-
Laboratories play significant roles in all the critical processes of detecting rapid infectious disease outbreaks, risk assessments, early warnings, early responses and notifications, and monitoring and surveillance[1,2]. Veterinary Laboratories (VLs) that rapidly identify, respond to and control rapidly spreading and emerging (or re-emerging) infectious and zoonotic diseases is critical to: (1) the financial performance of animal agriculture and international trade; (2) livelihoods of animal related industries; and (3) nutritional status, food security, and the socio-economic well-being of a country[3].
Over the years, the Global Health Security Agenda (GHSA) has supported collective and sustainable capacity-building at the international, regional and local levels in order to promote rapid detection, prevention or mitigation, and to support responses needed to control emerging infectious diseases (EIDs) outbreaks before they become epidemic. Capacity-building is additionally designed to reduce the impact of naturally occurring outbreaks, as well as intentional or accidental releases of dangerous pathogens[4]. The agenda spurs progress toward implementation of the World Health Organization’s International Health Regulations (2005) (WHO/IHR) and other global health security frameworks, such as the World Organization for Animal Health. Since the Ebola outbreak in West Africa in 2014, the GHSA has been committed to strengthening capacity in infrastructure, equipment, and skilled personnel across sectors, sustainable national biosafety, biosecurity, and especially laboratory systems in Africa, all to ensure a safer world.
The ability of VLs in developing countries, applicable to Nigeria, is frequently limited by many factors. These factors may be assessed along three dimensions: (1) skilled and competent personnel; (2) adequacy and upgrade of equipment/materials, and (3) the ability to mobilize technical support when needed[5]. To address these limitations in African countries, the Food and Agriculture Organization of the United Nations (FAO) developed a core laboratory mapping tool (LMT-core) to aid in pre-emptive laboratory assessment. Released for public use in May 2014, this instrument can determine and identify gaps in laboratory functions, define strategic pathways, and set targets for capacity building[2]. Currently, to the best of our knowledge, this is the first study in Nigeria to use the FAO LMT tool for veterinary laboratory assessments to determine the capacities and functional status in compliance with the GHSA requirements.
This study was conducted in the south-western states of Oyo and Ogun in Nigeria, selected due to the higher numbers of livestock populations and VLs in these areas. A cross-sectional survey was conducted at eleven laboratories. These included: (i) seven veterinary laboratories that were based at academic institutions (three microbiology, two parasitology and two pathology laboratories); (ii) two government veterinary clinics and laboratories in each state; (iii) one private veterinary laboratory; and (iv) one national veterinary laboratory were purposively selected. The inclusion criteria for laboratories were performance of veterinary diagnostics, location within the study area, and establishment of minimum standards against which assessment might be carried out. Before the commencement of the study consent was obtained from each laboratory. Positive responses indicating a willingness to participate in the study triggered the initiation of the assessment and questionnaire process.
The LMT-Core, is a standardized set of questions embedded in a Microsoft spreadsheet (Microsoft Excel 2007). Using such, we gathered information on five key aspects: (1) general laboratory profile; (2) infrastructure, equipment, and supplies; (3) laboratory performance; (4) quality assurance and bio-safety/bio-security; and (5) laboratory collaboration and networking. Within these areas were 17 categories and 108 subcategories, each of which addressed specific laboratory functions. For instance, the general laboratory profile aspect sought information on geographic location (in terms of strategic placement and accessibility, laboratory budget, basic electricity and potable water supplies, and sustainable personnel organization systems). The aspect of infrastructure, equipment, and supplies gathered information on containment facilities, laboratory biosafety, equipment for bacterial, viral, serological and parasitological diagnosis, and reagent supply. Every laboratory generated an individual profile or ‘map’ using automatic calculations embedded in the spreadsheet, thus allowing the laboratory to visualize their unique laboratory capability and capacity status for the five aspects assessed.
We scored laboratories based on observations and interviews with heads of respective units of laboratories and by strictly following the guidelines provided by FAO for assessment of laboratories (Supplementary Table S1, available in www.besjournal.com). Briefly, for each question of the assessment set of four options were provided as responses. The single best option describing the existing situation in the laboratory was recorded by the assessor. Where a suitable answer was not available or no answer was provided, the respective scoring area was marked not applicable (N/A). For all questions where ‘N/A’ was entered, the associated subcategory was omitted from the summary score. Where a laboratory struggled to select between two scoring options, a preferred score best representing the laboratory’s situation was selected, and the reason for hesitation was documented as a comment in the assessors’ column for comment/observation (column K). Additional information guiding assessors to determine the appropriate score was provided in column L of the spreadsheet (e.g. specific observations or documents needed to select from the 4 possible options), and was used by all assessors to provide consistency in scoring between the different laboratories.
The scoring within the LMT-core sums to 100% that is the ideal and achieved when a laboratory scores the maximum points (n = 4) in all the subcategories. Each laboratory was given a reliability score based on the number of questions answered, excluding N/A as a completed response. Questionnaires with 0%–69% completion rendered a low reliability score and completion of 70%–89% rendered a medium confidence score and, lastly, completion of 90%–100% rendered the questionnaire as confidently reliable.
Following verification for completeness, each LMT-core questionnaire was imported into the LMT automated analytic tool, available through an FAO interface. This process was supervised and eviewed by at least two researchers in every case. Outputs were generated in a ‘Summary sheet’ with scores as sub-values and graphs for the five core areas and seventeen different categories presented in a tabulated format. Graphic depictions of individual laboratory functionalities for each of the five key areas, including the specific strengths and weaknesses, were generated and analyzed. Scores were presented in percentages and compared with the theoretical ideal of 100%. The final laboratory capacity assessment was presented as either ‘advanced’ (67%–100%), ‘moderate’ (34%–66%) or ‘basic’ (0%–33%) (see Figure 1). Inter-laboratory comparisons, regarding performance, was conducted using ordinary one-way ANOVA and Tukey’s multiple comparisons test (P = 0.05). For confidentiality purposes, the study excluded any identifiers of individual laboratories, and facilities were further re-grouped into regions A (Oyo state) and B (Ogun state) to assure anonymity.
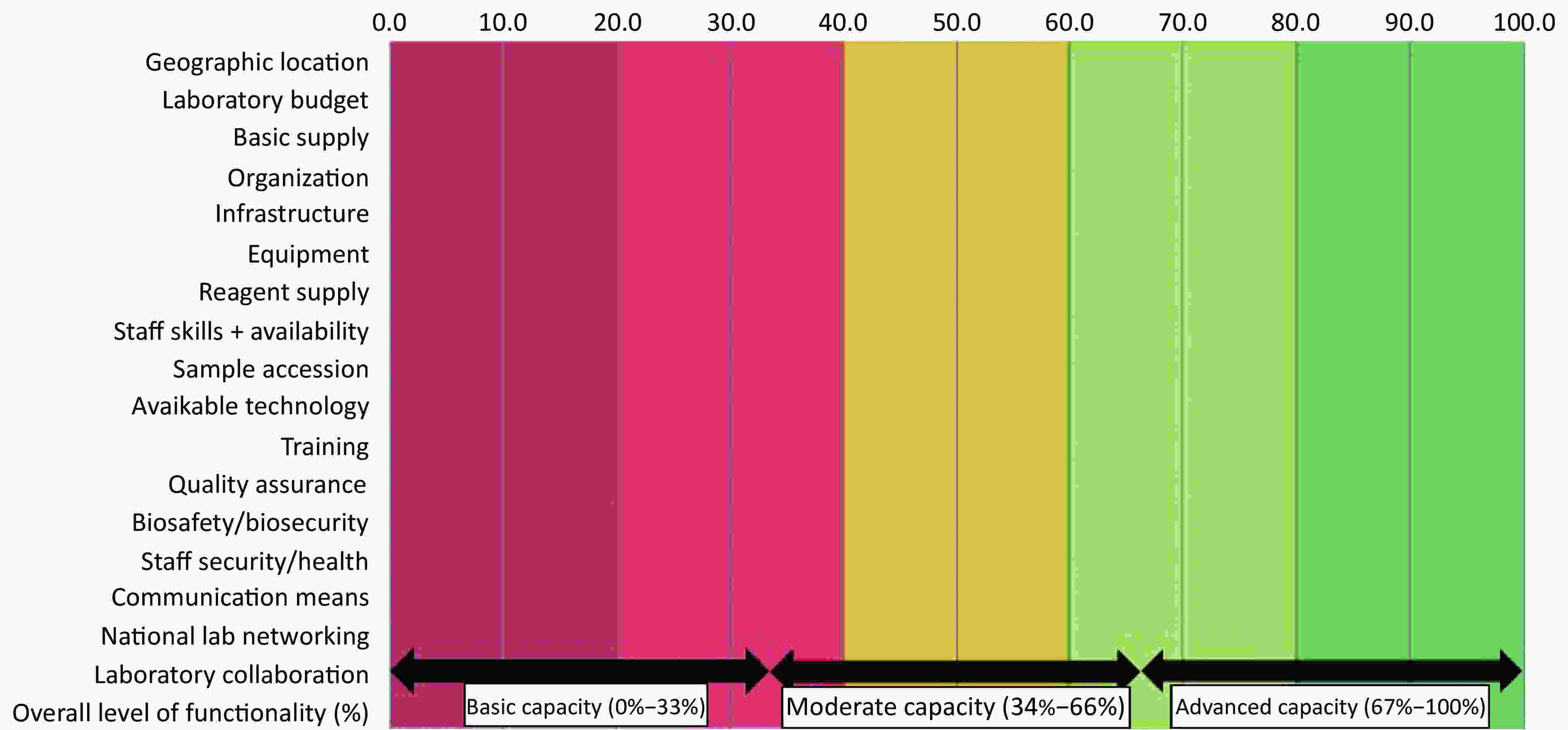
Figure 1. Final output of laboratory capacities and functionalities assessed against a sliding scale with advanced = (67%–100%), moderate = (34%–66%), or basic = (0%–33%).
The preliminary evaluation of eleven veterinary diagnostic laboratories located in selected staes of south-west Nigeria resulted in an average reliability score of 81%, per LMT-core. Teaching and research services were provided by 9 out of the 11 laboratories (81.8%); diagnostic, clinical and hospital services by 81.8%; and a single laboratory additionally offered public health services. Pathogen types handled by laboratories included bacteria (8/11, 72.7%), viruses (4/11, 36.3%), fungi or mucor (3/11, 27.2%), and parasites (6/11, 54.5%). Overall, the capacity and functionality score obtained was 24.3% (ranging from 9.7%–39.7%), with an average score of 24.5% ± 10.0% (ranging from 9.7%–39.0%) for Oyo State and 24.1% ± 9.3% (ranging from 15.7%–39.7%) for Ogun State (Table 1).
Table 1. Outputs from assessment of veterinary laboratories in south-west nigeria using the laboratory mapping tool-core, 2018
Facility GL LB BS O I E RS SS + A SA AT T QA BB SSH CM NLN LC Grand total Ogun State PC 66.7 66.7 66.7 66.7 44.4 50.0 57.1 61.1 52.4 37.0 27.8 15.2 29.2 22.2 55.6 22.2 18.5 39.7 Uni-Para 44.4 22.2 11.1 66.7 5.6 33.3 16.7 33.3 26.7 29.6 14.3 18.2 25.0 0.0 33.3 22.2 48.1 25.1 Uni-Path 33.3 22.2 11.1 0.0 14.3 50.0 27.8 26.7 8.3 73.3 9.5 21.2 20.0 0.0 41.7 0.0 3.7 21.0 Uni-Mic 33.3 22.2 22.2 66.7 20.8 20.0 28.6 16.7 16.7 14.8 22.2 16.7 25.0 0.0 41.7 0.0 3.7 19.1 Pub Vet C&L 44.4 11.1 0.0 66.7 4.2 0.0 0.0 33.3 N/A 13.3 N/A 5.6 41.7 11.1 33.3 N/A 23.8 15.7 Oyo State Inst-Mic 55.6 33.3 33.3 66.7 33.3 44.4 44.6 46.7 20.0 16.7 44.4 6.1 11.1 11.1 8.3 0.0 0.0 22.9 Pub 2 Vet C&L 44.4 0.0 0.0 66.7 8.3 4.8 16.7 0.0 11.1 16.7 11.1 9.1 11.1 0.0 0.0 11.1 0.0 9.7 Inst L & V 55.6 66.7 33.3 66.7 0.0 8.3 11.1 42.9 16.7 19.4 4.8 3.0 12.5 0.0 33.3 77.8 11.1 18.6 Uni2- Mic 44.4 16.7 22.2 66.7 45.8 66.7 57.1 83.3 38.9 25.9 33.3 12.1 28.6 0.0 41.7 11.1 59.3 39.0 Uni2- Para 44.4 44.4 44.4 66.7 33.3 50.0 53.3 44.4 26.7 23.8 19.0 23.3 25.0 0.0 41.7 0.0 3.7 28.0 Uni2- Path 44.4 33.3 44.4 66.7 28.6 66.7 38.9 61.1 33.3 36.4 28.6 18.2 33.3 0.0 8.3 0.0 3.7 28.6 Mean value 46.4 30.8 26.2 60.6 21.7 35.8 32.0 40.9 25.1 27.9 21.5 13.5 23.9 4.0 30.8 14.4 16.0 24.3 Median 44.4 22.2 22.2 66.7 33.3 44.4 27.8 33.3 23.3 19.4 25.0 18.2 25.0 0.0 41.7 0.0 23.8 22.9 SEM 2.9 6.4 6.2 6.1 4.9 7.3 5.9 6.9 4.3 5.2 3.9 2.0 2.9 2.3 5.3 7.6 6.1 2.8 95% Confidence Interval 37.1–55.8 16.5–45.1 12.4–40.1 47.1–74.1 10.7–32.7 19.6–52.1 18.8–45.2 25.5–56.2 15.4–34.8 16.4–39.4 12.8–30.2 9.0–18.1 17.4–30.3 0.0–9.1 19.0–42.6 −2.8–31.6 2.3–29.6 18.1–30.5 Note. Functionality categories assessed: GL = Geographic location; LB = Laboratory Budget; BS = Basic supply; O = Organization of the laboratory; I = Infrastructure; E = Equipment; RS = Reagent supply; SS + A = Staff skills + availability; SA = Sample accession; AT = Available technology; T = Training; QA = Quality Assurance; BB = Biosafety/Biosecurity; SSH = Staff Security/Health; CM = Communication means; NLN = National laboratory networking; LC = Laboratory collaboration. Categories of laboratories/facilities: PC = private clinic; Uni-Para = University parasitology; Uni-Path = University pathology; Uni-Mic = University microbiology; Pub Vet C&L = State Veterinary clinic and laboratory; Inst-Mic = Training institution microbiology; Pub 2 Vet C&L = 2nd State Veterinary clinic and laboratory; Inst L & V = Institution with laboratory and vaccine supply chain; Uni2-Para = 2nd University parasitology; Uni2-Path = 2nd University pathology; Uni2-Mic = 2nd University microbiology. SEM = Standard error of the mean. All values are expressed in percentages (%). The overall laboratory capacity scores were similar in both states (P = 0.73). The average scores were low (< 33%) across the different functionalities and capacities assessed (see Table 1). The LMT-core category ‘organization’ was generally strong across-the-board (average: 60.6%; 95% CI, 47.1–74.1) with the exception of a single pathology laboratory). Particularly low scores (≤ 25%) were obtained for the aspects of infrastructure, sample accessioning, on-the-job training, quality assurance, biosafety and biosecurity, staff security and health, and national laboratory networking and collaboration. The weakest scores were observed for staff security and health (e.g. regular health checks, protection against zoonoses through prophylactic immunizations, and medical health surveillance) (average 4.0%; 95% CI, 0.0–9.1). Other scores obtained were for the laboratory budgets in relation to finance, research autonomy and upgrading (30.8%; 95% CI, 16.5–45.1); basic electricity, water and deionized water supplies (26.2%; 95% CI, 12.4–40.1); reagent supplies (validity and affordability) (32.0%; 95% CI, 18.8–45.2); case sample throughputs (25.1%; 95% CI, 15.4–34.8); advanced technology for molecular and serological assays (27.9%; 95% CI, 16.4–39.4); communication including the availability of landlines, internet facilities, access to scientific publications and dissemination of data (30.8%; 95% CI, 19.0–42.6).
Only two of the laboratories, a private laboratory with an overall average score of 39.7% and a university microbiology laboratory with 39.0%, were rated by the LMT-core as having moderate diagnostic capability. The remaining nine laboratories received scores placing them within the basic range for diagnostic laboratory services (< 30.0%). None of the laboratories had comprehensively advanced facilities for disease diagnosis, active surveillance or early warning systems. Inter-laboratory comparison of capacities and functionalities showed significant variations in laboratory capacities with the private laboratory performing best (ranging from 9.7%–39.7%; P = 0.04).
In this study the performance, reported as functionality and capacity of veterinary laboratories, were systematically evaluated and documented using a standardized assessment tool, the FAO Core Laboratory Mapping Tool (LMT-core). The results provided for 11 veterinary laboratories in south-west Nigeria were quantitatively assessed and analyzed. The LMT-core has been tested and validated in at least fourteen African countries to date and resulting outputs have been used to recommend priority actions for laboratory improvements across Africa. Where such has been implemented, tremendous progress has been noticed in service delivery and laboratory outputs[2]. The use of the LMT-core to assess laboratories and assist them in identifying weaknesses and prioritize improvements is consistent with the Joint External Evaluation (JEE) protocol of the WHO/IHR[4].
Although low-performance scores were reported across many functionalities accessed in this study, the outputs should be viewed not as negative findings, but as a valuable guide towards individual laboratory level improvements. The ongoing use of the LMT-core would ultimately provide a means for tracking positive changes and new enhancements in the overall functionality and capacity of VLs. Almost all of the VLs reported good organizational structure that is the basic requirement and template on which improvements can be made. Our findings will aid the various laboratory managements to begin the process of correcting the identified weak areas and moving towards the implementation of quality laboratory systems.
Poor levels of laboratory infrastructure, sample accessioning, on-the-job training, quality assurance, biosafety and biosecurity, staff security and health, and national laboratory networking and collaboration hamper the functionalities and efficiencies of these laboratories. Poor infrastructure in particular, is a major drawback in animal disease detection, investigation, and control in many developing countries[6,7]. The lack of robust infrastructure, consistent with the findings documented for the small sub-set of laboratories reported here, is most often associated with insufficient funding for the purchase and maintenance of equipment, supplies, reagents and staff training; the designing and specification of proper sample collection, traceable accession methods, planned disease surveillance strategy, and regular appraisal of the performance of novel diagnostic techniques are all important.
A consistent weakness identified in the current study was in training programs implemented to ensure that each staff member is suitably trained to meet the skills required for undertaking their job responsibilities. Such training (e.g. skills and competencies) is critical for efficiency in the day-to-day performance of routine duties, the handling of hazardous biological agents, the acquirement of knowledge and the interpretation of results, epidemiology, pathogenicity, and human susceptibility to various biological materials[8]. Without sound quality assurance and quality control, as observed in this study, performing procedures to standard is limited and may result in non-comparable data for other international laboratories with established capacity[9,10].
In any laboratory, biosafety and biosecurity are key to ensure occupational health and safety of staff. However, the health and safety of staff in this study may be compromised routinely by the poor biosafety practices, the non-existence of medical health policies and surveillance, lack of adequate immunizations or emergency plans for control of laboratory-acquired diseases, and the absence of health and safety protocols. Only 1 of the 11 laboratories in the current study had networks and collaborations with other facilities at national, regional and international levels. We expect other VLs desire to enhanced their functions as per the WHO/IHR; the objective of the laboratory twinning and collaborations is to contribute to the sustainable improvement of public health services in developing countries through the establishment of partnerships between laboratories and institutions. Results of the LMT-core assessment indicated that more efforts to build collaborations and networking may be made a priority in order to facilitate future effective functioning of VLs in developing countries.
Our study was limited by the inadequate record keeping in many of the laboratories visited. Where records were unavailable, the authors’ judgement was strictly based on the assessment guidelines provided by the FAO. Record keeping in many developing countries is an issue and continuous training, monitoring and evaluation, and the supply of recorded materials for laboratory staff is critical.
The status of VLs in south-west, Nigeria is currently poor and falls below required standards. This is a major concern, especially when VLs are to ensure rapid responses to infectious diseases outbreaks and control. It is required that each VL should develop implementable actions plans aimed at progressively addressing the identified gaps. The weaknesses identified need timely interventions and political will to continuously invest in infrastructures that enhance research and disease surveillance in the country. VLs also need to contribute to the global health security agenda (GHSA) in combatting infections through the development of initiatives, projects, and partnerships with established international laboratories, that should promote capacitation for early detection and emergency response to infectious pathogens which threaten the public.
The authors gratefully acknowledge all of the participating laboratories and staff who shared their time and experiences with us.
Table S1. FAO lab capacitation.
# Area Category Sub Category 4 3 2 1 Assessments scores A B C Current 1 General Laboratory Profile Geographic location Strategic placing Isolated compound outside of any residential area Isolated compound in low populated area Single building in low populated area Building within residential area 2 Accessibility Proper containment + guard (24 hr) + Restricted access to building by use of Identity card (employees) only Restricted access, doors are locked + guard at the entrance for 24 h Doors are closed but not locked / low biosecurity level / guard is not always present Easy access to laboratory compound even by visitor / stranger / doors are open / no guard present 3 Location, access Access to highway, airport, harbor and / or station within 30 minutes Access to highway, airport, harbor or station within 60 minutes Access to road; but sometimes limitations (traffic, road condition, flooding) Regular limitations in access to transport means (traffic, bad road, airport is far) 4 Laboratory Budget Financial autonomy (allocation of funds) Lab is financially autonomous, lab funds (>90%) from public source and/or self-generated Lab is almost financially autonomous; lab funds from public source or self-generated (>60%) AND development programmes (<40%) Lab has insufficient own budget (<60%), activities dependant on development partners (>40%) Lab has no autonomous budget; all activities exclusively rely on external funding source 5 Research autonomy (n of publication / year) Lab budget allows ample opportunity for research (>10 publications/year) besides routine diagnostic/production Lab budget allows limited research (1 to 10 publications/year according to the lab context), but mainly routine diagnostic/production Lab budget is insufficient for research, but results from ongoing work are published in national journals/bulletin or regularly presented No research activity due to insufficient lab budget 6 Autonomous infrastructural upgrading (n of constructions / year) Lab budget sufficient for regular (1/year) upgrading / renovation of larger lab infrastructure Lab budget allows irregular (1 / 3 years) independent upgrading / renovation of infrastructure Constructions or renovation by use of lab budget irregular and only for minor infrastructural changes Construction or renovation in general only possible under external funding 7 Basic supply Regular (hours of) electricity supply Constant (24 hours) stable electricity supply + stabilizer and automatic stand-by generator 24 hours electricity supply + stabilizer and back-up generator (manually operated) Frequent electrical instability/voltage irregularity, manually/automatically operated generator + stabilizer Electricity supply (temporarily) less than 10 hours per day, generator does not run permanently (not existent, no fuel) 8 Regular water supply (access and quality) Daily and unlimited supply of good-quality (drinkable) water (pipe) through public source; no risk of water shortage Sufficient daily supply of low quality (not drinkable) water through pipe or tank; back-up tank available Supply of water through tank; sometimes insufficient or low quality (not drinkable) Irregular and insufficient water supply, also low quality (not drinkable) 9 General Laboratory Profile Basic supply Access to purified, deionized, distilled water Unlimited access to purified water; own production of deionised and distilled water Easy access to deionised and distilled water (external/internal source) Limited access to deionised and/or distilled water (external/internal source) Difficult or no access to deionised and/or distilled water 10 Organization Sustainable personnel organization system Organigram and organization system in place + written description of responsibilities according to personnel skill level Organigram and organization system in place; staff mostly know their roles, but not written down Organization system in place, but frequent changes, staff not always clear on their roles Frequent shifting of personnel, no stable organization system 11 Infrastructure, equipment, supplies Infrastructure Containment (means of containment) All lab departments (of different Biosafety levels) are clearly separated and well contained by airlock, personnel shower/changing room, sterilizing and storage rooms; use of disinfections, identity badges or other means (then describe). It can be accessed only by lab staff from the department Lab departments are separated (e.g. different buildings, different floors) with restricted access but can be accessed by lab staff from other department with change of lab clothes including shoes and use of disinfectants Lab departments are separated (e.g. different buildings or different floors for different departments), all lab staff can enter the different departments any time, change of lab clothes possible but not always required/followed No clear separation, e.g. one-room-lab or one floor harbours different departments (rooms) that can be easily accessed without changing clothes, without using disinfectants 12 Functionality of all departments (safety) Lab facilities of all departments are air locked, have a fire extinguisher, gas supply; emergency exit and alarm system; monitoring for staff adherence to safety rules and practices; there is room for upgrade Lab facilities of more than 70% of departments are well-maintained, have a fire extinguisher, gas supply; safety rules and practices are followed, but absence of monitoring system; there is room for upgrade Only some facilities (<70%) are well-maintained, e.g. virology, pathology section, are regularly maintained; room for upgrade is limited; safety rules and practices are not always required/followed >60% of labs of the departments are in poor status, only few temperature control system/mainly window ventilation, no fire extinguisher, there are hardly any safety rules and practices; room for upgrade is limited 13 Biosafe virology lab BSL-3/4 Virology lab (Biosafety Cabinets Class (BSC)-III) BSL-2/3 Virology Lab (BSC-II) BSL-1/2 Virology lab (one BSC-II) No biosafe Virology lab (BSC-II not functioning or not existent) 14 Biosafe post mortem room BSL-3/4 Postmortem (PM) room + BSC-III BSL-2/3 PM-room + BSC-II BSL-1/2 PM-room (BSC-II) No biosafe PM-room, no BSC 15 Infrastructure, equipment, supplies Infrastructure Biosafe Bacteriology lab BSL-3/4 Bacteriology lab + BSC-II BSL-2/3 Bacteriology lab + BSC-II BSL-1/2 Bacteriology lab + 1xBSC-II No biosafe Bacteriology lab (BSC-II not functioning or not existent) 16 Animal facilities BSL-3 Animal facility in use for experiments and bioproducts (diagnosis) Animal facility in use (BSL 1-2) for production of diagnostic reagents (SPF-eggs, sera etc.) Animal facilities available but not in use; and no BSL Animal facilities not available 17 Cleaning plan and checking process Cleaning plan and checking process in place, regular microbiological controls Cleaning plan and checking process in place, no microbiological controls Occasional cleaning, no cleaning plan drafted No cleaning, no cleaning plan drafted 18 Separated rooms for PCR (number of rooms and BSC/PCR hood/laminar flow cabinet) PCR set-up (extraction, master mix, template, machine) separated (2-3 rooms/workstations, air flow controlled, 1-2 BSC-II + 1 PCR-hood) including change of lab clothes (coats and shoes) + dedicated small PCR equipment (centrifuges, vortex, micropipettes) PCR set-up (extraction, master mix, template, machine) separated (2-3 rooms/workstation, 1-2 BSC-II + 1 PCR hood), including change of lab clothes (coats and shoes), but no dedicated small PCR equipment (centrifuges, vortex, micropipettes) Different rooms available (3 rooms or 2 rooms and 1 BSC-II + 1 laminar flow far from each in one room), but not in consequent use for PCR set-up separation, no change of lab clothes and no dedicated micropipettes No or insufficient separation of PCR set-up (1 room for all 4 activities or 0-1 BSC-II (not functional equals 0) 19 Biosafe and biosecure labs (no of labs without air-lock, AC) All labs are closed rooms and harbour Air Conditioner (AC) in all departments Labs of at least 3 departments (virology, bacteriology and molecular biological) are closed and lockable rooms and harbour AC Only few labs in selected departments (e.g. AI-Lab in virology department) have closed rooms and harbour AC, other rooms have windows or not functional (old) AC In the majority of labs doors or windows do not properly close, if AC in rooms they are not or rarely functional 20 Equipment Equipment for diagnosis of viral diseases Virology department sufficiently equipped to carry out biosafe and rapid diagnosis of various viral diseases (>10 viruses including TAD/zoonoses) by virus isolation and other tests (including cell culture, electronic microscopy, etc.); Samples received are processed within 24 hours Virology department sufficiently equipped to carry out biosafe and rapid diagnosis of only selected diseases (5 to 10 viruses) by virus isolation and other tests (including cell culture, electronic microscopy, etc.); Samples received are processed within 24 to 48 hours Virology department uses old equipment (more than 10 years). Some equipment may be broken or is not well-maintained. Only few diseases can be tested (less than 5) Virology department lacks functional equipment for appropriate diagnosis of viral diseases, for any technique 21 Equipment for serology department Serology department sufficiently equipped to carry out diagnosis of various diseases (>10 diseases including TAD/zoonoses) by various techniques. Serology department is equipped to carry out diagnosis of only selected diseases (5 to 10 diseases) by various techniques. Serology department uses old equipment (more than 10 years). Some equipment may be broken or is not well-maintained. Only few diseases can be tested (less than 5). Serology department lacks functional equipment. Only rough-and-ready techniques can be performed. 22 Equipment for molecular biological diagnosis Molecular section sufficiently equipped (functional BSC-II, PCR hood, PCR cycler…) to carry out diagnosis of various diseases (>15 pathogens); including real-time PCR and functional sequencer or access to efficient sequencing services (results within 48 hours) Molecular section sufficiently equipped to carry out diagnosis of 5 to 15 selected pathogens (including real-time PCR and access to sequencing machine/services) Molecular section lacks modern equipment but may harbour old PCR cyclers and gel-electrophoresis equipment for 3 to 15 selected pathogens Molecular section lacks even basic equipment, is not functional or is not existent 23 Pathology department equipment for necropsies Pathology department sufficiently equipped to carry out necropsies of variable size animals, including big animals. Biosafety and biosecurity conditions are good (protection against contamination of personnel and of the environment). Pathology department sufficiently equipped to carry out necropsies of variable size animals, including big animals. Biosafety and biosecurity conditions are not fully respected to protect against contamination of personnel and of the environment). Pathology department can carry out necropsies of some species. Some biosafety and biosecurity conditions are not respected (personal protective equipment, use of facilities, effluent management...) to protect against contamination of personnel and of the environment). Pathology department lacks basic equipment for appropriate necropsies. Biosafety and biosecurity conditions are not respected. 24 Pathology department equipment for histo-pathological techniques Pathology department sufficiently equipped to carry out up-to-date histo-pathological techniques (including immunohistochemistry) Pathology department is equipped to perform basic histopathological techniques. These techniques are routinely performed. Pathology department is equipped to perform basic histopathological techniques, but these techniques are performed rarely. Pathology department lacks basic equipment for appropriate histopathological analysis. 25 Equipment for diagnosis of bacterial diseases Bacteriology department sufficiently equipped to carry out biosafe and rapid diagnosis of a broad range of bacterial diseases (>10 bacterial diseases/ including TAD/zoonoses) Bacteriology department sufficiently equipped to carry out biosafe diagnosis of at least the most important bacterial diseases (5-10 TAD/zoonoses) Bacteriology department uses old (>8years) equipment for diagnosis of important bacterial diseases; only few (max 5) bacteria can be typed/cultivated Bacterial department lacks basic equipment for appropriate diagnosis of bacterial diseases (e.g. equipment not existent or not functional) 26 Equipment for diagnosis of parasitological diseases Parasitology department sufficiently equipped to carry out up-to-date diagnosis of a broad range of parasitological diseases (>10 including most important) Parasitology department sufficiently equipped to carry out up-to-date diagnosis of at least the most important parasitological diseases (5-10) Parasitology department uses old (>8years) equipment for diagnosis of important parasitological diseases; only few parasites (max 5) can be typed Parasitology department lacks basic equipment for appropriate diagnosis of parasitological diseases (e.g. equipment not existent or not functional) 27 Maintenance procedures for autoclave(s) Periodic maintenance procedures in place for all autoclave(s); annual maintenance by specialist the last 3 years; systematic use of steam autoclave indicator Periodic maintenance procedures not fully in place for all autoclave(s); annual maintenance by specialist the last 3 years; systematic use of steam autoclave indicator Periodic maintenance procedures not fully in place for all autoclave(s); at least one full maintenance by specialist done the last 3 years; use of steam autoclave indicator not systematic. Autoclave(s) never maintained; no constant use of steam autoclave indicator 28 Reagent supply Fresh reagent supply, procurement, affordability Reagents for all operating procedures can be autonomously procured; fresh supplies are always available for continuous service/work Only selected reagents can be autonomously procured for diagnostic use; fresh supplies are not always available Only few reagents can be autonomously procured for diagnostic use; always come with delay upon request -> reagents are often missing All reagents for diagnostic use must be procured by external funding/organization 29 Own production of diagnostic reagents (type, quality) Key diagnostic reagents/material (antigen, antisera, cell lines, buffer solution, red blood cells (RBC)…) is self-produced in good quality (according to standards and monitored, documented) in sufficient amount and validated; animals serving for diagnostic material are SPF Some reagents/material for diagnostic use (RBC, buffer solution, culture medium, chicken eggs) can be self-produced; efforts taken to produce in good quality according to standards (animals serving for reagent material are kept in quarantine and monitored for diseases (SAN) Limited reagents/material for diagnostic use are produced (e.g. RBC, buffer), quality questionable with regard to animal source for reagents (not quarantine, not monitored for diseases (not SPF, or not SAN) No self-production 30 Proper stocking/storage of reagents Separate storage and documentation (updated inventory log) of different material (reagents, sera, samples …) according to QA / QMS standards Separate storage of different material (reagents, sera, samples…), a list is available but no inventory log of all supplies and reagents No correct separation of storage of different material; expired reagents; a list may or may not be available No separation of storage of different material, storage conditions doubtful because of limitations in functionality or availability of appropriate rooms or freezer / fridges / electricity, insufficient documentation, expired supplies 31 Validity of reagents for virological investigations Reagents for daily virological diagnosis by several techniques (excluding serology) are available for a range of viral diseases, including cell culture (>10 viruses including TAD/zoonoses or all viral testing under their mission*) Reagents for daily virological diagnosis by several techniques (excluding serology) are available for a range of viral diseases, including cell culture (5-10 viruses including TAD/zoonoses or partial viral testing under their mission). Some reagents are expired. Limited reagents for diagnosis of few viral diseases (1-5) through rough-and-ready techniques; Reagents are usually not properly stored (no constant freezing), 40 to 70% of reagents are expired Virology (excluding serology) department hardly harbours any reagents, or reagents are expired or have not been properly stored (>70%). 32 Validity of reagents for daily immuno-serological diagnosis Reagents for daily immuno-serological diagnosis by several techniques are available for a range of diseases (>10 diseases including TAD/zoonoses or all diseases under their mission*) Reagents for daily immuno-serological diagnosis by several techniques by several techniques are available for a range of diseases (5-10 diseases including TAD/zoonoses or partial diseases under their mission*). Some reagents are expired. Limited reagents for immuno-serological diagnosis (1-5 diseases) through rough-and-ready techniques; Reagents are usualy not properly stored (no constant freezing), 40 to 70% of reagents are expired Immuno-serological department hardly harbours any reagents, or reagents are expired or have not been properly stored (>70%). 33 Validity of reagents for PCR and sequencing Reagents for daily molecular diagnosis are available in sufficient amount and stored in good condition (including for real-time PCR technology and for sequencing); enzymes are stored at constant -20°C Reagents for daily molecular diagnosis are available -and stored in good conditions- but with some limitations (e.g. no real-time PCR, but conventional PCR, reagents for sequencing limited) Reagents for daily molecular diagnosis are frequently limited (frequent shortages, especially in real-time PCR reagents and extraction kits; not properly stored; some are expired) Molecular diagnosis cannot be carried out due to constant or almost constant lack of valid reagents 34 Validity of reagents for bacteriological investigations All necessary reagents for daily diagnosis of a broad range of bacterial diseases (excluding serology) are available (>10 bacterial diseases including TAD and zoonosis or all diseases under their mission*) All reagents for diagnosis of a limited range but of the most important bacterial diseases (excluding serology) (5-10; zoonosis, TADs or partial bacterial diseases under their mission*) Limited reagents for diagnosis of bacterial diseases (1-5); reagents are not properly stored (no constant freezing), 40-90% of reagents are expired Bacteriology department harbours hardly any reagents for diagnosis of bacterial diseases, or reagents are expired, or have not been properly stored 35 Validity of reagents for pathological methods All reagents for histo-pathological investigations are daily available (staining, IFT-antibodies, fluorescent dyes…) Pathology department harbours all necessary reagents for necropsies / gross-pathology but often lacks some reagents for histo-phatology (e.g. staining, IFT-antibodies…) Pathology department harbours limited reagents for pathology; in general, histo-pathology is not functional due to a lack of valid and appropriate reagents Pathology department hardly harbours any reagents for histo-pathological exam, or reagents are expired, or have not been properly stored; only gross pathology possible 36 Validity of reagents for parasitic methods All necessary reagents for diagnosis of a broad range of parasitic diseases are daily available (>10 parasitic diseases including TAD and zoonosis or all diseases under their mission*) All reagents for diagnosis of a limited range but of the key parasitic diseases (5-10; zoonosis, TADs) Limited reagents for diagnosis of parasitic diseases (1-5); reagents are not properly stored 40-90% of reagents are expired Parasitology department hardly harbours any reagents, or reagents are expired, or have not been properly stored 37 Laboratory performance Staff skills + availability Number of trained and experienced staff per department Each department counts at least one senior and two junior scientists and at least 2 technicians/co-workers. All staff is trained and experienced Each department has at least one senior and one junior scientist and 2 or more technicians/ co-workers, but not all are well-trained or experienced or always available In each department, the number of staff is not sufficient (1 scientist, 1 technician) or are not available for more than 2 working days per week Serious lack of skilled (trained and experienced) personnel in most of the departments (<1 scientist or <1 technician per department); or their availability is of less than 2 working days per week 38 Expertise of staff in virology/serology (continuing education and accuracy of testing) All staff in virology/serology department is experienced, well-trained and continuously educated (>2 training opportunities per year); all tests are reported within 48 hours, test results are validated and recorded before release Well-trained and motivated but not very experienced staff in virology/serology department; <2 opportunities per year for continuing education; tests reported sometimes with delay; test results are recorded Trained staff in virology/serology department, but lack experience/ motivation, <1 training opportunity per year; tests reported sometimes with delay, results not always recorded Staff in virology/serology department not trained or motivated; samples often left uninvestigated, no prompt reporting, results not always recorded 39 Expertise of staff in molecular biology (continuing education and accuracy of testing) All staff in molecular department is experienced, well-trained and continuously educated (>2 training opportunities per year); all tests are reported within 48 hours, test results are validated and recorded before release Well-trained and motivated but not very experienced staff in molecular department; <2 opportunities per year for continuing education; tests reported sometimes with delay; test results are recorded Trained staff in molecular department, but lack experience/ motivation, <1 training opportunity per year; tests reported sometimes with delay, results not always recorded Molecular section does not exist or staff in molecular section not trained or motivated; samples often left uninvestigated, no prompt reporting, results not always recorded 40 Expertise of staff in bacteriology and parasitology (continuing education and accuracy of testing) All staff in bacteriology and parasitology departments is experienced, well-trained and continuously educated (>2 training opportunities per year); all tests are reported within 48 hours, test results are validated and recorded before release Well-trained and motivated but not very experienced staff in bacteriology and parasitology departments; <2 opportunities per year for continuing education; tests reported sometimes with delay; test results are recorded Trained staff in bacteriology and/or parasitology department, but lack experience/ motivation, <1 training opportunity per year; tests reported sometimes with delay, results not always recorded Staff in bacteriology and/or parasitology department not trained or motivated; samples often left uninvestigated, no prompt reporting, results not always recorded 41 Expertise of staff in pathology (continuing education and accuracy of testing) All staff in pathology department is experienced, well-trained and continuously educated (>2 training opportunities per year); all tests are reported within 48 hours, test results are validated and recorded before release Well-trained and motivated but not very experienced staff in pathology department; <2 opportunities per year for continuing education; tests reported sometimes with delay; test results are recorded Trained staff in pathology department, but lack experience/ motivation, <1 training opportunity per year; tests reported sometimes with delay, results not always recorded Staff in pathology department not trained or motivated; samples often left uninvestigated, no prompt reporting, results not always recorded 42 Staff emergency service Staff for emergency available for 24 hours + weekend shifts Irregular availability (only in special situation) of staff for emergency; weekend shifts are planned Irregular availability of staff for emergency and weekend shifts only rarely (<20 weekends per year) Less than 10 weekend shift per year, no staff for emergency available; 43 Availability of maintenance staff Maintenance staff (plumbing, electricity, mechanics etc.) employed and daily available, with emergency service Maintenance staff available during working hours Maintenance staff available with delay in arrival (sometimes hours, sometimes days of delay) Very difficult to get hold of maintenance staff 44 Sample accession Carcass accession (number of carcasses per week, month or year) Pathology department receives carcasses >25 weekly or >100 monthly or >1200 per year for post-mortem Pathology department receives 15-25 carcasses weekly or 60-100 monthly or 720-1200 yearly for post-mortem Pathology department receives 5-15 carcasses weekly or 20-59 monthly or 240-719 yearly for post-mortem Pathology department receives <5 carcasses weekly or <20 monthly or < 240 yearly for post-mortem 45 Case sample throughput in Immunoserology (nb of biological samples per year) Immunoserology laboratory receives more than 100,000 routine samples per year. Immunoserology laboratory receives between 50,000 to 100,000 routine samples per year. Immunoserology laboratory receives between 10,000 and 50,000 routine samples per year. Immunoserology laboratory receives less than 10 000 routine samples per year. 46 Case sample throughput in virology (nb of biological samples per year) Virology laboratory receives more than 5,000 routine samples per year. Virology laboratory receives between 2,500 and 5,000 routine samples per year. Virology laboratory receives between 1,000 and 2,500 routine samples per year. Virology laboratory receives less than 1,000 routine samples per year. 47 Case sample throughput in bacteriology (nb of biological samples per year) Bacteriology laboratory receives more than 5,000 routine samples per year. Bacteriology laboratory receives more between 2,500 and 5,000 routine samples per year. Bacteriology laboratory receives more between 1,000 and 2,500 routine samples per year. Bacteriology laboratory receives less than 1,000 routine samples per year. 48 Case sample throughput in paraistology (nb of biological samples per year) Parasitology laboratory receives more than 5,000 routine samples per year. Parasitology laboratory receives more between 2,500 and 5,000 routine samples per year. Parasitology laboratory receives more between 1,000 and 2,500 routine samples per year. Parasitology laboratory receives less than 1,000 routine samples per year. 49 Sample throughput by PCR Investigation of samples by PCR: >20 samples weekly or >80 monthly or >960 per year Investigation of samples by PCR: 10-20 samples weekly or 40-80 monthly or 480-960 per year Investigation of samples by PCR: 5-9 sample weekly or 20-39 monthly or 240-479 per year No PCR performed, or only in rare circumstances (<5 sample weekly, <20 monthly, <240 per year) 50 Sample throughput from surveillance or monitoring Lab is regularly involved in active surveillance or monitoring of >3 animal diseases that generates more than 20,000 samples per year Lab is involved in active surveillance of 2-3 animal diseases that generates 10,000-20,000 samples per year Lab is involved in surveillance of 1 animal disease and receives 1,000-9,999 samples per year Lab is currently not involved in active surveillance or monitoring 51 Prompt sample processing Processing of overall diagnostic samples is carried out same day of reception by trained and experienced lab staff Overall sample processing is usually carried out within 1-2 days by trained and experienced lab staff (e.g. days before the week end or holidays or when samples arrive in the afternoon) Samples are usually put into fridge or freezer for some days until investigation; or samples are stored for maximum 3 days and then shipped to another laboratory Diagnosis samples are usually put into fridge or freezer over 3 days before being processed because of lack of staff; OR: some samples are sent to another lab, but often not immediately 52 Available technology Post mortem capability (skills and experience of pathologists) in necropsies All necropsies conducted by specifically trained and/or experienced (>5 years) pathologists. The volume of activity allows to maintain this skill All necropsies conducted by specifically trained and/or experienced (>5 years) pathologists, but the volume of activity is not sufficient to maintain this skill Necropsies are not all conducted by specifically trained and/or experienced (>5 years) pathologists No available experience in necropsy in the laboratory 53 Skills and experience of histologists Specifically trained and/or experienced (>5 years) histologists. The volume of activity allows to maintain this skill Specifically trained and/or experienced (>5 years) histologists, but the volume of activity is not sufficient to maintain this skill No histology competence available in the laboratory, but has access to reliable and effective sub contracted services No competence in histology available in the laboratory, and no access to reliable and effective sub contracted services 54 Prompt bacteria identification All required operating methods (SOPs) available at the laboratory describing the method of identification / isolation of all important bacteria* causing disease in animals and/or zoonotic. The laboratory regularly performs (at least once per quarter) these methods Most SOPs describing the method of identification / isolation of all notifiable bacteria** causing disease in animals and/or zoonotic in the region are generally available in the laboratory. The laboratory regularly performs (at least once per quarter) these methods Only a few SOPs describing the method of identification / isolation of all notifiable bacteria** causing disease in animals and/or zoonotic in the region are available in the laboratory and/or the laboratory does not regularly performs (less than once per quarter) these methods No operating methods describing the method of identification / isolation of notifiable bacteria** causing disease in animals and/or zoonotic expected to be present in the region are available in the laboratory. 55 Prompt parasite identification All required operating methods (SOPs) available at the laboratory describing the method of identification of all important parasites* causing disease in animals and/or zoonotic. The laboratory regularly performs (at least once per quarter) these methods Most SOPs describing the method of identification of all parasites** causing notifiable disease in animals and/or zoonotic in the region are generally available in the laboratory. The laboratory regularly performs (at least once per quarter) these methods Only a few SOPs describing the method of identification of parasites** causing notifiable disease in animals and/or zoonotic in the region are available in the laboratory and/or the laboratory does not regularly perform (less than once per quarter) these methods No SOPs describing the method of identification of parasites** causing notifiable disease in animals and/or zoonotic expected to be present in the region are available in the laboratory. 56 Microscopy capacity Microscope functional and in regular (weekly) use Microscope functional but no operator or infrequently used (< 1 per months) Microscope available but not satisfactory No microscope 57 Cell culture (virology) capability Well-established (>5 years) and biosafe/clean cell culturing with >5 different cell lines Cell-culturing possible but with limited cell types (<5 cell lines) or established less than 5 years ago Cell-culturing possible with limited cell types (<2 cell lines), but limited expertise No cell-culture 58 Egg culture (virology) capability Biosafe (BSC-II and III or BSL-3 conditions) virus isolation with Embryonated chicken eggs (ECE) Culturing with Embryonated chicken eggs (ECE) under limited BSL (BSC-II only) Limited and irregular use of ECE for various reasons (limited access to SPF or SAN eggs, no experienced operator, no functioning incubator, no BSC-II…) No or rare (<1 per 6 months) use of ECE, and limited BSL 59 Serological capability Weekly use of serological assays; all following assays are implemented: ELISA, HI, Immuno-histochemistry, Immuno-Fluorescence, AGID for diagnosis of broad range of diseases including all key animal diseases (>10) Weekly use of serological assays; all following assays are implemented: ELISA, HI, Immuno-histochemistry, Immuno-Fluorescence, AGID for diagnosis of key animal diseases (5-10) Only use of basic serological assays like HI, AGID for a few key diseases (<5); limitation in use of ELISA or immunofluorescence No use or rare use (<1 assay per 4 months) of serological assays like ELISA, HI, Immuno-histochemistry, AGID 60 Molecular capability PCR technology (including realtime) weekly used for >10 genome targets PCR technology (including realtime) used for 5-10 genome targets Conventional PCR technology applied only for <5 genome targets No PCR technology applied 61 Annual maintenance of PCR cycler Annual maintenance of PCR cycler Irregular maintenance of PCR cycler No/or rare maintenance of PCR cycler No PCR 62 Sequencing capability Sequencing technology applied and frequently used (≥ 2 per month or more than 100 sequences per year) and maintained following manufacturer's instructions Sequencing technology is set up, but rarely used (<2 per month); infrequent maintenance; easy access to external sequencing services Sequencing technology available but not in use (not functional, no kits, no experienced operator…) and/or some access to external sequencing services No direct access to sequencing services 63 Animal experiment capability Animal experiments as diagnostic method (esp. ICPI, IVPI, mouse inoculation, inoculation of larger animals) applied under biosafe conditions Animal experiments as diagnostic method applied under unsatisfactory conditions (e.g. insufficient biosafety) A few animal experiments (e.g. mouse inoculation for rabies) may be used but lacks of appropriate expertise/other conditions (no biosafe conditions, no easy access to animals, no experienced operator) No experiments performed on animals 64 QA, Biosafety/Biosecurity Training External training in lab performance Staff (scientist, technicians) of each/every department receives at least 1 external training per year in lab diagnosis Not every staff, and/or not all department receives at least 1 external training per year in lab diagnosis Only few selected staff* receives at least 1 external training per year in lab diagnosis Only few selected staff* receive occasional (< 1 per year) external training in lab diagnosis 65 Internal training in lab performance/GLP Every staff of each department receives documented weekly in house [training / updates / meetings] on GLP Not every staff, and/or not all department receives at least 1 internal [update/training/meeting] on GLP per month Only selected staff* receive rare (<1) internal [update training/meeting] on GLP per month No weekly or monthly in house [training/updates/meeting] on GLP 66 Training in QA/QC Key staff receives documented and regular (>1 per year) training in quality assurance (QA)/quality control (QC) Key staff receives regular (1 per year) training in QA/QC; consistent documentation on QA/QC not always available Key staff rarely receives (< 1 per year) training in QA/QC; consistent documentation on QA/QC not available No (< 1 every 2 years) training opportunity in QA/QC 67 Training in maintenance and calibration Key staff receives documented and regular (≥ 1 per year) training in equipment maintenance and calibration Key staff receives irregular (<1 per year) training in equipment maintenance and calibration; consistent documentation on maintenance and calibration not always available Rare trainings (<1 every 2 years); consistent documentation on maintenance and calibration not available No training in equipment maintenance and calibration in the last 3 years 68 Training in lab management Directors/managers regularly (1/year) trained in lab management Directors/managers irregularly (1 every 2 years) trained in lab management Directors / managers irregularly train themselves through self-education or e-learning on lab management No training in lab management 69 Training in biosafety All staff receive regular documented training in biosafety practices (>1 per year) Selected* staff receive regular (1 per year) training in biosafety practices; and/or no consistent documentation Staff rarely trained but good level of awareness in biosafety No training opportunity for most of the staff in biosafety practices, low awareness level among the staff 70 Training in shipping of infectious substances Up-to-date certification for shipping of infectious substances (IATA standards), -for more than one person Up-to-date certification for shipping of infectious substances (IATA standards), for one person in the lab Out-of-date certification for shipping of infectious substances (IATA standards) No certification for shipping of infectious substances (IATA standards) (since 3 years) 71 Quality Assurance Standard requirements for the competence to carry out tests and calibrations Quality system applied in 80% of departments, accreditation of 80% of analytical parameters Quality system applied in 80% of departments, accreditation of 40% of analytical parameters No accreditation yet but in the process of adapting ISO 17025 standards in most departments for future accreditation Quality system not in place 72 Corrective and preventive actions management System established to manage corrective and preventive actions, registration in the quality management system, customer complaints management System established to manage corrective and preventive actions, registration in the quality management system, no customer complaints management System established to manage corrective and preventive actions, no registration in the quality management system No management of nonconforming testing 73 Best practice testing of particular diseases Satisfactory results (>75% correct) in Proficiency Testing for at least 3 selected diseases* in the past 18 months Participation to Proficiency Testing for at least 3 selected diseases* and satisfactory results (>75% correct) in at least one in the past 18 months Participation in Proficiency Testing in the past 18 months for selected diseases* No participation in Proficiency testing within the past 18 months 74 Methodology standardization SOPs for all performed tests prepared and in use; including available biosafety information relevant to the tests SOPs only for selected key diseases* in use including biosafety procedures SOPs for selected test under development, or SOPs developed and in use but biosafety information missing No or only a few SOPs available/under development 75 Correct performance of tests / methodology and kits Proper and documented validation of in house tests / commercial kits by using reference material before use on routine basis Validation of in house tests / commercial kits by using reference material before use on routine basis; but insufficient documentation available No regular validation of in house tests or kits by using reference material before use on routine basis; No documentation available No kits/test validation 76 Overall lab quality assurance Quality officer/manager assigned + quality manual fully applied No quality officer/manager assigned, or Quality manual only partially applied No quality officer/manager assigned, and/or Quality manual still under process No Quality officer/manager and no quality manual developed 77 Test quality assurance Use of internal test quality control (QC) in all tests according to international standards QC not always applied; not necessarily according to international standards QC rarely applied for key tests, but not in all runs/tests No experience in QC 78 Sample identification and follow-up Identification and tracking of each sample entering the lab by use of LIMS or bar-coding or comparable technology Identification and tracking of each sample entering the lab by use of well documented Log-Book or Excel file Identification and tracking of each samples for only a few diseases Irregular identification and tracking of samples 79 Application of international recommendations OIE/WHO/FAO/CDC guidelines and OIE Terrestrial Manual are applied OIE Manual and/or other guidelines are in place, but not sufficiently followed OIE Manual and/or other guidelines rarely used No notice of OIE Manual or other guidelines 80 Metrology procedures All departments have described and routinely implemented metrology procedures* Some departments have described and routinely implemented metrology procedures* All or some departments have metrology procedures described but not implemented routinely None of the departments have metrology procedures described 81 Maintenance procedures All departments have described and routinely implemented maintenance procedures Some departments have described and routinely implemented maintenance procedures All or some departments have maintenance procedures described but not implemented routinely None of the departments have maintenance procedures described 82 Biosafety/Biosecurity Biosafety / biosecurity application Biorisk officer officially assigned and SOPs for personnel biosafety / biosecurity well documented, available at the right places and applied Key-Staff is well-trained in biosafety/biosecurity; but SOPs for personnel biosafety / biosecurity not all finalized and applied Some staff is aware of biosafety/biosecurity principles. No or rare SOPs developed and applied Only vague knowledge of biosafety/biosecurity principles, no SOPs 83 Preparation for emerging diseases A risk assessment for biocontainment of all high consequence pathogens has been conducted A risk assessment for biocontainment of some high consequence pathogens has been conducted Biocontainment of high consequence pathogens has been discussed among the director and staff Biocontainment of high consequence pathogens has not been discussed 84 Biosafety cabinets tested Biosafety cabinets are tested (under recognized standard NSF49 or EN12469) and validated annually by NSF certified assessors Biosafety cabinets are tested (under recognized standard NSF49 or EN12469) at least every 2 years by NSF certified assessors, and corrective measures are taken if needed Biosafety cabinets are tested (under recognized standard NSF49 or EN12469) at least every 2 years by NSF certified assessors but insufficient corrective measures are taken Biosafety cabinets have not been tested (NSF49 or EN12469) by NSF certified assessors for 5 years or more 85 Biosafety cabinets in conformity with international recognized standards 100% biosafety cabinets (BSC) are in conformity with internationally recognized standards (NSF49 or EN12469) and are properly placed in the lab premises 100% BSC are in conformity with internationally recognized standards (NSF49 or EN12469) but 5-10% are not properly placed in the lab premises 100% BSC are in conformity with internationally recognized standards (NSF49 or EN12469) but more than 10% are not properly placed in the lab premises Some BSC are not in conformity with internationally recognized standards (NSF49 or EN12469) and/or more than 10% are not properly placed in the lab premises 86 Staff protection from biohazards PPE are available and used when required; consequent change of lab clothes including shoes according to requirements of respective biosafety level PPE are available but used under rare occasions (in outbreak / critical situations); when necessary change of lab coat but not shoes PPE are available, but not used; inconsequent use of lab clothes PPE are not sufficiently available, inconsequent use of lab clothes 87 Unintentional release of pathogens from the lab (quarantine) Lab staff has to follow an obligatory quarantine period of 3-7 days (depending on pathogens manipulated) before entering any animal holding and is never involved in taking samples during active surveillance Lab staff generally follows a quarantine period of 1-3 days (depending on pathogens manipulated) before entering any animal holding, is not (or only in emergency situations) involved in taking samples during active surveillance Laboratory staff is sometimes or often actively involved in taking samples from animals (esp. in outbreak times) but follows a quarantine period of 1-3 days prior entering any flock (depending on pathogens manipulated). Lab staff is actively involved in taking samples from farm / domestic animals without prior quarantine period 88 Unintentional release of pathogens from the lab (waste management) Proper waste management by compulsive use of incinerator, autoclave, chemical waste treatment, sharp disposal Frequent use of incinerator and autoclave, but sharp disposal rarely in the lab; chemical waste treatment partially addressed Incinerator, autoclave are available, but only used in situations where the lab deals with specific bio agents; no chemical waste treatment Improper waste management, no incinerator (or not functional) and/or no autoclaving (or not functional) of infectious material 89 Intentional release of pathogens from the lab (controlled access) Completely controlled and restricted access only for key staff to BS-Labs and freezer rooms by use of security system (biometric system, ID-badges, camera) Controlled and restricted access only for key staff to BS-Labs and freezer rooms by physical security (eg. lock, guard) Controlled and restricted access only for staff to BS-Labs and freezer rooms but no use of locks Easy access to all labs and freezers / fridges during working hours 90 Staff Security/Health Health Check Health check of all lab staff on regular basis (at least annually) Irregular health check of lab staff (< 1 per year) Health check of lab staff only on request or in case of an accident No health check, even in case of an accident : staff has to seek doctor at own expenses 91 Staff protection from zoonoses (vaccination) Annual vaccination of staff working with zoonotic agents according to WHO recommendations (rabies, influenza…) Vaccination on request but possibly at own expense Post-exposure vaccination or immunization in case of accident No vaccination available 92 Eye wash and emergency shower Eye wash and emergency shower available and functional in each department and after departing BSL-3 labs Eye wash and/or shower available and functional in some labs Eye wash and shower available in some labs but with limitations (not regular checked or not functional or only cold water etc.) Not available or not functional 93 Lab collaboration and networking Communication means Connectivity to landline phone/fax (hours of availability, use of cell phones) 24 hours of constant public cellphone/landline telephone and good fax connectivity Good public cellphone/landline telephone and/or fax connectivity, sometimes interrupted (not constant on 24 hours) Connectivity of public cellphone/landline phone and /or fax often interrupted, private cellphones are sometimes used Public cellphone/landline phone and/or fax not/badly working or not existent; private cell phones (if any) are exclusively used 94 Access to internet (hours per day or days/week) Good and speedy 24 h internet connection, all staff have access in all areas (labs, office…) Good internet connection (12-24 h) in offices; not all staff members have access Internet connection limited (< 12 hours daily), but in general working; only selected staff member have access Internet is slow and interrupted (availability <3 d/week); only selected staff member have access 95 Access to scientific publications All Staff have free access to library during or even outside of working hours providing current scientific publications and to online-journals including at least 3-5 restricted-access journals Free access to up-to-date library during working hours for all staff, but limited access to online-journals (free journals only) Library might be existent, but not up-to-date, limited access to freely accessible online-journals No access to library in the field of scientific interest, and only to few online journals (free access) 96 Distribution of scientific results (updates of website, frequency of publications) Institute have website with updates < 2 month old and produces periodic (>1/year) bulletin on generated scientific results and >10 publications in peer-reviewed international journals Institute has website (updates are older than 2 months) and irregularly (1-10 publications/year) publishes scientific information in peer-reviewed journals Annual reports are public documents and available on request; publications are rare (<1-5 publications per year in peer-reviewed international journals) No website, no regular information sharing 97 National lab networking Regular contacts with national laboratory network Close collaboration /communication (daily contact) with and constant support to/from members of the national lab network Regular contact /communication (1 per week) with members of the national lab network, but support limited due to insufficient lab budget Contact with members of the national lab network, but collaboration/communication difficult (irregular contact, <1 per month) Very scarce collaboration/contact/communication with members of the national lab network (<1 per 3 months) 98 Support to/by members of the national lab network by training (n trainings/year) Support to/by members of the national lab network by provision of training on regular basis (>2/year) Support to/by members of the national lab network by training on irregular basis (<2/year) Support to/by members of the national lab network by rare training (<1/year) No training provided by/to members of the national lab network 99 Support to/by members of the national lab network by provision of material Support to/by members of the national lab network by provision of material/reagents/kits upon request and also regularly without request Support to/by members of the national lab network by irregular provision of material/reagents/kits upon request only Rare provision of material/reagents/kits because of own limited resources No possibility for provision of material/reagents/kits from a central lab to the national lab network 100 Laboratory collaboration In-country lab networking Lab has regular (>1 per 3 months) contacts / collaborations with >3 labs/institutions within the country Lab has regular (1 per 3 months) contacts / collaborations with 1-3 labs/institutions within the country Lab has regular (1 per 3 months) contacts / collaborations with 1 lab/institution within the country Lab has no regular (<1 per 3 months) contacts / collaborations labs/institutions within the country 101 Regional lab networking Lab has key role in regional lab-networking (provision of material, expertise, training, meetings, PT) and takes this role very seriously Lab is actively involved in regional lab-networking (presence at all meetings, participation in PT, training…) and shares data Lab attends meetings (not always present) for regional lab-networking, but is not active in regional networking Lab is not involved in regional lab-networking 102 International collaboration Lab participates in >3 international projects for TAD/zoonotic diseases* of major importance Lab participates in 1-3 international projects for key TADs/zoonotic diseases of major importance* Lab participates in 1 international project for TAD/zoonotic diseases of major importance* Lab does not currently participate in any international project 103 Networking with international labs Lab communicates and has regular direct or bilateral contacts (once a month) with >3 international laboratories (including reference or regional support/leading labs) Lab has some contacts (1 per 6 months) with international laboratories (reference or regional support/leading labs) AND/OR regular contacts (1 per month) with 2 or 3 international labs Lab has irregular contacts (<1 per 6 months) with international laboratories (reference or regional support/leading labs) AND/OR regular contacts (1 per month) with 1 international lab Lab has hardly any contact (<1 per year) with international laboratories (including reference or regional support/leading labs) 104 International lab networking / twinning Lab participates in >2 twinning projects (OIE, EU) or offers twinning Lab participates in 1-2 twinning projects Lab considers/plans to participate in twinning No twinning (considerations) 105 Information retrieval from public sources Lab regularly (>1/month) consults (open-access) disease-related web pages (OIE, FAO, WHO, OFFLU, Promed…) Lab sometimes (1 per 6 months) uses (open-access) disease-related web pages Lab rarely (<1 per 6 months) uses (open-access) disease-related web pages Lab does not or cannot use (open-access) disease-related web pages 106 Information retrieval Databases (GenBank, EMPRES, WAHIS..) are regularly (>1/month) used to access information Databases (GenBank, EMPRES, WAHIS..) are irregularly (1/month) used to access information Databases (GenBank, EMPRES, WAHIS..) are rarely (<1 per month) used to access information Databases are not used 107 Information sharing Regular (>1/month) information shared by lab staff through web-based platforms or databases; and/or submission of more than 20 sequences from 3 pathogens to public sequence database (eg. GenBank) within the last 12 months Irregular Information (1/month) shared by lab staff through web-based platforms; and/or submission of 6 to 19 sequences to public sequence database (eg. GenBank) within the last 12 months Information only shared by lab staff very occasionally through web-based platforms; and/or submission of less than 6 sequences to public sequence database (eg. GenBank) within the last 12 months No information shared by lab staff through web-based platforms within the last 12 months; no sequence submission to a public sequence database (eg. GenBank) 108 Expertise in using e-platforms for epidemiological data analysis / risk assessments Lab / Epi department routinely works with e-platforms (TAD-Info, GIS, others) Lab / Epi department rarely uses e-platforms (TAD-Info, GIS, others) Lab / Epi department has e-platforms installed, but no expertise in usage No use of such platforms Note. The tool can be obtained on request from the FAO EMPRES Laboratory Unit.
doi: 10.3967/bes2020.062
Capacities and Functionalities Assessment of Veterinary Laboratories in South-west Nigeria Using the FAO Laboratory Mapping Tool
-
-
Table 1. Outputs from assessment of veterinary laboratories in south-west nigeria using the laboratory mapping tool-core, 2018
Facility GL LB BS O I E RS SS + A SA AT T QA BB SSH CM NLN LC Grand total Ogun State PC 66.7 66.7 66.7 66.7 44.4 50.0 57.1 61.1 52.4 37.0 27.8 15.2 29.2 22.2 55.6 22.2 18.5 39.7 Uni-Para 44.4 22.2 11.1 66.7 5.6 33.3 16.7 33.3 26.7 29.6 14.3 18.2 25.0 0.0 33.3 22.2 48.1 25.1 Uni-Path 33.3 22.2 11.1 0.0 14.3 50.0 27.8 26.7 8.3 73.3 9.5 21.2 20.0 0.0 41.7 0.0 3.7 21.0 Uni-Mic 33.3 22.2 22.2 66.7 20.8 20.0 28.6 16.7 16.7 14.8 22.2 16.7 25.0 0.0 41.7 0.0 3.7 19.1 Pub Vet C&L 44.4 11.1 0.0 66.7 4.2 0.0 0.0 33.3 N/A 13.3 N/A 5.6 41.7 11.1 33.3 N/A 23.8 15.7 Oyo State Inst-Mic 55.6 33.3 33.3 66.7 33.3 44.4 44.6 46.7 20.0 16.7 44.4 6.1 11.1 11.1 8.3 0.0 0.0 22.9 Pub 2 Vet C&L 44.4 0.0 0.0 66.7 8.3 4.8 16.7 0.0 11.1 16.7 11.1 9.1 11.1 0.0 0.0 11.1 0.0 9.7 Inst L & V 55.6 66.7 33.3 66.7 0.0 8.3 11.1 42.9 16.7 19.4 4.8 3.0 12.5 0.0 33.3 77.8 11.1 18.6 Uni2- Mic 44.4 16.7 22.2 66.7 45.8 66.7 57.1 83.3 38.9 25.9 33.3 12.1 28.6 0.0 41.7 11.1 59.3 39.0 Uni2- Para 44.4 44.4 44.4 66.7 33.3 50.0 53.3 44.4 26.7 23.8 19.0 23.3 25.0 0.0 41.7 0.0 3.7 28.0 Uni2- Path 44.4 33.3 44.4 66.7 28.6 66.7 38.9 61.1 33.3 36.4 28.6 18.2 33.3 0.0 8.3 0.0 3.7 28.6 Mean value 46.4 30.8 26.2 60.6 21.7 35.8 32.0 40.9 25.1 27.9 21.5 13.5 23.9 4.0 30.8 14.4 16.0 24.3 Median 44.4 22.2 22.2 66.7 33.3 44.4 27.8 33.3 23.3 19.4 25.0 18.2 25.0 0.0 41.7 0.0 23.8 22.9 SEM 2.9 6.4 6.2 6.1 4.9 7.3 5.9 6.9 4.3 5.2 3.9 2.0 2.9 2.3 5.3 7.6 6.1 2.8 95% Confidence Interval 37.1–55.8 16.5–45.1 12.4–40.1 47.1–74.1 10.7–32.7 19.6–52.1 18.8–45.2 25.5–56.2 15.4–34.8 16.4–39.4 12.8–30.2 9.0–18.1 17.4–30.3 0.0–9.1 19.0–42.6 −2.8–31.6 2.3–29.6 18.1–30.5 Note. Functionality categories assessed: GL = Geographic location; LB = Laboratory Budget; BS = Basic supply; O = Organization of the laboratory; I = Infrastructure; E = Equipment; RS = Reagent supply; SS + A = Staff skills + availability; SA = Sample accession; AT = Available technology; T = Training; QA = Quality Assurance; BB = Biosafety/Biosecurity; SSH = Staff Security/Health; CM = Communication means; NLN = National laboratory networking; LC = Laboratory collaboration. Categories of laboratories/facilities: PC = private clinic; Uni-Para = University parasitology; Uni-Path = University pathology; Uni-Mic = University microbiology; Pub Vet C&L = State Veterinary clinic and laboratory; Inst-Mic = Training institution microbiology; Pub 2 Vet C&L = 2nd State Veterinary clinic and laboratory; Inst L & V = Institution with laboratory and vaccine supply chain; Uni2-Para = 2nd University parasitology; Uni2-Path = 2nd University pathology; Uni2-Mic = 2nd University microbiology. SEM = Standard error of the mean. All values are expressed in percentages (%). S1. FAO lab capacitation.
# Area Category Sub Category 4 3 2 1 Assessments scores A B C Current 1 General Laboratory Profile Geographic location Strategic placing Isolated compound outside of any residential area Isolated compound in low populated area Single building in low populated area Building within residential area 2 Accessibility Proper containment + guard (24 hr) + Restricted access to building by use of Identity card (employees) only Restricted access, doors are locked + guard at the entrance for 24 h Doors are closed but not locked / low biosecurity level / guard is not always present Easy access to laboratory compound even by visitor / stranger / doors are open / no guard present 3 Location, access Access to highway, airport, harbor and / or station within 30 minutes Access to highway, airport, harbor or station within 60 minutes Access to road; but sometimes limitations (traffic, road condition, flooding) Regular limitations in access to transport means (traffic, bad road, airport is far) 4 Laboratory Budget Financial autonomy (allocation of funds) Lab is financially autonomous, lab funds (>90%) from public source and/or self-generated Lab is almost financially autonomous; lab funds from public source or self-generated (>60%) AND development programmes (<40%) Lab has insufficient own budget (<60%), activities dependant on development partners (>40%) Lab has no autonomous budget; all activities exclusively rely on external funding source 5 Research autonomy (n of publication / year) Lab budget allows ample opportunity for research (>10 publications/year) besides routine diagnostic/production Lab budget allows limited research (1 to 10 publications/year according to the lab context), but mainly routine diagnostic/production Lab budget is insufficient for research, but results from ongoing work are published in national journals/bulletin or regularly presented No research activity due to insufficient lab budget 6 Autonomous infrastructural upgrading (n of constructions / year) Lab budget sufficient for regular (1/year) upgrading / renovation of larger lab infrastructure Lab budget allows irregular (1 / 3 years) independent upgrading / renovation of infrastructure Constructions or renovation by use of lab budget irregular and only for minor infrastructural changes Construction or renovation in general only possible under external funding 7 Basic supply Regular (hours of) electricity supply Constant (24 hours) stable electricity supply + stabilizer and automatic stand-by generator 24 hours electricity supply + stabilizer and back-up generator (manually operated) Frequent electrical instability/voltage irregularity, manually/automatically operated generator + stabilizer Electricity supply (temporarily) less than 10 hours per day, generator does not run permanently (not existent, no fuel) 8 Regular water supply (access and quality) Daily and unlimited supply of good-quality (drinkable) water (pipe) through public source; no risk of water shortage Sufficient daily supply of low quality (not drinkable) water through pipe or tank; back-up tank available Supply of water through tank; sometimes insufficient or low quality (not drinkable) Irregular and insufficient water supply, also low quality (not drinkable) 9 General Laboratory Profile Basic supply Access to purified, deionized, distilled water Unlimited access to purified water; own production of deionised and distilled water Easy access to deionised and distilled water (external/internal source) Limited access to deionised and/or distilled water (external/internal source) Difficult or no access to deionised and/or distilled water 10 Organization Sustainable personnel organization system Organigram and organization system in place + written description of responsibilities according to personnel skill level Organigram and organization system in place; staff mostly know their roles, but not written down Organization system in place, but frequent changes, staff not always clear on their roles Frequent shifting of personnel, no stable organization system 11 Infrastructure, equipment, supplies Infrastructure Containment (means of containment) All lab departments (of different Biosafety levels) are clearly separated and well contained by airlock, personnel shower/changing room, sterilizing and storage rooms; use of disinfections, identity badges or other means (then describe). It can be accessed only by lab staff from the department Lab departments are separated (e.g. different buildings, different floors) with restricted access but can be accessed by lab staff from other department with change of lab clothes including shoes and use of disinfectants Lab departments are separated (e.g. different buildings or different floors for different departments), all lab staff can enter the different departments any time, change of lab clothes possible but not always required/followed No clear separation, e.g. one-room-lab or one floor harbours different departments (rooms) that can be easily accessed without changing clothes, without using disinfectants 12 Functionality of all departments (safety) Lab facilities of all departments are air locked, have a fire extinguisher, gas supply; emergency exit and alarm system; monitoring for staff adherence to safety rules and practices; there is room for upgrade Lab facilities of more than 70% of departments are well-maintained, have a fire extinguisher, gas supply; safety rules and practices are followed, but absence of monitoring system; there is room for upgrade Only some facilities (<70%) are well-maintained, e.g. virology, pathology section, are regularly maintained; room for upgrade is limited; safety rules and practices are not always required/followed >60% of labs of the departments are in poor status, only few temperature control system/mainly window ventilation, no fire extinguisher, there are hardly any safety rules and practices; room for upgrade is limited 13 Biosafe virology lab BSL-3/4 Virology lab (Biosafety Cabinets Class (BSC)-III) BSL-2/3 Virology Lab (BSC-II) BSL-1/2 Virology lab (one BSC-II) No biosafe Virology lab (BSC-II not functioning or not existent) 14 Biosafe post mortem room BSL-3/4 Postmortem (PM) room + BSC-III BSL-2/3 PM-room + BSC-II BSL-1/2 PM-room (BSC-II) No biosafe PM-room, no BSC 15 Infrastructure, equipment, supplies Infrastructure Biosafe Bacteriology lab BSL-3/4 Bacteriology lab + BSC-II BSL-2/3 Bacteriology lab + BSC-II BSL-1/2 Bacteriology lab + 1xBSC-II No biosafe Bacteriology lab (BSC-II not functioning or not existent) 16 Animal facilities BSL-3 Animal facility in use for experiments and bioproducts (diagnosis) Animal facility in use (BSL 1-2) for production of diagnostic reagents (SPF-eggs, sera etc.) Animal facilities available but not in use; and no BSL Animal facilities not available 17 Cleaning plan and checking process Cleaning plan and checking process in place, regular microbiological controls Cleaning plan and checking process in place, no microbiological controls Occasional cleaning, no cleaning plan drafted No cleaning, no cleaning plan drafted 18 Separated rooms for PCR (number of rooms and BSC/PCR hood/laminar flow cabinet) PCR set-up (extraction, master mix, template, machine) separated (2-3 rooms/workstations, air flow controlled, 1-2 BSC-II + 1 PCR-hood) including change of lab clothes (coats and shoes) + dedicated small PCR equipment (centrifuges, vortex, micropipettes) PCR set-up (extraction, master mix, template, machine) separated (2-3 rooms/workstation, 1-2 BSC-II + 1 PCR hood), including change of lab clothes (coats and shoes), but no dedicated small PCR equipment (centrifuges, vortex, micropipettes) Different rooms available (3 rooms or 2 rooms and 1 BSC-II + 1 laminar flow far from each in one room), but not in consequent use for PCR set-up separation, no change of lab clothes and no dedicated micropipettes No or insufficient separation of PCR set-up (1 room for all 4 activities or 0-1 BSC-II (not functional equals 0) 19 Biosafe and biosecure labs (no of labs without air-lock, AC) All labs are closed rooms and harbour Air Conditioner (AC) in all departments Labs of at least 3 departments (virology, bacteriology and molecular biological) are closed and lockable rooms and harbour AC Only few labs in selected departments (e.g. AI-Lab in virology department) have closed rooms and harbour AC, other rooms have windows or not functional (old) AC In the majority of labs doors or windows do not properly close, if AC in rooms they are not or rarely functional 20 Equipment Equipment for diagnosis of viral diseases Virology department sufficiently equipped to carry out biosafe and rapid diagnosis of various viral diseases (>10 viruses including TAD/zoonoses) by virus isolation and other tests (including cell culture, electronic microscopy, etc.); Samples received are processed within 24 hours Virology department sufficiently equipped to carry out biosafe and rapid diagnosis of only selected diseases (5 to 10 viruses) by virus isolation and other tests (including cell culture, electronic microscopy, etc.); Samples received are processed within 24 to 48 hours Virology department uses old equipment (more than 10 years). Some equipment may be broken or is not well-maintained. Only few diseases can be tested (less than 5) Virology department lacks functional equipment for appropriate diagnosis of viral diseases, for any technique 21 Equipment for serology department Serology department sufficiently equipped to carry out diagnosis of various diseases (>10 diseases including TAD/zoonoses) by various techniques. Serology department is equipped to carry out diagnosis of only selected diseases (5 to 10 diseases) by various techniques. Serology department uses old equipment (more than 10 years). Some equipment may be broken or is not well-maintained. Only few diseases can be tested (less than 5). Serology department lacks functional equipment. Only rough-and-ready techniques can be performed. 22 Equipment for molecular biological diagnosis Molecular section sufficiently equipped (functional BSC-II, PCR hood, PCR cycler…) to carry out diagnosis of various diseases (>15 pathogens); including real-time PCR and functional sequencer or access to efficient sequencing services (results within 48 hours) Molecular section sufficiently equipped to carry out diagnosis of 5 to 15 selected pathogens (including real-time PCR and access to sequencing machine/services) Molecular section lacks modern equipment but may harbour old PCR cyclers and gel-electrophoresis equipment for 3 to 15 selected pathogens Molecular section lacks even basic equipment, is not functional or is not existent 23 Pathology department equipment for necropsies Pathology department sufficiently equipped to carry out necropsies of variable size animals, including big animals. Biosafety and biosecurity conditions are good (protection against contamination of personnel and of the environment). Pathology department sufficiently equipped to carry out necropsies of variable size animals, including big animals. Biosafety and biosecurity conditions are not fully respected to protect against contamination of personnel and of the environment). Pathology department can carry out necropsies of some species. Some biosafety and biosecurity conditions are not respected (personal protective equipment, use of facilities, effluent management...) to protect against contamination of personnel and of the environment). Pathology department lacks basic equipment for appropriate necropsies. Biosafety and biosecurity conditions are not respected. 24 Pathology department equipment for histo-pathological techniques Pathology department sufficiently equipped to carry out up-to-date histo-pathological techniques (including immunohistochemistry) Pathology department is equipped to perform basic histopathological techniques. These techniques are routinely performed. Pathology department is equipped to perform basic histopathological techniques, but these techniques are performed rarely. Pathology department lacks basic equipment for appropriate histopathological analysis. 25 Equipment for diagnosis of bacterial diseases Bacteriology department sufficiently equipped to carry out biosafe and rapid diagnosis of a broad range of bacterial diseases (>10 bacterial diseases/ including TAD/zoonoses) Bacteriology department sufficiently equipped to carry out biosafe diagnosis of at least the most important bacterial diseases (5-10 TAD/zoonoses) Bacteriology department uses old (>8years) equipment for diagnosis of important bacterial diseases; only few (max 5) bacteria can be typed/cultivated Bacterial department lacks basic equipment for appropriate diagnosis of bacterial diseases (e.g. equipment not existent or not functional) 26 Equipment for diagnosis of parasitological diseases Parasitology department sufficiently equipped to carry out up-to-date diagnosis of a broad range of parasitological diseases (>10 including most important) Parasitology department sufficiently equipped to carry out up-to-date diagnosis of at least the most important parasitological diseases (5-10) Parasitology department uses old (>8years) equipment for diagnosis of important parasitological diseases; only few parasites (max 5) can be typed Parasitology department lacks basic equipment for appropriate diagnosis of parasitological diseases (e.g. equipment not existent or not functional) 27 Maintenance procedures for autoclave(s) Periodic maintenance procedures in place for all autoclave(s); annual maintenance by specialist the last 3 years; systematic use of steam autoclave indicator Periodic maintenance procedures not fully in place for all autoclave(s); annual maintenance by specialist the last 3 years; systematic use of steam autoclave indicator Periodic maintenance procedures not fully in place for all autoclave(s); at least one full maintenance by specialist done the last 3 years; use of steam autoclave indicator not systematic. Autoclave(s) never maintained; no constant use of steam autoclave indicator 28 Reagent supply Fresh reagent supply, procurement, affordability Reagents for all operating procedures can be autonomously procured; fresh supplies are always available for continuous service/work Only selected reagents can be autonomously procured for diagnostic use; fresh supplies are not always available Only few reagents can be autonomously procured for diagnostic use; always come with delay upon request -> reagents are often missing All reagents for diagnostic use must be procured by external funding/organization 29 Own production of diagnostic reagents (type, quality) Key diagnostic reagents/material (antigen, antisera, cell lines, buffer solution, red blood cells (RBC)…) is self-produced in good quality (according to standards and monitored, documented) in sufficient amount and validated; animals serving for diagnostic material are SPF Some reagents/material for diagnostic use (RBC, buffer solution, culture medium, chicken eggs) can be self-produced; efforts taken to produce in good quality according to standards (animals serving for reagent material are kept in quarantine and monitored for diseases (SAN) Limited reagents/material for diagnostic use are produced (e.g. RBC, buffer), quality questionable with regard to animal source for reagents (not quarantine, not monitored for diseases (not SPF, or not SAN) No self-production 30 Proper stocking/storage of reagents Separate storage and documentation (updated inventory log) of different material (reagents, sera, samples …) according to QA / QMS standards Separate storage of different material (reagents, sera, samples…), a list is available but no inventory log of all supplies and reagents No correct separation of storage of different material; expired reagents; a list may or may not be available No separation of storage of different material, storage conditions doubtful because of limitations in functionality or availability of appropriate rooms or freezer / fridges / electricity, insufficient documentation, expired supplies 31 Validity of reagents for virological investigations Reagents for daily virological diagnosis by several techniques (excluding serology) are available for a range of viral diseases, including cell culture (>10 viruses including TAD/zoonoses or all viral testing under their mission*) Reagents for daily virological diagnosis by several techniques (excluding serology) are available for a range of viral diseases, including cell culture (5-10 viruses including TAD/zoonoses or partial viral testing under their mission). Some reagents are expired. Limited reagents for diagnosis of few viral diseases (1-5) through rough-and-ready techniques; Reagents are usually not properly stored (no constant freezing), 40 to 70% of reagents are expired Virology (excluding serology) department hardly harbours any reagents, or reagents are expired or have not been properly stored (>70%). 32 Validity of reagents for daily immuno-serological diagnosis Reagents for daily immuno-serological diagnosis by several techniques are available for a range of diseases (>10 diseases including TAD/zoonoses or all diseases under their mission*) Reagents for daily immuno-serological diagnosis by several techniques by several techniques are available for a range of diseases (5-10 diseases including TAD/zoonoses or partial diseases under their mission*). Some reagents are expired. Limited reagents for immuno-serological diagnosis (1-5 diseases) through rough-and-ready techniques; Reagents are usualy not properly stored (no constant freezing), 40 to 70% of reagents are expired Immuno-serological department hardly harbours any reagents, or reagents are expired or have not been properly stored (>70%). 33 Validity of reagents for PCR and sequencing Reagents for daily molecular diagnosis are available in sufficient amount and stored in good condition (including for real-time PCR technology and for sequencing); enzymes are stored at constant -20°C Reagents for daily molecular diagnosis are available -and stored in good conditions- but with some limitations (e.g. no real-time PCR, but conventional PCR, reagents for sequencing limited) Reagents for daily molecular diagnosis are frequently limited (frequent shortages, especially in real-time PCR reagents and extraction kits; not properly stored; some are expired) Molecular diagnosis cannot be carried out due to constant or almost constant lack of valid reagents 34 Validity of reagents for bacteriological investigations All necessary reagents for daily diagnosis of a broad range of bacterial diseases (excluding serology) are available (>10 bacterial diseases including TAD and zoonosis or all diseases under their mission*) All reagents for diagnosis of a limited range but of the most important bacterial diseases (excluding serology) (5-10; zoonosis, TADs or partial bacterial diseases under their mission*) Limited reagents for diagnosis of bacterial diseases (1-5); reagents are not properly stored (no constant freezing), 40-90% of reagents are expired Bacteriology department harbours hardly any reagents for diagnosis of bacterial diseases, or reagents are expired, or have not been properly stored 35 Validity of reagents for pathological methods All reagents for histo-pathological investigations are daily available (staining, IFT-antibodies, fluorescent dyes…) Pathology department harbours all necessary reagents for necropsies / gross-pathology but often lacks some reagents for histo-phatology (e.g. staining, IFT-antibodies…) Pathology department harbours limited reagents for pathology; in general, histo-pathology is not functional due to a lack of valid and appropriate reagents Pathology department hardly harbours any reagents for histo-pathological exam, or reagents are expired, or have not been properly stored; only gross pathology possible 36 Validity of reagents for parasitic methods All necessary reagents for diagnosis of a broad range of parasitic diseases are daily available (>10 parasitic diseases including TAD and zoonosis or all diseases under their mission*) All reagents for diagnosis of a limited range but of the key parasitic diseases (5-10; zoonosis, TADs) Limited reagents for diagnosis of parasitic diseases (1-5); reagents are not properly stored 40-90% of reagents are expired Parasitology department hardly harbours any reagents, or reagents are expired, or have not been properly stored 37 Laboratory performance Staff skills + availability Number of trained and experienced staff per department Each department counts at least one senior and two junior scientists and at least 2 technicians/co-workers. All staff is trained and experienced Each department has at least one senior and one junior scientist and 2 or more technicians/ co-workers, but not all are well-trained or experienced or always available In each department, the number of staff is not sufficient (1 scientist, 1 technician) or are not available for more than 2 working days per week Serious lack of skilled (trained and experienced) personnel in most of the departments (<1 scientist or <1 technician per department); or their availability is of less than 2 working days per week 38 Expertise of staff in virology/serology (continuing education and accuracy of testing) All staff in virology/serology department is experienced, well-trained and continuously educated (>2 training opportunities per year); all tests are reported within 48 hours, test results are validated and recorded before release Well-trained and motivated but not very experienced staff in virology/serology department; <2 opportunities per year for continuing education; tests reported sometimes with delay; test results are recorded Trained staff in virology/serology department, but lack experience/ motivation, <1 training opportunity per year; tests reported sometimes with delay, results not always recorded Staff in virology/serology department not trained or motivated; samples often left uninvestigated, no prompt reporting, results not always recorded 39 Expertise of staff in molecular biology (continuing education and accuracy of testing) All staff in molecular department is experienced, well-trained and continuously educated (>2 training opportunities per year); all tests are reported within 48 hours, test results are validated and recorded before release Well-trained and motivated but not very experienced staff in molecular department; <2 opportunities per year for continuing education; tests reported sometimes with delay; test results are recorded Trained staff in molecular department, but lack experience/ motivation, <1 training opportunity per year; tests reported sometimes with delay, results not always recorded Molecular section does not exist or staff in molecular section not trained or motivated; samples often left uninvestigated, no prompt reporting, results not always recorded 40 Expertise of staff in bacteriology and parasitology (continuing education and accuracy of testing) All staff in bacteriology and parasitology departments is experienced, well-trained and continuously educated (>2 training opportunities per year); all tests are reported within 48 hours, test results are validated and recorded before release Well-trained and motivated but not very experienced staff in bacteriology and parasitology departments; <2 opportunities per year for continuing education; tests reported sometimes with delay; test results are recorded Trained staff in bacteriology and/or parasitology department, but lack experience/ motivation, <1 training opportunity per year; tests reported sometimes with delay, results not always recorded Staff in bacteriology and/or parasitology department not trained or motivated; samples often left uninvestigated, no prompt reporting, results not always recorded 41 Expertise of staff in pathology (continuing education and accuracy of testing) All staff in pathology department is experienced, well-trained and continuously educated (>2 training opportunities per year); all tests are reported within 48 hours, test results are validated and recorded before release Well-trained and motivated but not very experienced staff in pathology department; <2 opportunities per year for continuing education; tests reported sometimes with delay; test results are recorded Trained staff in pathology department, but lack experience/ motivation, <1 training opportunity per year; tests reported sometimes with delay, results not always recorded Staff in pathology department not trained or motivated; samples often left uninvestigated, no prompt reporting, results not always recorded 42 Staff emergency service Staff for emergency available for 24 hours + weekend shifts Irregular availability (only in special situation) of staff for emergency; weekend shifts are planned Irregular availability of staff for emergency and weekend shifts only rarely (<20 weekends per year) Less than 10 weekend shift per year, no staff for emergency available; 43 Availability of maintenance staff Maintenance staff (plumbing, electricity, mechanics etc.) employed and daily available, with emergency service Maintenance staff available during working hours Maintenance staff available with delay in arrival (sometimes hours, sometimes days of delay) Very difficult to get hold of maintenance staff 44 Sample accession Carcass accession (number of carcasses per week, month or year) Pathology department receives carcasses >25 weekly or >100 monthly or >1200 per year for post-mortem Pathology department receives 15-25 carcasses weekly or 60-100 monthly or 720-1200 yearly for post-mortem Pathology department receives 5-15 carcasses weekly or 20-59 monthly or 240-719 yearly for post-mortem Pathology department receives <5 carcasses weekly or <20 monthly or < 240 yearly for post-mortem 45 Case sample throughput in Immunoserology (nb of biological samples per year) Immunoserology laboratory receives more than 100,000 routine samples per year. Immunoserology laboratory receives between 50,000 to 100,000 routine samples per year. Immunoserology laboratory receives between 10,000 and 50,000 routine samples per year. Immunoserology laboratory receives less than 10 000 routine samples per year. 46 Case sample throughput in virology (nb of biological samples per year) Virology laboratory receives more than 5,000 routine samples per year. Virology laboratory receives between 2,500 and 5,000 routine samples per year. Virology laboratory receives between 1,000 and 2,500 routine samples per year. Virology laboratory receives less than 1,000 routine samples per year. 47 Case sample throughput in bacteriology (nb of biological samples per year) Bacteriology laboratory receives more than 5,000 routine samples per year. Bacteriology laboratory receives more between 2,500 and 5,000 routine samples per year. Bacteriology laboratory receives more between 1,000 and 2,500 routine samples per year. Bacteriology laboratory receives less than 1,000 routine samples per year. 48 Case sample throughput in paraistology (nb of biological samples per year) Parasitology laboratory receives more than 5,000 routine samples per year. Parasitology laboratory receives more between 2,500 and 5,000 routine samples per year. Parasitology laboratory receives more between 1,000 and 2,500 routine samples per year. Parasitology laboratory receives less than 1,000 routine samples per year. 49 Sample throughput by PCR Investigation of samples by PCR: >20 samples weekly or >80 monthly or >960 per year Investigation of samples by PCR: 10-20 samples weekly or 40-80 monthly or 480-960 per year Investigation of samples by PCR: 5-9 sample weekly or 20-39 monthly or 240-479 per year No PCR performed, or only in rare circumstances (<5 sample weekly, <20 monthly, <240 per year) 50 Sample throughput from surveillance or monitoring Lab is regularly involved in active surveillance or monitoring of >3 animal diseases that generates more than 20,000 samples per year Lab is involved in active surveillance of 2-3 animal diseases that generates 10,000-20,000 samples per year Lab is involved in surveillance of 1 animal disease and receives 1,000-9,999 samples per year Lab is currently not involved in active surveillance or monitoring 51 Prompt sample processing Processing of overall diagnostic samples is carried out same day of reception by trained and experienced lab staff Overall sample processing is usually carried out within 1-2 days by trained and experienced lab staff (e.g. days before the week end or holidays or when samples arrive in the afternoon) Samples are usually put into fridge or freezer for some days until investigation; or samples are stored for maximum 3 days and then shipped to another laboratory Diagnosis samples are usually put into fridge or freezer over 3 days before being processed because of lack of staff; OR: some samples are sent to another lab, but often not immediately 52 Available technology Post mortem capability (skills and experience of pathologists) in necropsies All necropsies conducted by specifically trained and/or experienced (>5 years) pathologists. The volume of activity allows to maintain this skill All necropsies conducted by specifically trained and/or experienced (>5 years) pathologists, but the volume of activity is not sufficient to maintain this skill Necropsies are not all conducted by specifically trained and/or experienced (>5 years) pathologists No available experience in necropsy in the laboratory 53 Skills and experience of histologists Specifically trained and/or experienced (>5 years) histologists. The volume of activity allows to maintain this skill Specifically trained and/or experienced (>5 years) histologists, but the volume of activity is not sufficient to maintain this skill No histology competence available in the laboratory, but has access to reliable and effective sub contracted services No competence in histology available in the laboratory, and no access to reliable and effective sub contracted services 54 Prompt bacteria identification All required operating methods (SOPs) available at the laboratory describing the method of identification / isolation of all important bacteria* causing disease in animals and/or zoonotic. The laboratory regularly performs (at least once per quarter) these methods Most SOPs describing the method of identification / isolation of all notifiable bacteria** causing disease in animals and/or zoonotic in the region are generally available in the laboratory. The laboratory regularly performs (at least once per quarter) these methods Only a few SOPs describing the method of identification / isolation of all notifiable bacteria** causing disease in animals and/or zoonotic in the region are available in the laboratory and/or the laboratory does not regularly performs (less than once per quarter) these methods No operating methods describing the method of identification / isolation of notifiable bacteria** causing disease in animals and/or zoonotic expected to be present in the region are available in the laboratory. 55 Prompt parasite identification All required operating methods (SOPs) available at the laboratory describing the method of identification of all important parasites* causing disease in animals and/or zoonotic. The laboratory regularly performs (at least once per quarter) these methods Most SOPs describing the method of identification of all parasites** causing notifiable disease in animals and/or zoonotic in the region are generally available in the laboratory. The laboratory regularly performs (at least once per quarter) these methods Only a few SOPs describing the method of identification of parasites** causing notifiable disease in animals and/or zoonotic in the region are available in the laboratory and/or the laboratory does not regularly perform (less than once per quarter) these methods No SOPs describing the method of identification of parasites** causing notifiable disease in animals and/or zoonotic expected to be present in the region are available in the laboratory. 56 Microscopy capacity Microscope functional and in regular (weekly) use Microscope functional but no operator or infrequently used (< 1 per months) Microscope available but not satisfactory No microscope 57 Cell culture (virology) capability Well-established (>5 years) and biosafe/clean cell culturing with >5 different cell lines Cell-culturing possible but with limited cell types (<5 cell lines) or established less than 5 years ago Cell-culturing possible with limited cell types (<2 cell lines), but limited expertise No cell-culture 58 Egg culture (virology) capability Biosafe (BSC-II and III or BSL-3 conditions) virus isolation with Embryonated chicken eggs (ECE) Culturing with Embryonated chicken eggs (ECE) under limited BSL (BSC-II only) Limited and irregular use of ECE for various reasons (limited access to SPF or SAN eggs, no experienced operator, no functioning incubator, no BSC-II…) No or rare (<1 per 6 months) use of ECE, and limited BSL 59 Serological capability Weekly use of serological assays; all following assays are implemented: ELISA, HI, Immuno-histochemistry, Immuno-Fluorescence, AGID for diagnosis of broad range of diseases including all key animal diseases (>10) Weekly use of serological assays; all following assays are implemented: ELISA, HI, Immuno-histochemistry, Immuno-Fluorescence, AGID for diagnosis of key animal diseases (5-10) Only use of basic serological assays like HI, AGID for a few key diseases (<5); limitation in use of ELISA or immunofluorescence No use or rare use (<1 assay per 4 months) of serological assays like ELISA, HI, Immuno-histochemistry, AGID 60 Molecular capability PCR technology (including realtime) weekly used for >10 genome targets PCR technology (including realtime) used for 5-10 genome targets Conventional PCR technology applied only for <5 genome targets No PCR technology applied 61 Annual maintenance of PCR cycler Annual maintenance of PCR cycler Irregular maintenance of PCR cycler No/or rare maintenance of PCR cycler No PCR 62 Sequencing capability Sequencing technology applied and frequently used (≥ 2 per month or more than 100 sequences per year) and maintained following manufacturer's instructions Sequencing technology is set up, but rarely used (<2 per month); infrequent maintenance; easy access to external sequencing services Sequencing technology available but not in use (not functional, no kits, no experienced operator…) and/or some access to external sequencing services No direct access to sequencing services 63 Animal experiment capability Animal experiments as diagnostic method (esp. ICPI, IVPI, mouse inoculation, inoculation of larger animals) applied under biosafe conditions Animal experiments as diagnostic method applied under unsatisfactory conditions (e.g. insufficient biosafety) A few animal experiments (e.g. mouse inoculation for rabies) may be used but lacks of appropriate expertise/other conditions (no biosafe conditions, no easy access to animals, no experienced operator) No experiments performed on animals 64 QA, Biosafety/Biosecurity Training External training in lab performance Staff (scientist, technicians) of each/every department receives at least 1 external training per year in lab diagnosis Not every staff, and/or not all department receives at least 1 external training per year in lab diagnosis Only few selected staff* receives at least 1 external training per year in lab diagnosis Only few selected staff* receive occasional (< 1 per year) external training in lab diagnosis 65 Internal training in lab performance/GLP Every staff of each department receives documented weekly in house [training / updates / meetings] on GLP Not every staff, and/or not all department receives at least 1 internal [update/training/meeting] on GLP per month Only selected staff* receive rare (<1) internal [update training/meeting] on GLP per month No weekly or monthly in house [training/updates/meeting] on GLP 66 Training in QA/QC Key staff receives documented and regular (>1 per year) training in quality assurance (QA)/quality control (QC) Key staff receives regular (1 per year) training in QA/QC; consistent documentation on QA/QC not always available Key staff rarely receives (< 1 per year) training in QA/QC; consistent documentation on QA/QC not available No (< 1 every 2 years) training opportunity in QA/QC 67 Training in maintenance and calibration Key staff receives documented and regular (≥ 1 per year) training in equipment maintenance and calibration Key staff receives irregular (<1 per year) training in equipment maintenance and calibration; consistent documentation on maintenance and calibration not always available Rare trainings (<1 every 2 years); consistent documentation on maintenance and calibration not available No training in equipment maintenance and calibration in the last 3 years 68 Training in lab management Directors/managers regularly (1/year) trained in lab management Directors/managers irregularly (1 every 2 years) trained in lab management Directors / managers irregularly train themselves through self-education or e-learning on lab management No training in lab management 69 Training in biosafety All staff receive regular documented training in biosafety practices (>1 per year) Selected* staff receive regular (1 per year) training in biosafety practices; and/or no consistent documentation Staff rarely trained but good level of awareness in biosafety No training opportunity for most of the staff in biosafety practices, low awareness level among the staff 70 Training in shipping of infectious substances Up-to-date certification for shipping of infectious substances (IATA standards), -for more than one person Up-to-date certification for shipping of infectious substances (IATA standards), for one person in the lab Out-of-date certification for shipping of infectious substances (IATA standards) No certification for shipping of infectious substances (IATA standards) (since 3 years) 71 Quality Assurance Standard requirements for the competence to carry out tests and calibrations Quality system applied in 80% of departments, accreditation of 80% of analytical parameters Quality system applied in 80% of departments, accreditation of 40% of analytical parameters No accreditation yet but in the process of adapting ISO 17025 standards in most departments for future accreditation Quality system not in place 72 Corrective and preventive actions management System established to manage corrective and preventive actions, registration in the quality management system, customer complaints management System established to manage corrective and preventive actions, registration in the quality management system, no customer complaints management System established to manage corrective and preventive actions, no registration in the quality management system No management of nonconforming testing 73 Best practice testing of particular diseases Satisfactory results (>75% correct) in Proficiency Testing for at least 3 selected diseases* in the past 18 months Participation to Proficiency Testing for at least 3 selected diseases* and satisfactory results (>75% correct) in at least one in the past 18 months Participation in Proficiency Testing in the past 18 months for selected diseases* No participation in Proficiency testing within the past 18 months 74 Methodology standardization SOPs for all performed tests prepared and in use; including available biosafety information relevant to the tests SOPs only for selected key diseases* in use including biosafety procedures SOPs for selected test under development, or SOPs developed and in use but biosafety information missing No or only a few SOPs available/under development 75 Correct performance of tests / methodology and kits Proper and documented validation of in house tests / commercial kits by using reference material before use on routine basis Validation of in house tests / commercial kits by using reference material before use on routine basis; but insufficient documentation available No regular validation of in house tests or kits by using reference material before use on routine basis; No documentation available No kits/test validation 76 Overall lab quality assurance Quality officer/manager assigned + quality manual fully applied No quality officer/manager assigned, or Quality manual only partially applied No quality officer/manager assigned, and/or Quality manual still under process No Quality officer/manager and no quality manual developed 77 Test quality assurance Use of internal test quality control (QC) in all tests according to international standards QC not always applied; not necessarily according to international standards QC rarely applied for key tests, but not in all runs/tests No experience in QC 78 Sample identification and follow-up Identification and tracking of each sample entering the lab by use of LIMS or bar-coding or comparable technology Identification and tracking of each sample entering the lab by use of well documented Log-Book or Excel file Identification and tracking of each samples for only a few diseases Irregular identification and tracking of samples 79 Application of international recommendations OIE/WHO/FAO/CDC guidelines and OIE Terrestrial Manual are applied OIE Manual and/or other guidelines are in place, but not sufficiently followed OIE Manual and/or other guidelines rarely used No notice of OIE Manual or other guidelines 80 Metrology procedures All departments have described and routinely implemented metrology procedures* Some departments have described and routinely implemented metrology procedures* All or some departments have metrology procedures described but not implemented routinely None of the departments have metrology procedures described 81 Maintenance procedures All departments have described and routinely implemented maintenance procedures Some departments have described and routinely implemented maintenance procedures All or some departments have maintenance procedures described but not implemented routinely None of the departments have maintenance procedures described 82 Biosafety/Biosecurity Biosafety / biosecurity application Biorisk officer officially assigned and SOPs for personnel biosafety / biosecurity well documented, available at the right places and applied Key-Staff is well-trained in biosafety/biosecurity; but SOPs for personnel biosafety / biosecurity not all finalized and applied Some staff is aware of biosafety/biosecurity principles. No or rare SOPs developed and applied Only vague knowledge of biosafety/biosecurity principles, no SOPs 83 Preparation for emerging diseases A risk assessment for biocontainment of all high consequence pathogens has been conducted A risk assessment for biocontainment of some high consequence pathogens has been conducted Biocontainment of high consequence pathogens has been discussed among the director and staff Biocontainment of high consequence pathogens has not been discussed 84 Biosafety cabinets tested Biosafety cabinets are tested (under recognized standard NSF49 or EN12469) and validated annually by NSF certified assessors Biosafety cabinets are tested (under recognized standard NSF49 or EN12469) at least every 2 years by NSF certified assessors, and corrective measures are taken if needed Biosafety cabinets are tested (under recognized standard NSF49 or EN12469) at least every 2 years by NSF certified assessors but insufficient corrective measures are taken Biosafety cabinets have not been tested (NSF49 or EN12469) by NSF certified assessors for 5 years or more 85 Biosafety cabinets in conformity with international recognized standards 100% biosafety cabinets (BSC) are in conformity with internationally recognized standards (NSF49 or EN12469) and are properly placed in the lab premises 100% BSC are in conformity with internationally recognized standards (NSF49 or EN12469) but 5-10% are not properly placed in the lab premises 100% BSC are in conformity with internationally recognized standards (NSF49 or EN12469) but more than 10% are not properly placed in the lab premises Some BSC are not in conformity with internationally recognized standards (NSF49 or EN12469) and/or more than 10% are not properly placed in the lab premises 86 Staff protection from biohazards PPE are available and used when required; consequent change of lab clothes including shoes according to requirements of respective biosafety level PPE are available but used under rare occasions (in outbreak / critical situations); when necessary change of lab coat but not shoes PPE are available, but not used; inconsequent use of lab clothes PPE are not sufficiently available, inconsequent use of lab clothes 87 Unintentional release of pathogens from the lab (quarantine) Lab staff has to follow an obligatory quarantine period of 3-7 days (depending on pathogens manipulated) before entering any animal holding and is never involved in taking samples during active surveillance Lab staff generally follows a quarantine period of 1-3 days (depending on pathogens manipulated) before entering any animal holding, is not (or only in emergency situations) involved in taking samples during active surveillance Laboratory staff is sometimes or often actively involved in taking samples from animals (esp. in outbreak times) but follows a quarantine period of 1-3 days prior entering any flock (depending on pathogens manipulated). Lab staff is actively involved in taking samples from farm / domestic animals without prior quarantine period 88 Unintentional release of pathogens from the lab (waste management) Proper waste management by compulsive use of incinerator, autoclave, chemical waste treatment, sharp disposal Frequent use of incinerator and autoclave, but sharp disposal rarely in the lab; chemical waste treatment partially addressed Incinerator, autoclave are available, but only used in situations where the lab deals with specific bio agents; no chemical waste treatment Improper waste management, no incinerator (or not functional) and/or no autoclaving (or not functional) of infectious material 89 Intentional release of pathogens from the lab (controlled access) Completely controlled and restricted access only for key staff to BS-Labs and freezer rooms by use of security system (biometric system, ID-badges, camera) Controlled and restricted access only for key staff to BS-Labs and freezer rooms by physical security (eg. lock, guard) Controlled and restricted access only for staff to BS-Labs and freezer rooms but no use of locks Easy access to all labs and freezers / fridges during working hours 90 Staff Security/Health Health Check Health check of all lab staff on regular basis (at least annually) Irregular health check of lab staff (< 1 per year) Health check of lab staff only on request or in case of an accident No health check, even in case of an accident : staff has to seek doctor at own expenses 91 Staff protection from zoonoses (vaccination) Annual vaccination of staff working with zoonotic agents according to WHO recommendations (rabies, influenza…) Vaccination on request but possibly at own expense Post-exposure vaccination or immunization in case of accident No vaccination available 92 Eye wash and emergency shower Eye wash and emergency shower available and functional in each department and after departing BSL-3 labs Eye wash and/or shower available and functional in some labs Eye wash and shower available in some labs but with limitations (not regular checked or not functional or only cold water etc.) Not available or not functional 93 Lab collaboration and networking Communication means Connectivity to landline phone/fax (hours of availability, use of cell phones) 24 hours of constant public cellphone/landline telephone and good fax connectivity Good public cellphone/landline telephone and/or fax connectivity, sometimes interrupted (not constant on 24 hours) Connectivity of public cellphone/landline phone and /or fax often interrupted, private cellphones are sometimes used Public cellphone/landline phone and/or fax not/badly working or not existent; private cell phones (if any) are exclusively used 94 Access to internet (hours per day or days/week) Good and speedy 24 h internet connection, all staff have access in all areas (labs, office…) Good internet connection (12-24 h) in offices; not all staff members have access Internet connection limited (< 12 hours daily), but in general working; only selected staff member have access Internet is slow and interrupted (availability <3 d/week); only selected staff member have access 95 Access to scientific publications All Staff have free access to library during or even outside of working hours providing current scientific publications and to online-journals including at least 3-5 restricted-access journals Free access to up-to-date library during working hours for all staff, but limited access to online-journals (free journals only) Library might be existent, but not up-to-date, limited access to freely accessible online-journals No access to library in the field of scientific interest, and only to few online journals (free access) 96 Distribution of scientific results (updates of website, frequency of publications) Institute have website with updates < 2 month old and produces periodic (>1/year) bulletin on generated scientific results and >10 publications in peer-reviewed international journals Institute has website (updates are older than 2 months) and irregularly (1-10 publications/year) publishes scientific information in peer-reviewed journals Annual reports are public documents and available on request; publications are rare (<1-5 publications per year in peer-reviewed international journals) No website, no regular information sharing 97 National lab networking Regular contacts with national laboratory network Close collaboration /communication (daily contact) with and constant support to/from members of the national lab network Regular contact /communication (1 per week) with members of the national lab network, but support limited due to insufficient lab budget Contact with members of the national lab network, but collaboration/communication difficult (irregular contact, <1 per month) Very scarce collaboration/contact/communication with members of the national lab network (<1 per 3 months) 98 Support to/by members of the national lab network by training (n trainings/year) Support to/by members of the national lab network by provision of training on regular basis (>2/year) Support to/by members of the national lab network by training on irregular basis (<2/year) Support to/by members of the national lab network by rare training (<1/year) No training provided by/to members of the national lab network 99 Support to/by members of the national lab network by provision of material Support to/by members of the national lab network by provision of material/reagents/kits upon request and also regularly without request Support to/by members of the national lab network by irregular provision of material/reagents/kits upon request only Rare provision of material/reagents/kits because of own limited resources No possibility for provision of material/reagents/kits from a central lab to the national lab network 100 Laboratory collaboration In-country lab networking Lab has regular (>1 per 3 months) contacts / collaborations with >3 labs/institutions within the country Lab has regular (1 per 3 months) contacts / collaborations with 1-3 labs/institutions within the country Lab has regular (1 per 3 months) contacts / collaborations with 1 lab/institution within the country Lab has no regular (<1 per 3 months) contacts / collaborations labs/institutions within the country 101 Regional lab networking Lab has key role in regional lab-networking (provision of material, expertise, training, meetings, PT) and takes this role very seriously Lab is actively involved in regional lab-networking (presence at all meetings, participation in PT, training…) and shares data Lab attends meetings (not always present) for regional lab-networking, but is not active in regional networking Lab is not involved in regional lab-networking 102 International collaboration Lab participates in >3 international projects for TAD/zoonotic diseases* of major importance Lab participates in 1-3 international projects for key TADs/zoonotic diseases of major importance* Lab participates in 1 international project for TAD/zoonotic diseases of major importance* Lab does not currently participate in any international project 103 Networking with international labs Lab communicates and has regular direct or bilateral contacts (once a month) with >3 international laboratories (including reference or regional support/leading labs) Lab has some contacts (1 per 6 months) with international laboratories (reference or regional support/leading labs) AND/OR regular contacts (1 per month) with 2 or 3 international labs Lab has irregular contacts (<1 per 6 months) with international laboratories (reference or regional support/leading labs) AND/OR regular contacts (1 per month) with 1 international lab Lab has hardly any contact (<1 per year) with international laboratories (including reference or regional support/leading labs) 104 International lab networking / twinning Lab participates in >2 twinning projects (OIE, EU) or offers twinning Lab participates in 1-2 twinning projects Lab considers/plans to participate in twinning No twinning (considerations) 105 Information retrieval from public sources Lab regularly (>1/month) consults (open-access) disease-related web pages (OIE, FAO, WHO, OFFLU, Promed…) Lab sometimes (1 per 6 months) uses (open-access) disease-related web pages Lab rarely (<1 per 6 months) uses (open-access) disease-related web pages Lab does not or cannot use (open-access) disease-related web pages 106 Information retrieval Databases (GenBank, EMPRES, WAHIS..) are regularly (>1/month) used to access information Databases (GenBank, EMPRES, WAHIS..) are irregularly (1/month) used to access information Databases (GenBank, EMPRES, WAHIS..) are rarely (<1 per month) used to access information Databases are not used 107 Information sharing Regular (>1/month) information shared by lab staff through web-based platforms or databases; and/or submission of more than 20 sequences from 3 pathogens to public sequence database (eg. GenBank) within the last 12 months Irregular Information (1/month) shared by lab staff through web-based platforms; and/or submission of 6 to 19 sequences to public sequence database (eg. GenBank) within the last 12 months Information only shared by lab staff very occasionally through web-based platforms; and/or submission of less than 6 sequences to public sequence database (eg. GenBank) within the last 12 months No information shared by lab staff through web-based platforms within the last 12 months; no sequence submission to a public sequence database (eg. GenBank) 108 Expertise in using e-platforms for epidemiological data analysis / risk assessments Lab / Epi department routinely works with e-platforms (TAD-Info, GIS, others) Lab / Epi department rarely uses e-platforms (TAD-Info, GIS, others) Lab / Epi department has e-platforms installed, but no expertise in usage No use of such platforms Note. The tool can be obtained on request from the FAO EMPRES Laboratory Unit. -
[1] Masanza MM, Nqobile N, Mukanga D, et al. Laboratory capacity building for the International Health Regulations [IHR(2005)] in resource-poor countries: the experience of the African Field Epidemiology Network (AFENET). BMC Public Health, 2010; 10, 1−7. doi: 10.1186/1471-2458-10-1 [2] Food and Agriculture Organization of the United Nations. Strengthening veterinary diagnostic capacities: the FAO laboratory mapping tool, 2016. http://www.fao.org/emergencies/resources/documents/resources%20detail/en/c/1039809/. [2019-2-13]. [3] Schulz LL, Hayes DJ, Holtkamp DJ, et al. Economic impact of university veterinary diagnostic laboratories: A case study. Prev Vet Med, 2018; 151, 5−12. doi: 10.1016/j.prevetmed.2017.12.018 [4] The Global Health Security Agenda. Fact sheet: the Global Health Security Agenda. 2015. https://geneva.usmission.gov/2015/07/28/fact-sheet-the-global-health-security-agenda/. [2019-2-13] [5] Adamu AM, Alimi YA, Akefe IO, et al. Veterinary diagnostic services for the future: Nigeria’s pathway to progress. Biomed J Sci Tech Res, 2018; 6, 5−7. [6] Blood DC, Brightling P. National Animal Disease Databanks. In: veterinary information management. Bailliere Tindall, London, 1998; 153-60. [7] Kebede H, Melaku A, Kebede E. Constraints in animal health service delivery and sustainable improvement alternatives in North Gondar, Ethiopia. Onderstepoort J Vet Res, 2014; 81, 713−23. [8] Manickam TS, Ankanagari S. Evaluation of quality management systems implementation in medical diagnostic laboratories benchmarked for accreditation. J Med Lab Diagn, 2015; 6, 27−35. doi: 10.5897/JMLD2015.0104 [9] Pacey AA. Quality assurance and quality control in the laboratory andrology. Asian J Androl, 2010; 12, 21−5. doi: 10.1038/aja.2009.16 [10] Ahmed MM. Quality assurance & quality control in laboratories: a review. J Pharm Qual Assur, 2016; 2, 26−31. -




 下载:
下载:



 Quick Links
Quick Links