-
Kashin-Beck disease (KBD) is a regionally endemic chronic osteoarthropathy, while osteoarthritis (OA) is a degenerative joint disease characterized by progressive articular cartilage degradation and extracellular matrix remodeling. Although KBD and OA share overlapping clinical and pathological features, key differences exist in their etiology and disease progression. KBD preferentially affects children aged 3–12 years, whereas OA predominantly affects older individuals between the age of 40–60 years. KBD cartilage necrosis originates in the deep layers of the epiphyseal plate and articular cartilage, progressing toward the cartilage surface. In contrast, OA cartilage destruction initiates at the articular cartilage surface and gradually progresses to expose the subchondral bone[1,2].
Cytokines (CK) are small-protein molecules that plays a key role in the pathogenesis and development of OA. Biomarkers (BMs), which reflect metabolic changes in articular cartilage, synovium, and bone, are potential indicators for early diagnosis, disease monitoring, prognosis assessment, and drug efficacy evaluation in OA[2,3]. These inflammatory CKs and BMs also exhibit significantly altered levels in the joint synovium, articular cartilage, serum, or urine of patients with KBD.
Histological and morphological changes in KBD articular cartilage differ from those observed in OA cartilage, including significant alterations in chondrocyte phenotype, chondrocyte necrosis/apoptosis, and aberrant terminal chondrocyte differentiation[4]. Therefore, this study aimed to compare the serum concentrations of interleukin-1 β (IL-1β), tumor necrosis factor-α (TNF-α), hyaluronic acid (HA), cartilage oligomeric matrix protein (COMP), carboxyl-terminal peptide of type Ⅱ collagen (CTX-Ⅱ), and type IIA procollagen amino-terminal peptide (PIIANP) in adult patients with KBD and OA in Qinghai, and to elucidate the molecular mechanisms underlying cartilage injury in KBD and OA.
The study was conducted in compliance with the ethical principles outlined in the Declaration of Helsinki and approved by the Ethics committee of the Qinghai Institute for Endemic Disease Prevention and Control (2024-007). A total of 63 patients diagnosed with knee osteoarthritis (KOA) and treated as outpatients and inpatients at the Affiliated Hospital of Qinghai university in 2020 were selected as the OA group. The KBD group included 67 adult patients with KBD from Qinghai Province recruited in October 2019. The control group consisted of 53 patients from the physical examination center of Qinghai University Affiliated Hospital.
All participants were aged 40–70 years. Patients with KOA met the KOA diagnostic criteria of the KOA as specified in the 2021 guidelines of the Osteoarthritis Research Society International (OARSI). KBD diagnosis followed the Diagnostic Criteria for Kashin-Beck Disease (WS/T 207-2010). The control group consisted of individuals without no history of KBD or OA and free of chronic infectious diseases, metabolic diseases, connective tissue disease, tumor or cancer, joint infections, endocrine diseases, abnormal liver and kidney function. Sample size calculations were based on previous studies, with power analysis (α = 0.05, β = 0.20) indicating sufficient statistical power (80%) to detect intergroup differences. Participants were stratified by age (40–70 years) and disease severity (KBD: Grade I/II; OA: end-stage requiring joint replacement).
Three milliliters of venous blood were collected from each participant. Serum concentrations of IL-1β, TNF-α, HA, COMP, CTX-II, and PIIANP were measured using enzyme linked immunosorbent assay (ELISA) kits provided by Beijing AIR-HX biological technology Co., Ltd., following the manufacturer’s instructions. Categorical data were expressed as composition ratios and compared using chi-squared (χ2) test. Continuous variables were expressed as mean ± standard deviation for normally distributed data and analyzed using one-way analysis of variance (ANOVA) for intergroup comparisons and the SNK-q test for pairwise comparisons. Non-normally distributed continuous data were presented as median (interquartile range [IQR]) and analyzed using the Kruskal-Wallis H test, with pairwise comparisons via the Nemenyi method. A significance level of α = 0.05 was adopted.
As illustrated in Table 1, the mean ages and BMI in the OA group were 59.43 ± 7.03 years and 24.62 ± 2.55 kg/m2, respectively; in the KBD group, 56.46 ± 8.19 years and 24.01 ± 4.79 kg/m2; and in the control group, 56.12 ± 9.31 years and 24.34 ± 3.72 kg/m2. There were no statistically significant differences in age, gender, or BMI among the three groups (χ2gender = 0.291, P = 0.864; Fage = 3.305, P = 0.0501; FBMI = 0.444, P = 0.642).
Table 1. Comparison of general data among three groups
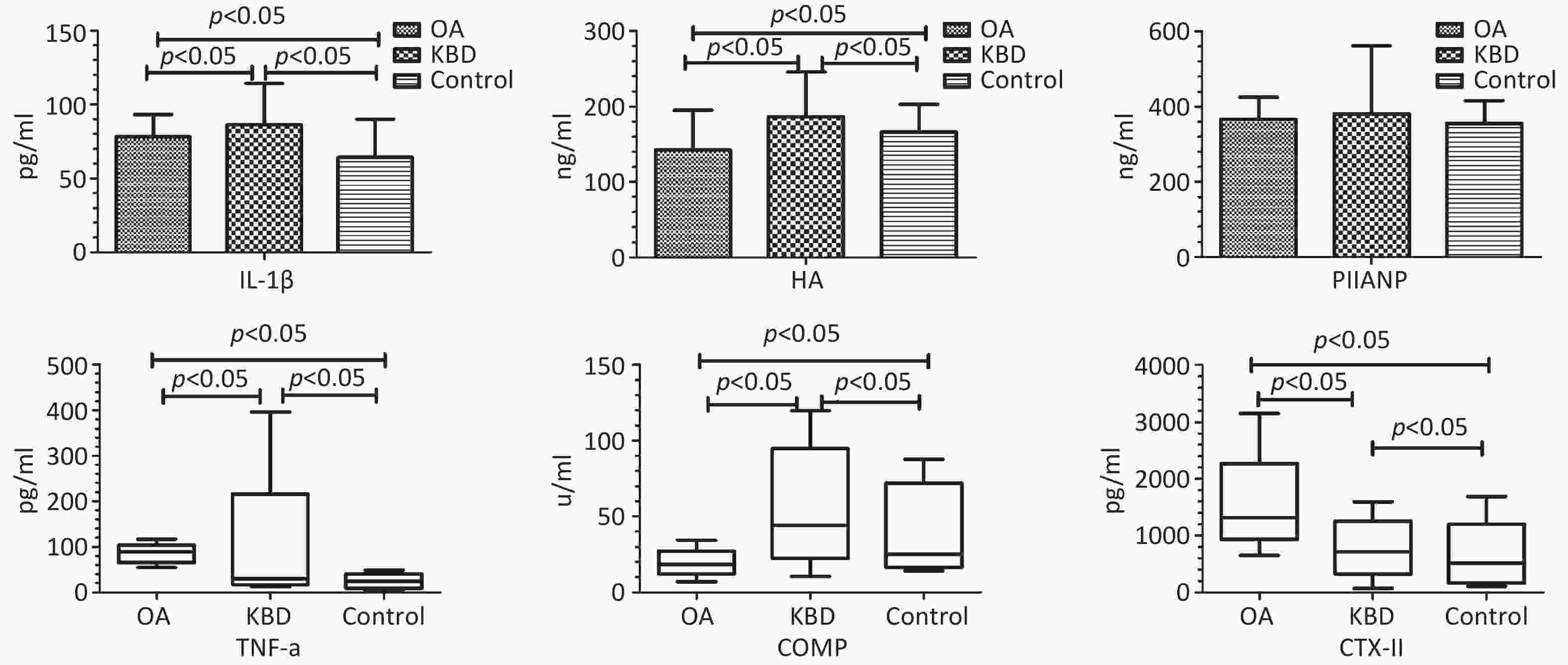
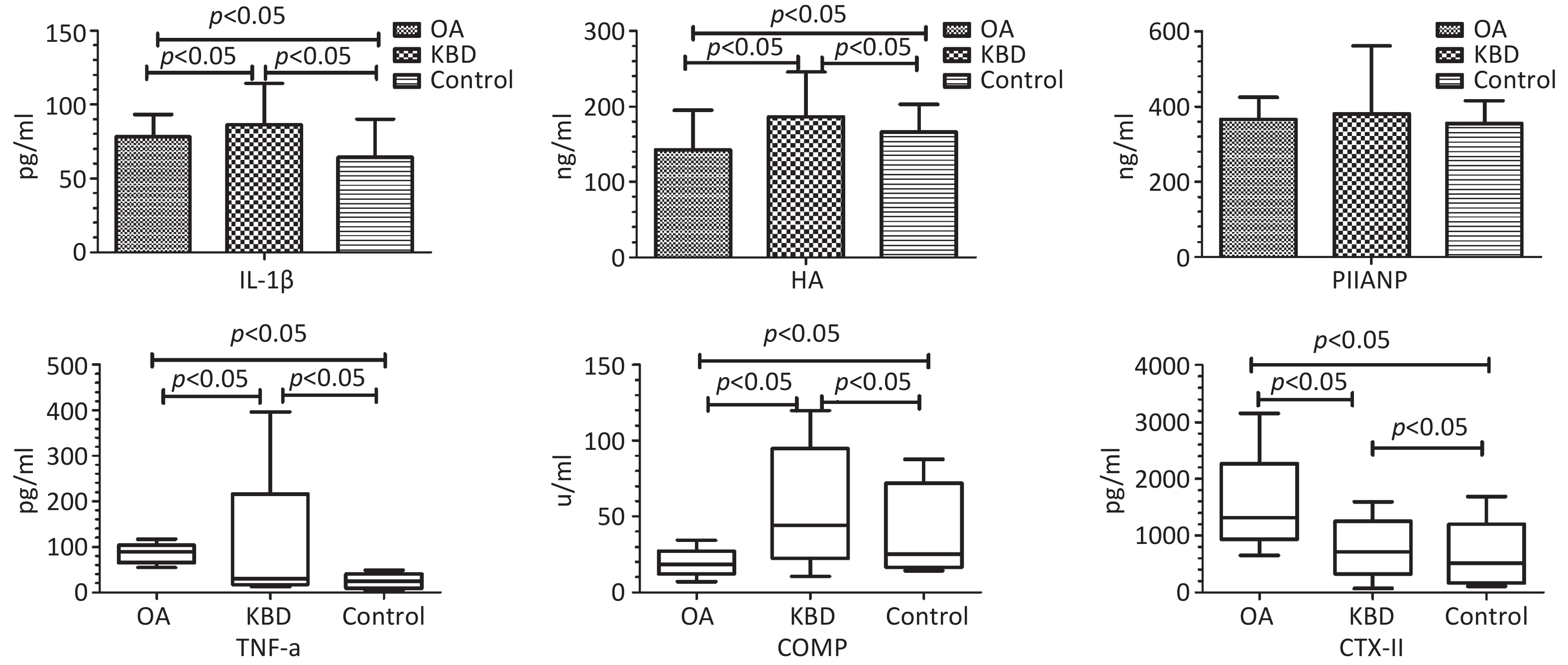
Groups n Gender (n) Age (Y) (X± S) BMI (kg/m2) (X± S) Male Female OA 63 32 31 59.43 ± 7.03 24.62 ± 2.55 KBD 67 31 36 56.46 ± 8.19 24.01 ± 4.79 Control 53 25 28 56.12 ± 9.31 24.34 ± 3.72 F/χ2 0.291 2.875 0.444 P 0.864 0.065 0.642 Note. BMI, body mass index; KBD, Kashin-Beck disease; OA, osteoarthritis. As shown in Table 2 and Figure 1, the average serum concentrations of IL-1β, HA, and PIIANP in the OA group were 78.07 ± 15.21 pg/mL, 142.62 ± 52.55 ng/mL, and 366.10 ± 59.31 ng/mL, respectively. In the KBD group, IL-1β, HA, and PIIANP levels were 86.37 ± 28.06 pg/mL, 186.64 ± 58.89 ng/mL, and 381.25 ± 179.75 ng/mL, respectively. In the control group, corresponding levels were 64.30 ± 25.93 pg/mL, 166.07 ± 36.72 ng/mL, and 355.57 ± 60.62 ng/mL, respectively. There were statistical differences in serum IL-1β and HA among the groups (FIL-1β = 12.992, P < 0.001; FHA = 12.803, P < 0.001), but no significant difference in serum PIIANP levels (FPIIANP = 0.942, P = 0.391).
Table 2. ???
Groups IL-1β X± S (pg/mL) HA X± S (ng/mL) PIIANPX± S (ng/mL) OA 78.07 ± 15.21ab 142.62 ± 52.55ab 366.10 ± 59.31 KBD 86.37 ± 28.06a 186.64 ± 58.89a 381.25 ± 179.75 Control 64.30 ± 25.93 166.07 ± 36.72 355.57 ± 60.62 F 12.992 12.803 0.942 p 0.000 0.000 0.391 As shown in Table 3 and Figure 1, the median serum levels of TNF-α, COMP, and CTX-II in the OA group were 81.86 pg/mL, 18.56 U/mL, and 1319.08 pg/mL, respectively. The KBD group showed TNF-α, COMP, and CTX-II levels of 29.80 pg/mL, 44.43 U/mL, and 715.23 pg/mL, respectively, the control group had levels of 24.42 pg/mL, 25.23 U/mL, and 519.37 pg/mL, respectively. Significant intergroup differences were observed for all three markers (HTNF-α = 104.464, HCOMP = 75.366, HCTX-II = 117.519, all P < 0.001).
Table 3. Comparison of serum TNF-α, COMP and CTX-II levels among three groups
Groups Median Range P25 P75 H P TNF-α
(pg/mL)OA 81.86ab 54.20 – 116.74 75.16 89.33 104.464 < 0.001 KBD 29.80a 12.27 – 396.78 21.87 34.97 Control 24.42 4.45 – 48.83 14.18 31.15 COMP
(u/mL)OA 18.56ab 7.13 – 34.31 17.15 20.20 75.366 < 0.001 KBD 44.43a 10.55 – 119.90 34.16 69.24 Control 25.23 14.13 – 87.79 19.21 55.54 CTX-II
(pg/mL))OA 1319.08ab 653.73 – 3158.23 1221.26 1 378.72 117.519 < 0.001 KBD 715.23a 72.25 – 1594.85 582.35 916.25 Control 519.37 109.72 – 1689.05 222.57 714.25 Note. Compared to control group, aP < 0.05; compared to the KBD group, bP < 0.05; BMI, body mass index; KBD, Kashin-Beck disease; OA, osteoarthritis; HA, hyaluronic acid; PIIANP, procollagen amino-terminal peptide; COMP. cartilage oligomeric matrix protein. Accurately distinguish the two diseases using medical history, clinical symptoms, physical examination, and other conventional methods remains challenging. The pathogenesis of both OA and KBD involves progressive cartilage degradation, though their underlying mechanisms of cartilage injury differ significantly. Inflammatory CKs and BMs are produced during this degenerative process.
CKs are important mediators of the imbalance between articular cartilage synthesis and catabolism. IL-1β, a pro-inflammatory CK, is significantly elevated in the joint cavity of patients with OA than that in the control group, and correlates positively with the severity of inflammation[2,5]. TNF-α induces chondrocyte apoptosis and matrix degradation, forming a vicious cycle that accelerates OA progression. IL-1β and TNF-α levels in the joint synovial fluid, serum, or cartilage of both patients with KBD and OA were elevated compared with the control group, though difference between KBD and OA groups were not statistically significant. In this study, serum concentrations of IL-1β and TNF-α in the KBD and OA groups were significantly elevated compared with the control group, indicating their critical roles in the pathogenesis of articular cartilage injury. However, serum concentrations of IL-1β in the KBD group were higher than that in the OA group, whereas serum concentrations of TNF-α in the KBD group were lower than that in the OA group. Because patients with OA in this trial were scheduled for joint replacement surgery, representing end-stage disease with severe symptoms, whereas patients with KBD were primarily in milder manifestations. Consequently, the joint inflammatory response in KBD was relatively attenuated compared with OA, contributing to lower serum TNF-α levels. Additionally, in advanced OA, reduced proteoglycan content in articular cartilage might affect the degradation of ADAMTS-4 and ADAMTS-5, with relatively high levels of these enzymes potentially inhibiting IL-1β secretion[5,6]. The observed lower serum IL-1β levels in patients with OA compared with that in patients with KBD might reflect disease-stage heterogeneity. Further mechanistic studies are warranted to explore whether advanced OA cartilage degradation modulates IL-1β expression through pathways involving ADAMTS-4/5 or other catabolic enzymes.
HA is an important component of proteoglycan in the extracellular matrix (ECM). COMP is an important non-collagen glycoprotein in the ECM of articular cartilage. Previous studies have demonstrated elevated serum concentrations of HA in both patients with OA and KBD compared with the control group[7,8], and serum COMP concentrations in the KBD group were significantly higher than those in the control and OA groups. In this study, serum HA and COMP in the KBD group remained significantly higher than those in the control and OA groups, meanwhile, serum HA and COMP in OA group were lower than those in the control group. Contrary to previous reports of elevated HA in OA[7], our study found lower HA levels in patients with OA compared to controls. This discrepancy might stem from differences in disease stages. OA cohort represented end-stage cases with severe proteoglycan loss, potentially reducing HA synthesis capacity.
CTX-II, a carboxyl-terminal peptide, represents a major degradation product of type II collagen. Serum CTX-II in patients with KOA are significantly elevated compared with the control group and correlates with cartilage damage severity. Serum CTX-II in patients with KBD are also elevated compared with the control group[9]. The results of this study demonstrated that serum CTX-II levels in both patients with OA and KBD were elevated compared with the control group. Notably, serum CTX-II levels in the OA group were significantly higher than those in the KBD group.
PIIANP is a specific BM for type II collagen synthesis. Serum PIIANP concentrations in patients with OA have been reported to be significantly lower than those in the control group and to be negatively correlated with knee joint K-L grade, indicating reduced type II collagen synthesis in OA[10]. Likewise, serum PIIANP levels in patients with KBD have been shown to be lower than those in controls, indicating reduced synthetic capacity for type II collagen. In this study, serum PIIANP concentrations followed the order KBD group > OA group > control group without statistical differences.
The pathogenesis of KBD and OA remains incompletely understood. By comparing these inflammatory CKs and metabolic BMs, the distinct characteristics of cartilage damage in KBD and OA could be further elucidated. This study provided a theoretical basis for differential diagnosis. In this study, patients with OA scheduled for artificial joint replacement represented end-stage disease, whereas the KBD cohort comprised primarily grade I/II cases. Additionally, the relatively limited sample size highlights the need for future studies involving larger cohorts, optimized protocols, and more in-depth mechanistic investigations.
In summary, these findings demonstrated distinct inflammatory and cartilage metabolic profiles changes in patients with KBD and OA. Both conditions are characterized by elevated serum IL-1β, TNF-α, and CTX-II levels, indicating shared inflammatory responses and type II collagen degradation. However, patients with KBD uniquely exhibited upregulated HA and COMP levels, whereas patients with OA displayed reduced levels of these proteoglycans/glycoproteins, reflecting divergent ECM remodeling patterns. The distinct serum profiles of IL-1β, HA, COMP, TNF-α, and CTX-II between patients with KBD and OA highlighted their potential as candidate BMs for further investigation. Although no statistically significant differences were observed in serum PIIANP levels between the groups, its role in differential diagnosis warrants further exploration. For clinical application, these BMs must be rigorously validated for diagnostic accuracy (e.g., receiver operating characteristic curve analysis, sensitivity/specificity) in future studies. These findings provide a foundation for understanding the divergent pathophysiological mechanisms underlying KBD and OA.
doi: 10.3967/bes2025.111
Analysis of Serum Metabolic Biomarkers in Adult Patients with Kashin–Beck Disease and Degenerative Osteoarthritis in Qinghai Province
-
Conceptualization, Investigation, Data curation, Methodology, Formal analysis, Visualization, Writing-original draft: Jiale Xu, Qiang Li, and Chuan Lu wrote this paper and carried out epidemical investigation and the statistical analysis
All authors declare that there are no conflicts of interest.
The study was carried out in compliance with the ethical principles outlined in the world medical association Helsinki’s declaration. This study was approved by the Ethics committee of the Qinghai institute for endemic disease prevention and control (2024-007).
&These authors contributed equally to this work.
注释:1) Authors’ Contributions: 2) Competing Interests: 3) Ethics: -
Table 1. Comparison of general data among three groups
Groups n Gender (n) Age (Y) (X± S) BMI (kg/m2) (X± S) Male Female OA 63 32 31 59.43 ± 7.03 24.62 ± 2.55 KBD 67 31 36 56.46 ± 8.19 24.01 ± 4.79 Control 53 25 28 56.12 ± 9.31 24.34 ± 3.72 F/χ2 0.291 2.875 0.444 P 0.864 0.065 0.642 Note. BMI, body mass index; KBD, Kashin-Beck disease; OA, osteoarthritis. Table 2. ???
Groups IL-1β X± S (pg/mL) HA X± S (ng/mL) PIIANPX± S (ng/mL) OA 78.07 ± 15.21ab 142.62 ± 52.55ab 366.10 ± 59.31 KBD 86.37 ± 28.06a 186.64 ± 58.89a 381.25 ± 179.75 Control 64.30 ± 25.93 166.07 ± 36.72 355.57 ± 60.62 F 12.992 12.803 0.942 p 0.000 0.000 0.391 Table 3. Comparison of serum TNF-α, COMP and CTX-II levels among three groups
Groups Median Range P25 P75 H P TNF-α
(pg/mL)OA 81.86ab 54.20 – 116.74 75.16 89.33 104.464 < 0.001 KBD 29.80a 12.27 – 396.78 21.87 34.97 Control 24.42 4.45 – 48.83 14.18 31.15 COMP
(u/mL)OA 18.56ab 7.13 – 34.31 17.15 20.20 75.366 < 0.001 KBD 44.43a 10.55 – 119.90 34.16 69.24 Control 25.23 14.13 – 87.79 19.21 55.54 CTX-II
(pg/mL))OA 1319.08ab 653.73 – 3158.23 1221.26 1 378.72 117.519 < 0.001 KBD 715.23a 72.25 – 1594.85 582.35 916.25 Control 519.37 109.72 – 1689.05 222.57 714.25 Note. Compared to control group, aP < 0.05; compared to the KBD group, bP < 0.05; BMI, body mass index; KBD, Kashin-Beck disease; OA, osteoarthritis; HA, hyaluronic acid; PIIANP, procollagen amino-terminal peptide; COMP. cartilage oligomeric matrix protein. -
[1] Song QQ, Sun LY, Li CH, et al. The urinary levels of CTX-II, C2C, PYD, and Helix-II increased among adults with KBD: a cross-sectional study. J Orthop Surg Res, 2019; 14, 328. doi: 10.1186/s13018-019-1392-6 [2] Molnar V, Matišić V, Kodvanj I, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci, 2021; 22, 9208. doi: 10.3390/ijms22179208 [3] Mobasheri A, Thudium CS, Bay-Jensen AC, et al. Biomarkers for osteoarthritis: current status and future prospects. Best Pract Res Clin Rheumatol, 2023; 37, 101852. doi: 10.1016/j.berh.2023.101852 [4] Yang L, Sun JW, Zhang Y, et al. Comprehensive comparative analysis of histopathology and gene expression in subchondral bone between kashin-beck disease and primary osteoarthritis. Front Genet, 2022; 13, 942326. doi: 10.3389/fgene.2022.942326 [5] Tang X, Zhou ZK, Shen B, et al. Serum levels of TNF-α, IL-1β, COMP, and CTX-II in patients with Kashin-Beck disease in Sichuan, China. Rheumatol Int, 2012; 32, 3503−9. doi: 10.1007/s00296-011-2172-8 [6] Jiang LJ, Lin JC, Zhao S, et al. ADAMTS5 in osteoarthritis: biological functions, regulatory network, and potential targeting therapies. Front Mol Biosci, 2021; 8, 703110. doi: 10.3389/fmolb.2021.703110 [7] Band PA, Heeter J, Wisniewski HG, et al. Hyaluronan molecular weight distribution is associated with the risk of knee osteoarthritis progression. Osteoarthritis Cartilage, 2015; 23, 70−6. doi: 10.1016/j.joca.2014.09.017 [8] Jiao Q, Wei L, Chen CW, et al. Cartilage oligomeric matrix protein and hyaluronic acid are sensitive serum biomarkers for early cartilage lesions in the knee joint. Biomarkers, 2016; 21, 146−51. doi: 10.3109/1354750X.2015.1118547 [9] Luo YY, He Y, Karsdal M, et al. Serological CTX-II does not measure the same as urinary CTX-II. Osteoarthr Cartil Open, 2020; 2, 100082. doi: 10.1016/j.ocarto.2020.100082 [10] Kraus VB, Collins JE, Hargrove D, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis, 2017; 76, 186−95. -




 下载:
下载:



 Quick Links
Quick Links