-
Photothermal therapy (PTT) uses heat generated by photothermal agents to efficiently kill cancer cells in the least invasive manner. Inorganic nanoparticles, such as precious metals and carbon nanoparticles, have been extensively studied for their ability to convert near-infrared (NIR) light (700–900 nm) into heat[1]. However, these nanoparticles are easily captured by the reticuloendothelial system, which severely limits their clinical applications[2]. Despite targeted and stealth modifications, these issues remain unresolved; therefore, researchers are seeking more effective methods.
Certain immune cells can target tumors and serve as vectors for cancer treatment. Despite the high interstitial barriers and pressure in tumors, these cells can infiltrate tumor tissues[3]. Macrophages, which are easily harvested from patients, can be ex vivo loaded with drugs or nanoparticles and reinjected into the bloodstream. However, their natural accumulation in tumors presents a limited risk. Studies have demonstrated that Indocyanine green (ICG)-loaded macrophages enhance PTT by improving photothermal conversion efficiency and targeting specificity[4]. Advances in ICG-based nanoparticles further optimized their design and functionality for PTT applications[5].
In this study, (W18O49+ICG)@mSiO2 nanoparticles were successfully prepared. The (W18O49+ICG)@mSiO2@ macrophages delivery system consists of four parts: 1) Tungsten oxide (W18O49) with high photothermal conversion efficiency was used as photothermal agent for photodynamic therapy[6]; 2) Indocyanine green (ICG) was used as a NIR tracker to dynamically monitor the PTT process[7]; 3) Mesoporous silica with high load capacity and good biocompatibility can be used as a three-dimensional robust frame work for loading W18O49 and ICG[8]; and 4) Macrophages carry (W18O49+ICG)@mSiO2 nanoparticles for targeted PTT therapy[9].
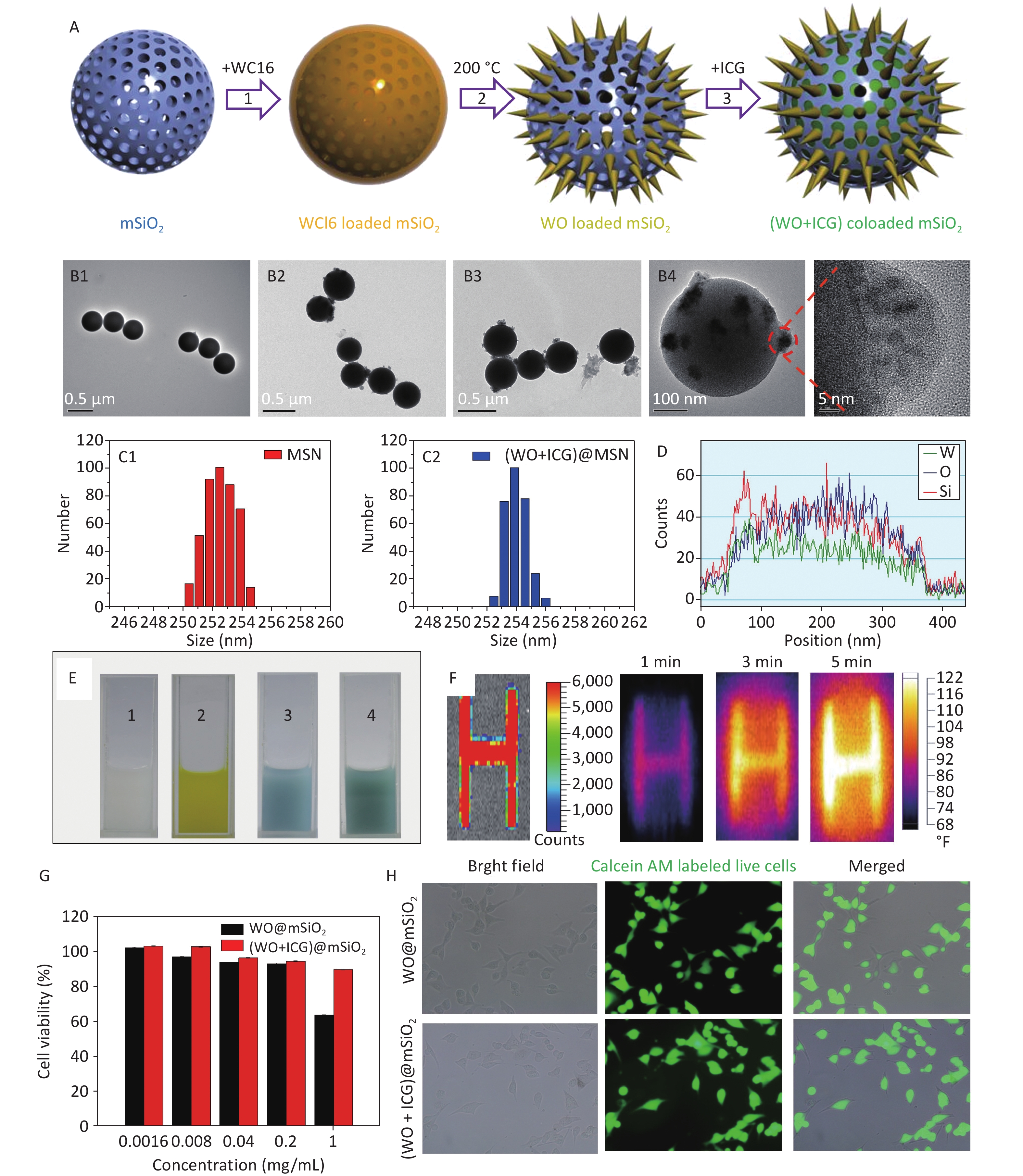
The synthesis process was generally divided into four parts (Figure 1A). First, mesoporous silica (mSiO2) was synthesized using a sol-gel method. Subsequently, tungsten (VI) chloride (WCl6) was encapsulated within the mSiO2 template[10]. Subsequently, WCl6 formed a W18O49 crystal in the mSiO2 by hydrothermal method. Finally, ICG, a fluorescent probe, was loaded onto the W18O49 loaded mSiO2 nanoparticles to form (W18O49+ICG)@mSiO2 nanoparticles. The transmission electron microscope (TEM) images showed that the mSiO2 nanoparticles were spherical and uniform (Figure 1B1). After encapsulation with WCl6, there were no significant changes in the particle size and morphology compared with the original mSiO2. After the hydrothermal reaction, W18O49 crystals were clearly observed inside the mSiO2 nanoparticles (Figure 1B2–B4). Furthermore, Figure 1C1–C2 reveals no significant difference in diameter between the mSiO2 and (W18O49+ICG)@mSiO2 nanoparticles. Energy-dispersive X-ray spectroscopy (EDX) was used to investigate the distribution of internal elements. The internal signals for tungsten (W), oxygen (O), and silicon (Si) were substantially stronger than those on the nanoparticle surfaces, indicating that the majority of the W18O49 crystals were formed within the mSiO2 matrix (Figure 1D). These characterizations confirmed the successful synthesis of the (W18O49+ICG)@mSiO2 nanoparticles.
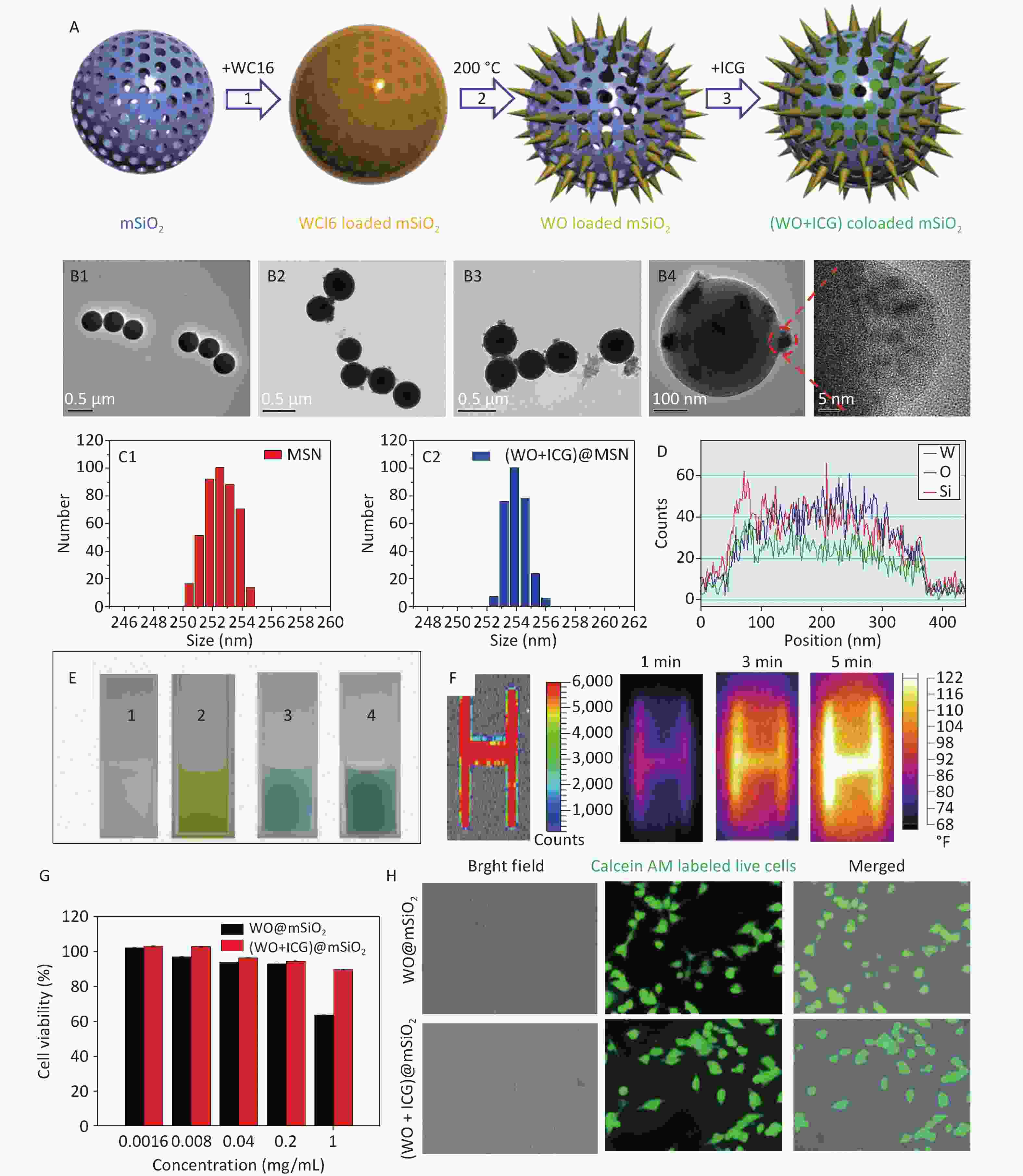
Figure 1. Characterization and performance of (W18O49+ICG)@mSiO2. (A) a schematic illustration showing the synthesis process of (W18O49+ICG)@mSiO2 nanoparticle; (B1–B4) TEM images of mSiO2 (Scale bar: 0.5 μm), WCl6@mSiO2 (Scale bar: 0.5 μm), W18O49@mSiO2 (Scale bar: 0.5 μm) and (W18O49+ICG)@mSiO2 (Scale bar: 100 μm); (C1–C2) the particle size data of mSiO2 and (W18O49+ICG)@mSiO2; (D) EELS line scan of the single (W18O49+ICG)@mSiO2 nanoparticle; (E) bright field images of mSiO2 (1), WCl6@mSiO2 (2), W18O49@mSiO2 (3), and (W18O49+ICG)@mSiO2 (4); (F) thermal imaging photos of a letter sample consisted by (W18O49+ICG)@mSiO2. (G) Cytotoxicity of the nanoparticles tested using the MTT assay. (H) Cell activity test using calcium-AM fluorescence staining (scale bar: 100 μm).
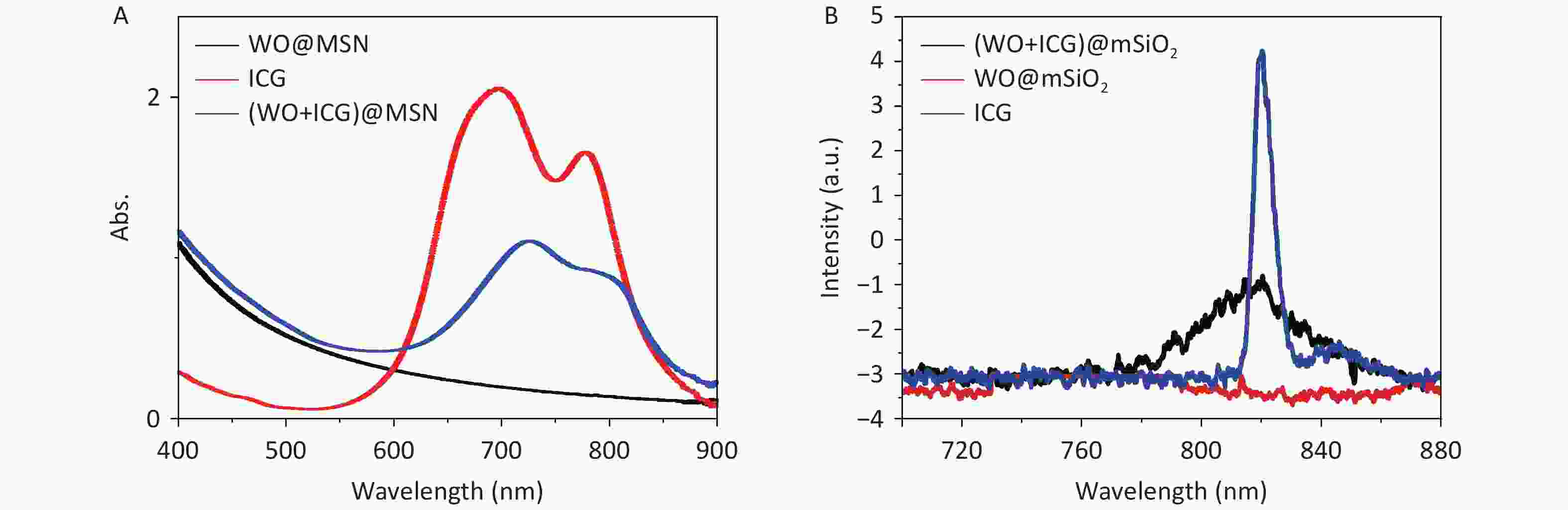
Subsequent analyses focused on the fluorescence imaging and photothermal efficiency. We examined the UV-Vis absorption and fluorescence spectra of each group of samples, and the results showed that ICG was successfully loaded into (W18O49+ICG)@mSiO2, proving that it could be used for NIR imaging (Figure 1E, Supplementary Figure S1, available in www.besjournal.com). Furthermore, samples were prepared by screen-printing three-letter patterns using the (W18O49+ICG)@mSiO2 nanoparticles as the nanoink. Upon excitation at 808 nm, a strong fluorescence signal from the samples was detected using an in vivo imaging system, demonstrating the potential of the nanoparticles as tracers for the real-time monitoring of the PTT process (Figure 1F). In summary, the rapid conversion of NIR into heat by (W18O49+ICG)@mSiO2 indicates its efficacy in photothermal therapy against tumor cells.
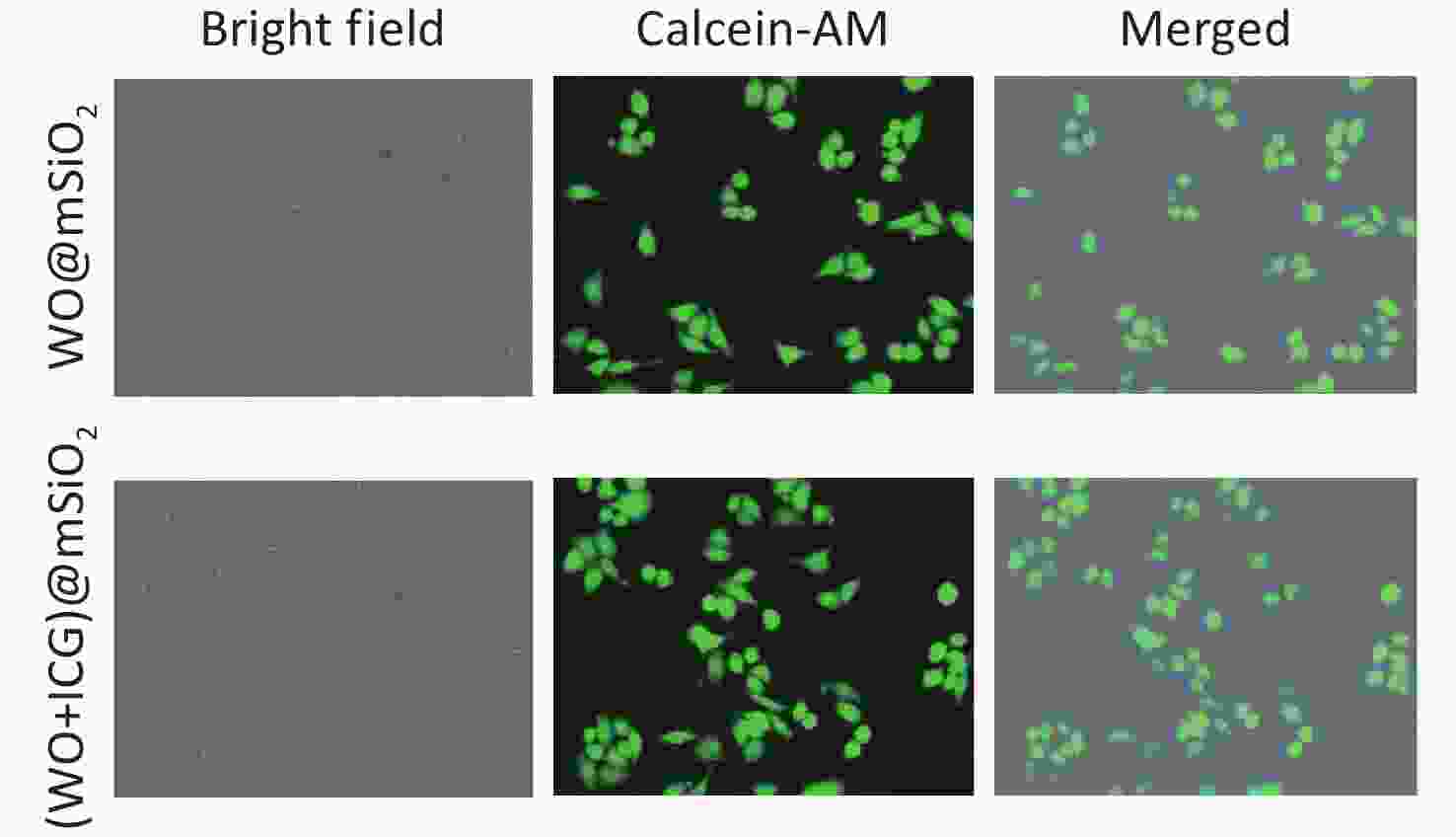
The 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazoliumromide (MTT) assay and fluorescence staining of living cells were used to test cytotoxicity. The survival rate of B16 cells exceeded 90% at concentrations below 0.2 mg/mL (Figure 1G). B16 and HeLa cells incubated with nanoparticles (0.2 mg/mL were stained with Calcein-AM, a fluorescent probe for live cells (Figure 1H, Supplementary Figure S2, available in www.besjournal.com). Most cells exhibited strong green fluorescence, indicating that the nanoparticles had no significant impact on cell viability at this concentration.
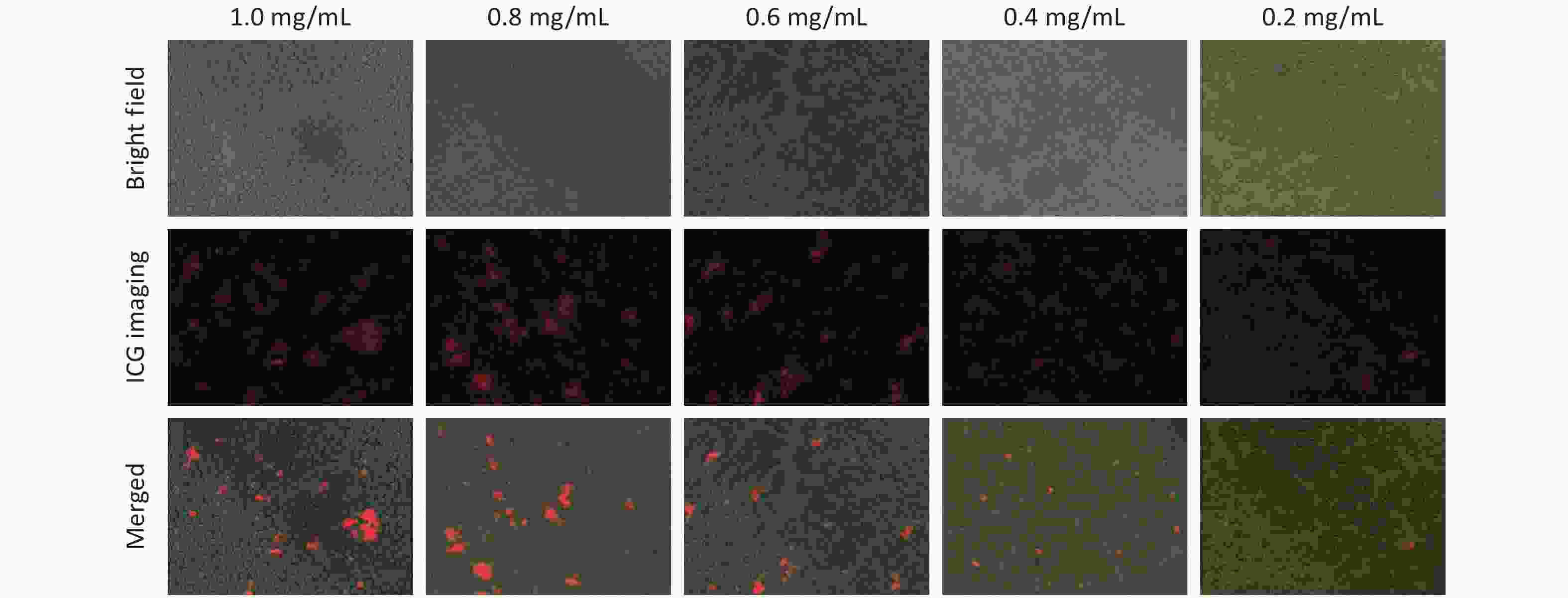
The interaction between the macrophages and (W18O49+ICG)@mSiO2 was analyzed using fluorescence microscopy. Macrophages were incubated with the nanoparticles at four concentrations: 0.0625, 0.125, 0.1875, and 0.2 mg/mL. Red fluorescence was observed in the macrophages under UV excitation, suggesting successful uptake of the nanoparticles (Figure 2A). The intensity of red fluorescence increased with increasing nanoparticle concentration. Considering both nanoparticle uptake and cell viability, the optimal concentration for macrophage uptake was determined to be 0.1875 mg/mL.
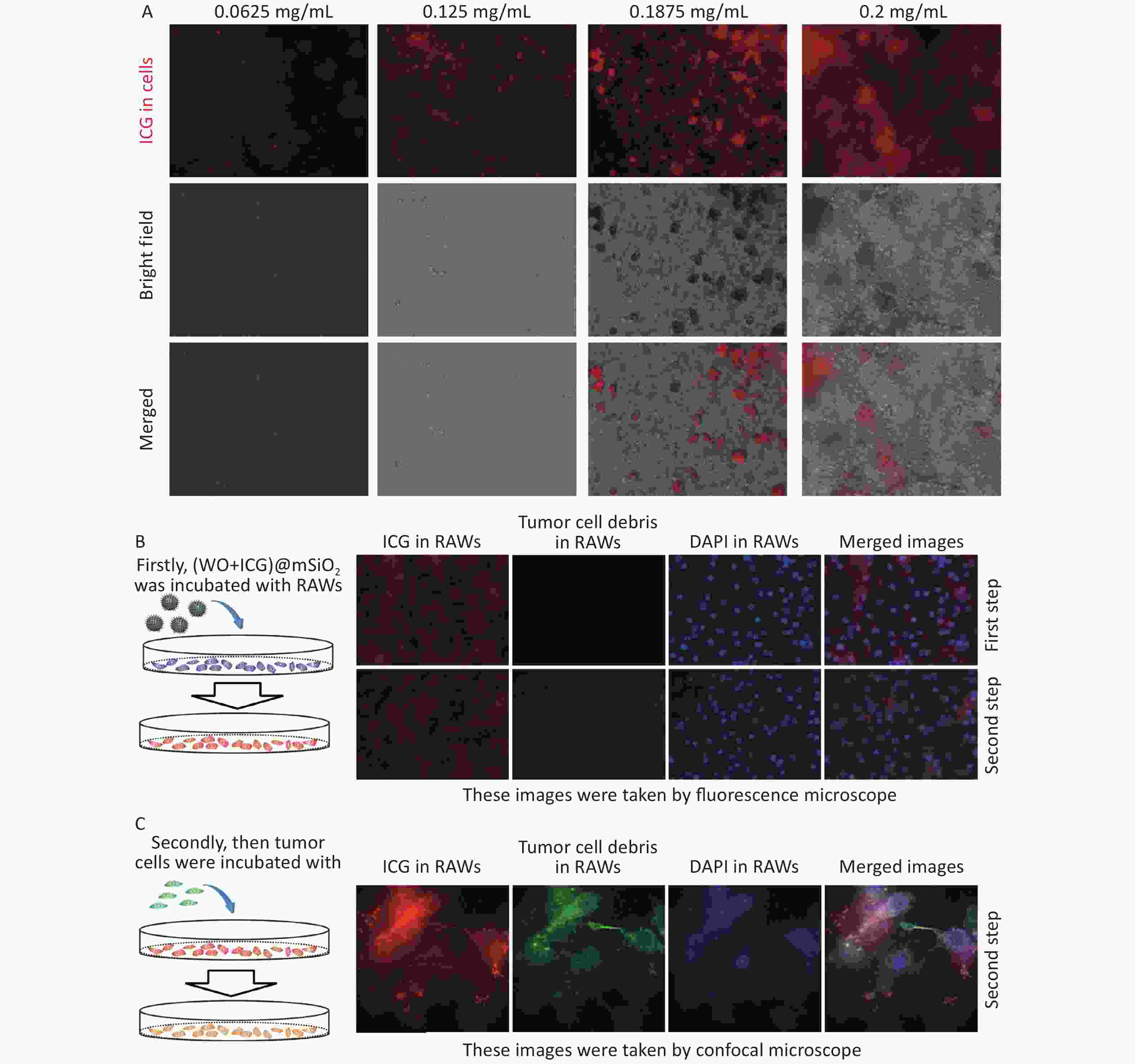
Figure 2. Macrophage uptake and phagocytosis of (W18O49+ICG)@mSiO2. (A) Macrophage uptake of (W18O49+ICG)@mSiO2 at different concentrations. The scale bars were 100 μm. (B–C) The macrophages phagocytosis activity test of (W18O49+ICG)@mSiO2 nanoparticles by fluorescence microscope and confocal microscope. (Scale bar for B: 100 μm; Scale bar for C: 10 μm).
Phagocytosis is a critical function of the macrophages. We assessed whether macrophages remained phagocytic after internalization of (W18O49+ICG)@mSiO2. Macrophages were first incubated with the nanoparticles, followed by the addition of B16 cells labeled with Fluorescein Isothiocyanate (FITC)-phalloidin (a green fluorescent probe) to the culture dish. Strong red fluorescence was observed in the macrophages after uptake of the nanoparticles (Figure 2B). Subsequently, FITC-phalloidin-labelled B16 cells were added. After incubation for 2 h, green fluorescence was detected within the macrophages, indicating that the B16 cell debris was phagocytosed (Figure 2C, Supplementary Figure S3, available in www.besjournal.com). These results suggest that macrophages still have a phagocytic function after uptalking (W18O49+ICG)@mSiO2.
Figure S3. The fluorescence imagines of B16 cells stained by FITC-phalloidin, which was used for testing the macrophages phagocytosis activity.
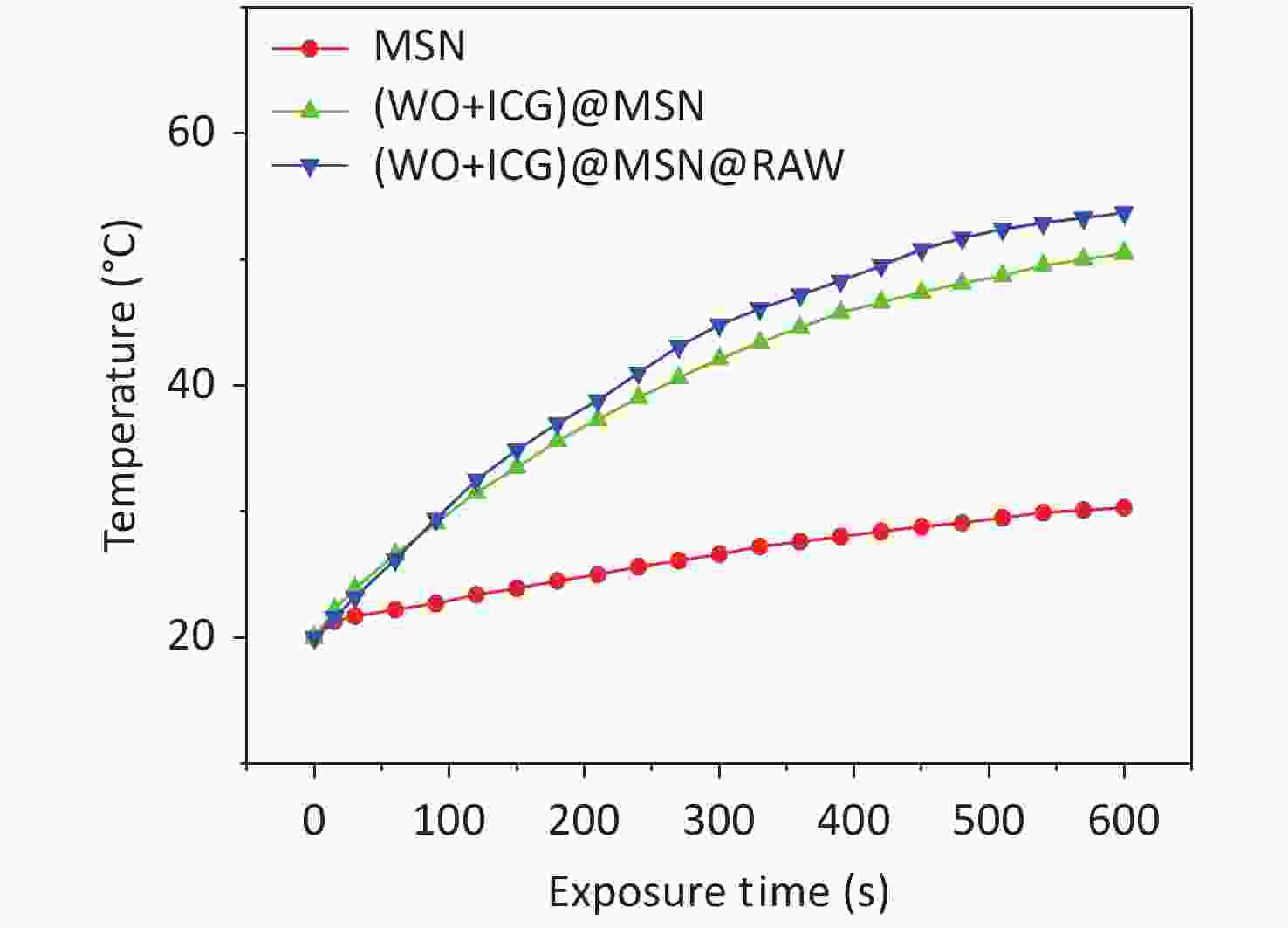
The fluorescence imaging and photothermal properties of (W18O49+ICG)@mSiO2-loaded RAW264.7 ((W18O49+ICG)@mSiO2@RAW) were evaluated using a fluorescence imaging system and a thermal imager. The (W18O49+ICG)@mSiO2@RAW group exhibited a strong red fluorescence (Figure 3A). The intensity was comparable to that of the (W18O49+ICG)@mSiO2 group at the same ICG concentration both in vitro and in vivo. Photothermal performance was also tested using a thermal imaging instrument. After 600 s of NIR light irradiation, the heat production efficiency of the (W18O49+ICG)@mSiO2@RAW group was similar to that of the (W18O49+ICG)@mSiO2 group both in vitro and in vivo (Figures 3B–C, Supplementary Figure S4, available in www.besjournal.com). These results suggest that macrophage phagocytosis has a minimal impact on the photothermal conversion efficiency of the nanoparticles.
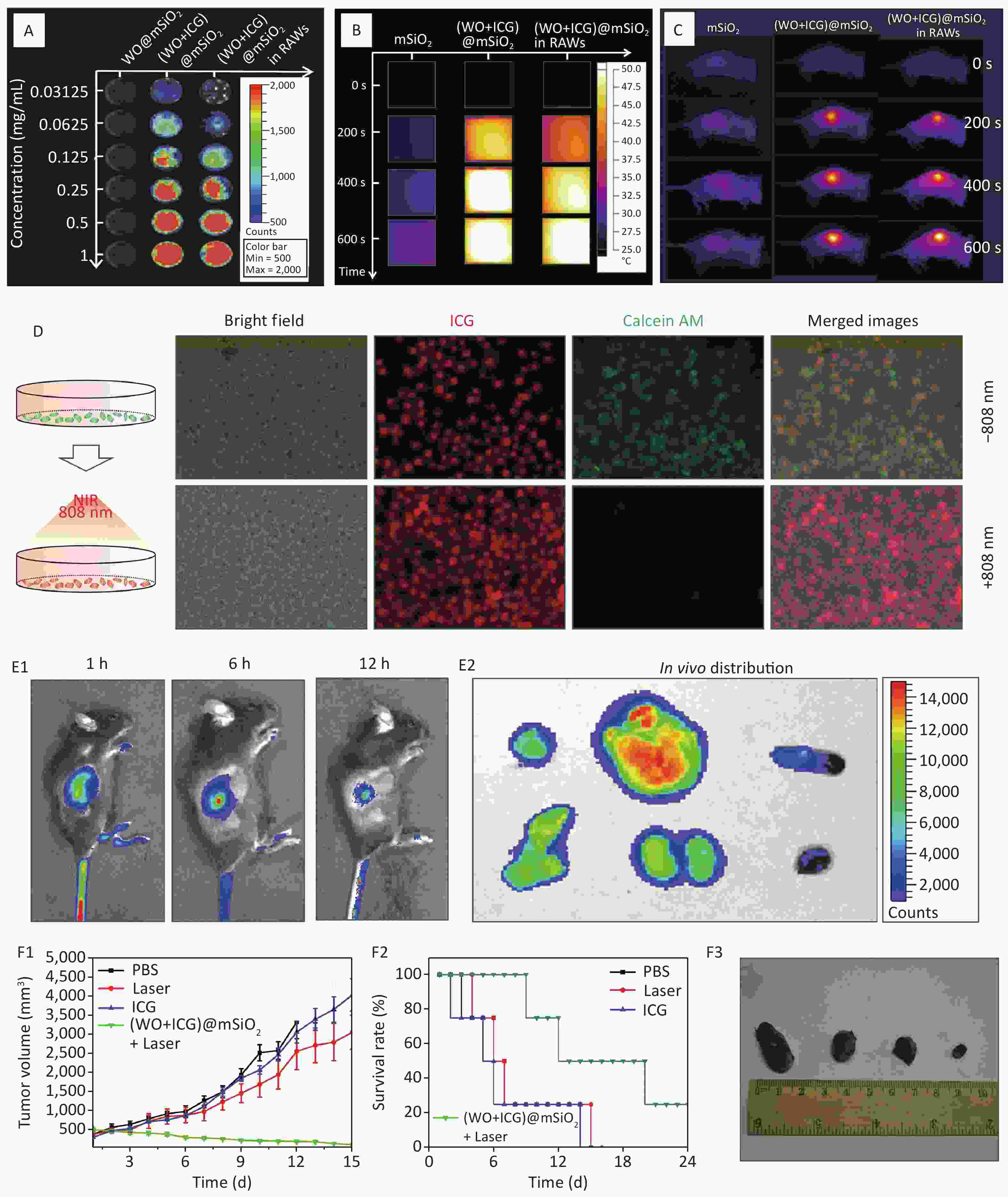
Figure 3. Fluorescence imaging and photothermal performance of (W18O49+ICG)@mSiO2@RAW. (A) Fluorescence images and (B–C) photothermal performance of (W18O49+ICG)@mSiO2@ RAW in vitro and vivo; (D) PTT effect in vitro. (Scale bar: 100 μm); (E1) the fluorescence imagines of the mice injected with (W18O49+ICG)@mSiO2@ RAW in vivo; (E2) the distribution of (W18O49+ICG)@mSiO2@ RAW in various tissues including heart, liver, spleen, lung, kidney and tumor (from top to bottom); (F1) the tumor volume changes, (F2) survival rate and (F3) the tumor size images of Group1, Group2, Group3 and Group4 (from left to right).
In vitro and tumor inhibition test in vivo were used to test the effect of PTT on nanoparticles. These two groups were used for the cell activity assay. The RAW264.7 macrophages incubated with (W18O49+ICG)@mSiO2 were set as Group 1 and the RAW264.7 macrophages incubated with (W18O49+ICG)@mSiO2 and exposed to NIR light irradiation were set as Group 2, respectively. In Group 1, significant green fluorescence was observed within the cells, indicating high cell viability after incubation with the nanoparticles (Figure 3D). In contrast, Group 2 exhibited a marked reduction in green fluorescence, indicating the onset of apoptosis after NIR irradiation. For the in vivo PTT evaluation, (W18O49+ICG)@mSiO2@RAW was administered via caudal vein injection into a murine tumor model. As illustrated in Figure 3E, a robust fluorescence signal was detected at the tumor site one-hour post-injection, which slightly diminished after 12 h. Autopsies of the treated mice after 12 h revealed a strong fluorescent signal at the tumor site, which confirmed the successful aggregation of (W18O49+ICG)@mSiO2@RAW within the tumor (Figure 3E).
The effect of PTT in vivo was tested using the growth curve of tumor volume, survival curve, and tumor size images. Four experimental groups were established: Group 1 was injected with PBS, Group 2 was exposed to an 808 nm laser for 5 min without any injection, Group 3 was injected with ICG, and Group 4 was injected with (W18O49+ICG)@mSiO2@RAW and exposed to an 808 nm laser. Group 3 showed no significant antitumor effect compared with Group 2, indicating that the NIR laser and pure ICG alone did not cause substantial damage (Figure 3F). In contrast, mice in Group 4 showed significantly reduced tumor growth (Supplementary Figures S5–S6, available in www.besjournal.com). This result indicates good tumor growth inhibition by (W18O49+ICG)@mSiO2@RAW under 808 nm laser irradiation. Additionally, the hemolysis assay and HE staining results indicated that PTT with (W18O49+ICG)@mSiO2@RAW was tolerable and safe in vivo (Supplementary Figures S7–S8, available in www.besjournal.com).
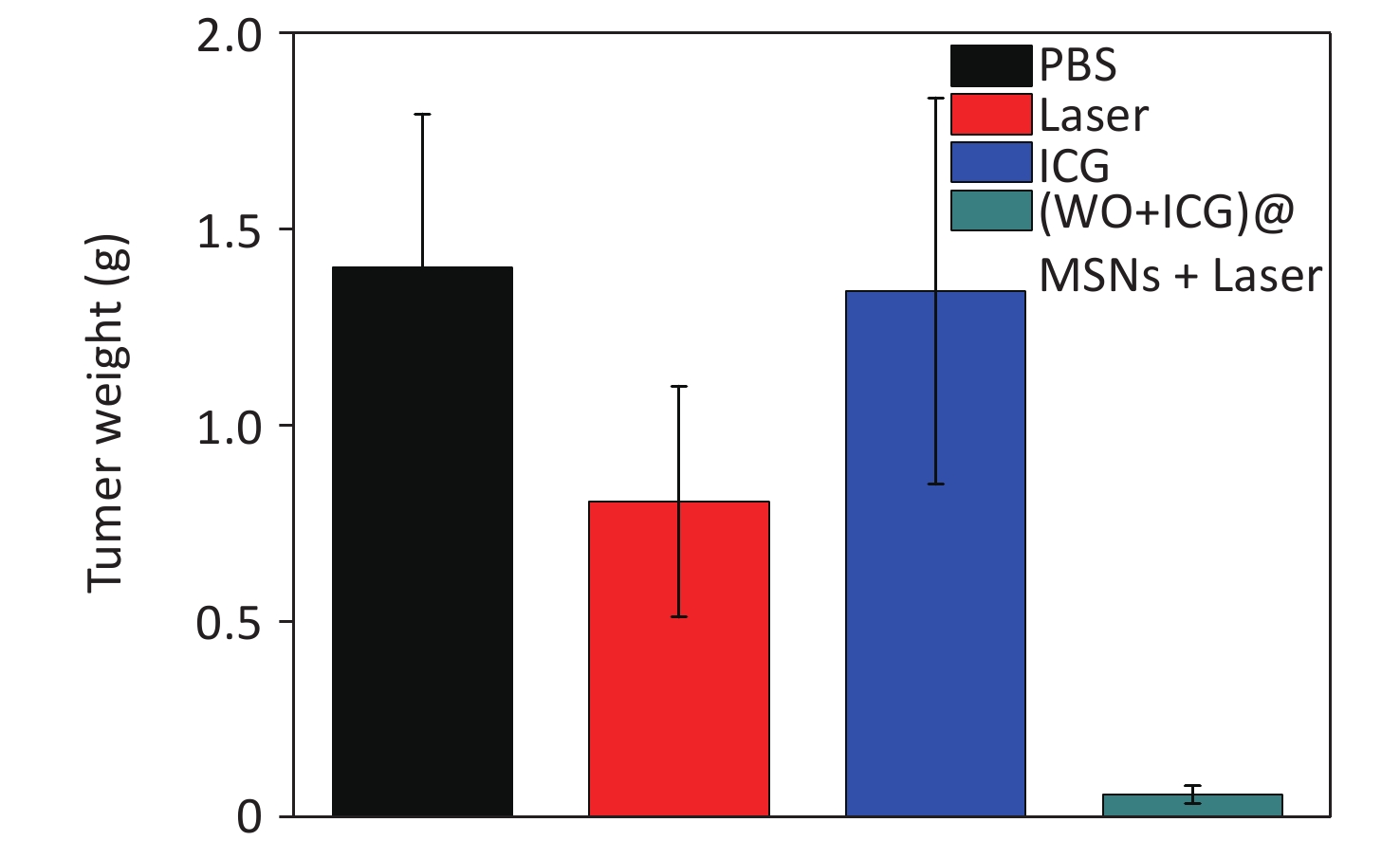
Figure S5. The tumor weight changes of the mice treated with different groups (the tumors were obtained at the 15 days).
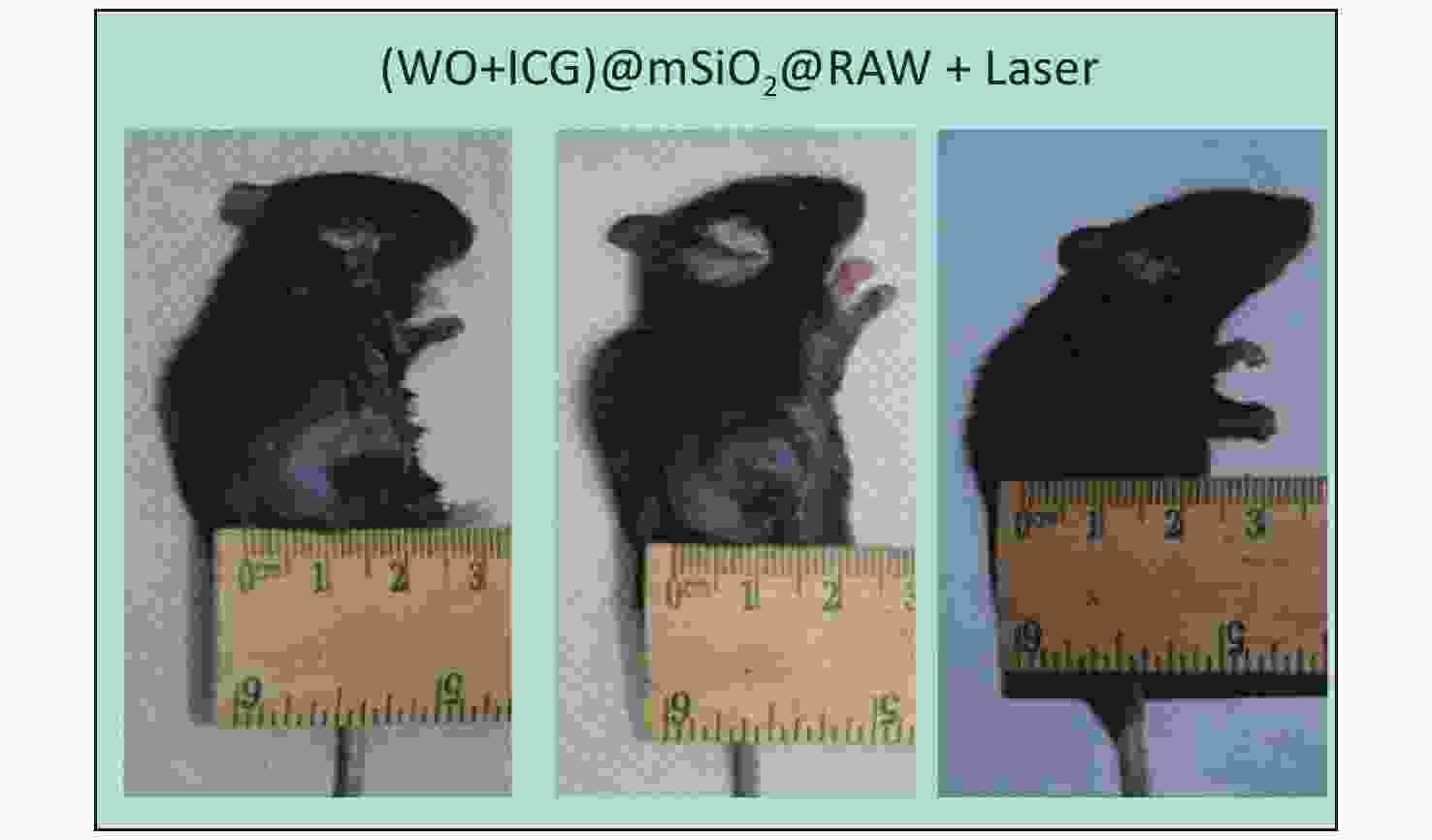
Figure S6. The tumor size changes of the mousr treated with (W18O49+ICG)@mSiO2@RAW under NIR laser irradiation.
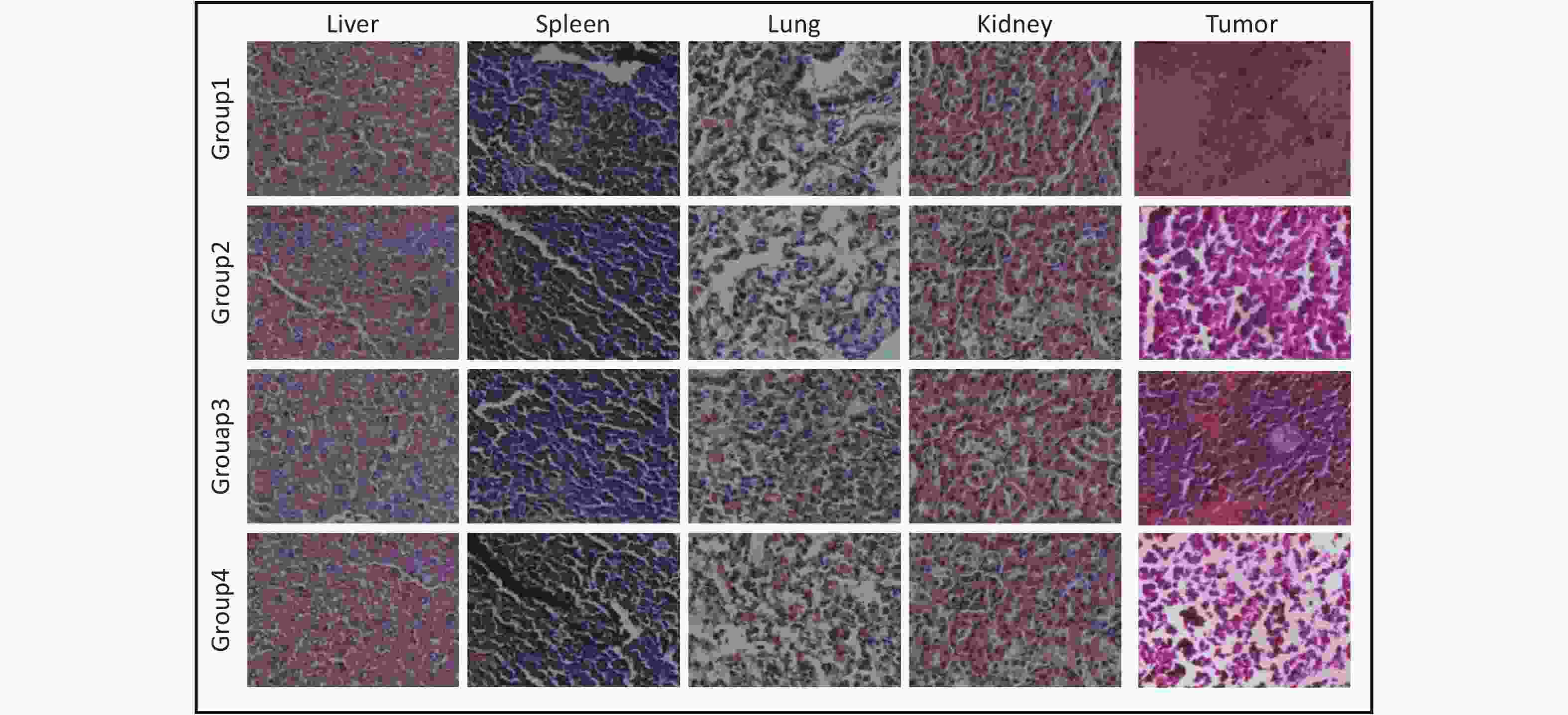
Figure S8. Hematoxylin and eosin (H&E) stained sections of tumors and organs from different treatment groups. All sections were stained using the H&E method. Sections were fixed, dehydrated, cleared, stained first with hematoxylin, followed by eosin, and finally mounted. The scale bars were 100 μm.
In conclusion, the photothermal agents W18O49 and ICG-co-loaded mesoporous silica (W18O49+ICG)@mSiO2 nanoparticles were designed. The fluorescence imaging and photothermal therapeutic efficiency of this system were perfectly integrated. Based on these properties, the new (W18O49+ICG)@mSiO2@RAW delivery system exhibited excellent fluorescence performance and enhanced temperature response during fluorescence imaging-guided PTT. Hence, this (W18O49+ICG)@mSiO2@RAW delivery system has potential applications as a phosphorescent drug carrier for various tumor treatments.
doi: 10.3967/bes2024.171
W18O49 Crystal and ICG Labeled Macrophage: An Efficient Targeting Vector for Fluorescence Imaging-guided Photothermal Therapy
-
The authors declare no conflicts of interest.
&These authors contributed equally to this work.
注释:1) Competing Interests: -
Figure 1. Characterization and performance of (W18O49+ICG)@mSiO2. (A) a schematic illustration showing the synthesis process of (W18O49+ICG)@mSiO2 nanoparticle; (B1–B4) TEM images of mSiO2 (Scale bar: 0.5 μm), WCl6@mSiO2 (Scale bar: 0.5 μm), W18O49@mSiO2 (Scale bar: 0.5 μm) and (W18O49+ICG)@mSiO2 (Scale bar: 100 μm); (C1–C2) the particle size data of mSiO2 and (W18O49+ICG)@mSiO2; (D) EELS line scan of the single (W18O49+ICG)@mSiO2 nanoparticle; (E) bright field images of mSiO2 (1), WCl6@mSiO2 (2), W18O49@mSiO2 (3), and (W18O49+ICG)@mSiO2 (4); (F) thermal imaging photos of a letter sample consisted by (W18O49+ICG)@mSiO2. (G) Cytotoxicity of the nanoparticles tested using the MTT assay. (H) Cell activity test using calcium-AM fluorescence staining (scale bar: 100 μm).
Figure 2. Macrophage uptake and phagocytosis of (W18O49+ICG)@mSiO2. (A) Macrophage uptake of (W18O49+ICG)@mSiO2 at different concentrations. The scale bars were 100 μm. (B–C) The macrophages phagocytosis activity test of (W18O49+ICG)@mSiO2 nanoparticles by fluorescence microscope and confocal microscope. (Scale bar for B: 100 μm; Scale bar for C: 10 μm).
Figure 3. Fluorescence imaging and photothermal performance of (W18O49+ICG)@mSiO2@RAW. (A) Fluorescence images and (B–C) photothermal performance of (W18O49+ICG)@mSiO2@ RAW in vitro and vivo; (D) PTT effect in vitro. (Scale bar: 100 μm); (E1) the fluorescence imagines of the mice injected with (W18O49+ICG)@mSiO2@ RAW in vivo; (E2) the distribution of (W18O49+ICG)@mSiO2@ RAW in various tissues including heart, liver, spleen, lung, kidney and tumor (from top to bottom); (F1) the tumor volume changes, (F2) survival rate and (F3) the tumor size images of Group1, Group2, Group3 and Group4 (from left to right).
-
[1] Qi J, Fang Y, Kwok RTK, et al. Highly stable organic small molecular nanoparticles as an advanced and biocompatible phototheranostic agent of tumor in living mice. ACS Nano, 2017; 11, 7177−88. doi: 10.1021/acsnano.7b03062 [2] Chen YY, Syed AM, MacMillan P, et al. Flow rate affects nanoparticle uptake into endothelial cells. Adv Mater, 2020; 32, 1906274. doi: 10.1002/adma.201906274 [3] Fu YH, Ye F, Zhang XW, et al. Decrease in tumor interstitial pressure for enhanced drug intratumoral delivery and synergistic tumor therapy. ACS Nano, 2022; 16, 18376−89. [4] Wang XD, Lu JY, Mao YL, et al. A mutually beneficial macrophages-mediated delivery system realizing photo/immune therapy. J Control Release, 2022; 347, 14−26. doi: 10.1016/j.jconrel.2022.04.038 [5] Zhao H, Xu JB, Feng C, et al. Tailoring aggregation extent of photosensitizers to boost phototherapy potency for eliciting systemic antitumor immunity. Adv Mater, 2022; 34, 2106390. doi: 10.1002/adma.202106390 [6] Sheng JP, Zhang L, Deng L, et al. Fabrication of dopamine enveloped WO3−x quantum dots as single-NIR laser activated photonic nanodrug for synergistic photothermal/photodynamic therapy against cancer. Chem Eng J, 2020; 383, 123071. doi: 10.1016/j.cej.2019.123071 [7] Li ZQ, Li Z, Ramos A, et al. Detection of pancreatic cancer by indocyanine green-assisted fluorescence imaging in the first and second near-infrared windows. Cancer Commun (Lond), 2021; 41, 1431−4. doi: 10.1002/cac2.12236 [8] Xu DL, Ge M, Zong M, et al. Revisiting the impacts of silica nanoparticles on endothelial cell junctions and tumor metastasis. Chem, 2023; 9, 1865−81. doi: 10.1016/j.chempr.2023.03.004 [9] Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer, 2004; 4, 437−47. doi: 10.1038/nrc1367 [10] Hou BB, Zheng B, Gong XQ, et al. A UCN@mSiO2@cross-linked lipid with high steric stability as a NIR remote controlled-release nanocarrier for photodynamic therapy. J Mater Chem B, 2015; 3, 3531−40. -




 下载:
下载:



 Quick Links
Quick Links