-
Hepatocellular carcinoma (HCC) is the second most common cancer, with 700,000 annual deaths recorded globally [1]. Surgical resection remains the primary treatment[2]. However, the prognosis of hepatectomy is not optimistic, and the postoperative recurrence rate is as high as 70%[3].
As a highly selective α2-adrenergic receptor agonist, Dexmedetomidine (DEX) is widely used in clinic and demonstrated favorable properties such as sedation, analgesia, anti-anxiety, anti-inflammatory, anti-apoptotic, and anti-oxidant. Although DEX is highly safe and has ideal pharmacological properties, we still need to be aware of the potential risks of DEX to avoid serious adverse reactions. New evidence suggests that anesthetic drugs may play a key role in the progression of malignant tumors[4-8].
With the growth of malignant solid tumors, hypoxia in tumor tissues promotes angiogenesis, thereby increasing the probability of metastasis[9,10]. Hypoxia-inducible factor-1 alpha (HIF-1α) regulates stress responses such as inflammation, metabolism, oxygen transfer, and cell survival by mediating the adaptive response of tissues to hypoxia[11-13]. Studies have confirmed[14-16] that HIF-1α is highly expressed in highly aggressive tumors, such as liver cancer, and is closely related to various biological activities in tumors. Liver cancer and other malignant tumor cells adapt to low-oxygen environments by expressing HIF-1α protein at high levels.
In addition to hypoxia, tumor angiogenesis also relies on the perception of hypoxia signals by the tumor-related microenvironment, and promotes the proliferation of vascular endothelial cells by producing chemokines and pro-angiogenic factors such as vascular endothelial growth factor a (VEGFA)[17]. VEGFA is an important regulator of angiogenesis. Several studies have shown that the increase in VEGFA concentration in the peripheral blood of hypoxic patients is closely related to the severity of the disease[18,19]. Researchers have also found that the transcription of HIF-1α can adjust VEGFA and plays a crucial role in activating vascular growth and improving oxygen supply in response to oxygen deficiency, including reduction of tissue deficiency, prevention of apoptosis, and promotion of macrophage migration and inflammatory development[20,21]. VEGFA is an important growth factor that stimulates and induces the formation of blood and lymphatic vessels in malignant tumors and is closely related to the hematogenous and lymphatic metastasis of some malignant tumors [22,23].
Recently, in ischemia-reperfusion injury (IRI) studies, it was found that DEX can upregulate HIF-1α expression to protect ischemic and hypoxic tissues [24-27]. However, its effect on HIF-1α and its downstream VEGFA in response to an oxygen-deficient environment in tumor tissues remains poorly understood.
In the present study, we used SMMC-7721 cells, MHCC97-H cells, and a mouse model of orthotopic hepatic carcinoma to explore the effect of DEX on angiogenesis and VM in HCC and the induction of cellular HIF-1α and VEGFA expression. We assumed that the risk impact on the HIF-1α/VEGFA pathway mediated by DEX might promote tumor angiogenesis and VM formation.
-
SMMC-7721 cells (the Chinese Academy of Sciences, China) and MHCC97-H cells (HangZhou Hibio Technology Co.,LTD, China) were maintained in the 1640 (Gibco, USA) and DMEM medium (Gibco, USA) respectively, with 10% FBS (Gibco, USA) and 1% penicillin-streptomycin solution (Meilunbio, China) as the supplement. Cell lines were cultured in a humidified environment with 5% CO2 and 20% O2 (normoxic groups) or 1% O2 (hypoxic groups) at 37 °C. DEX (Yangtze River, China) with a concentration of 0.5 μg/mL was designated for subsequent experiments, and the α2-AR antagonist yohimbine (Aladdin, China) was used in this study.
-
Animal experiments were carried out in accordance with the National Institutes of Health guidelines and were approved in advance by the Animal Ethical and Welfare Committee of Hibiotech Company (China). Experimental BALB/c nude mice (male, 4–6 weeks, 16–22 g) were purchased from Sleek Experimental Animal Center (China) and fed in the SPF-grade animal center of Hibiotech company. SMMC7721 cells were subcutaneously injected into the mice (5 × 106 cells per mouse). The tumor volume was monitored every three days until the 14th day. The subcutaneous tumor was cut into 1 mm3 sized tissue and inoculated under the left liver envelope of BALB/c nude mice[28]. The mice with successful modeling were randomly divided into low-dose, high-dose, and control groups (n = 8/per group) and intraperitoneally injected with an equal volume of 10 μg/kg, 25 μg/kg DEX, and normal saline respectively each day for consecutive 14 days .
-
Individual wells of the tube formation slide (ibid, Germany) were coated with 250 µL Matrigel (Corning, USA) for 30 min before cell seeding. MHCC97-H and SMMC-7721 cells (2 × 104 cells/well) were seeded in coated wells and incubated for 12 h, 24 h, and 48 h. The tube shapes were photographed using an inverted microscope (Olympus, Japan).
-
When the cell density reached 30%–50%, MHCC97-H and SMMC-7721 cells were cultured in Opti-MEM (Gibco, USA). The siRNA targeting HIF-1α and the negative control sequence were purchased from RiBobio (China) for transfection with ExFect2000, according to the manufacturer’s specifications. qPCR and western blotting were performed to detect HIF-1α expression. Optimal cell groups were determined for subsequent experiments. To mimic the effect of DEX on human hepatocellular carcinoma angiogenesis in vitro, cells were cultured with 0.5 μg/mL DEX for 12 h, 24 h, and 48 h in a medium containing a series of concentrations of yohimbine. Cultured cells were collected to analyze the expression of protein and RNA, and supernatants were harvested for ELISA.
-
According to the extracted using TRIzol reagent (Generay Biotech, China), and HiScript® II qRT SuperMix (Vazyme Biotech, China) was used to extract total RNA and transcribe it into cDNA. The reaction was performed at 25 °C, 42 °C, and 70 °C for 10 min, 60 min, and 10 min, respectively. The ChamQ SYBR Color qPCR Master Mix (Vazyme Biotech, China) was used to perform quantitative reverse transcriptase PCR (qRT-PCR). Actin was used as the internal reference gene listed in Table 1. The relative mRNA expression of the target genes was analyzed using the 2−ΔΔCt method[19]. The data were analyzed using the comparative Ct method. Primers (Sunny Biotech, China) for PCR were as follows:
Table 1. PCR primers sequences and length of fragments
Primer Sequence bp (s) Size of product (bp) Homo Actin F TGACGTGGACATCCGCAAAG 20 205 Homo Actin R CTGGAAGGTGGACAGCGAG 19 Homo HIF-1α F CACCACAGGACAGTACAGGAT 21 146 Homo HIF-1α R CGTGCTGAATAATACCACTCACA 23 -
First, cells were lysed in RIPA buffer (Beyotime, China) containing PMSF (Beyotime, China). Second, a bicinchoninic acid assay (Sigma, USA) was performed to quantify the protein concentrations. Third, the protein extracts (30 μg) were heated, denatured, loaded on 10% SDS-PAGE for electrophoresis, and then transferred to a PVDF membrane (Merck Millipore, Ireland). Thereafter, the membrane was blocked with 5% skim milk in TBS-T for 2 h at 37 °C. After that, the membrane was probed with primary and secondary antibodies at 4 °C overnight, aiming to detect the proteins of interest, and anti-β-actin (1:4,000, ab008), anti-HIF-1α (1:1,000, pA1-16601) were included. After four washes, the membrane was developed with the appropriate horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. HRP-conjugated secondary antibodies (Multi Sciences, China, 1:5,000). Staining was then observed using a micro ultraviolet spectrophotometer (Merinton, China) with ECL solution (Beyotime, China). Finally, the loading control was the constitutively expressed protein β-actin, and the blots were detected using an enhanced chemiluminescence system (Bio-Rad) and Image J.
-
The concentration of VEGFA secreted by MHCC97-H and SMMC-7721 cells was determined by ELISA using the corresponding ELISA kit (Multi Sciences, China), following the manufacturer’s instructions. The samples collected from the supernatants and the standard products were placed into plates with the anti-VEGFA antibody (Invitrogen, USA) at room temperature for 2 h. Then, the substrate solution and stop solution were added to react gradually. The protein content was calculated according to the OD values at a wavelength of 450 nm.
-
Paraffin-embedded mice hepatocellular carcinoma tissues were cut into slices. The slides were deparaffinized in xylene, hydrated in gradient alcohol, treated with 3% H2O2 to block endogenous peroxidase activity, and fixed with a citrate buffer for high-pressure thermal treatment. The tissues were cultured with mouse anti-CD31, anti-HIF-1α (Invitrogen, USA), and anti-VEGFA (Invitrogen, USA) antibodies at 4 ℃ overnight, followed by a setting with HRP-conjugated secondary antibodies (Multi Sciences, China) 37 ℃ for 30 min. The slices were successively stained with DAB, counterstained with hematoxylin, differentiated, turned blue, dehydrated, and sealed. The graphs were captured using a microscope under the same conditions.
-
After 48-hour fixation in 4% paraformaldehyde and paraffin embedding, the tumor tissue was conventionally cut into 5 μm portions. Then, the portions were dewaxed and rehydrated, and the antigen was retrieved by PH 9.0. Slices were sealed at 37 ℃ for 30 min and hydrated for incubation after washing 3 times with TBS for 5 min each time. Then Rabbit anti-CD31 polyclonal antibody (Abcam, AB281583, 1:100 dilution) and anti-CD147 antibody (Proteinatech, 66443-1-IG, 1:100 dilution) were incubated overnight at 4 ℃. After washing 3 times in TBS-T, sections were incubated with diluted fluorescent-labeled secondary antibodies for 30 min at room temperature and then stained in darkness with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min to indicate the nuclei. Images were captured using a fluorescence microscope (U-25ND25; Olympus, Tokyo, Japan).
-
Statistical analysis was performed using Graphpad Prism 9.0 (Graphpad, USA). Quantitative measurement data were displayed as the mean ± SEM. Two-way and one-way ANOVA procedures were used in the cell and animal models experiments respectively. Statistical significance was set at P < 0.05.
-
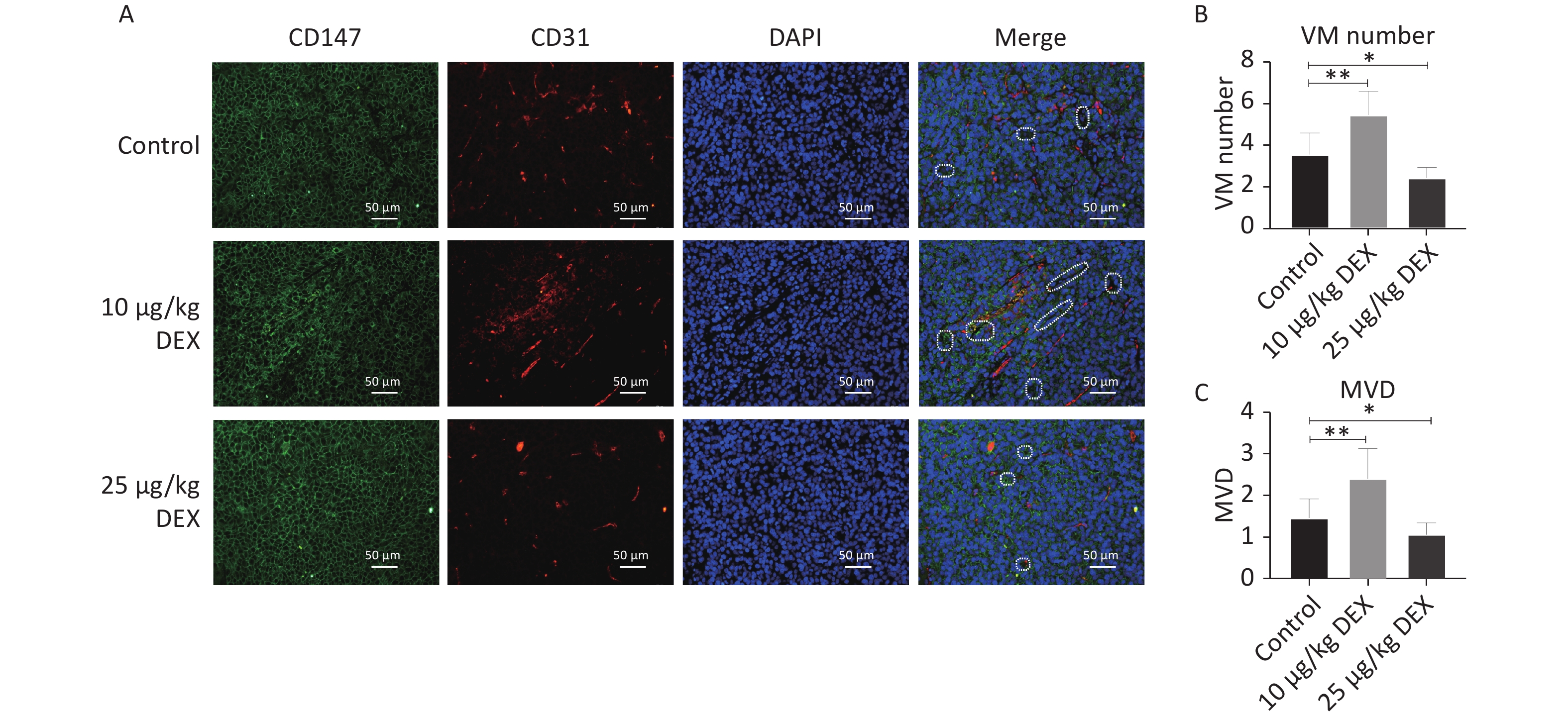
In this study, we used an orthotopic mouse model with an SMMC-7721 HCC cell line. CD147, CD31, and DAPI staining showed the development of Vasculogenic Mimicry and angiogenesis in tumor tissues. CD31-negative/CD147- and DAPI-positive lumen structures were identified as VM channels, and CD31-positive vessels were identified to calculate the microvessel density (MVD). As shown in Figure 1A–C, compared with the control group, our immunofluorescence staining revealed that both the CD31-positive rate and the number of CD147- and DAPI-positive channels significantly increased in the tumor specimen samples in the low-dose (10 μg/kg) DEX group, with a P-value < 0.05. These results showed that low-dose DEX increased angiogenesis and VM. In contrast, high-dose DEX (25 μg/kg) showed the opposite effects. This may be because of the pharmacological toxicity of DEX.
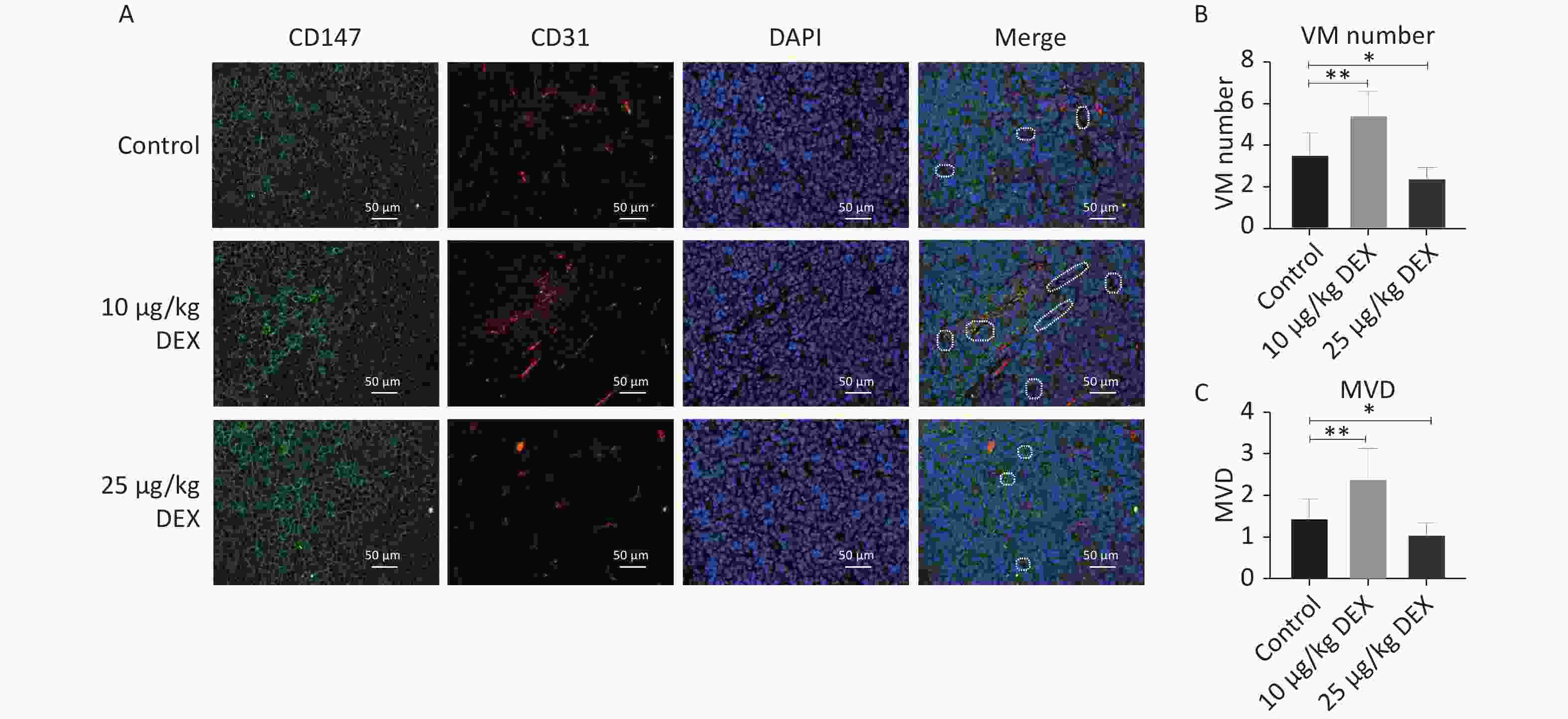
Figure 1. Low-dose DEX promotes angiogenesis and VM in a mouse HCC orthotopic model. (A) CD147, CD31 and DAPI immunofluorescence staining showed the formation of angiogenesis and VM in tumor tissues. (A) White dotted circle indicated the VM lumen structures. Individual and merged images of CD147, CD31, and DAPI staining show that MVD and the number of VM in the low-dose group were significantly increased. But in the high-dose group, MVD and the number of VM was lower than in the control group. Scale bar: 50 μm. (B–C) Number of VM channels and MVD in each group. 0.01 < *P < 0.05, **P < 0.01. DEX: Dexmedetomidine; VM: vasculogenic mimicry; HCC: hepatocellular carcinoma.
-
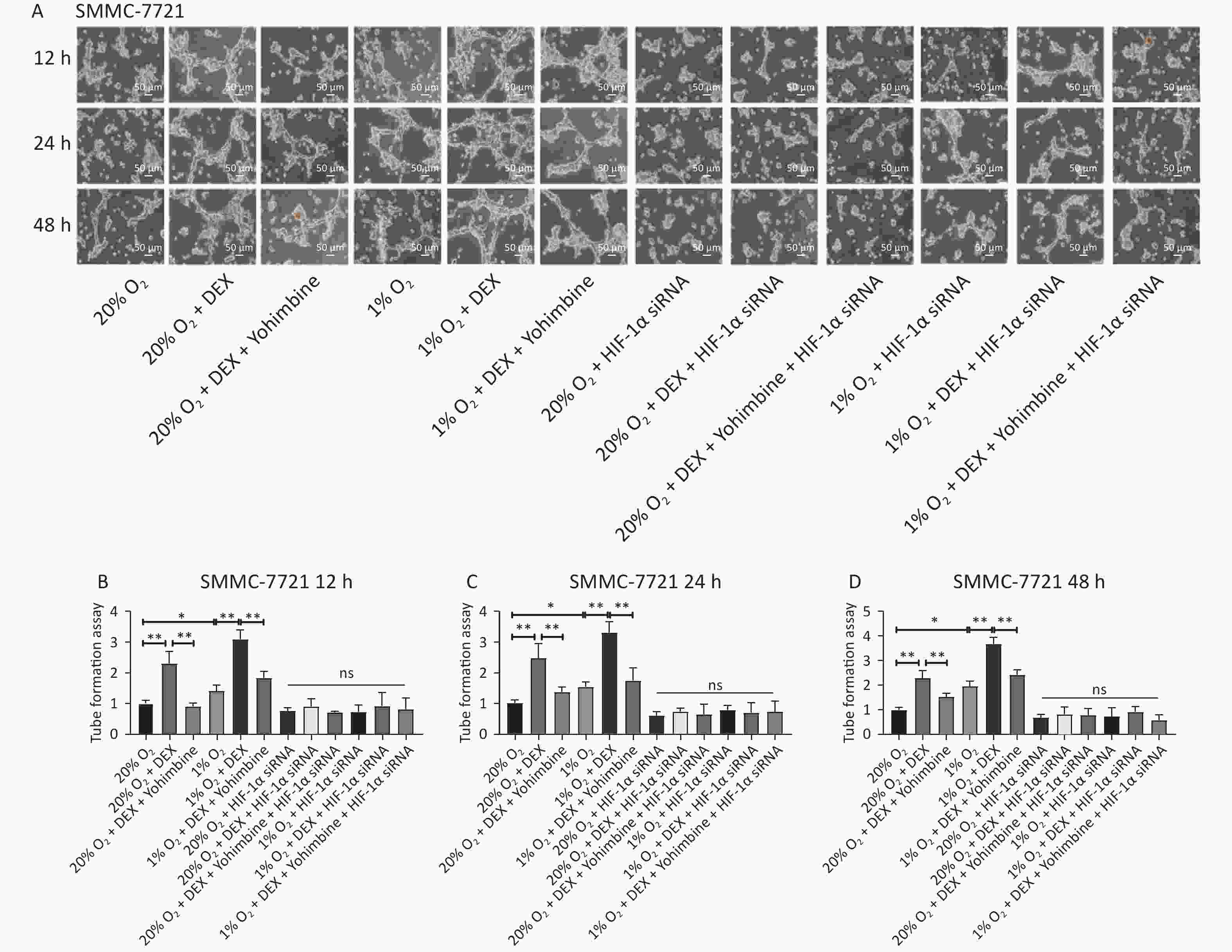
VM formation is a dynamic and common process conducive to tumor cell growth and metastasis in solid malignant tumors. To further evaluate the effects and possible mechanism of DEX on VM in HCC cells, we performed tube formation assays in SMMC-7721 and MHCC97-H cells treated with 0.5 μg/mL DEX under normoxic and hypoxic conditions (20% and 1% O2) at three different time points (12 h, 24 h, and 48 h).
As shown in Figure 2, 0.5 μg/mL DEX significantly increased the tube cavity formation capacity of SMMC-7721 and MHCC97-H liver cancer cells (P-value < 0.01). Moreover, our study found that yohimbine, an α2-adrenoceptor antagonist used to treat erectile dysfunction, attenuated this effect significantly under both normoxic and hypoxic conditions (20% and 1% O2), with a P-value < 0.05.
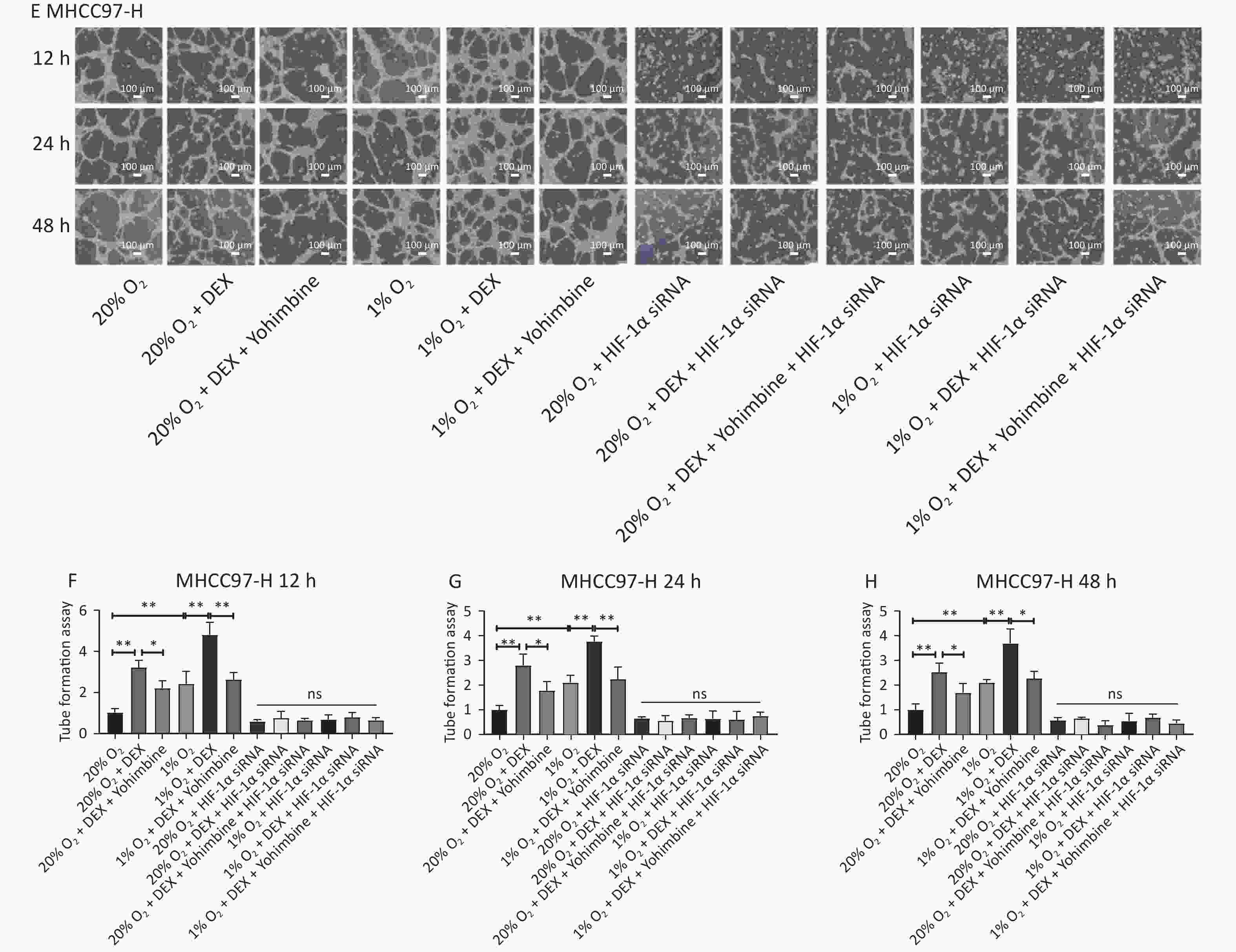
Figure 2. VM formation in SMMC-7721 and MHCC97-H cell lines in vitro. (A) The tube formation assay in SMMC-7721 cells in each group. Scale bar, 50 μm. (B–D) The number of tubes formed by SMMC-7721 cells in each group. (E) The tube formation assay in MHCC97-H cells in each group. Scale bar, 100 μm. (F–H) The number of tubes formed by MHCC97-H cells in each group. Both under normoxia and hypoxia conditions, 0.5 μg/mL DEX promoted VM formation in SMMC-7721 cell and MHCC97-H lines in vitro. Yohimbine could inhibit this effect. 0.01 < *P < 0.05, **P < 0.01. NS means there is no statistically significant difference between groups.
-
Previous studies have shown that HIF-1α expression is associated with invasion and metastasis of malignant tumors. The results of other studies also pointed out that high expression of VEGFA, the downstream protein of HIF-1α, often leads to poor prognosis in patients with HCC. To further explore whether the effect of DEX on angiogenesis is related to HIF-1α and VEGFA expression, we used anti-CD31, anti-HIF-1α, and anti-VEGFA monoclonal antibodies for immunohistochemical staining in a nude mouse liver orthotopic tumorigenesis model with SMMC-7721 HCC cells.
The mice were randomized into three groups (control, low-dose DEX, and high-dose DEX). Figure 3A–B show that CD31-positive cells indicate microvessels. After continued intraperitoneal injection for 14 days, DEX significantly increased microvessel formation and upregulated HIF-1α and VEGFA expression in the low-dose (10 μg/kg) DEX group (Figure 3A–D, P-value < 0.01). However, the expression of CD31, VEGFA, and HIF-1α in the high-dose (25 μg/kg) DEX group was lower than that in the control group (Figure 3A–D, P-value < 0.01).
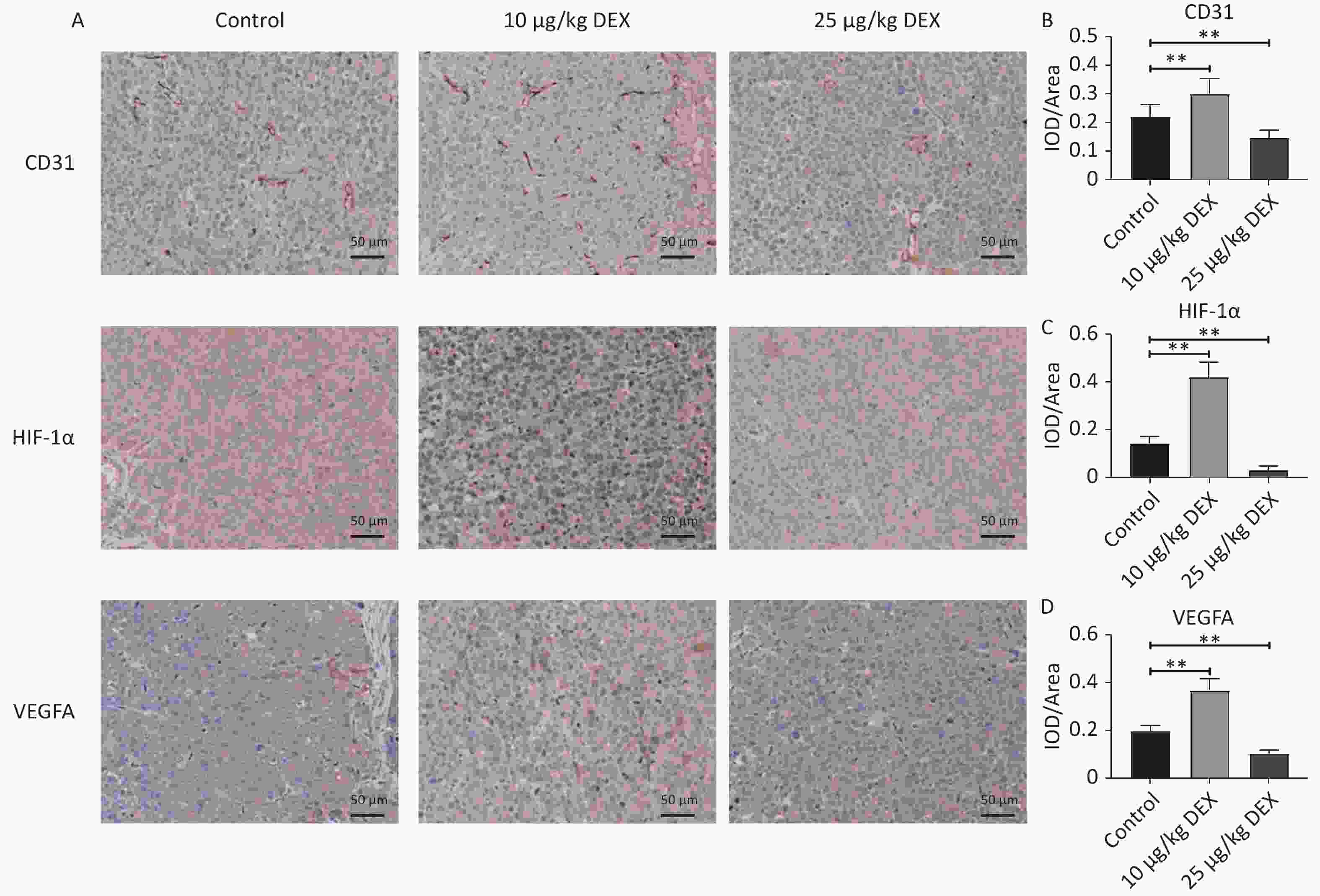
Figure 3. Immunohistochemical staining of CD31, HIF-1α, and VEGFA. (A) Immunohistochemistry staining for CD31, HIF-1α, and VEGFA expression of HCC tissue. (B–D) The Optical Density Value of the immunohistochemistry staining for CD31, HIF-1α, and VEGFA. After 14 days of continuous intraperitoneal injection of low-dose and high doses of DEX, Immunohistochemistry staining demonstrated that CD31, HIF-1α, and VEGFA expression levels were enhanced remarkedly in the low dose groups but dropped in high dose group. Scale bar: 50 μm. 0.01 < *P < 0.05, **P < 0.01.
-
HIF-1α is a critical transcription factor that regulates tumor metabolism, particularly under hypoxic conditions. In this study, we used western blotting and qRT-PCR assays to investigate the regulatory effects of DEX on HIF-1α protein (Figure 4) and mRNA levels (Figure 5) in HCC-derived cell lines (MHCC97-H and SMMC-7721). We carried out two conditions, normoxia and hypoxia, and three different time points, including 12 h, 24 h, and 48 h, to measure HIF-1α expression at the gene and protein levels. DEX (0.5 μg/mL) was used to intervene in cells, and yohimbine was used to rescue them. As shown in Figures 4–5, significant upregulation of HIF-1α under normoxic and hypoxic conditions at each time point was observed with DEX treatment (P-value < 0.05 or 0.01). Yohimbine rescue significantly attenuated the effects of DEX.
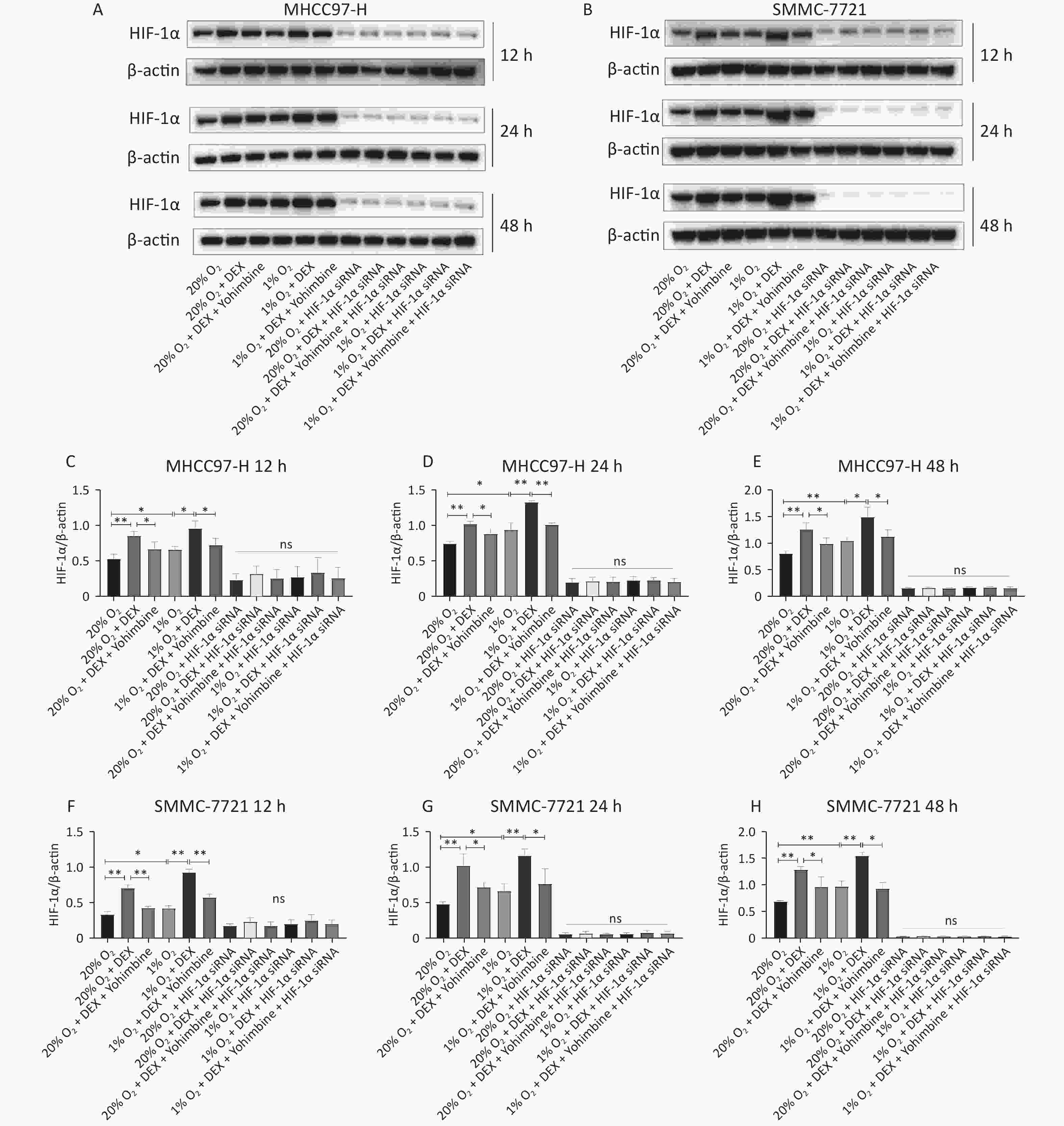
Figure 4. DEX upregulated the HIF-1α protein expression level in MHCC97-H and SMMC-7721 cells. (A–B) HIF-1α protein expression were analyzed with western blot in MHCC97-H and SMMC-7721 cells under 20% and 1% O2 conditions after treatment with DEX for 12 h, 24 h, and 48 h respectively, and β-actin was used as a protein loading control in western blot assay. (C–H) The gray value ratio of the western blot.
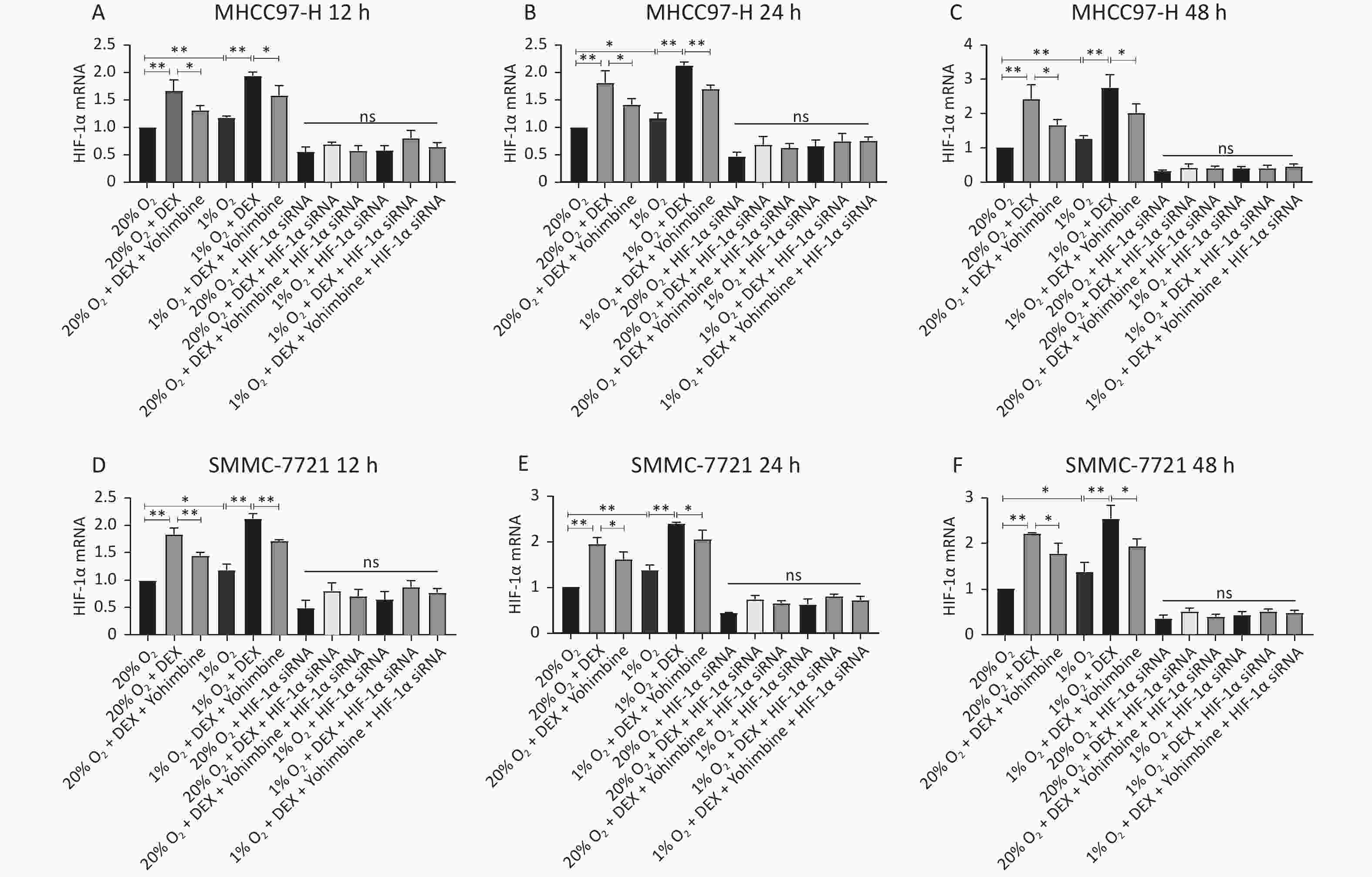
Figure 5. DEX upregulated the HIF-1α expression in MHCC97-H and SMMC-7721 cells at gene levels. (A–F) qRT-PCR analysis of mRNA levels of HIF-1α in MHCC97-H and SMMC-7721 cells. The mRNA levels of HIF-1α in the two cancer cell lines were substantially elevated after DEX treatment. Furthermore, hypoxia insult could aggravate, and Yohimbine could reverse it. The regulatory effect of DEX or Yohimbine was suppressed in HIF-1α si-RNA groups. Error bars represent SD. *P < 0.05, **P < 0.01. NS represent no statistically significant difference between the groups.
-
VEGFA is one of the most effective growth factors affecting angiogenesis and mediates vasculogenic mimicry in tumor cells to participate in the proliferation and migration of tumor cells. It has also been pointed out that regulated by HIF-1α transcription, VEGFA plays a pivotal role in activating vascular growth and improving the oxygen supply in response to hypoxia.
An ELISA assay was carried out to investigate the effect of DEX on the synthesis of the downstream protein VEGFA of HIF-1α in the two HCC-derived cell lines (MHCC97-H and SMMC-7721) to detect VEGFA synthesis after 12 h, 24 h, and 48 h at 0.5 μg/mL DEX. Yohimbine was used to rescue the mice. As shown in Figure 6, DEX increased VEGFA expression in MHCC97-H and SMMC-7721 cells under normoxic and hypoxic conditions at each time point. Yohimbine rescue significantly reduced the effect of DEX on VEGFA synthesis under normoxic and hypoxic conditions at every time point. The expression level of VEGFA was consistent with that of HIF-1α (all P < 0.01).
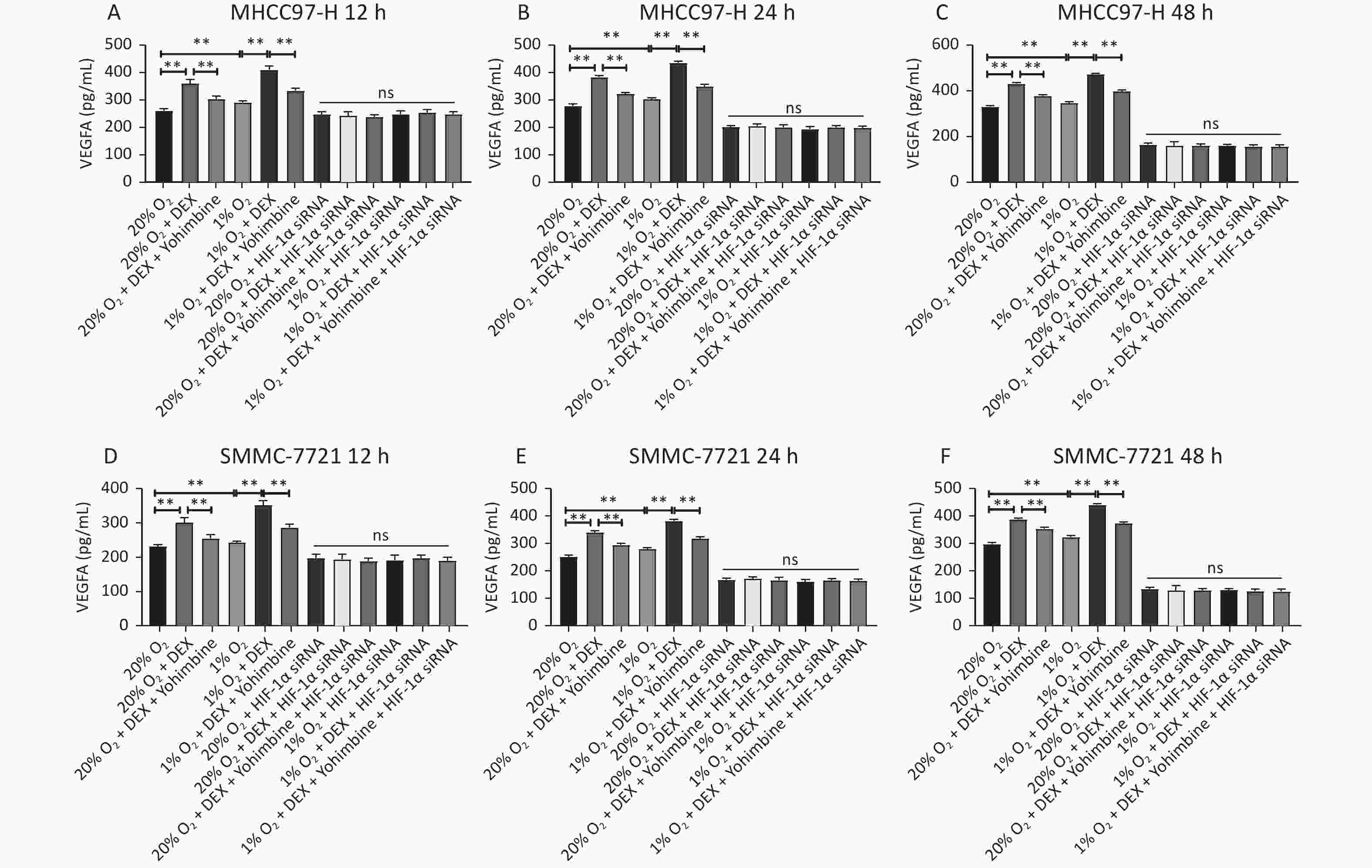
Figure 6. Determination of the VEGFA expression in the ELISA test. Two different conditions (20% O2 and 1% O2), three different time points, 12 h, 24 h, and 48 h in MHCC97-H (A–C) and SMMC-7721 cells (D–F), were tested in ELASA. The experiment was conducted with negative control. The VEGFA concentration (pg/mL) is shown on the horizontal axis. Error bars display the standard deviation of the mean of three experiments. *P < 0.05, **P < 0.01. NS represent there were no significant differences between groups.
-
MTo further verify whether DEX acts through the HIF-1α pathway, we designed three HIF-1α interference sequences and transfected them into SMMC-7721 and MHCC97-H cells. PCR was used to detect the knockdown effect of each sequence pair on HIF-1α expression. When HIF-1α was knocked down via siRNA and then treated with DEX (0.5 μg/mL) or DEX combined with yohimbine, western blotting, qRT-PCR normalized to β-actin, and ELISA were conducted to measure the expression of HIF-1α and the synthesis of VEGFA, respectively. Tube formation assays were performed to determine the VM capacity of the SMMC-7721 and MHCC97-H cells. We found that when we knocked it down, the expression of HIF-1α and VEGFA would be abolished under normoxic and hypoxic conditions at each time point (12 h, 24 h, and 48 h). (Figures 4–6). The tube cavity formation capacity of SMMC-7721 and MHCC97-H cells was suppressed, which was consistent with that of HIF-1α and VEGFA (Figure 2). These results demonstrated that after HIF-1α was silenced, DEX intervention and the addition of yohimbine did not significantly affect HIF-1α and VEGFA expression or the VM behaviors of cells. Thus, we speculated that the HIF-1α/VEGFA pathway might be an important target of DEX.
-
Oxygen deficiency is prevalent in fast-growing, highly invasive tumor cells, and is one of the characteristics of the microenvironment on which their proliferation and metastasis depend. For example, the average O2 partial pressure in human liver cancer tissue is 6 mmHg, compared to 30 mmHg in normal liver tissue. So we performed in vitro experiments in two different conditions, oxygen concentration was set at 1% in the hypoxic groups and 20% in the normoxic groups.
VM is a mechanism described recently for the formation of channels by tumor cells rather than endothelial cells in certain high-aggression solid tumors, such as liver, esophagus, ovarian, and breast cancer, characterized by the absence of vascular inner skin cells in the formation process, thus providing nutrition and oxygen for the growth of tumor cells and eliminating their metabolic waste[28-30]. HIF-1α is a critical transcription factor dependent on oxygen deficiency. It has been shown that HIF-1α is closely related to various biological activities of the tumor[31,32]. Hypoxia can induce the high expression of HIF-1α mRNA and protein in HCC and other malignant tumor cells. As the most important growth factor affecting vascular formation, regulated by HIF-1α transcription, VEGFA plays a key role in activating vascular growth and improving oxygen supply in response to hypoxia, including the reduction of tissue hypoxia, prevention of apoptosis, promotion of macrophage migration, and inflammatory development. In addition to specifically inducing vascular endothelial cells to promote microangiogenesis, a traditional angiogenesis mode, many studies have also shown[33,34] that: VEGFA may act directly on tumor cells, mediating the formation of VM channels to accelerate the proliferation and migration of tumor cells.
We found in vitro experiments that HIF-1α and VEGFA expressions in the hypoxia groups was significantly higher than that in the normoxic groups, and VM formation was markedly higher in the normoxic group. After silencing HIF-1α, the expression of HIF-1α and VEGFA, and the number of VM in all the groups were reduced largely, and there was no statistically significant difference between the groups. These results are in line with those of previous studies: where hypoxia induced HIF-1α expression, activated the signal transduction of VEGFA, and thus promoted angiogenesis in hypoxic tissues. Many other studies have shown that the HIF-1α/VEGFA signaling pathway has become the target of many novel drugs, including antitumor drugs [35-37].
DEX, a highly selective α2-adrenergic receptor agonist, has exhibited paradoxical effects on the progression of highly aggressive tumors [38-40]. We speculated that DEX may promote the vascular formation of HCC by activating the HIF-1α/VEGFA pathway and performed in vitro experiments under two different conditions: normoxia (20% O2) and anoxic (1% O2), for human liver cancer MHCC97-H and SMMC-7721 cells. After treatment with 0.5 μg/mL DEX at 12 h, 24 h, and 48 h, we found that DEX significantly upregulated HIF-1α and VEGFA expression and contributed to an increase in the number of lumen formations in both HCC cells under normoxic and anoxic conditions at each time point. In line with our in vitro data, our in vivo study showed that after intraperitoneal injection of 10 μg/kg DEX for 14 consecutive days, the positive rate of HIF-1α and VEGFAwas enhanced significantly, and MVD and the number of VM channels were also increased significantly in tumor tissues. The results of in vitro and in vivo experiments indicated that DEX might activate the HIF-1α/VEGFA pathway in HCC, thereby promoting the generation of microvessels and quasi-vascular ducts, and thus creating conditions for the proliferation, migration, and invasion of cancer cells.
However, we noticed a paradoxical effect after the intraperitoneal injection of 25 μg/kg DEX for 14 consecutive days. DEX can significantly inhibit HIF-1α and VEGFA expression and decrease the number of VM and MVD in tumor tissues, indicating that DEX leads to carcinogenesis within a certain dose range, and its mechanism needs to be further studied.
In the in vitro study, we used small interfering RNA (siRNA) to silence HIF-1α expression. Silencing results were confirmed by qPCR. After silencing, HIF-1α and VEGFA expression decreased sharply in SMMC-7721 and MHCC97-H cells, and VM formation was significantly suppressed. Moreover, the effects of DEX or yohimbine rescue were abolished in vitro. These results further confirmed that the effect of DEX on angiogenesis and VM in HCC was mediated through the HIF-1α/VEGFA pathway.
Human hepatoma SMMC-7721 and MHCC97-H cells were also pretreated with yohimbine, a selective α2-adrenergic receptor antagonist, and then treated with 0.5 μg/mL DEX in vitro. We found that the addition of yohimbine significantly attenuated the effect of DEX on the HIF-1α/VEGFA pathway and VM in both tumor cell lines. These results suggest that the α2-adrenergic receptor agonist may be one of the fundamental mechanisms by which DEX increases HIF-1α/VEGFA expression and induces vasculature and VM formation. Therefore, these data indicated that further clinical and basic studies are needed to elucidate the role and related mechanisms of dexmedetomidine in tumor progression.
-
In summary, this study investigated the effects of DEX on angiogenesis and vasculogenic mimicry in HCC in vivo and in vitro. These observations suggested that Hif-1α/VEGFA might be a critical pathway through which DEX promotes angiogenesis and VM formation in human hepatocellular carcinoma. α2-adrenergic receptor mediation might be one of the crucial mechanisms by which DEX activates HIF-1α/VEGFA and thus promotes vasculature. Our study asks for prospective clinical trials to evaluate the effect of perioperative dexmedetomidine on the prognosis of patients with HCC.
doi: 10.3967/bes2022.120
Dexmedetomidine Promotes Angiogenesis and Vasculogenic Mimicry in Human Hepatocellular Carcinoma through α2-AR/HIF-1α/VEGFA Pathway
-
Abstract:
Objective Dexmedetomidine (DEX), the most specific α2-adrenergic receptor agonist widely used for its sedative and analgesic properties, has been reported to upregulate HIF-1α expression to protect hypoxic and ischemic tissues. However, it is largely unclear whether DEX can also upregulate Hypoxia-inducible factor-1 alpha (HIF-1α) expression and its downstream vascular endothelial growth factor-A (VEGFA) in cancer tissues with oxygen-deficient tumor microenvironment. Methods We used SMMC-7721 cells, MHCC97-H cells, and a mouse model of orthotopic hepatic carcinoma to explore the effect of DEX on angiogenesis and vasculogenic mimicry (VM) and its mechanism. Under normoxic (20% O2) and hypoxic (1% O2) conditions, DEX was used to intervene cells, and yohimbine was used to rescue them. Results The results showed that DEX promoted angiogenesis and VM in human liver cancer cells within a certain dose range, and the addition of yohimbine inhibited this effect. DEX could activate HIF-1α/VEGFA pathway, which was further verified by silencing HIF-1α. Consistently, in vivo results also showed that DEX can up-regulate HIF-1α/VEGFA expression, and enhance the number of VM channels and microvessel density (MVD). Conclusion We believe that HIF-1α/VEGFA might be an important signaling pathway by which DEX promotes angiogenesis and VM formation in human hepatocellular carcinoma, whereas α2-adrenergic receptor mediation might be the critical mechanisms. -
Key words:
- Hepatocellular carcinoma /
- Dexmedetomidine /
- Yohimbine /
- α2-adrenergic receptor /
- HIF-1a /
- VEGFA /
- Vascular mimicry /
- Angiogenesis
The authors declared no conflict of interest.
&These authors contributed equally to this work.
注释:1) AUTHOR CONTRIBUTIONS: 2) CONFLICT OF INTEREST: -
Figure 1. Low-dose DEX promotes angiogenesis and VM in a mouse HCC orthotopic model. (A) CD147, CD31 and DAPI immunofluorescence staining showed the formation of angiogenesis and VM in tumor tissues. (A) White dotted circle indicated the VM lumen structures. Individual and merged images of CD147, CD31, and DAPI staining show that MVD and the number of VM in the low-dose group were significantly increased. But in the high-dose group, MVD and the number of VM was lower than in the control group. Scale bar: 50 μm. (B–C) Number of VM channels and MVD in each group. 0.01 < *P < 0.05, **P < 0.01. DEX: Dexmedetomidine; VM: vasculogenic mimicry; HCC: hepatocellular carcinoma.
Figure 2. VM formation in SMMC-7721 and MHCC97-H cell lines in vitro. (A) The tube formation assay in SMMC-7721 cells in each group. Scale bar, 50 μm. (B–D) The number of tubes formed by SMMC-7721 cells in each group. (E) The tube formation assay in MHCC97-H cells in each group. Scale bar, 100 μm. (F–H) The number of tubes formed by MHCC97-H cells in each group. Both under normoxia and hypoxia conditions, 0.5 μg/mL DEX promoted VM formation in SMMC-7721 cell and MHCC97-H lines in vitro. Yohimbine could inhibit this effect. 0.01 < *P < 0.05, **P < 0.01. NS means there is no statistically significant difference between groups.
Figure 3. Immunohistochemical staining of CD31, HIF-1α, and VEGFA. (A) Immunohistochemistry staining for CD31, HIF-1α, and VEGFA expression of HCC tissue. (B–D) The Optical Density Value of the immunohistochemistry staining for CD31, HIF-1α, and VEGFA. After 14 days of continuous intraperitoneal injection of low-dose and high doses of DEX, Immunohistochemistry staining demonstrated that CD31, HIF-1α, and VEGFA expression levels were enhanced remarkedly in the low dose groups but dropped in high dose group. Scale bar: 50 μm. 0.01 < *P < 0.05, **P < 0.01.
Figure 4. DEX upregulated the HIF-1α protein expression level in MHCC97-H and SMMC-7721 cells. (A–B) HIF-1α protein expression were analyzed with western blot in MHCC97-H and SMMC-7721 cells under 20% and 1% O2 conditions after treatment with DEX for 12 h, 24 h, and 48 h respectively, and β-actin was used as a protein loading control in western blot assay. (C–H) The gray value ratio of the western blot.
Figure 5. DEX upregulated the HIF-1α expression in MHCC97-H and SMMC-7721 cells at gene levels. (A–F) qRT-PCR analysis of mRNA levels of HIF-1α in MHCC97-H and SMMC-7721 cells. The mRNA levels of HIF-1α in the two cancer cell lines were substantially elevated after DEX treatment. Furthermore, hypoxia insult could aggravate, and Yohimbine could reverse it. The regulatory effect of DEX or Yohimbine was suppressed in HIF-1α si-RNA groups. Error bars represent SD. *P < 0.05, **P < 0.01. NS represent no statistically significant difference between the groups.
Figure 6. Determination of the VEGFA expression in the ELISA test. Two different conditions (20% O2 and 1% O2), three different time points, 12 h, 24 h, and 48 h in MHCC97-H (A–C) and SMMC-7721 cells (D–F), were tested in ELASA. The experiment was conducted with negative control. The VEGFA concentration (pg/mL) is shown on the horizontal axis. Error bars display the standard deviation of the mean of three experiments. *P < 0.05, **P < 0.01. NS represent there were no significant differences between groups.
Table 1. PCR primers sequences and length of fragments
Primer Sequence bp (s) Size of product (bp) Homo Actin F TGACGTGGACATCCGCAAAG 20 205 Homo Actin R CTGGAAGGTGGACAGCGAG 19 Homo HIF-1α F CACCACAGGACAGTACAGGAT 21 146 Homo HIF-1α R CGTGCTGAATAATACCACTCACA 23 -
[1] Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer, 2015; 136, E359−86. doi: 10.1002/ijc.29210 [2] Liu CY, Chen KF, Chen PJ. Treatment of liver cancer. Cold Spring Harb Perspect Med, 2015; 5, a021535. doi: 10.1101/cshperspect.a021535 [3] Xia YX, Zhang F, Li XC, et al. Surgical treatment of primary liver cancer: a report of 10966 cases. Chin J Surg, 2021; 59, 6−17. (In Chinese [4] Chen P, Luo XJ, Dai GQ, et al. Dexmedetomidine promotes the progression of hepatocellular carcinoma through hepatic stellate cell activation. Exp Mol Med, 2020; 52, 1062−74. doi: 10.1038/s12276-020-0461-6 [5] Zhang Y, Li HJ, Wang DX, et al. Impact of inhalational versus intravenous anaesthesia on early delirium and long-term survival in elderly patients after cancer surgery: study protocol of a multicentre, open-label, and randomised controlled trial. BMJ Open, 2017; 7, e018607. doi: 10.1136/bmjopen-2017-018607 [6] Li KH, Yang JX, Han XC. Lidocaine sensitizes the cytotoxicity of cisplatin in breast cancer cells via up-regulation of RARβ2 and RASSF1A demethylation. Int J Mol Sci, 2014; 15, 23519−36. doi: 10.3390/ijms151223519 [7] Ling Q, Wu SY, Liao XZ, et al. Anesthetic propofol enhances cisplatin-sensitivity of non-small cell lung cancer cells through N6-methyladenosine-dependently regulating the miR-486-5p/RAP1-NF-κB axis. BMC Cancer, 2022; 22, 765. doi: 10.1186/s12885-022-09848-y [8] Huang H, Benzonana LL, Zhao H, et al. Prostate cancer cell malignancy via modulation of HIF-1α pathway with isoflurane and propofol alone and in combination. Br J Cancer, 2014; 111, 1338−49. doi: 10.1038/bjc.2014.426 [9] Vaupel P, Kelleher DK, Höckel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol, 2001; 28, 29−35. [10] Daniele M Gilkes, Gregg L Semenza, Denis Wirtz. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer, 2014; 14, 430−9. [11] Chin BY, Jiang G, Wegiel B, et al. Hypoxia-inducible factor 1α stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci USA, 2007; 104, 5109−14. doi: 10.1073/pnas.0609611104 [12] Palazon A, Goldrath AW, Nizet V, et al. HIF transcription factors, inflammation, and immunity. Immunity, 2014; 41, 518−28. doi: 10.1016/j.immuni.2014.09.008 [13] Huang SH, Zhao YY, Liu J. HIF-1α enhances autophagy to alleviate apoptosis in marginal cells in the stria vascular in neonatal rats under hypoxia. Int J Biochem Cell Biol, 2022; 149, 106259. doi: 10.1016/j.biocel.2022.106259 [14] Quintero M, Mackenzie N, Brennan PA. Hypoxia-inducible factor 1 (HIF-1) in cancer. Eur J Surg Oncol, 2004; 30, 465−8. doi: 10.1016/j.ejso.2004.03.008 [15] Zhuang Y, Li X, Zhan P, et al. HIF-1α and MBP1 are associated with the progression of breast cancer cells by repressing β-catenin transcription. Oncol Rep, 2022; 48, 149. doi: 10.3892/or.2022.8361 [16] Janji B, Chouaib S. The promise of targeting hypoxia to improve cancer immunotherapy: mirage or reality? Front Immunol, 2022; 13, 880810. [17] Florentin J, O'Neil SP, Ohayon LL, et al. VEGF receptor 1 promotes hypoxia-induced hematopoietic progenitor proliferation and differentiation. Front Immunol, 2022; 13, 882484. doi: 10.3389/fimmu.2022.882484 [18] Hosseini RF, Azad FJ, Yousefzadeh H, et al. Serum levels of vascular endothelial growth factor in chronic obstructive pulmonary disease. Med J Islam Repub Iran, 2014; 28, 85. [19] Balakrishnan S, Kumar BS. Correlation of serum vascular endothelial growth factor and cardiovascular risk factors on collateral formation in patients with acute coronary artery syndrome. Clin Anat, 2022; 35, 673−8. doi: 10.1002/ca.23890 [20] Krenn K, Klepetko W, Taghavi S, et al. Vascular endothelial growth factor increases pulmonary vascular permeability in cystic fibrosis patients undergoing lung transplantation. Eur J Cardiothorac Surg, 2007; 32, 35−41. doi: 10.1016/j.ejcts.2007.04.006 [21] Al-Salam S, Balalaa N, Faour I, et al. HIF-1α, VEGF and WT-1 are protagonists in bilateral primary angiosarcoma of breast: a case report and review of literature. Int J Clin Exp Pathol, 2012; 5, 247−53. [22] Wei D, Xin YC, Rong Y, et al. Correlation between the expression of VEGF and Ki67 and lymph node metastasis in non-small-cell lung cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med, 2022; 2022, 9693746. [23] Tian L, Chen XX, Cao L, et al. Effects of plant-based medicinal food on postoperative recurrence and lung metastasis of gastric cancer regulated by Wnt/β-catenin-EMT signaling pathway and VEGF-C/D-VEGFR-3 cascade in a mouse model. BMC Complement Med Ther, 2022; 22, 233. doi: 10.1186/s12906-022-03703-0 [24] Luo C, Ouyang MW, Fang YY, et al. Dexmedetomidine protects mouse brain from ischemia-reperfusion injury via inhibiting neuronal autophagy through up-regulating hiF-1α. Front Cell Neurosci, 2017; 11, 197. [25] Li BY, Liu Y, Li ZH, et al. Dexmedetomidine promotes the recovery of renal function and reduces the inflammatory level in renal ischemia-reperfusion injury rats through PI3K/Akt/HIF-1α signaling pathway. Eur Rev Med Pharmacol Sci, 2020; 24, 12400−7. [26] Chen SY, Wu JJ, Yang L, et al. Dexmedetomidine leads to the mitigation of myocardial ischemia/reperfusion-induced acute lung injury in diabetic rats via modulation of hypoxia-inducible factor-1α. Braz J Cardiovasc Surg, 2022; 37, 370−9. [27] Peng K, Chen WR, Xia F, et al. Dexmedetomidine post-treatment attenuates cardiac ischaemia/reperfusion injury by inhibiting apoptosis through HIF-1α signalling. J Cell Mol Med, 2020; 24, 850−61. doi: 10.1111/jcmm.14795 [28] Rao Q, You AB, Guo ZL, et al. Intrahepatic tissue implantation represents a favorable approach for establishing orthotopic transplantation hepatocellular carcinoma mouse models. PLoS One, 2016; 11, e0148263. doi: 10.1371/journal.pone.0148263 [29] Maniotis AJ, Folberg R, Hess A, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol, 1999; 155, 739−52. doi: 10.1016/S0002-9440(10)65173-5 [30] Luo QX, Wang J, Zhao WY, et al. Vasculogenic mimicry in carcinogenesis and clinical applications. J Hematol Oncol, 2020; 13, 19. doi: 10.1186/s13045-020-00858-6 [31] Niu K, Chen XW, Qin Y, et al. Celecoxib blocks vasculogenic mimicry via an off-target effect to radiosensitize lung cancer cells: an experimental study. Front Oncol, 2021; 11, 697227. doi: 10.3389/fonc.2021.697227 [32] Li Q, Ni Y, Zhang LR, et al. HIF-1α-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Target Ther, 2021; 6, 76. doi: 10.1038/s41392-020-00453-8 [33] You L, Wu WD, Wang X, et al. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med Res Rev, 2021; 41, 1622−43. doi: 10.1002/med.21771 [34] Li FF, Shi YY, Zhang Y, et al. Investigating the mechanism of Xian-ling-lian-xia-fang for inhibiting vasculogenic mimicry in triple negative breast cancer via blocking VEGF/MMPs pathway. Chin Med, 2022; 17, 44. doi: 10.1186/s13020-022-00597-5 [35] Yuan YY, Geng BC, Xu XY, et al. Dual VEGF/PDGF knockdown suppresses vasculogenic mimicry formation in choroidal melanoma cells via the Wnt5a/β-catenin/AKT signaling pathway. Acta Histochem, 2022; 124, 151842. doi: 10.1016/j.acthis.2021.151842 [36] Zhao YS, Guo SP, Deng J, et al. VEGF/VEGFR-targeted therapy and immunotherapy in non-small cell lung cancer: targeting the tumor microenvironment. Int J Biol Sci, 2022; 18, 3845−58. doi: 10.7150/ijbs.70958 [37] Zeng CY, Wang XF, Hua FZ, et al. HIF-1α in osteoarthritis: from pathogenesis to therapeutic implications. Front Pharmacol, 2022; 13, 927126. doi: 10.3389/fphar.2022.927126 [38] Zhang CC, Niu H, Wan CY, et al. Drug D, a diosgenin derive, inhibits L-arginine-induced acute pancreatitis through meditating GSDMD in the endoplasmic reticulum via the TXNIP/HIF-1α pathway. Nutrients, 2022; 14, 2591. doi: 10.3390/nu14132591 [39] Cai Q, Liu GQ, Huang LS, et al. The role of dexmedetomidine in tumor-progressive factors in the perioperative period and cancer recurrence: a narrative review. Drug Des Devel Ther, 2022; 16, 2161−75. doi: 10.2147/DDDT.S358042 [40] Che JP, Liu MM, Lv HW. Dexmedetomidine disrupts esophagus cancer tumorigenesis by modulating circ_0003340/miR-198/HMGA2 axis. Anticancer Drugs, 2022; 33, 448−58. doi: 10.1097/CAD.0000000000001284 -




 下载:
下载:



 Quick Links
Quick Links