantly higher than in other age groups. In 1973, Tianjin began to supply MCV in one dose as part of the EPI program. In 1986, MCV supply was modified to two doses. Therefore, the majority of the population aged between 30 and 39 years received the single dose MCV. It was difficult to find documented vaccination history of patients aged ≥ 20 years. As a result, the majority of vaccination histories obtained in adults were from anecdotal accounts. With a high percentage of high avidity (70.07%), patients aged between 30 and 39 years had a high proportion of SVF in our study. This suggests that, in several instances, it is possible that a single MCV dose did not confer life-long immunity.
Measles avidity increased with time of exposure or immunization[15], so we examined acute phase serum samples of all measles cases. Equivocal results were excluded in the distinction between PVF and SVF. In our study, SVF occurred in 78.85% of vaccinated patients in Tianjin. In a study conducted in Iran, SVF occurred in only 24.28% in vaccinated patients. However, 64.38% of total measles cases in their study were in patients aged < 20 years[21]. SVF was first reliably described as recently as 1987[22]. Rather than waning immunity, it was often attributed to the poor heat stability associated with older vaccines[23]. However, a study during a US measles resurgence between 1989 and 1991 showed that waning immunity played an important role in measles vaccine failures[24]. An investigation into a measles outbreak in a middle school with high vaccination coverage in Beijing, China showed that the length of time since the last MCV was significantly associated with risk of measles. Compared to an MCV dose received within the previous 5 years, the risk of developing measles was 4.6 and 5.5 times higher if the last MCV dose was 5–9 years and ≥ 10 years prior, respectively[25]. In waning immunity, a higher incidence of high avidity cases would be expected with a longer period since vaccination, as was the case in our study. A higher proportion of high avidity cases occurred in participants aged 30–39 years, indicating that that vaccine-induced immunity likely waned after 30 years, especially in participants who received the single dose MCV before 1986 in Tianjin. As a result, to achieve better results in the quest for elimination of the MV, a further dose of vaccines should be recommended for adults aged between 30 and 39 years in Tianjin.
Measles in previously vaccinated patients is reported to be associated with modified disease. Severe clinical features were 2.8 times more likely in unvaccinated cases than in vaccinated cases (OR = 2.8, 95% CI 1.5–5.0)[26]. In our study, no significant differences were observed in clinical symptoms based on vaccination status. Furthermore, in vaccinated patients, there was no significant difference observed in clinical symptoms between high avidity and low avidity cases. This result was associated with a lack of documented vaccination history in adults. Disregarding vaccination status, clinical symptoms were significantly milder in high avidity measles cases than in low avidity cases.
Previously vaccinated children who develop measles are likely to have less severe clinical symptoms, as well as inconclusive serology results, particularly for IgM antibody, if tested in the first few days following rash onset[26]. The occurrence of a primary antibody response, a delayed IgG response with IgM production, is an indication of PVF in confirmed measles cases found in participants with a history of vaccination. A secondary or anamnestic response, a rapid and heightened IgG response with absent or few IgM, is an indication of SVF. Prior to the introduction of measles IgG avidity testing, IgM negativity was used as a surrogate marker for SVF. Our study, which found that 66.28% of measles cases confirmed by a positive IgM exhibited a secondary immune response, and a positive IgM rate of 24.32% in high avidity measles on day 0 post rash onset, provided further evidence that the presence of IgM cannot be used as a reliable indicator of SVF[27].
Because SVF cases have generally mild clinical features and inconclusive IgM serology results, they may be missed unless considered within the context of an outbreak, or linked to an acute, severe case of measles. Measles resulting from SVF may be less contagious than cases resulting from PVF. Theoretical calculations of the critical community size in which endemic measles transmission can be sustained yield values larger than those seen in reality, suggesting that subclinical infections may contribute to transmission of the measles virus[28]. Subclinical cases of measles, as well as cases due to waning immunity have been described among vaccinated individuals in England, especially following long periods without natural boosters[29]. In 2011, a measles outbreak among previously immunized individuals was reported in New York City, including the first report of transmission from a twice-vaccinated individual with documented SVF[30]. Recently, measles outbreaks in Beijing and Tianjin have suggested that SVF plays a significant role in measles transmission[25, 31].
This study has several limitations. Firstly, vaccination status was unknown in 59.15% of cases examined, all such patients were aged ≥ 20 years. This made analysis of clinical symptoms in vaccinated and unvaccinated measles cases unreliable. Therefore, measles IgG avidity testing was used to assess clinical severity again. In addition, all confirmed measles cases were defined by serological or virological evidence of acute measles infection. Therefore, some subclinical cases may have been missed. In periods between measles outbreaks, surveillance is increasingly crucial, as both clinical and serological diagnoses using specific IgM antibodies may be non-specific in epidemic periods. By testing IgM and IgG avidity testing simultaneously, specificity can be significantly improved[32]. IgG avidity testing can play an important role in distinguishing between PVF and SVF[33]. Furthermore, avidity testing may be coupled with conventional humoral techniques to understand how immunity can be induced in various settings and under future revaccination programs.
-
Understanding PVF and SVF is important for assessing the success of measles control programs in developing countries. There is enough evidence to necessitate monitoring for possible waning immunity in vaccine failure patients, particularly in areas where measles cases have been confirmed in vaccinated adults. Therefore a further dose of vaccination should be recommended for adults aged 30–39 years in Tianjin. Given the consistency of our results, measles IgG avidity testing is a reliable and feasible tool for studying the determinants of quality and duration of immunity after measles vaccinations.
-
We acknowledge the valuable contributions of laboratory staff at the National Institute for Viral Disease Control and Prevention. We thank QU Jiang Wen, SUN Jing, CHEN Wei, LIU Yang, and TIAN Hong from the Tianjin Center for Disease Control and Prevention for their assistance in field investigation and laboratory testing. We are grateful to all of the staff in local hospitals and Centers for Disease Control and Prevention who arranged the collection and shipment of samples and helped clarify details of suspected measles cases. We also thank all study participants for their enthusiasm and cooperatively participating in the investigation.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Tianjin Center for Disease Control and Prevention.
-
The authors declare no conflict of interest.
doi: 10.3967/bes2019.102
Measles Virus IgG Avidity Assay for Use in Identification of Measles Vaccine Failures in Tianjin, China
-
Abstract:
Objective To identify measles vaccine failures in Tianjin, China using a measles virus IgG avidity assay. Methods The China Information System for Disease Control and Prevention (CISDCP) was used to collect information about measles cases and blood specimens in Tianjin from 2013 to 2015. Measles-specific IgM and IgG antibodies were detected using Enzyme-Linked Immunosorbent Assay (ELISA). Avidity testing for measles IgG was performed using a commercial enzyme immunoassay (EIA). Results A total of 284 confirmed measles cases were identified. Of this total, 262 (92.25%) were in patients aged ≥ 20 years. High avidity was exhibited in 172 (60.56%) cases, while 80 (28.17%) cases demonstrated low avidity. High avidity was detected in only 21.43% of cases in patients aged < 1 year. The proportion of high avidity increased with age, and was significantly higher in patients aged 30–39 years at 70.07% (χ2 = 17.27, P = 0.002). Of the 52 measles cases in patients with a history of vaccinations, 41 (78.85%) cases showed high avidity, indicating secondary vaccine failures (SVF). In these vaccinations, there was no significant difference (P > 0.05) in clinical severity between high avidity and low avidity cases. However, regardless of vaccination status, clinical severity was significantly lower in high avidity cases (P < 0.001) than in low avidity cases. The percentages of positive measles IgM results in high avidity and low avidity cases were 66.28% and 91.25%, respectively. Geometric Mean Concentration (GMC) was significantly lower in high avidity cases at 33.73 U/mL, compared to 166.07 U/mL in low avidity cases. Conclusions Low clinical severity and inconclusive IgM antibody results are more likely in high avidity measles cases. Measles cases were more common in adults. Therefore, a further dose of vaccines should be recommended for 30–39 years in Tianjin. -
Key words:
- Measles /
- IgG avidity /
- China /
- Primary vaccine failures /
- Secondary vaccine failures
-
Table 1. Measles IgG avidity testing results by age group
Age groups (years) Cases No. (%) of MCV doses No. (%) Avidity testing classifications 0 1 ≥ 2 Unknown High avidity Equivocal Low avidity < 1 14 8 (57.14) 6 (42.86) 0 (0) 0 (0) 3 (21.43) 3 (21.43) 8 (57.14) 1–19 8 2 (25.00) 2 (25.00) 4 (50.00) 0 (0) 4 (50.00) 1 (12.50) 3 (37.50) 20–29 58 8 (13.79) 6 (10.34) 3 (5.17) 41 (70.69) 29 (50.00) 7 (12.07) 22 (37.93) 30–39 137 28 (20.44) 27 (19.71) 0 (0) 82 (59.85) 96 (70.07) 12 (8.76) 29 (21.17) ≥ 40 67 18 (26.87) 4 (5.97) 0 (0) 45 (67.16) 40 (59.70) 9 (13.43) 18 (26.87) Total 284 64 (22.54) 45 (15.85) 7 (2.46) 168 (59.15) 172 (60.56) 32 (11.27) 80 (28.17) Table 2. Measles IgG avidity testing results by MCV vaccination status
No. of
MCV dosesCases No. (%) Avidity testing classifications High avidity Equivocal Low avidity 0 64 32 (50.00) 7 (10.94) 25 (39.06) 1 45 36 (80.00) 1 (2.22) 8 (17.78) ≥ 2 7 5 (71.43) 1 (14.29) 1 (14.29) Unknown 168 99 (58.93) 23 (13.69) 46 (27.38) Total 284 172 (60.56) 32 (11.27) 80 (28.17) Table 3. Clinical symptoms in measles cases classified by vaccination status
Symptoms Cases Vaccinated patients Unvaccinated patients Patients with unknown status Chi-square P No. % No. % No. % Fever 284 52 100.00 64 100.00 168 100.00 − − Rash 284 52 100.00 64 100.00 168 100.00 − − Cough 217 32 61.54 49 76.56 136 80.95 8.30 0.016 Coryza 132 17 32.69 32 50.00 83 49.40 4.87 0.088 Conjunctivitis 144 21 40.38 34 53.13 89 52.98 2.27 0.258 Koplik spots 115 17 32.69 28 43.75 70 41.67 1.69 0.429 Table 4. Clinical symptoms in high avidity and low avidity measles cases
Symptoms Vaccinated patients All cases High avidity Low avidity Chi-square P High avidity Low avidity Chi-square P No. % No. % No. % No. % Cough 22 53.66 8 88.89 2.49 0.115 111 64.53 76 95.00 26.48 < 0.001 Coryza 10 24.39 5 55.56 2.09 0.148 57 33.14 54 67.50 26.16 < 0.001 Conjunctivitis 14 34.15 5 55.56 0.67 0.413 67 38.95 54 67.50 17.83 < 0.001 Koplik spots 10 24.39 5 55.56 2.09 0.148 52 30.23 44 55.00 14.20 < 0.001 -
[1] Watson JC, Hadler SC, Dykewicz CA, et al. Measles, mumps, and rubella--vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep, 1998; 47, 1−57. [2] Hayman DTS. Measles vaccination in an increasingly immunized and developed world. Hum Vaccin Immunother, 2019; 15, 28−33. doi: 10.1080/21645515.2018.1517074 [3] Dabbagh A, Patel MK, Dumolard L, et al. Progress Toward Regional Measles Elimination - Worldwide, 2000-2016. MMWR Morb Mortal Wkly Rep, 2017; 66, 1148−53. doi: 10.15585/mmwr.mm6642a6 [4] Ma C, Hao L, Zhang Y, et al. Monitoring progress towards the elimination of measles in China: an analysis of measles surveillance data. Bull World Health Organ, 2014; 92, 340−7. doi: 10.2471/BLT.13.130195 [5] Wang X, Boulton ML, Montgomery JP, et al. The epidemiology of measles in Tianjin, China, 2005-2014. Vaccine, 2015; 33, 6186−91. doi: 10.1016/j.vaccine.2015.10.008 [6] Wang Y, Liu R, Lu M, et al. Enhancement of safety and immunogenicity of the Chinese Hu191 measles virus vaccine by alteration of the S-adenosylmethionine (SAM) binding site in the large polymerase protein. Virology, 2018; 518, 210−20. doi: 10.1016/j.virol.2018.02.022 [7] Zheng J, Zhou Y, Wang H, et al. The role of the China Experts Advisory Committee on Immunization Program. Vaccine, 2010; 28, A84−7. [8] Lixia W, Guang Z, Lee LA, et al. Progress in accelerated measles control in the People's Republic of China, 1991-2000. J Infect Dis, 2003; 187(Suppl 1), S252−7. [9] Wagner AL, Zhang Y, Mukherjee B, et al. The impact of supplementary immunization activities on the epidemiology of measles in Tianjin, China. Int J Infect Dis, 2016; 45, 103−8. [10] Paunio M, Hedman K, Davidkin I, et al. IgG avidity to distinguish secondary from primary measles vaccination failures: prospects for a more effective global measles elimination strategy. Expert Opin Pharmacother, 2003; 4, 1215−25. doi: 10.1517/14656566.4.8.1215 [11] Inouye S, Hasegawa A, Matsuno S, et al. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. J Clin Microbiol, 1984; 20, 525−9. [12] Paunio M, Hedman K, Davidkin I, et al. Secondary measles vaccine failures identified by measurement of IgG avidity: high occurrence among teenagers vaccinated at a young age. Epidemiol Infect, 2000; 124, 263−71. doi: 10.1017/S0950268899003222 [13] de Souza VA, Pannuti CS, Sumita LM, et al. Enzyme-linked immunosorbent assay-IgG antibody avidity test for single sample serologic evaluation of measles vaccines. J Med Virol, 1997; 52, 275−9. doi: 10.1002/(SICI)1096-9071(199707)52:3<275::AID-JMV7>3.0.CO;2-# [14] Goldblatt D, Vaz AR, Miller E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J Infect Dis, 1998; 177, 1112−5. doi: 10.1086/517407 [15] Mercader S, Garcia P, Bellini WJ. Measles virus IgG avidity assay for use in classification of measles vaccine failure in measles elimination settings. Clin Vaccine Immunol, 2012; 19, 1810−7. doi: 10.1128/CVI.00406-12 [16] National measles surveillance guideline. Beijing: Chinese Ministry of Health. http://www.moh.gov.cn/jkj/s3581/200902/9ea8e400444c45b0a48e3e55d929c9b5.shtml. [2018-10-13]. (In Chinese) [17] Fu J, Jiang C, Wang J, et al. A hospital-associated measles outbreak in health workers in Beijing: implications for measles elimination in China, 2018. Int J Infect Dis, 2019; 78, 85−92. doi: 10.1016/j.ijid.2018.10.023 [18] Hagan JE, Kriss JL, Takashima Y, et al. Progress Toward Measles Elimination - Western Pacific Region, 2013-2017. MMWR Morb Mortal Wkly Rep, 2018; 67, 491−5. doi: 10.15585/mmwr.mm6717a3 [19] Ma C, Su QR, Wen N, et al. Measles epidemiology in China, 2014. Disease Surveillance, 2015; 30, 818−23. [20] Boulton ML, Wang X, Zhang Y, et al. A population profile of measles susceptibility in Tianjin, China. Vaccine, 2016; 34, 3037−43. doi: 10.1016/j.vaccine.2016.04.094 [21] Hamkar R, Mahmoodi M, Nategh R, et al. Distinguishing between primary measles infection and vaccine failure reinfection by IgG avidity assay. East Mediterr Health J, 2006; 12, 775−82. [22] Reyes MA, de Borrero MF, Roa J, et al. Measles vaccine failure after documented seroconversion. Pediatr Infect Dis J, 1987; 6, 848−51. doi: 10.1097/00006454-198709000-00012 [23] Markowitz LE, Preblud SR, Fine PE, et al. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J, 1990; 9, 101−10. doi: 10.1097/00006454-199002000-00008 [24] Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis, 1990; 162, 1036−42. doi: 10.1093/infdis/162.5.1036 [25] Ma R, Lu L, Zhangzhu J, et al. A measles outbreak in a middle school with high vaccination coverage and evidence of prior immunity among cases, Beijing, China. Vaccine, 2016; 34, 1853−60. doi: 10.1016/j.vaccine.2015.11.006 [26] Mitchell P, Turner N, Jennings L, et al. Previous vaccination modifies both the clinical disease and immunological features in children with measles. J Prim Health Care, 2013; 5, 93−8. doi: 10.1071/HC13093 [27] Pannuti CS, Morello RJ, Moraes JC, et al. Identification of primary and secondary measles vaccine failures by measurement of immunoglobulin G avidity in measles cases during the 1997 Sao Paulo epidemic. Clin Diagn Lab Immunol, 2004; 11, 119−22. [28] Keeling MJ, Grenfell BT. Understanding the persistence of measles: reconciling theory, simulation and observation. Proc Biol Sci, 2002; 269, 335−43. doi: 10.1098/rspb.2001.1898 [29] Glass K, Grenfell BT. Waning immunity and subclinical measles infections in England. Vaccine, 2004; 22, 4110−6. doi: 10.1016/j.vaccine.2004.02.047 [30] Rosen JB, Rota JS, Hickman CJ, et al. Outbreak of measles among persons with prior evidence of immunity, New York City, 2011. Clin Infect Dis, 2014; 58, 1205−10. doi: 10.1093/cid/ciu105 [31] Yaxing Ding, Yimin Sun, Yang Liu, et al. A measles outbreak in a middle school with high vaccination coverage in Tianjin,2016. Chinese Journal of Vaccines and Immunization. 2016; 23, 62-6. (In chinese) [32] Sowers SB, Rota JS, Hickman CJ, et al. High Concentrations of Measles Neutralizing Antibodies and High-Avidity Measles IgG Accurately Identify Measles Reinfection Cases. Clin Vaccine Immunol, 2016; 23, 707−16. doi: 10.1128/CVI.00268-16 [33] Hickman CJ, Hyde TB, Sowers SB, et al. Laboratory characterization of measles virus infection in previously vaccinated and unvaccinated individuals. J Infect Dis, 2011; 204(Suppl 1), S549−58. -




 下载:
下载:



 Quick Links
Quick Links