-
In recent years, due to the increased use of ozone-depleting substances (ODSs), ozone depletion has become one of the most prominent environmental problems in the past 40 years[1]. Even though there are recent reports indicating that the global ozone layer is starting to recover from the depletion caused by ODSs, however, it will take several decades for ozone to return to 1980s levels[2]. Ozone layer destruction causes a large amount of ultraviolet radiation to reach the surface of the earth, which is a major factor in the damage to the skin. The cause for most skin cancers is the interaction of non-modifiable and modifiable risk factors with the most common modifiable risk factor for skin cancer being exposure to ultraviolet radiation[3]. Each year, around 5 million people are diagnosed with skin cancer and that is just in the United States[3]. Due to the persistent rising of diagnosis, skin cancer is considered a major public health problem[3].
Ultraviolet radiation, especially ultraviolet radiation B (UVB) wave band is still responsible for the deleterious effects of sun exposure on human[4,5]. Even though UVB irradiation is able to induce the production of vitamin D which is beneficial to the health of human beings, unfortunately, UVB is also the main reason for skin cancer[6]. Excessive UVB irradiation is able to induce skin oxidative stress, which further triggers inflammation, apoptosis and endoplasmic reticulum stress, resulting in the damage of epidermal keratinocytes through various signaling pathways, including NF-κB[7,8].
Among the intrinsic protective mechanisms in skin against ultraviolet injury, the vitamin D system plays a role[9]. It is revealed that vitamin D compound can prevent ultraviolet-induced cell death and DNA damage in human skin cells [10]. In vitro studies also suggest that the damage to keratinocytes was prevented by an application of 1,25(OH)2D3[11,12]. Moreover, it is reported that 1,25(OH)2D3 can prevent oxidative stress and decrease the production of reactive oxygen species (ROS) and the phosphorylation of NF-κB[13].
Toll-like receptors (TLRs), which are able to recognize a wide variety of pathogen-associated molecular patterns and are centrally involved in the initiation and adaptive immune responses, are implicated in the pathogenesis of multiple chronic inflammatory diseases [14]. The damage induced by UVB is mainly through toll-like receptor-4 (TLR-4) signaling pathway [4, 15]. Moreover, NF-κB signaling pathways, involved in the damage of epidermal keratinocytes caused by UVB and in the prevention of UV-induced cell death and DNA damage in human skin cells, were regulated by TLR4 signaling pathway [16-19]. TLR-4 deficiency or TLR-4 inhibition will prevent the development of oxidative stress, inflammation and apoptotic responses [14, 20].
Both 1,25(OH)2D3 and TLR-4 play important roles in the damage to skin cells caused by UVB irradiation. However, there is no reported research which explore the effects of combination of 1,25(OH)2D3 and TLR-4 inhibitor on the damage of HaCaT cells caused by UVB irradiation, therefore, in the study, we explore the effects of combination of vitamin D and TLR inhibitor (TAK-242) on the damage caused by UVB to keratinocytes. In the present study, we applied both 1,25(OH)2D3 and TAK-242 to the HaCaT cells, an immortalized keratinocyte, prior to UVB irradiation to explore whether combination of 1,25(OH)2D3 and TAK-242 would exert better protection on HaCaT cells irradiated with UVB and relevant mechanisms. The present study will offer a new idea and novel method for the prevention and treatment of skin damage caused by UVB, which is of great significance for the prevention of health damage of the population induced by UVB.
-
UVB peak at 313 nm was manufactured by Beijing Normal University Photoelectric Instrumentation Plant. The UVB light was set up 8 cm from the cells, the detected irradiation intensity was 220 μw/cm2, the time for irradiation was determined based on the formula, 1 J = 2.778 × 10−7 × kw × h.
-
HaCaT cells were cultured in Dulbecco’s Modified Eagle’s Medium (high glucose DMEM, GIBCO USA) supplemented with 10% fetal bovine serum (FBS, Gemini USA), penicillin 100 IU/mL (North China Pharmaceutical Corporation Limited), streptomycin 100 μg/mL (North China Pharmaceutical Corporation Limited) at 37 ℃ with 5% CO2. And 1.2 × 104 HaCaT cells (purchased from Shanghai Cell Bank) were planted into a well in a 96-well plate. The UVB intensity was set at 0 J/m2, 0.5 J/m2, 1 J/m2, 1.5 J/m2, 2 J/m2, 2.5 J/m2, 3 J/m2, 3.5 J/m2, 4 J/m2, and 4.5 J/m2 respectively, each intensity triplicate wells. The cells were cultured for 24 h, then the culture medium was discarded, phosphate buffer saline (PBS) was used to wash the cells twice. 20 μL PBS was added to each well and the cells were irradiated with UVB light at the set intensity. After the irradiation, PBS was used to wash the cells twice, then culture medium was added to the cells and the cells were further cultured for 24 h. Then 20 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrasoliumbromide, MTT) was added to each well and mixed with cells, then the cells were cultured for 4 h. 4 h later, the culture medium was removed, 150 μL dimethyl sulfoxide (DMSO) was added to each well, the adsorbance value of the cells were determined by BioTek- ELX-800 at the wavelength of 490 nm.
-
Totally 1.2 × 104 HaCaT cells were seeded into a well in a 96-well plate and cultured for 24 h, then, the culture medium was removed and the cells were washed with PBS twice. TAK-242 (Medchem Express, China) was added to the cells at the final concentration of 0, 0.01, 0.1, 1, 2, 5, 10, 12, 15, and 20 μmol/L respectively, with each concentration triplicate wells. The cells were cultured with TAK-242 for 1 h, then were washed with PBS twice before being irradiated with 2 J/m2 UVB. After irradiation, the cells were washed with PBS twice, cultured in medium for 24 h. Then 20 μL MTT was added to the cells, then further cultured for 4 h and the culture medium was removed, 150 μL DMSO was added to each well. The adsorbance value of the cells were determined by BioTek- ELX-800 at the wavelength of 490 nm.
-
Totally 0.8 × 104 HaCaT cells were seeded into a well in a 96-well plate and cultured for 24 h, then the culture medium was removed and the cells were washed with PBS twice. 1,25(OH)2D3 (TCI, Shanghai) was added to the cells at the final concentration of 0, 0.0001, 0.001, 0.01, 0.1, 1, 10, 100, 1,000, and 10,000 nmol/L respectively, and co-cultured with the cells for 24 h. Then the culture medium was removed and the cells were washed with PBS twice, then 20 μL PBS was added to the cells and the cells were irradiated with UVB at the intensity of 2 J/m2. After the irradiation, the cells were washed with PBS twice, after that the cells were further cultured for 24 h. Then 20 μL MTT was added to the cells and then further cultured for 4 h. Then the culture medium was removed and 150 μL DMSO was added to the cells. The adsorbance value of the cells were determined by BioTek- ELX-800 at the wavelength of 490 nm.
-
Totally 3 × 106 HaCaT cells were seeded into a Petri dish and when the confluence of the cells was 80%–90%, the cells were randomly assigned to five different groups: 1) untreated controls; 2) cells irradiated with UVB (UVB); 3) cells pre-treated with 1,25(OH)2D3 prior to irradiation with UVB [1,25(OH)2D3+UVB]; 4) cells pre-treated with TAK-242 prior to irradiation with UVB (TAK-242+UVB); and 5) cells pre-treated with 1,25(OH)2D3 and TAK-242 prior to irradiation with UVB [1,25(OH)2D3 + TAK-242+UVB]. Each group was set triplicates.
-
Totally 4.5 × 105 cells were seeded into a well of a 96-well plate with triplicate wells for each group. When the confluence of the cells was 70%–80%, the cells were pretreated with 1,25(OH)2D3 and/or TAK-242 and then irradiated with UVB. After irradiation, the cells were washed with PBS twice and were further cultured for 24 h in medium. Then, the culture medium was removed and the cells were washed with PBS twice, 95% ethanol was added to the cells and incubated for 15 min, then the cells were washed with PBS three times, followed by dips in 1% acid ethanol (1% HCl in 70% ethanol) and then rinsed in distilled water. Then the cells were stained with eosin solution for 3 min and followed by dehydration with graded alcohol and clearing in xylene. The cells were examined and photographed using an Olympus BX53 fluorescence microscope (Tokyo, Japan).
-
HaCaT cells were pre-treated with 1,25(OH)2D3, or/and TAK-242 before being irradiated with UVB, then further cultured for 24 h. According to the protocol offered by the manufacturer (Solarbio LIFE SCIENCES, China), the cells were collected and 7 × 105 cells were put in an EP tube, 200 μL Annexin-V-PE was added to the tube and gently mixed, incubated at room temperature (20−25 Degree Celsius) for 20 min in darkness, then detected by flow cytometry.
-
Based on their different groups with each group triplicates, HaCaT cells were collected and put into an EP tube, 1 mL DCFH-DA was added to the cells and cultured in darkness at 37 ℃ for 20 min. Then the cells were washed with PBS, after being washed, the cells were resuspended with PBS. Then, the fluorescence intensity was detected by Cytation 3 Cell Imaging Multi-Mode Reader (BioTek, USA). The excitation wavelength and emission wavelength were 488 nm and 525 nm, respectively.
-
Each well of a six-well plate was pre-drawn a line. And 4.5 × 105 HaCaT cells were plated into a well with triplicate wells for each group. When the confluence of the cells was 70%–80%, the cells were pre-treated with 1,25(OH)2D3, or/and TAK-242 according to the different groups. Then the cells were washed with PBS twice, a tip was used to scratch the cells on the pre-drawn line, the suspended cells were washed by PBS and the scratch was photographed before being irradiated with UVB. After irradiation, the cells were washed with PBS twice, then were further cultured for 12 h and 24 h. The migration of cells was photographed at 12 h and 24 h, respectively. Software Image J 1.8.0 was used to measure the scratch.
-
The expression of TLR4, NF-κB, IκBα, PERK, p-PERK, Bip, Cytc, Caspase-8, and Caspase-3 was determined according to the procedure of our previous study[10] (Dixon et al., 2013). The antibody information was TLR4 (1:1,000, Abcam USA, ab13556), NF-κB (1:1,000, CST USA, 8242S), IκB-α (1:1,000, CST USA, 9242S), p-IκB-α (1:1,000, CST USA, 2859S), PERK (1:1,000, CST USA, 5683S), p-PERK (1:1,000, CST USA, 3179S), Bip (1:1,000, CST USA, 3177S), Cytc (1:1,000, Santa Cruz USA, sc-13156), Caspase-8 (1:1,000, CST USA, 4790S), Caspase-3 (1:1,000, CST USA, 9662S).
The images were obtained by Chemiluminescence imaging analysis system (ECL from Tanon 5200, Shanghai, China) and band intensity were analyzed by Tanon Gis analytical software (Shanghai, China).
-
The data were represented as mean ± SD and statistical analysis was conducted with SPSS 21.0. The comparison among different groups was conducted with ANOVA, the difference between two groups was performed using SNK. P < 0.05 was considered significant.
-
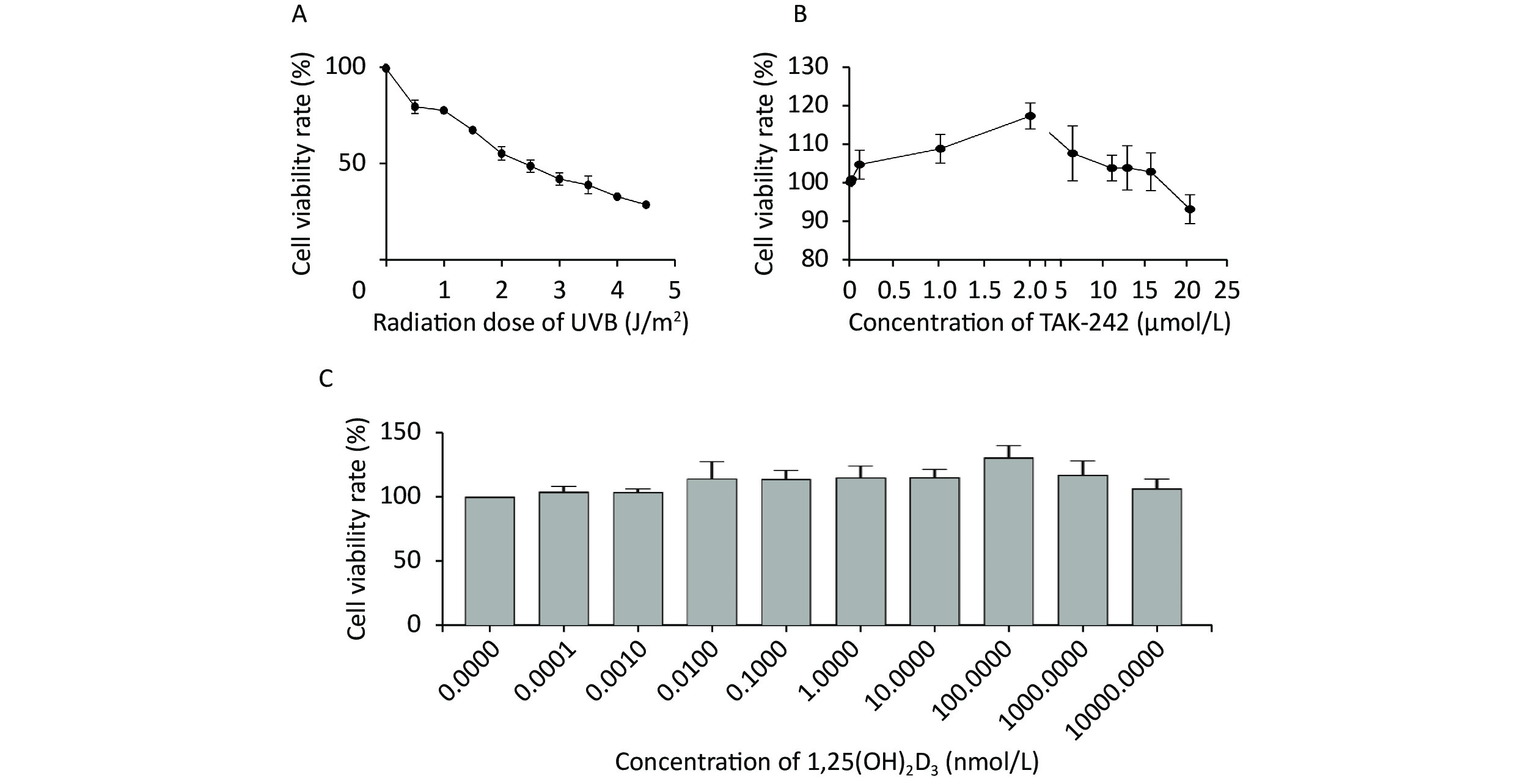
When the HaCaT cells were irradiated with UVB at the intensity of 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, and 4.5 J/m2, the mean survival rate of the cells was 100.00%, 80.02%, 78.08%, 67.82%, 55.61%, 49.10%, 42.33%, 39.17%, 33.14%, and 28.96%, respectively (Figure 1A). We selected the intensity of UVB 2 J/m2, the mean survival rate of the cells was 55.61% for the rest of our experiments.
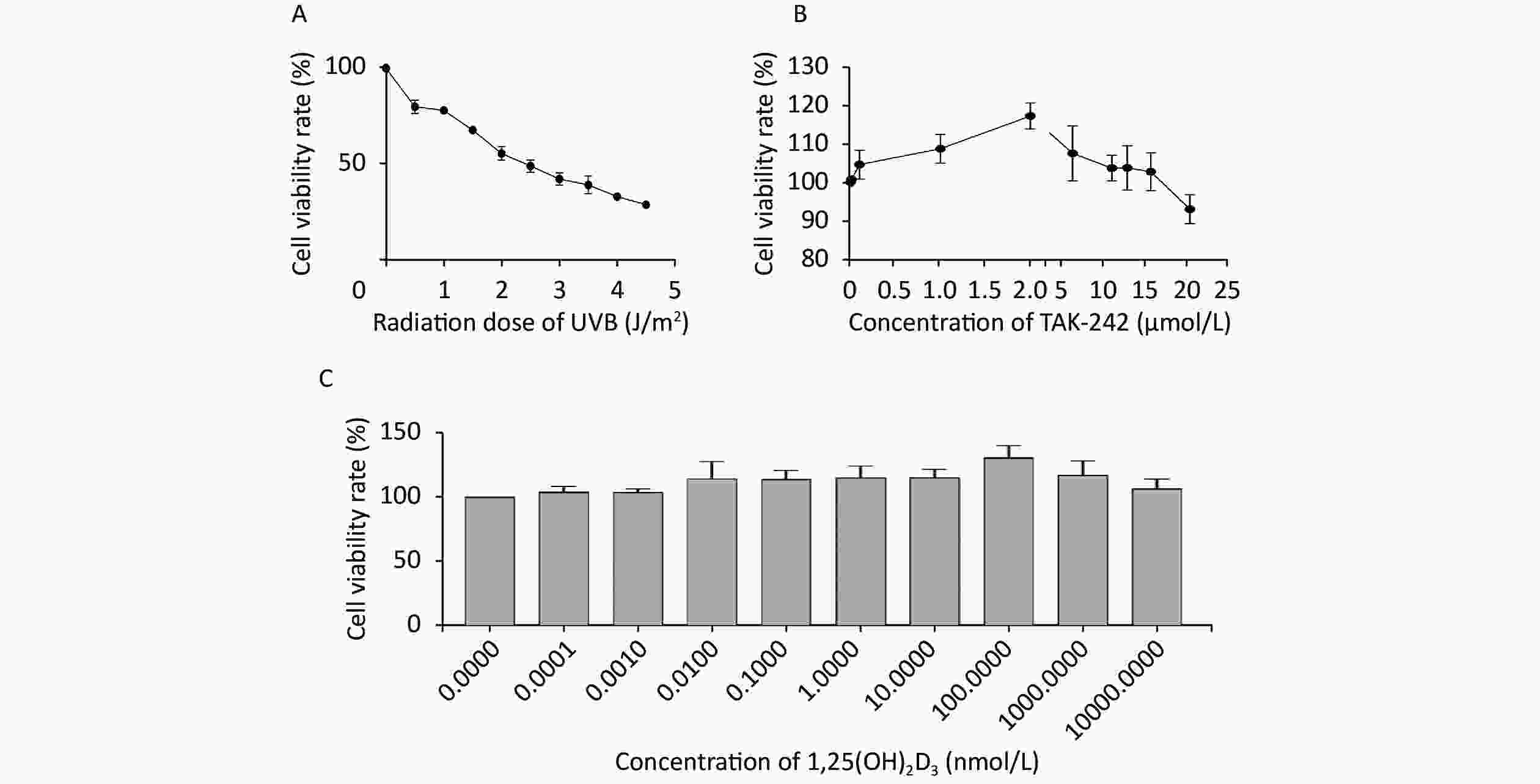
Figure 1. Determination of UVB intensity, TAK-242 and 1,25(OH)2D3 for subsequent experiment HaCaT cells were seeded into a 96-well plate and cultured for 24 hours, and TAK-242, or/and 1,25(OH)2D3 were added to the cells prior to UVB irradiation. MTT method was used to determine the cell viability. (A) UVB intensity; (B) TAK-242; (C) 1,25(OH)2D3.
-
When the concentration of TAK-242 was at 0, 0.010, 0.100, 1.000, 2.000, 5.000, 10.000, 12.000, 15.000, and 20.000 μmol/L, the mean survival rate of the cells was 100.00%, 100.81%, 104.64%, 108.75%, 117.31%, 107.57%, 103.71%, 103.77%, 102.75%, and 93.04%, respectively (Figure 1B). We selected the TAK-242 concentration of 2.000 μmol/L, the mean survival rate was 117.31% for our experiment.
-
When the concentration of 1,25(OH)2D3 was at 0, 0.0001, 0.001, 0.01, 0.1, 1, 10, 100, 1,000, and 10,000 nmol/L, the mean survival rate of the cells was 100.00%, 103.88%, 103.32%, 113.84%, 113.61%, 114.50%, 115.02%, 130.49%, 116.96%, and 106.51%, respectively (Figure 1C). We selected the concentration of 1,25(OH)2D3 at 100 nmol/L, the mean survival rate was 130.49% for our experiment.
-
HaCaT cells from untreated control, 1,25(OH)2D3 + UVB group, TAK-242 + UVB group or 1,25(OH)2D3 + TAK-242 + UVB group had complete cell membrane, appropriate cell density, even dye of the nuclei with rare nulcear fragmentation, membrane rupture, or unclear sketch of cell structure. HaCaT cells from UVB group had lower cell density, membrane rupture, nuclear fragmentation and unclear cell sketch(Figure 2A). We used Image J software to perform the quantification of the number of cells surviving. As shown in Figure 2B, there were more cells surviving in the untreated control, 1,25(OH)2D3 + UVB group, TAK-242 + UVB group or 1,25(OH)2D3 + TAK-242 + UVB group than those in the UVB group (P < 0.05).
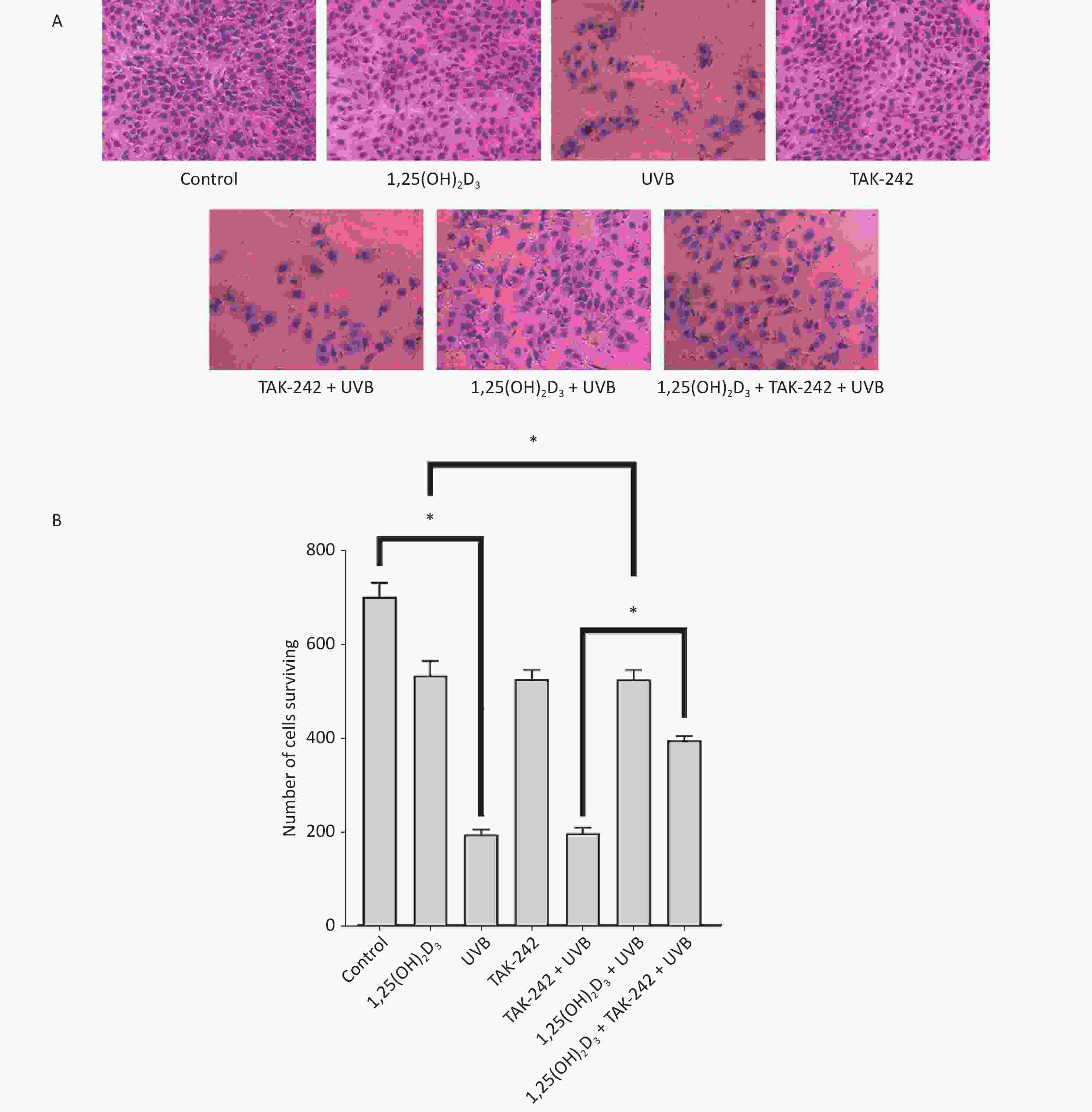
Figure 2. (A) Effects of 1,25(OH)2D3 or/and TAK-242 on the morphology of HaCaT cells HaCaT cells were pre-treated with 1,25(OH)2D3 or/and TAK-242 prior to UVB irradiation. Then, the cells were stained with eosin solution and photographed using an Olympus BX 53 microscope. (B) Quantification of the number of cells surviving among different groups.
-
As shown in Figure 3A, the cells from UVB group had significantly higher number of apoptotic bodies than those from TAK-242 + UVB group, 1,25(OH)2D3 + UVB, or 1,25(OH)2D3 + TAK-242 + UVB group. In flow cytometry determination, the cells from 1,25(OH)2D3 + TAK-242 + UVB group, 1,25(OH)2D3 + UVB group or TAK-242 + UVB group had significantly lower number of apoptotic cells than those from UVB group (P < 0.05), Figure 3B.
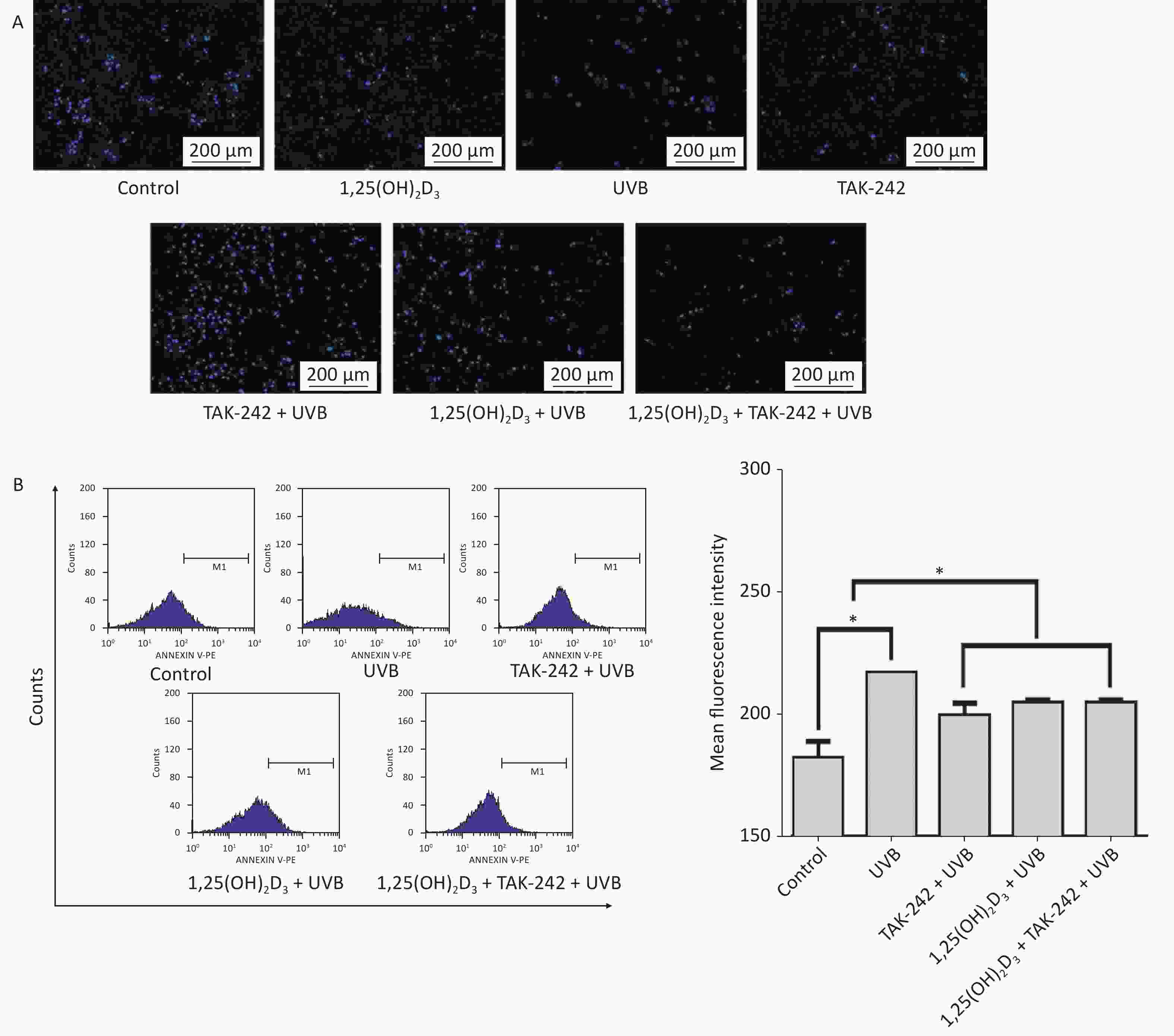
Figure 3. (A) Effects of 1,25(OH)2D3 or/and TAK-242 on the apoptosis of HaCaT cells A. HaCaT cells were pre-treated with 1,25(OH)2D3 (100 nmol/L) or/and TAK-242 (2.0 μmol/L) prior to UVB irradiation. Then, 200 μL DAPI was added to the cells and co-cultured with the cells for 10 minutes, and the cells were washed with PBS and photographed under fluorescent microscope. (B) HaCaT cells were pre-treated with 1,25(OH)2D3 or/and TAK-242 prior to UVB irradiation. Then, 200 μL Annexin-V-PE was added to the cells and co-cultured with the cells for 20 minutes in darkness, and the cells were detected by flow cytometry.
-
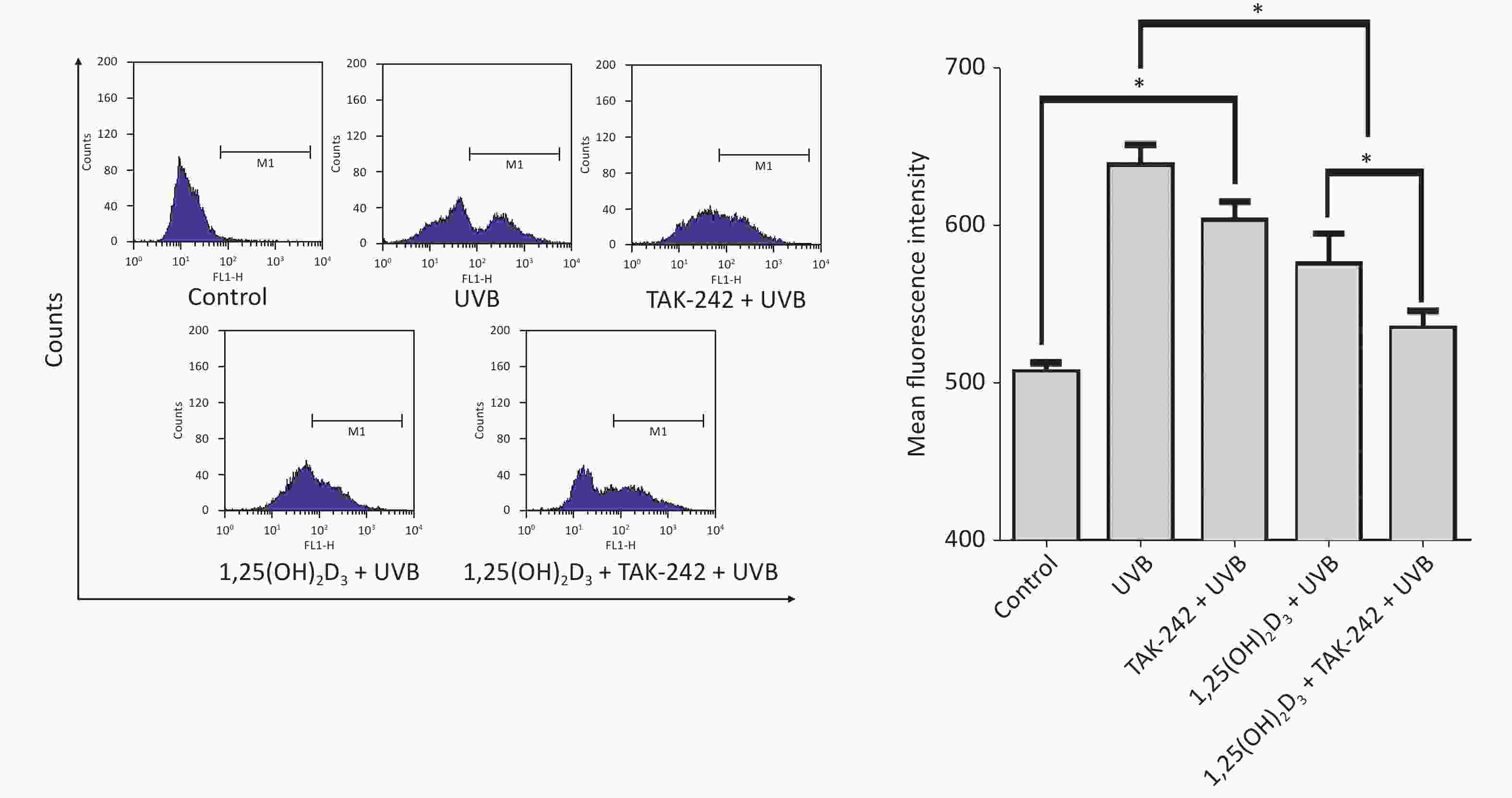
Compared with the UVB group cells, the 1,25(OH)2D3 + UVB group cells, TAK-242 + UVB group or 1,25(OH)2D3 + TAK-242 + UVB group cells had significantly lower production of ROS (P < 0.05); moreover, the 1,25(OH)2D3 + TAK-242 + UVB group cells had lower production of ROS than those from 1,25(OH)2D3 + UVB group, or TAK-242 + UVB group (P < 0.05), Figure 4.
-
HaCaT cells from 1,25(OH)2D3 + UVB group, TAK-242 + UVB group, or 1,25(OH)2D3 + TAK-242 + UVB group had significant closure of the scratch than those from UVB group on 24 h, moreover, the 1,25(OH)2D3 group cells or 1,25(OH)2D3 + TAK-242 group cells had significant closure than those from TAK-242 group on 24 h (P < 0.05), but there was no significant difference in the closure of scratch between 1,25(OH)2D3 + UVB and 1,25(OH)2D3 + TAK-242 + UVB group cells, Figure 5.
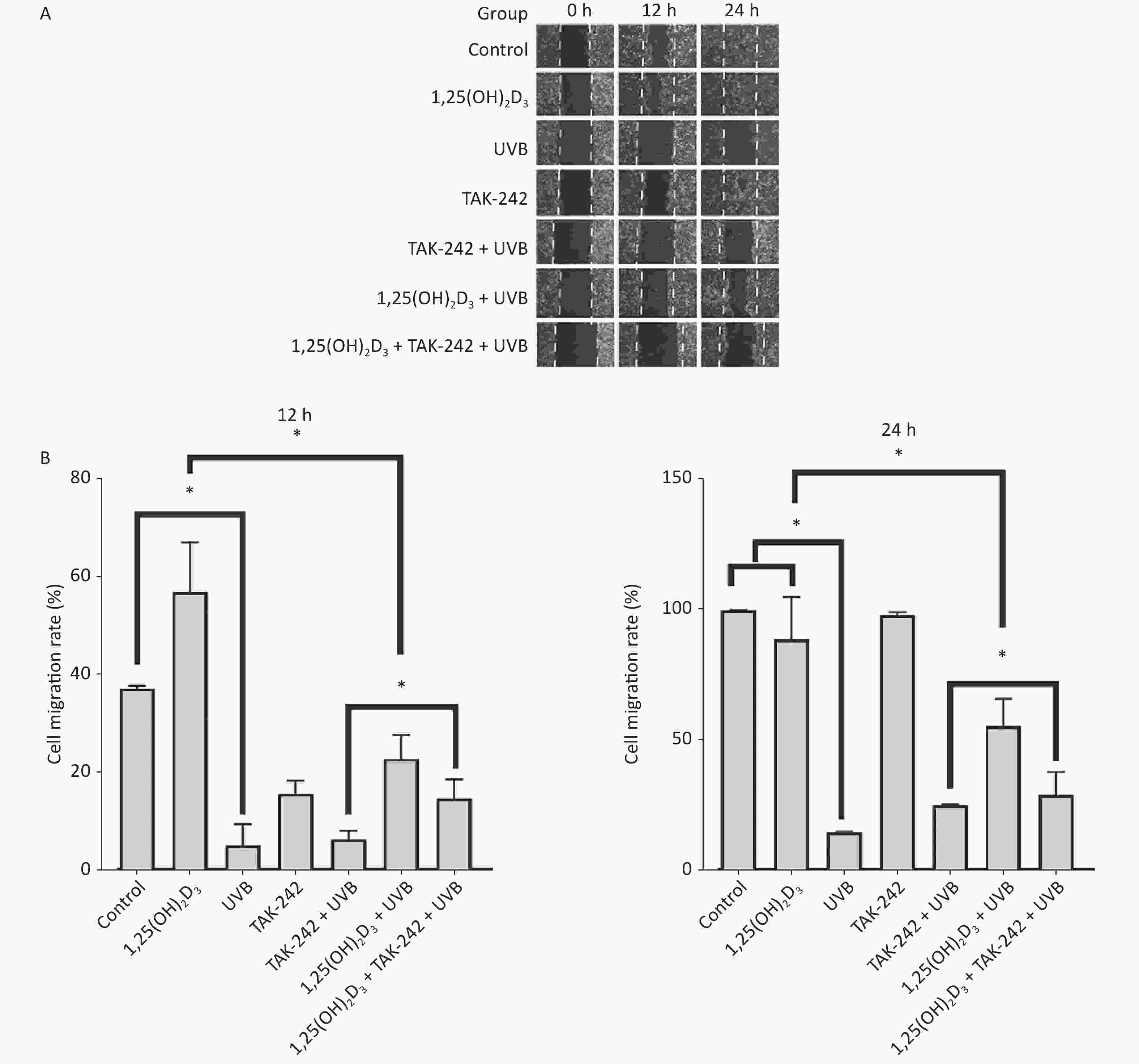
Figure 5. Effects of 1,25(OH)2D3 or/and TAK-242 on the cell migration. (A) Representative images of scratch assay at selected times. (B) Comparison of cell migration rate among different groups. HaCaT cells were plated into a 6-well plate with triplicate wells for each group. The cells were pre-treated with 1,25(OH)2D3 (100 nmol/L), or/and TAK-242 (2.0 μmol/L) according to the different groups. The scratch was photographed before and after UVB irradiation. Software Image J 1.8.0 was used to measure the scratch.
-
For the expression of NF-κB, HaCaT cells from 1,25(OH)2D3 + UVB group, or TAK-242 + UVB group, or 1,25(OH)2D3 + TAK-242 + UVB group had significantly lower expression of NF-κB than those from UVB group (P < 0.05). In addition, the expression of NF-κB from 1,25(OH)2D3 + TAK-242 + UVB group was significantly lower than that from the TAK-242 + UVB group (P < 0.05), Figure 6.
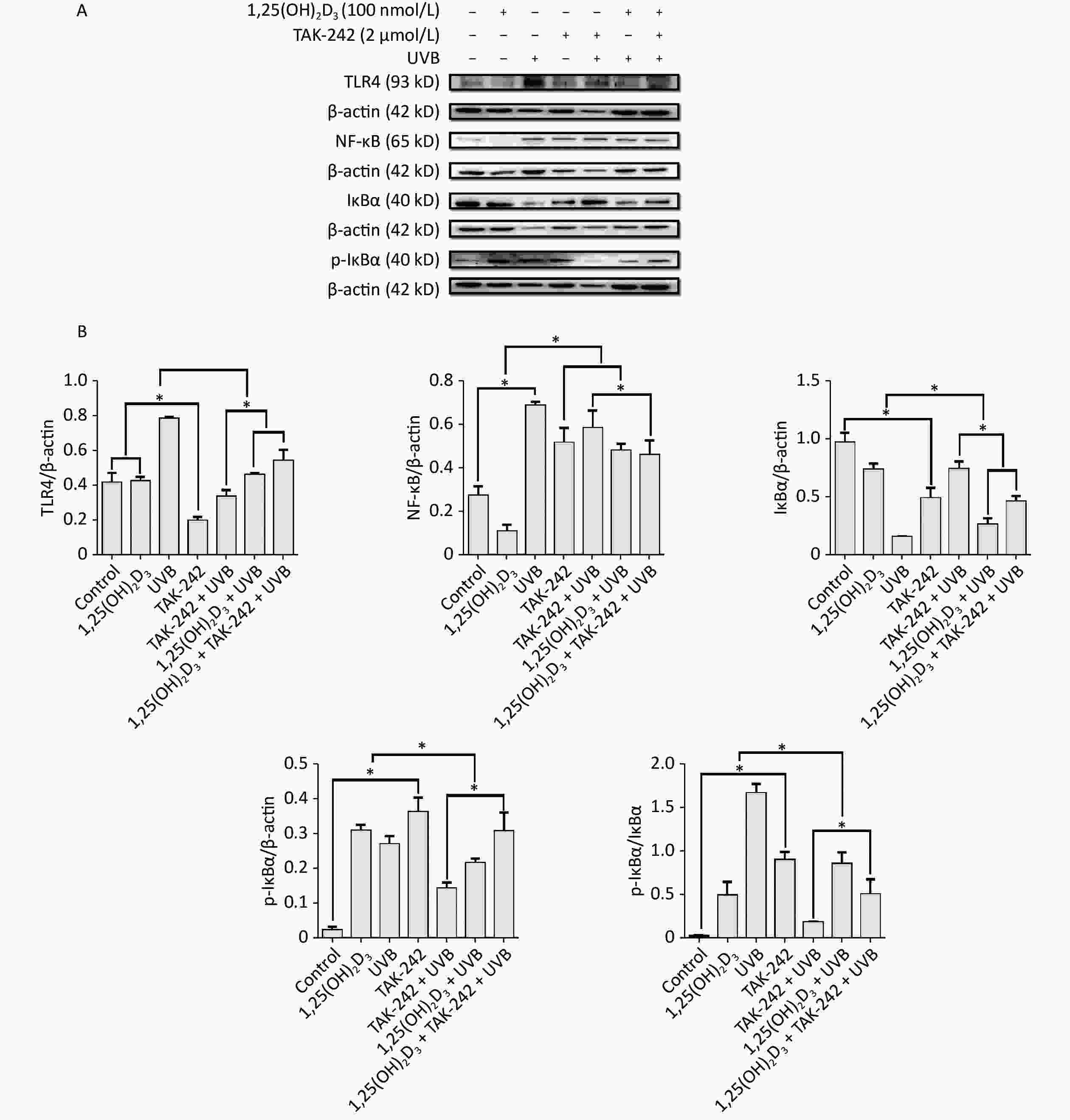
Figure 6. Effects of 1,25(OH)2D3 or/and TAK-242 on the expression of oxidative stress related proteins. (A) Western blot analysis of the expression of oxidative stress related proteins. (B) Comparison of the expression of oxidative stress related proteins. Results are means ± SD determined from three experiments. *P < 0.05.
For the expression of TLR-4, HaCaT cells from 1,25(OH)2D3 + UVB group, or TAK-242 + UVB group, or 1,25(OH)2D3 + TAK-242 + UVB group had significantly lower expression of TLR-4 than those from UVB group (P < 0.05), moreover, the cells from TAK-242 + UVB group had lower expression of TLR-4 than those from 1,25(OH)2D3 + UVB, or 1,25(OH)2D3 + TAK-242 + UVB group (P < 0.05), Figure 6.
For the expression of IkB-ɑ, the cells from UVB group had significantly higher ratio of phosphorylated IkB-ɑ to IkB-ɑ than those from TAK-242 + UVB group, 1,25(OH)2D3 + UVB, or 1,25(OH)2D3 + TAK-242 + UVB group (P < 0.05); the cells from TAK-242 + UVB group had significantly lower ratio of phosphorylated IkB-ɑ to IkB-ɑ than those from 1,25(OH)2D3 + TAK-242 + UVB group (P < 0.05), Figure 6.
For the expression of Bip, HaCaT cells from 1,25(OH)2D3 + UVB group, or TAK-242 + UVB group, or 1,25(OH)2D3 + TAK-242 + UVB group had significantly lower expression of Bip than those from UVB group (P < 0.05); moreover, the cells from 1,25(OH)2D3 + TAK-242 + UVB group had significantly lower expression of Bip than those from the 1,25(OH)2D3 + UVB, or TAK-242 + UVB group (P < 0.05). Figure 7.

Figure 7. Effects of 1,25(OH)2D3 or/and TAK-242 on the expression of endoplasmic reticulum stress related proteins. (A) Western blot analysis of the expression of oxidative stress related proteins; (B) Comparison of the expression of oxidative stress related proteins. Results are means ± SD determined from three experiments. *P < 0.05.
For the expression of PERK, HaCaT cells from 1,25(OH)2D3 + UVB group, or TAK-242 + UVB group, or 1,25(OH)2D3 + TAK-242 + UVB group had significantly lower expression of p-PERK than those from UVB group (P < 0.05), moreover, the cells from 1,25(OH)2D3 + UVB group had lower expression of p-PERK than those from TAK-242 + UVB group, or 1,25(OH)2D3 + TAK-242 + UVB group (P < 0.05). Figure 7.
-
For the expression of apoptosis related proteins, the cells from 1,25(OH)2D3 + UVB group, or TAK-242 + UVB group, or 1,25(OH)2D3 + TAK-242 + UVB group had significantly lower expression of Caspase-3, Caspase-8, and Cytc than those from UVB group (P < 0.05). The cells from 1,25(OH)2D3 + TAK-242 + UVB group had significantly lower expression of Caspase-8, Caspase-3 and Cytc than those from 1,25(OH)2D3 + UVB group, or TAK-242 + UVB group (P < 0.05). Figure 8.
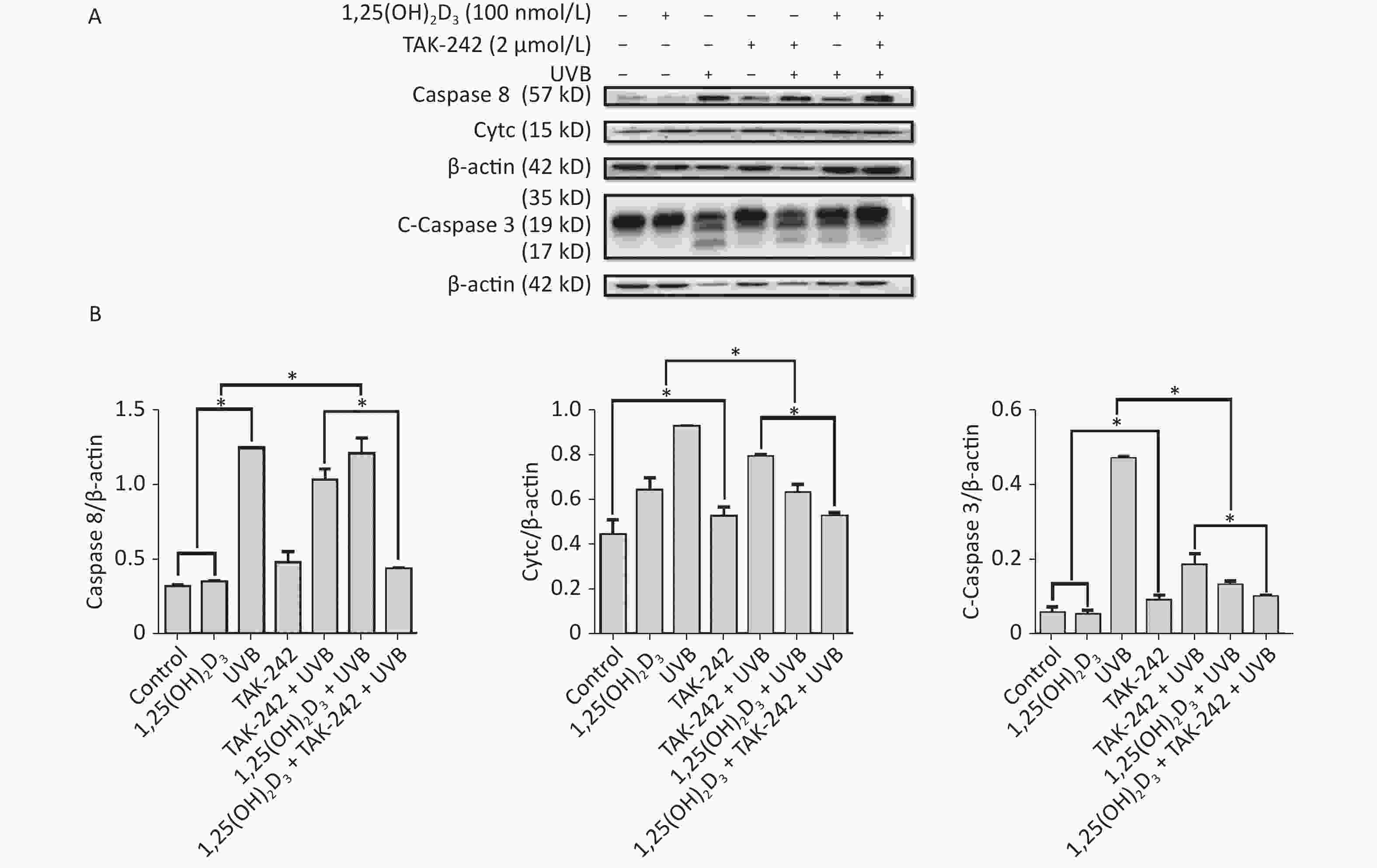
Figure 8. Effects of 1,25(OH)2D3 or/and TAK-242 on the expression of apoptosis related proteins. A. Western blot analysis of the expression of oxidative stress related proteins. B. Comparison of the expression of oxidative stress related proteins. Results are means ± SD determined from three experiments. *P < 0.05.
-
The skin injury caused by UVB irradiation is associated with the ability of UVB to induce the changes of skin cell morphology, increased production of ROS, decreased cell migration and promoting skin cell apoptosis[7, 8, 21].
In our study, with the increasing of UVB irradiation intensity, the cell viability decreased and displayed a dose-response relationship, which indicate that UVB irradiation can cause the death of HaCaT cells.
The dose of UVB irradiation in our study was 2 J/m2, which is similar to previous studies [22]. At this dose, UVB irradiation decreased the cell density and caused the membrane rupture, moreover, it triggered the production of ROS and apoptosis of cells as well as decreased migration of the cells. Our findings are consistent with previous studies [14].
TLR-4 is expressed in a variety of cells, including keratinocytes, Langerhans cells (LCs), skin fibroblasts and melanocytes [23]. TLR-4/NF-kB signaling pathway is associated with the damage to keratinocytes caused by UVB [12], UVB irradiation can induce the production of ROS which will cause oxidative stress, consequently leading to inflammation, apoptosis and endoplasmic reticulum stress, finally resulting in the damage of epidermal keratinocytes [8, 9]. Ultraviolet radiation regulates skin immunity by upregulating TLR4 expression in human LCs and inducing vitamin D synthesis [23]. TLR4 signal transduction in fibroblasts plays a crucial role in skin senescence and aging [23]. Human skin melanocytes express a variety of TLRs, including TLR1, TLR2, TLR3, TLR4, TLR7 and TLR9, and their stimulation activates NF-κB dependent proinflammatory immune response [24].
For the HaCaT cells, if we block the TLR-4 or apply some agent which is similar to intrinsic agent, and the agent has the ability to prevent UV-induced cell death in human skin cells and can prevent oxidative stress and decrease the production of ROS, we will be able to protect the skin from the damage of UVB irradiation.
In our study, we applied TAK-242, an inhibitor of TLR-4, and vitamin D, a product by UVB irradiation, to explore the combined effects on the damage to HaCaT cells caused by UVB.
Our findings show that the production of ROS and cell apoptosis were reduced by 1,25(OH)2D3 or TAK-242, and this reduction is significant by combination of 1,25(OH)2D3 and TAK-242 than 1,25(OH)2D3, or TAK-242 alone.
Moreover, we determined the expression of NF-kB and ER stress related protein. Our results showed that the expression levels of NF-kB and ER stress related proteins were reduced by 1,25(OH)2D3 or TAK-242, and the reduced effect was significant by combination of 1,25(OH)2D3 and TAK-242 than by 1,25(OH)2D3 or TAK-242, alone, which indicated 1,25(OH)2D3 and TAK-242 could produce effects on the NF- kB pathway and ER stress to protect HaCaT cells from UVB irradiation.
This study had limitations. Firstly, we set up one single dose of UVB, did not perform the dose-response relationship. Secondly, skin is complex system, what happened to one type of cell can not explain the real mechanisms and changes, further study is needed to use other skin cells or animal model to investigate the combined effects of 1,25(OH)2D3 and TAK-242 on the skin from the damage by UVB.
-
Combination of 1,25(OH)2D3 and TAK-242 will provide a better protection against UVB and decrease the risk of development of skin problems via inhibiting the oxidative stress, endoplasmic reticulum stress and apoptosis. Our study provides a new strategy for protection of skin from the effects of UVB through combination of 1,25(OH)2D3 and TAK-242.
-
The authors declare that they have no competing interests.
doi: 10.3967/bes2022.133
Effects of Combination of 1,25(OH)2D3 and TLR-4 Inhibitor on the Damage to HaCaT Cells Caused by UVB Irradiation
-
Abstract:
Objective Vitamin D and Toll-like receptor-4 (TLR-4) inhibition are involved in the protection of keratinocytes. The effects of combination of 1,25(OH)2D3 and TLR-4 inhibitor on the protection of keratinocytes against ultraviolet radiation B (UVB) irradiation remain unclear. This study was undertaken to explore the effects of combination of 1,25(OH)2D3 and TAK-242 (TLR-4 inhibitor) on the damage to HaCaT cells caused by UVB irradiation. Methods In vitro, HaCaT cells were treated with 1,25(OH)2D3 or/and TAK-242 prior to UVB irradiation at the intensity of 20 mJ/cm2, then the production of reactive oxygen species (ROS), cell migration, apoptosis of cells, and the expression of oxidative stress, endoplasmic reticulum stress, and apoptosis related proteins were determined. Results Compared with the HaCaT cells treated with 1,25(OH)2D3 or TAK-242, the cells treated with both 1,25(OH)2D3 and TAK-242 showed, 1) significantly lower production of ROS (P < 0.05); 2) significantly less apoptosis of HaCaT cells (P < 0.05); 3) significantly lower expression of NF-κB, Caspase-8, Cyto-C, Caspase-3 (P < 0.05). Conclusion The combination of 1,25(OH)2D3 and TAK-242 could produce a better protection for HaCaT cells via inhibiting the oxidative stress, endoplasmic reticulum stress and apoptosis than 1,25(OH)2D3 or TAK-242 alone. -
Key words:
- UVB /
- 1,25(OH)2D3 /
- Toll-like receptor 4 /
- HaCaT cell /
- Combined effects
注释:1) AUTHOR CONTRIBUTIONS: -
Figure 1. Determination of UVB intensity, TAK-242 and 1,25(OH)2D3 for subsequent experiment HaCaT cells were seeded into a 96-well plate and cultured for 24 hours, and TAK-242, or/and 1,25(OH)2D3 were added to the cells prior to UVB irradiation. MTT method was used to determine the cell viability. (A) UVB intensity; (B) TAK-242; (C) 1,25(OH)2D3.
Figure 2. (A) Effects of 1,25(OH)2D3 or/and TAK-242 on the morphology of HaCaT cells HaCaT cells were pre-treated with 1,25(OH)2D3 or/and TAK-242 prior to UVB irradiation. Then, the cells were stained with eosin solution and photographed using an Olympus BX 53 microscope. (B) Quantification of the number of cells surviving among different groups.
Figure 3. (A) Effects of 1,25(OH)2D3 or/and TAK-242 on the apoptosis of HaCaT cells A. HaCaT cells were pre-treated with 1,25(OH)2D3 (100 nmol/L) or/and TAK-242 (2.0 μmol/L) prior to UVB irradiation. Then, 200 μL DAPI was added to the cells and co-cultured with the cells for 10 minutes, and the cells were washed with PBS and photographed under fluorescent microscope. (B) HaCaT cells were pre-treated with 1,25(OH)2D3 or/and TAK-242 prior to UVB irradiation. Then, 200 μL Annexin-V-PE was added to the cells and co-cultured with the cells for 20 minutes in darkness, and the cells were detected by flow cytometry.
Figure 5. Effects of 1,25(OH)2D3 or/and TAK-242 on the cell migration. (A) Representative images of scratch assay at selected times. (B) Comparison of cell migration rate among different groups. HaCaT cells were plated into a 6-well plate with triplicate wells for each group. The cells were pre-treated with 1,25(OH)2D3 (100 nmol/L), or/and TAK-242 (2.0 μmol/L) according to the different groups. The scratch was photographed before and after UVB irradiation. Software Image J 1.8.0 was used to measure the scratch.
Figure 6. Effects of 1,25(OH)2D3 or/and TAK-242 on the expression of oxidative stress related proteins. (A) Western blot analysis of the expression of oxidative stress related proteins. (B) Comparison of the expression of oxidative stress related proteins. Results are means ± SD determined from three experiments. *P < 0.05.
Figure 7. Effects of 1,25(OH)2D3 or/and TAK-242 on the expression of endoplasmic reticulum stress related proteins. (A) Western blot analysis of the expression of oxidative stress related proteins; (B) Comparison of the expression of oxidative stress related proteins. Results are means ± SD determined from three experiments. *P < 0.05.
Figure 8. Effects of 1,25(OH)2D3 or/and TAK-242 on the expression of apoptosis related proteins. A. Western blot analysis of the expression of oxidative stress related proteins. B. Comparison of the expression of oxidative stress related proteins. Results are means ± SD determined from three experiments. *P < 0.05.
-
[1] Koenig TK, Volkamer R, Apel EC, et al. Ozone depletion due to dust release of iodine in the free troposphere. Sci Adv, 2021; 7, eabj6544. doi: 10.1126/sciadv.abj6544 [2] Bais AF, McKenzie RL, Bernhard G, et al. Ozone depletion and climate change: impacts on UV radiation. Photochem Photobiol Sci, 2015; 14, 19−52. doi: 10.1039/C4PP90032D [3] Watson M, Holman DM, Maguire-Eisen M. Ultraviolet radiation exposure and its impact on skin cancer risk. Semin Oncol Nurs, 2016; 32, 241−54. doi: 10.1016/j.soncn.2016.05.005 [4] Ahmad I, Simanyi E, Guroji P, et al. Toll-like receptor-4 deficiency enhances repair of UVR-induced cutaneous DNA damage by nucleotide excision repair mechanism. J Invest Dermatol, 2014; 134, 1710−7. doi: 10.1038/jid.2013.530 [5] Diffey B. Climate change, ozone depletion and the impact on ultraviolet exposure of human skin. Phys Med Biol, 2004; 49, R1−11. doi: 10.1088/0031-9155/49/1/R01 [6] Piotrowska A, Wierzbicka J, Żmijewski MA. Vitamin D in the skin physiology and pathology. Acta Biochim Pol, 2016; 63, 17−29. [7] Mera K, Kawahara KI, Tada KI, et al. ER signaling is activated to protect human HaCaT keratinocytes from ER stress induced by environmental doses of UVB. Biochem Biophys Res Commun, 2010; 397, 350−4. doi: 10.1016/j.bbrc.2010.05.128 [8] Mishra V, Pathak C. Human toll-like receptor 4 (hTLR4): structural and functional dynamics in cancer. Int J Biol Macromol, 2019; 122, 425−51. doi: 10.1016/j.ijbiomac.2018.10.142 [9] Gupta R, Dixon KM, Deo SS, et al. Photoprotection by 1, 25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol, 2007; 127, 707−15. doi: 10.1038/sj.jid.5700597 [10] Dixon KM, Tongkao-On W, Sequeira VB, et al. Vitamin D and death by sunshine. Int J Mol Sci, 2013; 14, 1964−77. doi: 10.3390/ijms14011964 [11] Chaiprasongsuk A, Janjetovic Z, Kim TK, et al. Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol, 2019; 24, 101206. doi: 10.1016/j.redox.2019.101206 [12] Mason RS, Sequeira VB, Dixon KM, et al. Photoprotection by 1α, 25-dihydroxyvitamin D and analogs: further studies on mechanisms and implications for UV-damage. J Steroid Biochem Mol Biol, 2010; 121, 164−8. doi: 10.1016/j.jsbmb.2010.03.082 [13] Manna P, Achari AE, Jain SK. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch Biochem Biophys, 2017; 615, 22−34. doi: 10.1016/j.abb.2017.01.002 [14] Li XQ, Cai LM, Liu J, et al. Liquiritin suppresses UVB-induced skin injury through prevention of inflammation, oxidative stress and apoptosis through the TLR4/MyD88/NF-κB and MAPK/caspase signaling pathways. Int J Mol Med, 2018; 42, 1445−59. [15] Sherwani MA, Abdelgawad A, Chung M, et al. Toll-like receptor-4 antagonist enhances the repair of ultraviolet radiation-induced DNA damage and augments anti-tumor immune responses in mice. Cancers (Basel), 2021; 13, 5406. doi: 10.3390/cancers13215406 [16] Jiang SJ, Chu AW, Lu ZF, et al. Ultraviolet B-induced alterations of the skin barrier and epidermal calcium gradient. Exp Dermatol, 2007; 16, 985−92. doi: 10.1111/j.1600-0625.2007.00619.x [17] Kim HM, Oh S, Yang JY, et al. Evaluating whether radiofrequency irradiation attenuated UV-B-induced skin pigmentation by increasing melanosomal autophagy and decreasing melanin synthesis. Int J Mol Sci, 2021; 22, 10724. doi: 10.3390/ijms221910724 [18] Shah P, He YY. Molecular regulation of UV-induced DNA repair. Photochem Photobiol, 2015; 91, 254−64. doi: 10.1111/php.12406 [19] Wang J, Ke J, Wu X, et al. Astragaloside prevents UV-induced keratinocyte injury by regulating TLR4/NF-κB pathway. J Cosmet Dermatol, 2022; 21, 1163−70. doi: 10.1111/jocd.14174 [20] Zhang QQ, Feng AZ, Zeng MN, et al. Chrysosplenol D protects mice against LPS-induced acute lung injury by inhibiting oxidative stress, inflammation, and apoptosis via TLR4-MAPKs/NF-κB signaling pathways. Innate Immun, 2021; 27, 514−24. doi: 10.1177/17534259211051069 [21] Feng GZ, Wei L, Che HL, et al. Cathelicidin-NV from Nanorana ventripunctata effectively protects HaCaT cells, ameliorating ultraviolet B-induced skin photoaging. Peptides, 2022; 150, 170712. doi: 10.1016/j.peptides.2021.170712 [22] Farrukh MR, Nissar UA, Afnan Q, et al. Oxidative stress mediated Ca2+ release manifests endoplasmic reticulum stress leading to unfolded protein response in UV-B irradiated human skin cells. J Dermatol Sci, 2014; 75, 24−35. doi: 10.1016/j.jdermsci.2014.03.005 [23] Kumar V. Going, toll-like receptors in skin inflammation and inflammatory diseases. EXCLI J, 2021; 20, 52−79. [24] Yu N, Zhang SJ, Zuo FG, et al. Cultured human melanocytes express functional toll-like receptors 2-4, 7 and 9. J Dermatol Sci, 2009; 56, 113−20. doi: 10.1016/j.jdermsci.2009.08.003 -




 下载:
下载:



 Quick Links
Quick Links