-
On December 14, 2017, a faculty member of a university in Hunan Province reported that an anthrax vaccine strain might have recovered virulence during an undergraduate experiment and potential exposure could not be ruled out for the students involved. Upon receiving the case report, the CDC, health bureaus, and local governments at the county, prefectural, and provincial levels promptly organized experts in different fields (including epidemiologists, biosafety experts, and laboratory testing experts) for case investigation, evaluation, and response. As the investigation results showed, no virulence recovery was identified in the involved anthrax vaccine strain; and no contamination of Bacillus anthracis was detected at the involved areas. Thus, the university returned to normal functioning.
Bacillus anthracis is the pathogenic microorganism that causes anthrax, an acute anthropozoonosis. According to its infectivity and potential hazards to individuals or groups, it is listed and managed as a highly pathogenic microorganism, i.e., a Class 2 pathogen in China[1].
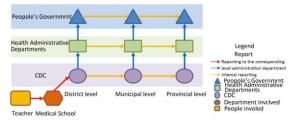
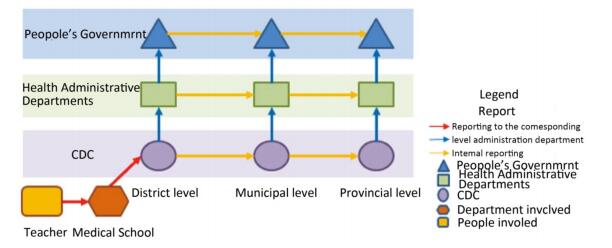
In December 2017, during an undergraduate experiment in a university in Hunan Province, one non-toxic Bacillus anthrax strain was cultured on a chocolate culture medium containing sheep blood and sodium bicarbonate. After incubation in 5% CO2 for 20 h, a faculty member found a smooth colony on the plate and suspected that the strain recovered its virulence. The experiment was performed in a common laboratory without a biological safety cabinet and other necessary biosafety equipment. Students and teachers were usually dressed in gowns, gloves, masks, but had no other personal protection. Since the experiment was not conducted in a laboratory of biosafety level 2 or higher, if the anthrax vaccine strain recovered its virulence, the Personal Protective Equipment (PPE) used in the experiment were not sufficient to protect the operators as well as the environment. At 12 o'clock, on December 14, the university reported the incident to the county CDC, whose jurisdiction covered the school. Next, the incident was reported to the prefectural CDC, and then further to the provincial CDC in a timely manner. At the same time, health bureaus and local governments at each corresponding administrative level also received reports from the CDC at the same level. Upon receiving the first incident report, a rapid risk assessment was conducted, and comprehensive emergency measures were taken, including emergency closure of the laboratory, epidemiological investigation, field sampling, laboratory identification and analysis of the involved virulence genes, prophylaxis, medical observation, site disinfection, and biosafety survey of close contacts.
During the emergency response, 12 samples were collected. Laboratory testing of the samples as described in more detail in another paper[2]. As the results showed, no virulence recovery was identified in the involved anthrax vaccine strain, and none was detected in the involved areas. Based on the results of laboratory testing and other investigations, all emergency response measures were relieved, and the university returned to normal functioning.
Looking back on the entire emergency response process, the tiered CDC system, the whole health administrative system, and the governments at all administrative levels that were involved in this case all took prompt action. The incident was reported level by level with no concealment or omission, and each reporting time was significantly less than the 2-hour requirement of the Regulations for the management of pathogenic microorganisms Laboratory biosafety[3] (Figure 1).
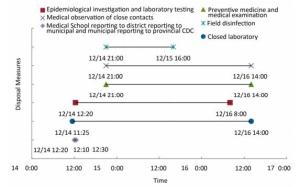
Since receiving the first incident report by the county CDC, the whole process of emergency response took less than 50 h. Upon receipt of the first report, the CDC system immediately launched both an epidemiological investigation and laboratory testing. Nine hours after the first report, because of post-exposure evaluation, comprehensive control measures were taken simultaneously, including prophylaxis, medical examination, medical observation of close contacts, and site disinfection. After 20 h of epidemiological investigation and laboratory identification, the response team performed a follow-up evaluation. After 6 h, based on the evaluation results, the isolation of potentially exposed individuals was terminated, and the emergency response was confirmed to be completed (Figure 2). All measures were taken, and procedures performed in response to this incident were timely and complete. They were in full compliance with the stipulated requirements both for incident management of laboratory personnel exposure and pathogenic microorganism release[3].
As could be seen from the timeline of the incident response, before the implementation of the control measures, including site disinfection, the post-exposure evaluation of the incident was completed in 9 h. After the epidemiological investigation and laboratory testing, the follow-up evaluation was completed within 6 h. The total evaluation took 15 h or 30% of the whole incident response time, and implementation of the control measures took 70%. It best exemplified the critical role of risk assessment for emergency response. (Figure 2)
Bacillus anthracis has the characteristics of easy cultivation, strong pathogenicity, and spore resistance. The United States has included it in the Select Agents List and given high attention and strict management on its handling and utilization[4]. In 2001, terrorist attacks using mail containing Bacillus anthracis white powder in the United States caused widespread concern nationwide. In 2014, a Bacillus anthracis exposure incident occurred in a US CDC laboratory when the laboratory staff transported inactivated Bacillus anthracis from a BSL-3 to a BSL-2 laboratory. In response to the incident, the US CDC took emergency measures including monitoring potentially exposed personnel and implementing prophylactic procedures. After the incident, the US CDC strengthened biosafety management for anthrax-involved experiments and laboratory activity application and proficiency training[5]. A series of incidents have raised public awareness about Bacillus anthracis and made it a focus of laboratory biosafety management.
In 2004, the State Council of the People's Republic of China issued Regulations for the Biosafety Management of Pathogenic Microorganism Laboratory. It further strengthened laboratory biosafety management by the publication of the classification of pathogenic microorganisms and the specification of laboratory biosafety levels. It also stipulated the time limits for emergency reporting and control during incident management involving laboratory personnel exposure and pathogenic microorganism release. In the decade that followed, laboratory biosafety in China made considerable progress in management standardization, technology development, personnel training, team building, research and development capacity, and emergency management, which has provided the most substantial support for both prevention and control of emerging infectious diseases in recent years[6].
doi: 10.3967/bes2019.032
-
-
[1] MOH. List of Human Pathogenic Microorganisms. Beijing: Ministry of Health of the PRC, 2006. [2] Zhang EM, Zhan ZF, Wei Q, et al. Analysis and identification on Bacillus anthracis virulence in the event of the anthrax vaccine suspected virulence recovery. Disease Surveillance, 2019; 34, 76-9. [3] State Council. Regulations for the Management of Pathogenic Microorganisms Laboratory Biosafety. Beijing: State Council of the PRC, 2004. [4] Federal Select Agent Program, USA. Select Agents and Toxins List. 2002. https://www.selectagents.gov/SelectAgentsand Toxins.html.[2018-06-13] [5] Centers for Disease Control and Prevention, USA. CDC Lab Incident: Anthrax. 2014. https://www.cdc.gov/anthrax/news-multimedia/lab-incident/index.html.[2018-06-13] [6] Wei Qiang, Wu Guizhen. Consideration on Microbiological Laboratory Biosafety of China in the New Era. Chinese J of Exp Clin Virol, 2018; 32, 113-5. http://d.old.wanfangdata.com.cn/Periodical/zhsyhlcbdx201802001 -




 下载:
下载:



 Quick Links
Quick Links