-
Pseudorabies virus (PRV), a member of the Alphaherpesvirinae subfamily within the family Herpesviridae, is a pathogen can infect numerous mammals including carnivores, ruminants, rodents and pigs. Pigs, as the natural host of PRV, show central nervous system disorders, respiratory and reproductive failure after infection. For other animal species, PRV infection leads to fever, nervous system disorder and severe itching with high mortality. The PRV major glycoprotein gB induces protective humoral immunity and also plays an important role for both viral entry and cell-to-cell spread. PRV was once well controlled previously using gene-deleted modified-live vaccines such as Bartha-K61 in China. However, a PRV variant started to prevail in vaccinated pig herds with more than 30% mortality since 2011[1], which caused huge losses to the pig industry.
PRV was rarely reported to infect human. The presence of PRV antibodies was previously reported in immunocompetent human encephalitis cases, but the virus was failed to be isolated[2]. Recently, PRV infections were confirmed in several human endophthalmitis and/or encephalitis cases by identifying virus genome with high-throughput deep-sequencing[3,4]. In this study, we aimed to determine a PRV retrospective seroprevalence among patients with encephalitis in 9 provinces in China.
1,335 serum samples of patients with encephalitis were selected from 9 provinces in 2012, 2013, and 2017 (Table 1). These samples were then sent to WHO regional reference Japanese Encephalitis (JE) laboratory in Chinese Center for Disease Control and Prevention (Beijing), for Japanese Encephalitis virus (JEV) IgM serological test. JEV IgM detection was performed as previously described[5]. Among of these samples, 45.47% were confirmed to be JEV serological positive, yet the etiology of other encephalitis cases remained unknown.
Table 1. PRV ELISA results of 9 provinces in 2012, 2013, and 2017
Province 2012 2013 2017 Serum sample PRV+ sample (n, %) Serum sample PRV+ sample (n, %) Serum sample PRV+ sample (n, %) Henan 135 17 (12.59) 69 11 (15.94) 52 2 (3.85) Shanghai 29 7 (24.14) 24 0 (0.00) 7 1 (14.29) Guizhou 73 13 (17.81) 43 11 (25.58) 29 1 (3.45) Chongqing 17 2 (11.76) 21 1 (4.76) 20 4 (20.00) Guangxi 77 6 (7.79) 11 0 (0.00) 9 4 (44.44) Shandong 46 5 (10.87) 55 1 (1.82) 39 1 (2.56) Zhejiang 36 7 (19.44) 108 12 (11.11) 49 3 (6.12) Sichuan 63 11 (17.46) 74 19 (25.68) 38 1 (2.63) Guangdong 141 7 (4.96) 37 8 (21.62) 33 1 (3.03) Total 617 75 (12.16) 442 63 (14.25) 276 18 (6.52) To detect PRV antibodies in above serum samples, IDEXX PRV gB Antibody Test Kit (IDEXX Laboratories, Westbrook, ME, USA) was applied which has previously been used for human serum samples as described[3]. The quantity of PRV gB antibodies in this blocking ELISA were inversely proportional to the A650, and a sample/negative (S/N) value ≤ 0.6 was considered positive. Thirty-three PRV gB positive serum samples with different S/N values were subjected to virus neutralizing antibody test as previously described[6]. Briefly, serum samples were heat-inactivated and serial dilutions were mixed with PRV HN1201 strain (200 TCID50) for 1 h. After incubation, the mixtures were transferred to the monolayers of porcine kidney (PK-15) cells and incubated for 72 h. Cytopathic effect (CPE) was used to determine the end-point titers that were calculated as the reciprocal of the highest serum dilution to neutralize.
Of the 617 serum samples collected in 2012, 75 (12.16%) tested positive for PRV antibodies (Supplementary Table S1 available in www.besjournal.com). 7 out of 9 provinces had PRV positive rates more than 10% with Shanghai ranked the top with 24.14% (Table 1). Of the 442 serum samples collected in 2013, 63 (14.25%) tested positive for PRV. 6 out of 9 provinces had PRV positives rates more than 10% with Guizhou ranked the top with 25.58%. Samples collected from Shanghai and Guangxi were tested negative of PRV antibodies in 2013. In 2017, 6 out of 9 provinces had dramatic decrease of PRV positive rates ranging from 2.56% to 6.12%. The average PRV positive rate in 2017 was 6.52%, which decreased significantly compared with those in 2012 and 2013 (Figure 1, Table 1).
Table S1. PRV ELISA result summary of serum samples from patients with encephalitis
Year No. of serum
samplesNo. of PRV+
serum samplesPRV+ serum
samples (%)No. of PRV+ samples in different S/N values 0.2–0.3 0.3–0.4 0.4–0.5 0.5–0.6 2012 617 75 12.16 1 2 19 53 2013 442 63 14.25 1 3 10 49 2017 276 18 6.52 1 1 4 12 Total 1,335 156 11.69 3 6 33 114 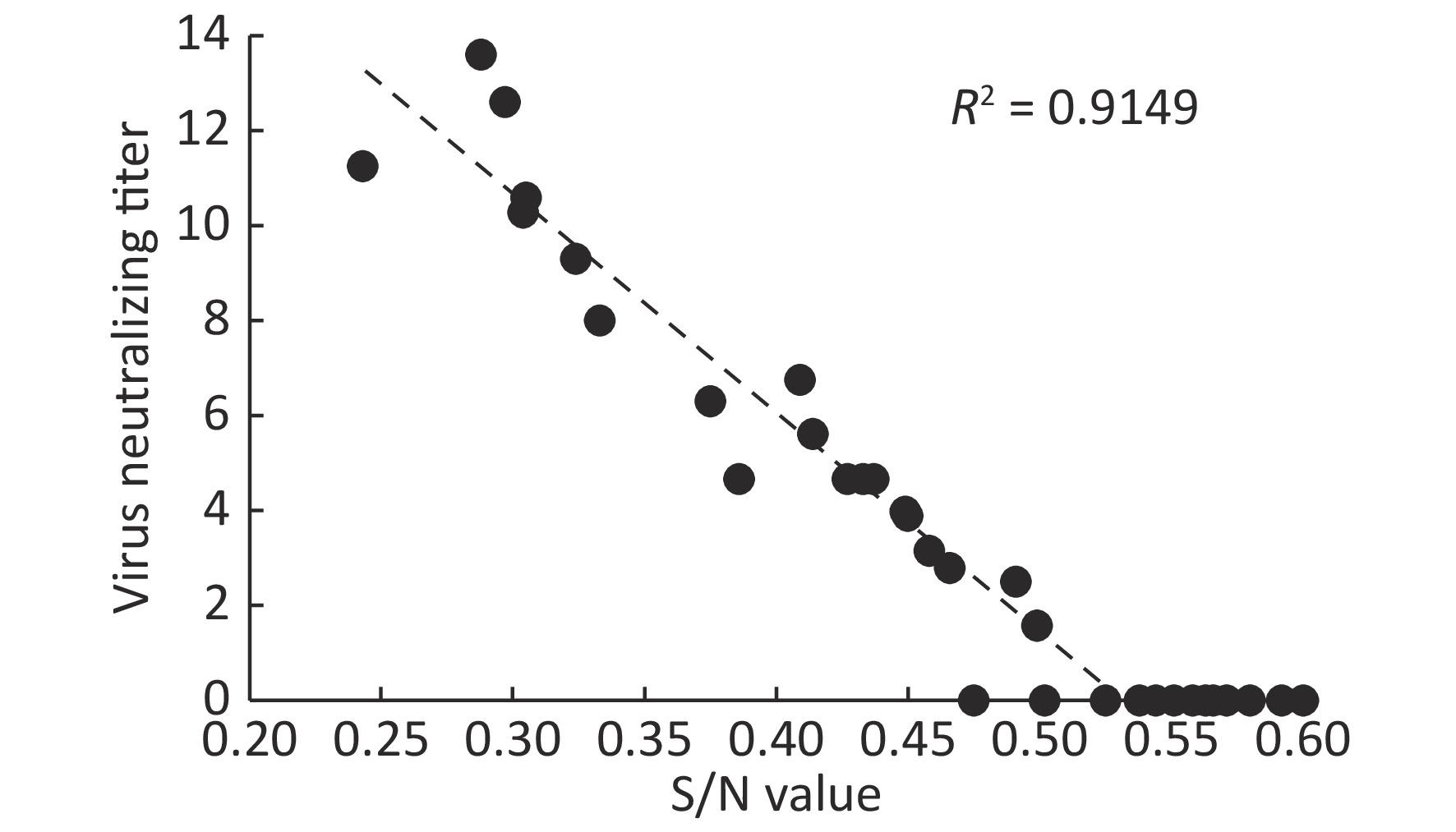
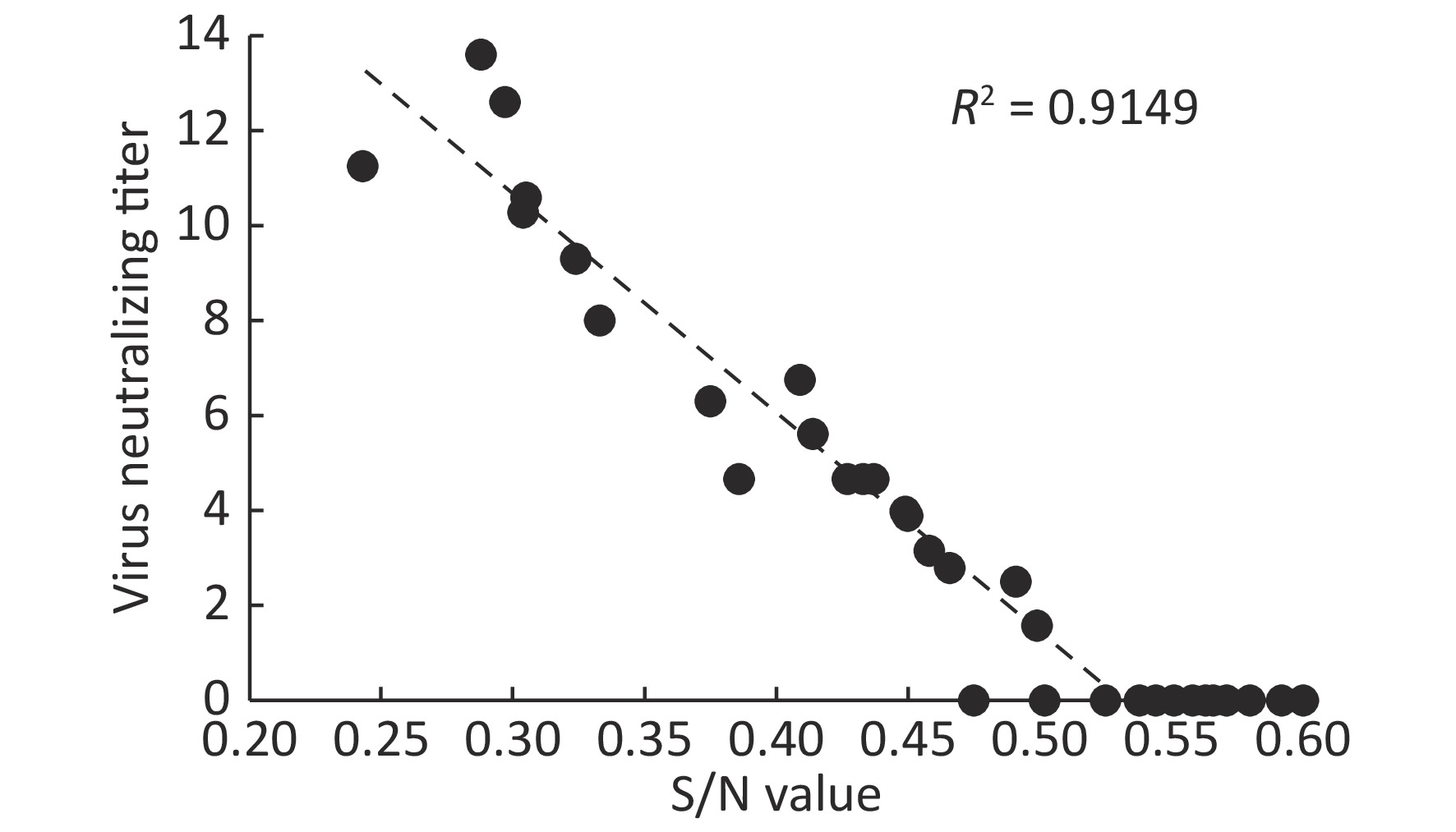
Figure 1. Correlation between PRV ELISA S/N values and virus neutralizing antibody titers of serum samples
Virus neutralizing antibodies to PRV HN1201, a PRV variant isolated in 2012, were tested from selected ELISA positive serum samples (three samples with S/N 0.2–0.3, six samples with S/N 0.3–0.4, twelve samples with S/N 0.4–0.5, and twelve samples with S/N 0.5–0.6). As shown in Figure 1, the virus neutralizing antibodies ranged from 12.59 to 16, 6.31 to 10, and 0 to 6.76 with respect to ELISA S/N values 0.2–0.3, 0.3–0.4, and 0.4–0.5. By contrast, no neutralizing antibodies were detected in 12 serum samples with S/N values ranging from 0.5–0.6. No PRV DNA in these virus neutralizing antibody positive samples was detected using Real-time PCR (data not shown)[3]. We next tested if PRV positive serum samples have any correlation with JEV infection. As shown in Table 2, there was no significant difference of PRV positive rates between JEV-positive and JEV-negative serum samples.
Table 2. Summary of PRV and JEV positive serum samples
Year No. of serum samples No. of JEV+ serum samples JEV+ serum
samples (%)JEV+PRV+ serum
samples (%)JEV-PRV+ serum
samples (%)2012 617 285 46.19 12.98 (37/285) 11.45 (38/332) 2013 442 240 54.30 12.92 (31/240) 15.84 (32/202) 2017 276 82 29.71 4.88 (4/82) 7.22 (14/194) Total 1,335 607 45.47 11.86 (72/607) 11.54 (84/728) The positive PRV ELISA antibodies and neutralizing antibodies in these encephalitis cases indicate the patients may infect with PRV but cannot conclude that the encephalitis was caused by PRV infection, since the patients with acute infections of the CNS should not rule out other treatable disease[7]. To compare PRV antibody positive rates between healthy people and patients with encephalitis, 778 serum samples from healthy people were collected from the same 9 provinces in 2017 to detect PRV ELISA antibodies. As shown in Supplementary Table S2 (available in www.besjournal.com), the total PRV positive rate was 3.86%, which was lower compared with encephalitis patients in 2017.
Table S2. PRV ELISA result summary of serum samples from healthy people
Year No. of serum
samplesNo. of PRV+
serum samplesPRV+ serum
samples (%)No. of PRV+ samples in different S/N values 0.2–0.3 0.3–0.4 0.4–0.5 0.5–0.6 2017 778 30 3.86 0 0 6 24 IDEXX PRV gB Antibody Test Kit is widely used to detect PRV antibodies. The principal of this kit is that PRV gB antibodies in serum samples will bind to the antigen coated in the microwell, competing with enzyme-conjugated monoclonal antibodies. Therefore, it can be applied to detect PRV antibodies in serum samples of different species. The S/N values of ELISA in PRV-positive healthy people were exclusively ranged from 0.4–0.5 (6 people) and 0.5–0.6 (24 people). The above results may raise the concern that if PRV ELISA values ranging from 0.5–0.6 were false positive for PRV infection. We could not exclude this possibility since the PRV infection could not confirmed by other virological or immunological methods in these PRV serological positive people. However, in Ai’s study, the same ELISA kit was used and the S/N values were 0.3–0.4 and 0.5–0.6 in plasma samples of confirmed PRV-infected patient[3]. Also, they excluded the cross-reaction with other human herpesviruses including EBV, CMV, HSV-1, HSV-2, and VZV. Recently, one research group reported that the S/N value using the same ELISA for a confirmed PRV-infected human patient was 0.215[8]. Another research group reported that the S/N values for a confirmed PRV-infected human patient were 0.248, 0.132, and 0.130 at 14-, 21-, and 28-d post-infection, respectively[9]. Based on above data and reports, we reason that the cutoff S/N value of the ELISA for human samples will be appropriate to set as 0.4, and the results have to be parallelly validated by neutralizing antibody test.
The information of age and sex of PRV ELISA positive patient in 2012 and 2013 were missing. We summarized the age and sex information of PRV positive serum samples from patients with encephalitis and healthy people in 2017. The age of PRV serological positive patients with encephalitis ranged from 20 to 44 with average age of 30.25. By contrast, the age of healthy people ranged from 19 to 46 with average age of 31.45. There was also no disparity as for the sex between patients with encephalitis (male, 10/18) and healthy people (male 14/30).
The previously reported human endophthalmitis and encephalitis cases with PRV infection had close contact with pigs or exposure to pig’s blood/tissue or sewage[3,5,9]. In China, people have more chance to contact pigs especially raising pigs in the backyard in countryside. Since 2011, there were massive outbreaks of PRV in vaccinated pig herds in China. The high PRV serological positive rates in patients with encephalitis since 2012 in this study may have some relevance with the outbreaks of PRV variants in Chinese swine herds at that time. After that, vaccines based on PRV variants in China were developed and massively used. Sun Y reported that the swine PRV serological positive rates in 27 provinces from 2012 to 2017 were 11.92% (153/1,284), 12.19% (225/1,846), 6.70% (169/2,523), 11.10% (269/2,424), 5.57% (147/2,640), and 6.90% (382/5,539), respectively[10]. The decreased PRV infection rates in pig herds were consistent with the decrease of PRV positive rates in serum samples of patient with encephalitis in 6 provinces except for Shanghai, Chongqing, and Guangxi in 2017. The discrepancy could be due to the limited numbers of sampling since there were only 7, 20, and 9 samples from Shanghai, Chongqing, and Guangxi in 2017, respectively (Table 1).
In summary, PRV antibodies were found in encephalitis patients with decreased positive rates since 2012. The positive rates were higher in patients with encephalitis than healthy population. Although there is no direct evidence that encephalitis was caused by PRV infection, the occupation-related workers such as abattoir workers, swine farmers, slaughterers and swine herders should increase the awareness of self-protection.
WANG Huan Yu and TIAN Ke Gong were responsible for the concept and design of the analysis, the acquisition, analyses and interpretation of the data. LI Xiang Dong, FU Shi Hong, CHEN Ling Yan, LI Fan, DEND Jun Hua, and LU Xuan Cheng performed the experiments. WANG Huan Yu and TIAN Ke Gong drafted the manuscript.
No conflict of interest to declare.
Ethical and institutional review boards for animal serum samples investigation at National Research Center for Veterinary Medicine.
doi: 10.3967/bes2020.059
-
Abstract: Pseudorabies virus (PRV), a veterinary pathogen that infects domestic animals as well as wild animals such as wild boar and feral swine, was recently reported to infect human and led to endophthalmitis and encephalitis. A retrospective seroepidemiologic survey was conducted using 1,335 serum samples collected from patients with encephalitis and ELISA positive rates were 12.16%, 14.25%, and 6.52% in 2012, 2013, and 2017, respectively. The virus neutralizing antibody titers of positive samples correlated well with ELISA results. The pseudorabies virus antibody positive rate of patients with encephalitis were higher than that of healthy people in 2017. The above results suggest that some undefined human encephalitis cases may be caused by PRV infection.
-
Key words:
- Pseudorabies virus /
- Human infection /
- Encephalitis
注释: -
Table 1. PRV ELISA results of 9 provinces in 2012, 2013, and 2017
Province 2012 2013 2017 Serum sample PRV+ sample (n, %) Serum sample PRV+ sample (n, %) Serum sample PRV+ sample (n, %) Henan 135 17 (12.59) 69 11 (15.94) 52 2 (3.85) Shanghai 29 7 (24.14) 24 0 (0.00) 7 1 (14.29) Guizhou 73 13 (17.81) 43 11 (25.58) 29 1 (3.45) Chongqing 17 2 (11.76) 21 1 (4.76) 20 4 (20.00) Guangxi 77 6 (7.79) 11 0 (0.00) 9 4 (44.44) Shandong 46 5 (10.87) 55 1 (1.82) 39 1 (2.56) Zhejiang 36 7 (19.44) 108 12 (11.11) 49 3 (6.12) Sichuan 63 11 (17.46) 74 19 (25.68) 38 1 (2.63) Guangdong 141 7 (4.96) 37 8 (21.62) 33 1 (3.03) Total 617 75 (12.16) 442 63 (14.25) 276 18 (6.52) S1. PRV ELISA result summary of serum samples from patients with encephalitis
Year No. of serum
samplesNo. of PRV+
serum samplesPRV+ serum
samples (%)No. of PRV+ samples in different S/N values 0.2–0.3 0.3–0.4 0.4–0.5 0.5–0.6 2012 617 75 12.16 1 2 19 53 2013 442 63 14.25 1 3 10 49 2017 276 18 6.52 1 1 4 12 Total 1,335 156 11.69 3 6 33 114 Table 2. Summary of PRV and JEV positive serum samples
Year No. of serum samples No. of JEV+ serum samples JEV+ serum
samples (%)JEV+PRV+ serum
samples (%)JEV-PRV+ serum
samples (%)2012 617 285 46.19 12.98 (37/285) 11.45 (38/332) 2013 442 240 54.30 12.92 (31/240) 15.84 (32/202) 2017 276 82 29.71 4.88 (4/82) 7.22 (14/194) Total 1,335 607 45.47 11.86 (72/607) 11.54 (84/728) S2. PRV ELISA result summary of serum samples from healthy people
Year No. of serum
samplesNo. of PRV+
serum samplesPRV+ serum
samples (%)No. of PRV+ samples in different S/N values 0.2–0.3 0.3–0.4 0.4–0.5 0.5–0.6 2017 778 30 3.86 0 0 6 24 -
[1] Yu X, Zhou Z, Hu D, et al. Pathogenic pseudorabies virus, China, 2012. Emerg Infect Dis, 2014; 20, 102−4. doi: 10.3201/eid2001.130531 [2] Mravak S, Bienzle U, Feldmeier H, et al. Pseudorabies in man. Lancet, 1987; 8531, 501−2. [3] Ai JW, Weng SS, Cheng Q, et al. Human endophthalmitis caused by pseudorabies virus infection, China. 2017, Emerg Infect Dis, 2018; 24, 1087−10. [4] Zhao WY, Li HF, Li SY, et al. Clinical experience and next-generation sequecing analysis of encephalitis caused by pseudotabies. Natl Med J China, 2018; 98, 1152−57. [5] Ye XF, Wang HY, Fu SH, et al. Etiological spectrum of clinically diagnosed Japanese encephalitis cases reported in Guizhou province, China, in 2006. J of clini microbiol, 2010; 48, 1343−49. doi: 10.1128/JCM.01009-09 [6] Li X, Yang F, Hu X, et al. Two classes of protective antibodies against pseudorabies virus variant glycoprotein B: implications for vaccine design. PLoS Pathogens, 2017; 13, e1006777. doi: 10.1371/journal.ppat.1006777 [7] Dubot-Pérès A, Sengvilaipaseuth O, Chanthongthip A, et al. How many patients with anti-JEV IgM in cerebrospinal fluid really have Japanese encephalitis? Lancet Infect Dis, 2015; 15, 1376−7. doi: 10.1016/S1473-3099(15)00405-3 [8] Zheng L, Liu X, Yuan D, et al. Dynamic cerebrospinal fluid analyses of severe pseudorabies encephalitis. Transbound Emerg Dis, 2019; 66, 2562−5. doi: 10.1111/tbed.13297 [9] Yang H, Han H, Wang H, et al. A case of human viral encephalitis caused by pseudorabies virus infection in China. Front Neurol. 2019, 4z: 10: 534. [10] Sun Y, Liang W, Liu Q, et al. Epidemiological and genetic characteristics of swine pseudorabies virus in mainland China between 2012 and 2017. Peer J, 2018; 23, e5785. -




 下载:
下载:



 Quick Links
Quick Links