-
Zika virus (ZIKV) is a mosquito-borne virus that was first identified in a monkey in Uganda in 1947[1,2]. Subsequently, ZIKV spread eastward across the Pacific Ocean to the Americas, resulting in the 2015–2016 epidemic[3]. ZIKV has been detected in many species of Aedes mosquito, along with Anopheles coustani, Mansonia uniformis, and Culex perfuscus; however, this alone does not incriminate these mosquitoes as vectors of ZIKV[4]. The infection often causes no symptoms or symptoms similar to a very mild form of dengue fever[1]. ZIKV is mainly transmitted through infected mosquito bites (Aedes spp.), but it can also spread through sexual contact and from a pregnant woman to her fetus[4,5]. ZIKV infections in adults can trigger Guillain–Barré syndrome[6] and cause microcephaly, and other severe brain anomalies in infants[7]. ZIKV belongs to the genus Flavivirus of family Flaviviridae, and is related to dengue, Japanese encephalitis (JE), yellow fever, and West Nile viruses. Like other flaviviruses, ZIKV is enveloped and icosahedral with a non-segmented, single-stranded, 11-kilobase, positive-sense RNA genome[8].
China has reported several cases of imported ZIKV infection in 2016, and the risk of autochthonous circulation following the imported ZIKV spreading will be inevitable due to the distribution of Aedes mosquitoes in southern China[9,10]. There were no previous reports of ZIKV being isolated from a vector in China until the isolation of two strains of ZIKV from Culex pipiens quinquefasciatus and Armigeres subalbatus in 2016 from Dejiang prefecture (107°36′–108°28′ E, 28°00′–28°38′ N) in northeast Guizhou Province in China[11]. Guizhou is located on the Yunnan Guizhou Plateau in southwest China and has a sub-tropical climate with abundant rainfall that is suitable for breeding for a variety of hematophagous insects. Guizhou Province has had a high prevalence of JE[12,13], and many JE viruses (JEVs) and Getah viruses have been isolated from mosquito samples collected from Guizhou[14]. It is not clear whether ZIKV circulates in local population and animals. In this study, we examined serum from healthy humans and domestic animals for ZIKV infection to determine the local ZIKV transmission risk.
-
This study was approved by the institutional review board of the National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention. All study participants provided informed consent before blood collection. No animals were killed to conduct this study.
-
Baby Syrian hamster kidney (BHK-21) cells were preserved in our laboratory and maintained in Minimal Essential Medium (MEM) supplemented with 10% fetal bovine serum (FBS) and 20,000 U/mL penicillin-streptomycin (PS) at 37 °C with 5% CO2. The cells were cultivated in PS-MEM with 2% FBS when infected with the virus. The ZIKV strain GZDJ1685 (GenBank MF099651) used in this study was isolated in our laboratory from C. p. quinquefasciatus specimens collected in 2016 from Dejiang prefecture in Guizhou Province[11]. The JEV P3 strain (GenBank JEU47032) was preserved in our laboratory[15].
-
Blood from humans and livestock was collected in October 2017 at Shidatou Village in Dejiang prefecture. Blood samples were collected from residents of all ages. Blood was collected from the livestock that were mainly raised locally. The blood samples were centrifuged to obtain serum and refrigerated for future analysis after aliquoting.
-
We used the 90% plaque reduction neutralization test (PRNT90) to detect neutralizing antibodies against ZIKV. The serum was inactivated at 50 °C for 30 min, and then diluted to 1:10 for prescreening ZIKV-neutralizing antibodies. Then, it was mixed with an equal volume of 200 pfu ZIKV suspension and incubated at 37 °C for 1 h. The mixture was added to BHK-21 cells plated in 6-well culture plates and incubated at 37 °C for 1 h, and then overlaid with 1.1% methylcellulose-MEM culture medium with 2% FBS for 4–5 days. Crystal Violet staining was performed to calculate the number of plaques. Samples that showed protective capacity against ZIKV infection in prescreening were diluted from 1:10 to 1:1,280 in two parallel wells to determine the antibody titers. A PRNT90 titer is the dilution of a sample at which 90% reduction of plaques is observed as compared to a virus backdropping control. The neutralizing antibody titers of JEV were detected in parallel to any ZIKV protective antibody-positive specimens. The specimens were judged seropositive when PRNT90-neutralizing antibody titer was ≥ 1:10. When the PRNT90 anti-ZIKV antibody titer was at least 4-fold greater than that of the corresponding anti-JEV antibody titer in the same specimen, it was identified as ZIKV infection rather than JEV infection. If the PRNT90 anti-JEV antibody titer was at least 4-fold greater than that of the corresponding anti-ZIKV antibody titer in the same specimen, it was identified as JEV infection[16]. If the antibody titers of the two viruses differed by less than 4-fold in the same specimen, it was identified as coinfection with ZIKV and JEV.
-
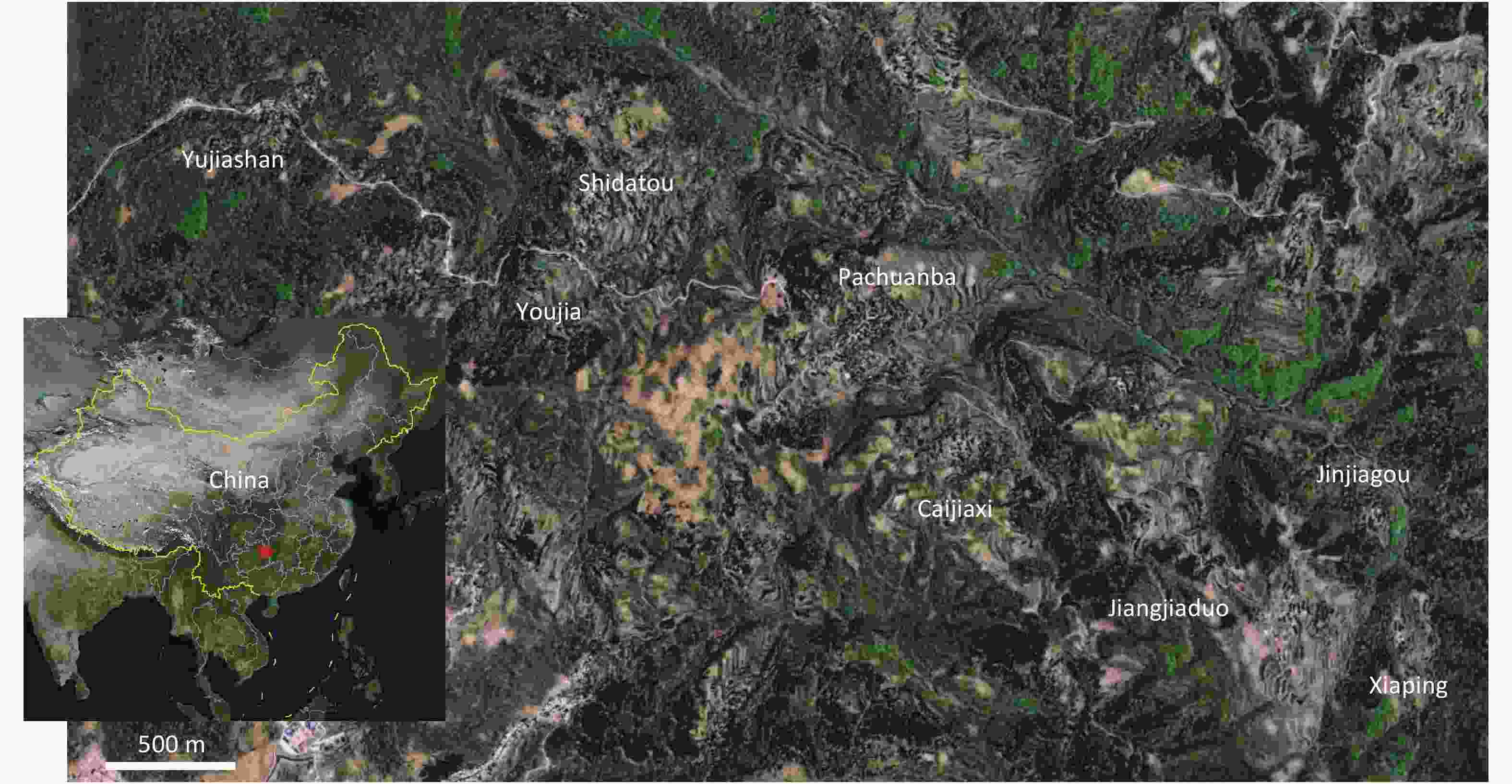
Blood was collected from October 27 to November 2, 2017 at Shidatou village in Dejiang prefecture in Guizhou Province. This study analyzed the samples from 366 healthy individuals collected from eight groups in Shidatou (the Yujiashan, Youjia, Shidatou, Pachuanba, Caijiaxi, Jinjiagou, Jiangjiatuo, and Xiaping groups) and 104 domestic animals (63 chickens, 11 pigs, and 30 sheep) from the Yujiashan, Shidatou, and Jiangjiatuo groups (Figure 1, Table 1). Of the human subjects, 29.9% were 0–19 years old, 28.1% were 20–49 years old, and 42.0% were ≥ 50 years old.
Table 1. Summary of the collected serum of human health and domestic animal
Variable Number (n) Constituent ratio (n%) Human Age group (Year) 0−9 69 18.8 10−19 40 10.9 20−29 26 7.1 30−39 31 8.5 40−49 46 12.5 50−59 54 14.7 60−69 63 17.2 > 70 37 10.1 Sex Man 171 46.7 Woman 195 53.2 Nationality Tu 364 99.4 Han 2 0.6 Total 366 Animal Chicken 63 60.5 pig 11 10.5 sheep 30 28.8 Total 104 -
None of the human serum specimens tested positive for ZIKV PRNT90, while three animals (chicken D32, sheep D56, and sheep D61) tested positive, with neutralizing antibody titers of 1:10, 1:20, and 1:40, respectively (Table 2). The three positive animal specimens were further tested for JEV neutralizing antibody titers: D32 was negative, while D56 and D61 were positive for JEV PRNT90 neutralizing antibody, with titers of 1:40 and 1:80, respectively (Table 2). As the titers of the two viruses did not reach a 4-fold difference in the same specimen, D56 and D61 were identified as being coinfected with ZIKV and JEV.
Table 2. Neutralizing antibody titer to ZIKA and JEV infection among domestic animals in Shidatou village
Sample ID Collection site Host species PRNT90 titer* ZIKV JEV D32 Yujiashan chicken 1:10 − D56 Shidatou sheep 1:20 1:40 D61 Shidatou sheep 1:40 1:80 Note.*PRNT 90 titer, 90% plaque reduction neutralization test titer. In summary, all 366 residents and 11 pigs whose samples were collected, tested negative for ZIKV neutralizing antibody; 1 of 63 chickens (1.59%) was positive for ZIKV neutralizing antibody but negative for JEV, and 2 of 30 sheep (6.67%) were positive for both ZIKV and JEV neutralizing antibodies (Table 3).
Table 3. The seroprevalence of ZIKV in human and domestic animals in Shidatou village
Host species No. case No. PRNT90 positive Seroprevalence (%) ZIKV JEV Human 366 − − − Chicken 63 1 − 1.59 Pig 11 − − − Sheep 30 2 2 6.67 -
This study follows up on the first isolation of a ZIKV strain in 2016 from Guizhou Province in China. It assessed the local risk of ZIKV infection in Dejiang prefecture in Guizhou Province. Shidatou Village, the region from where the ZIKV GZDJ1685 was isolated from a mosquito and this serological survey was conducted, covers an area of 8.3 square kilometers dominated by karst landforms[11]. Because of poor local infrastructure, the residents live in dispersed groups (the Yujiashan, Youjia, Shidatou, Pachuanba, Caijiaxi, Jinjiagou, Jiangjiatuo, and Xiaping groups). Most derive their incomes from agriculture, farm animals (pigs, sheep, and chickens) and aquaculture. The registered population of the village is 1,278, but young and middle-aged laborers have moved to urban areas and the local population is decreasing. The residents are mainly the elderly and children under 15 years of age. In this study, no ZIKV infection was seen in humans in the Shidatou region, while three of the animals sampled had suspected ZIKV infection.
ZIKV belongs to the flaviviruses, and its antigenic structure is very similar to that of the other flaviviruses[17], whose main antibody-binding sites are prM protein, E protein, and secretory non-structural protein 1 (NS1); this also applies to ZIKV[18]. Antibodies to ZIKV persist in the serum of infected individuals after ZIKV infection. There may be cross-reactivity between different flaviviruses, so an accurate serological diagnosis of ZIKV has always been problematic. The criterion classifies recent infection as samples with a 4-fold difference between two flaviviruses in the same specimen[19].
We conducted PRNT90 test only for ZIKV and JEV in this study as Guizhou Province is a traditionally epidemic region for JE[12,13], and no dengue virus or other flaviviruses were detected in local arbovirus survey in 2016 except JEV and ZIKV [11]. The results showed that only one animal (D32) was suspected of having ZIKV infection and showed no protection against JEV. Another two animals (D56 and D61) might have had ZIKV and JEV coinfection.
While virus detection from field vector samples is important for identifying putative vectors of the virus, the natural blood feeding pattern of the candidate vector species is also critical for understanding the role of a putative vector species in pathogen transmission. The two ZIKV strains isolated in Shidatou were from C. p. quinquefasciatus and A. subalbatus. In this area, the three dominant mosquito species are Anopheles sinensis, C. p. quinquefasciatus, and A. subalbatus while no Aedes mosquitoes have been found locally. The strain used for the PRNT90 test in this study was GZDJ1685 strain isolated from C. p. quinquefasciatus in 2016 [11]. Our results showed an extremely low seropositive rate in local animals. The local Center for Disease Control and Prevention is also investigating Guillain-Barré syndrome and microcephaly in Dejiang prefecture, but no cases have been discovered (data not shown). Therefore, we consider that ZIKV has not developed an effective transmission cycle between mosquitos and local animals or humans. In nature, C. p. quinquefasciatus and A. subalbatus may be incompetent vectors for ZIKV transmission[20].
The primary mosquito vectors for ZIKV belong to the genus Aedes, most notably Ae. aegypti and Ae. albopictus[21-24]. ZIKV is believed to be mainly transmitted by the human-biting mosquito Ae. aegypti[25,26]. Experimental infections with epidemic ZIKV strains have demonstrated that populations of Ae. aegypti and Ae. albopictus are associated with the risk of local transmission[27]. ZIKV has been isolated from many other mosquito species and genera, but those mosquito species were incompetent for ZIKV transmission[20,28-32]. The biological characteristics of different mosquito species differ markedly. The anthropophilic mosquitoes of Aedes feed during the day, while Culex and Armigeres feed at dawn and dusk. Aedes likes fresh water while Culex likes dirty water and Armigeres likes manure pits. Aedes specializes in feeding on humans while Armigeres prefers cows and humans and Culex prefers birds. A highly competent vector that does not feed on a competent host species will not contribute to virus transmission in nature[33]. With regard to ZIKV, for which humans are competent hosts, since both C. p. quinquefasciatus and A. subalbatus obtain a relatively low percentage of blood meals from humans, they are unlikely to serve as the principal transmission vectors of the virus. As there is no competent vector to support the replication of ZIKV and the risk of ZIKV transmission in the target region, we conclude that there is a low risk of ZIKV transmission in the Dejiang prefecture and even in Guizhou. The results of this ZIKV serological survey support this view.
-
In summary, this study conducted a serological survey of ZIKV infection in animals and humans in Shidatou village, where ZIKV-positive C. p. quinquefasciatus and A. subalbatus mosquitoes were reported previously. None of the humans tested positive for ZIKV PRNT90 while three livestock specimens tested positive for ZIKV PRNT90. The results revealed a low risk of local ZIKV transmission in Dejiang prefecture in Guizhou province in China. However, further mosquito surveillance should be performed locally on an annual basis to better understand the transmission dynamics and public health threat of ZIKV in this community.
-
We thank the staff of the Disease Control and Prevention of Dejiang and Maternal & Child Care Service Centre of Dejiang for their hard work during the sample-collection process.
-
LI Fan, WANG Huan Yu, and ZHANG Yan Ping designed the study; WANG Ding Ming, LI Fan, ZHOU Jing Zhu, TIAN Zhen Zao, SHAO Nan, and TANG Guang Peng collected the serum samples; LI Fan and FU Shi Hong performed the PRNT experiments; FU Shi Hong, LEI Wen Wen, and HE Ying performed virus culture; LI Fan, WANG Qi, and LI Dan processed the data and created the figure; LI Fan, ZHOU Jing Zhu, ZHOU Lei, and LIANG Guo Dong contributed to drafting and editing the paper; all authors reviewed the manuscript.
-
All authors declare that they have no conflicts of interest.
doi: 10.3967/bes2019.108
Serological Survey of Zika Virus in Humans and Animals in Dejiang Prefecture, Guizhou Province, China
-
Abstract:
Objective The current outbreak of Zika virus (ZIKV) poses a severe threat to human health. Two ZIKV strains were isolated from mosquitoes collected from the Dejiang prefecture in China in 2016, which was the first isolation of ZIKV in nature in China. Methods In this study, serum samples were collected from 366 healthy individuals and 104 animals from Dejiang prefecture in 2017, and the plaque reduction neutralization test (PRNT) was used to evaluate the seroprevalence of ZIKV. Results None of the 366 residents from whom the samples were collected were seropositive for ZIKV. None of the 11 pigs from whom the samples were collected were seropositive for ZIKV, while 1 of 63 (1.59%) chickens and 2 of 30 (6.67%) sheep were seropositive for ZIKV. Conclusions The extremely low seropositivity rate of ZIKV antibodies in animals in the Dejiang prefecture, Guizhou province in this study indicates that ZIKV can infect animals; however, there is a low risk of ZIKV circulating in the local population. -
Key words:
- Zika Virus /
- Serological survey /
- Humans /
- Animals /
- China /
- Plague reduction neutralization test
-
Table 1. Summary of the collected serum of human health and domestic animal
Variable Number (n) Constituent ratio (n%) Human Age group (Year) 0−9 69 18.8 10−19 40 10.9 20−29 26 7.1 30−39 31 8.5 40−49 46 12.5 50−59 54 14.7 60−69 63 17.2 > 70 37 10.1 Sex Man 171 46.7 Woman 195 53.2 Nationality Tu 364 99.4 Han 2 0.6 Total 366 Animal Chicken 63 60.5 pig 11 10.5 sheep 30 28.8 Total 104 Table 2. Neutralizing antibody titer to ZIKA and JEV infection among domestic animals in Shidatou village
Sample ID Collection site Host species PRNT90 titer* ZIKV JEV D32 Yujiashan chicken 1:10 − D56 Shidatou sheep 1:20 1:40 D61 Shidatou sheep 1:40 1:80 Note.*PRNT 90 titer, 90% plaque reduction neutralization test titer. Table 3. The seroprevalence of ZIKV in human and domestic animals in Shidatou village
Host species No. case No. PRNT90 positive Seroprevalence (%) ZIKV JEV Human 366 − − − Chicken 63 1 − 1.59 Pig 11 − − − Sheep 30 2 2 6.67 -
[1] Malone RW, Homan J, Callahan MV, et al. Zika virus: medical countermeasure development challenges. PLoS Negl Trop Dis, 2016; 10, e0004530. doi: 10.1371/journal.pntd.0004530 [2] Sikka V, Chattu VK, Popli RK, et al. The emergence of zika virus as a global health security threat: a review and a consensus statement of the INDUSEM joint working group (JWG). J Glob Infect Dis, 2016; 8, 3−15. doi: 10.4103/0974-777X.176140 [3] Mehrjardi MZ. Is Zika virus an emerging TORCH agent? an invited commentary. Virology (Auckl), 2017; 8, 1178122X17708993. [4] Ayres CF. Identification of Zika virus vectors and implications for control. Lancet Infect Dis, 2016; 16, 278−9. doi: 10.1016/S1473-3099(16)00073-6 [5] Abushouk AI, Negida A, Ahmed H. An updated review of Zika virus. J Clin Virol, 2016; 84, 53−8. doi: 10.1016/j.jcv.2016.09.012 [6] Malkki H. Zika virus infection could trigger Guillain–Barré syndrome. Nat Rev Neurol, 2016; 12, 187. doi: 10.1038/nrneurol.2016.30 [7] Rasmussen SA, Jamieson DJ, Honein MA, et al. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med, 2016; 374, 1981−7. doi: 10.1056/NEJMsr1604338 [8] Faye O, Freire CC, Iamarino A, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis, 2014; 8, e2636. doi: 10.1371/journal.pntd.0002636 [9] Deng YQ, Zhao H, Li XF, et al. Isolation, identification and genomic characterization of the Asian lineage Zika virus imported to China. Sci China Life Sci, 2016; 59, 428−30. [10] Bogoch II, Brady OJ, Kraemer MUG, et al. Anticipating the international spread of Zika virus from Brazil. Lancet, 2016; 387, 335−6. doi: 10.1016/S0140-6736(16)00080-5 [11] Fu S, Song S, Liu H, et al. ZIKA virus isolated from mosquitoes: a field and laboratory investigation in China, 2016. Sci China Life Sci, 2017; 60, 1364−71. doi: 10.1007/s11427-017-9196-8 [12] Gao X, Nasci R, Liang G. The neglected arboviral infections in mainland China. PLoS Negl Trop Dis, 2010; 4, e624. doi: 10.1371/journal.pntd.0000624 [13] Ye C, Lan R, Xia S, et al. Emergence of a new multidrug-resistant serotype X variant in an epidemic clone of Shigella flexneri. J Clin Microbiol, 2010; 48, 419−26. doi: 10.1128/JCM.00614-09 [14] Wang HY, Takasaki T, Fu SH, et al. Molecular epidemiological analysis of Japanese encephalitis virus in China. J Gen Virol, 2007; 88, 885−94. doi: 10.1099/vir.0.82185-0 [15] Lu Z, Fu SH, Cao L, et al. Human infection with West Nile Virus, Xinjiang, China, 2011. Emerg Infect Dis, 2014; 20, 1421−3. doi: 10.3201/eid2008.131433 [16] Li XL, Fu SH, Liu WB, et al. West nile virus infection in Xinjiang, China. Vector Borne Zoonotic Dis, 2013; 13, 131−3. doi: 10.1089/vbz.2012.0995 [17] Heinz FX, Stiasny K. Flaviviruses and their antigenic structure. J Clin Virol, 2012; 55, 289−95. doi: 10.1016/j.jcv.2012.08.024 [18] Olson JG, Ksiazek TG, Suhandiman, et al. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg, 1981; 75, 389−93. doi: 10.1016/0035-9203(81)90100-0 [19] Ward MJ, Alger J, Berrueta M, et al. Zika Virus and the World Health Organization Criteria for determining recent infection using plaque reduction neutralization testing. Am J Trop Med Hyg, 2018; 99, 780−2. doi: 10.4269/ajtmh.18-0237 [20] Fernandes RS, Campos SS, Ferreira-de-Brito A, et al. Culex quinquefasciatus from Rio de Janeiro Is Not Competent to Transmit the Local Zika Virus. PLoS Negl Trop Dis, 2016; 10, e0004993. doi: 10.1371/journal.pntd.0004993 [21] Wong PS, Li MZ, Chong CS, et al. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis, 2013; 7, e2348. doi: 10.1371/journal.pntd.0002348 [22] Diallo D, Sall AA, Diagne CT, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One, 2014; 9, e109442. doi: 10.1371/journal.pone.0109442 [23] Grard G, Caron M, Mombo IM, et al. Zika virus in Gabon (Central Africa)--2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis, 2014; 8, e2681. doi: 10.1371/journal.pntd.0002681 [24] Gendernalik A, Weger-Lucarelli J, Garcia Luna SM, et al. American Aedes vexans Mosquitoes are Competent Vectors of Zika Virus. Am J Trop Med Hyg, 2017; 96, 1338−40. doi: 10.4269/ajtmh.16-0963 [25] Ferreira-de-Brito A, Ribeiro IP, Miranda RM, et al. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem Inst Oswaldo Cruz, 2016; 111, 655−8. doi: 10.1590/0074-02760160332 [26] Guerbois M, Fernandez-Salas I, Azar SR, et al. Outbreak of Zika Virus Infection, Chiapas State, Mexico, 2015, and First Confirmed Transmission by Aedes aegypti Mosquitoes in the Americas. J Infect Dis, 2016; 214, 1349−56. doi: 10.1093/infdis/jiw302 [27] Gardner L, Chen N, Sarkar S. Vector status of Aedes species determines geographical risk of autochthonous Zika virus establishment. PLoS Negl Trop Dis, 2017; 11, e0005487. doi: 10.1371/journal.pntd.0005487 [28] Chouin-Carneiro T, Vega-Rua A, Vazeille M, et al. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Negl Trop Dis, 2016; 10, e0004543. doi: 10.1371/journal.pntd.0004543 [29] Ciota AT, Bialosuknia SM, Zink SD, et al. Effects of Zika Virus Strain and Aedes Mosquito Species on Vector Competence. Emerg Infect Dis, 2017; 23, 1110−7. doi: 10.3201/eid2307.161633 [30] Liu Z, Zhou T, Lai Z, et al. Competence of Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus Mosquitoes as Zika Virus Vectors, China. Emerg Infect Dis, 2017; 23, 1085−91. doi: 10.3201/eid2307.161528 [31] Faye O, Diallo D, Diallo M, et al. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J, 2013; 10, 311. doi: 10.1186/1743-422X-10-311 [32] Waddell LA, Greig JD. Scoping Review of the Zika Virus Literature. PLoS One, 2016; 11, e0156376. doi: 10.1371/journal.pone.0156376 [33] Hardy JL, Houk EJ, Kramer LD, et al. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol, 1983; 28, 229−62. doi: 10.1146/annurev.en.28.010183.001305 -




 下载:
下载:




 Quick Links
Quick Links