-
To investigate the genotoxicity and reveal the potential toxicological mechanisms of Hexabromocyclododecane (HBCD), human breast cells HBL-100 were exposed to a sequence of HBCD concentrations (0, 5, 10, and 50 mg/L) for 24 h. With a series of zymology and molecular biology methods, we found that HBCD induced dose-dependent oxidative stress on HBL-100 DNA. As revealed in qRT-PCR, activated prognostic factor ATM down-regulated tumor suppressor gene BRCA1 and prompted DNA repair genes hOGG1 and hMTH1 expression in lower concentrations of HBCD ( < 10 mg/L). However, DNA repair were inhibited as well as cell proliferation rate by higher concentrations of HBCD (50 mg/L). The results inferred that the genotoxicity of HBCD was dose-dependent and related to DNA repair pathway.
Hexabromocyclododecane (HBCD) is extensively used in polystyrene foam, interior decoration, textiles, and electronics[1]as an additive brominated flame retardant (BFR). After decades of consume, HBCD was globally distributed and detected in atmosphere, waters, wildlife, food staples, and human breast milk[2]. Moreover, the ubiquitous presence and lipophilic properties of HBCD facilitate its accumulation in biota[3]. Thus far, HBCD has a potential to initiate disease and threat human health. Recent studies have proved that HBCD exhibits neurotoxicity, endocrine-disrupting toxicity, growth and development toxicity, et al. However, the dispute on its genotoxicity still unsettled.
Genomes damaged by environmental chemicals, radiation and replication can be transmitted to offspring, which became causes of inheritable disease, cancer and aging[4]. However, repair of these damages are important in preventing mutations. Thus, detection of genetic expression change in DNA repair process can be used to indicate genotoxicity. Several related genes have been analyzed for human cancer risk in molecular epidemiological studies. Ataxia telangiectasia mutated (ATM) kinase is a critical regulator which responds to DNA damage in all eukaryotes from yeast to human[5]. Once activated, ATM phosphorylates its downstream-substrate breast cancer susceptibility gene 1 (BRCA1)[6], which is usually expressed significantly in breast cancer cells[7]. Moreover, human 8-oxoguanine DNA glycosylase 1 (hOGG1), and human MutT homologue 1 (hMTH1) enzyme have been reported to be able to remove transversion G:C→T:A from oxidated DNA[8]. Thus, DNA damage can be repaired through ATM, BRCA1, hOGG1, and hMTH1 expression.
With an increasing incidence of breast cancer in recent years, the potential genotoxicity of HBCD is expected to be detected to identify its impact on human breast. Human breast carcinoma cell line (HBL-100) was usually selected as exposure subjects for contaminants toxicity study. However, there is still few integrated mechanism elucidated the genotoxicity of HBCD in HBL-100 cells. This study was expected to investigate HBCD-induced genotoxicity in HBL-100 cells and reveal the potential mechanism. This preliminary study provided an insight into the relationship between HBCD and breast cancer, and evaluate the genetic risk of HBCD. Moreover, specific functional DNA-repair assays should be developed as promising tools for providing reliable data on the actual role of specific repair in cancer risk.
HBL-100 cells were purchased from the Cell Storage Center of Wuhan University (China), and cultured in RPMI 1640 medium with 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 10% fetal bovine serum (FBS) and stored at 37 ℃ in a humidified atmosphere of 5% CO2 and 95% air. For the cytotoxicity assay, the cells were seeded into 96-well microplates at a density of 5 × 104 cells/well. After 24 h of cell attachment, the plates were washed with a series of concentrations of HBCD (0, 5, 10, and 50 mg/L) for 24 h. Triplicate cultures were evaluated in each assay. After having been incubated for 24 h, the cells and culture media were used to detect HBCD toxicity.
MTT, lactate dehydrogenase (LDH), and reactive oxygen species (ROS) assays were performed to analyze the cytotoxicity of HBCD on the proliferation rate, LDH leakage, and the formation quantity of ROS. All the experiments were conducted according to the kits instructions (JIANCHENG, NANJING). LDH leakage was expressed as a percentage of total LDH activity, that is, a percentage of total LDH capable of leaking into the media. To reveal potential genotoxicity, a transmission electron microscopy (TEM) assay was used to observe the structure and function of organelles in HBL-100 cells with HBCD treatment. The comet assay was used to identify a DNA break in HBL-100 cells and discuss the potential genotoxicity of HBCD.
Analysis of ATM, BRCA1, hOGG1, hMTH1 expression levels were conducted by Quantitative real-time PCR (qRT-PCR). Total RNA was extracted with PCI (phenol:chloroform:isoamyl alcohol = 25:24:1) and CI (chloroform:isoamyl alcohol = 24:1), refined with ethanol from cells, and diluted in 1 mL of EASY Dilution (TaKaRa, Japan). First-strand cDNA was synthesized by reverse transcribing 5 μL of total RNA in a final reaction volume of 20 μL with the use of PrimeScript™ RT reagent kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Dalian). The produced cDNA was then subjected to qRT-PCR using specific primers for ATM (5'-ACTATCCCAATACACTGCTGGA GA-3' and 5'-TTTGAGCAACTGACTGGCAAAC-3', 152 bp), BRCA1 (5'-GCTGCTGCTCATACTACTG-3' and 5'-GCTGTCAATTCTGGCTTCT-3', 80 bp), hOGG1 (5'-CGTGGACTCCCACTTCCAAGA-3' and 5'-AAGCTGG ATGAGCCGAGGT-3', 175 bp), and hMTH1 (5'-CTACT GGTTTCCACTCCTGCTTC-3' and 5'-GCTTTATTTTCCCTT CCATCCA-3', 241 bp). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (5'-GCACCGTCAAGGCTGAG AAC-3' and 5'-TGGTGAAGACGCCAGTGGA-3', 138 bp) was selected as the housekeeping gene, and all samples were assayed in triplicate. Analyses were conducted using the 2−ΔΔCtmethod.
All data are presented as mean ± standard deviation (SD) when appropriate. The difference between the effects of different concentrations was considered statistically significant when P < 0.05 by using one-way analysis of variance (ANOVA) with a post-hoc Dunnett's test. For the comparison of multiple groups, ANOVA was used, followed by Newman-Keuls test. All statistical analyses were performed using SPSS 19.0 software.
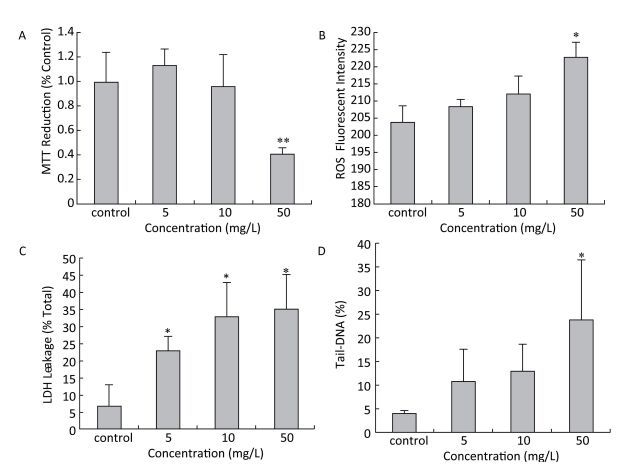
After exposed to a sequence of HBCD, the proliferation rate of HBL-100 cells was assessed by measuring succinodehydrogenase activity, which only acted in living cells. Compared with the control (Figure 1A), the proliferation rate of HBL-100 cells was promoted at 5 mg/L HBCD treatment but declined with HBCD increasing. Moreover, the cell viability was only significantly reduced at 50 mg/L HBCD, with a reduction of 60%.
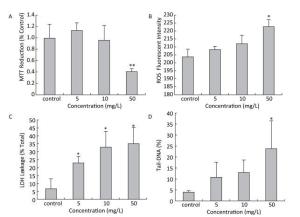
Figure 1. Cytotoxicity of HBCD determined by the MTT, ROS, LDH and comet assays.*and **represent significant (P < 0.05 and P < 0.01) differences compared with the control value.
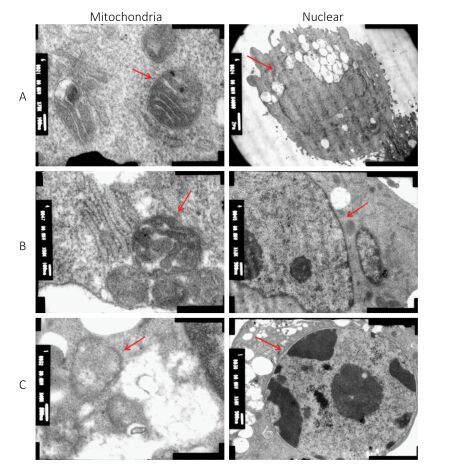
The oxidative stress induced by HBCD in HBL-100 cells was analyzed by measuring ROS content. As shown in Figure 1B, the production of ROS was promoted significantly (P < 0.05) only at 50 mg/L HBCD for an increase of 9% compared with that of the control value. However, LDH release was significantly increased in all exposure groups and presented a concentration-dependent trend (Figure 1C), which indicated that HBCD produced cytotoxic injury to HBL-100 cells by damaging membranes. Moreover, HBCD induced a dose-dependent effect on the membranes and organelles of HBL-100 cells (Figure S1, available in www.besjournal.com). This result was corroborated by TEM assay. When the concentration of HBCD reached 50 mg/L, the mitochondrial cristae degenerated badly and even disappeared, and the mitochondria became small cavities. The nuclei membranes were severely damaged, with chromatin deeply stained and karyopyknosis.
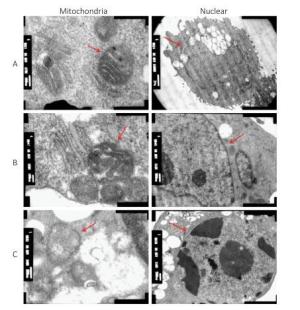
Figure Figure S1. Cytotoxicity of HBCD determined by transmission electron microscopy assay. (A), (B), and (C) Morphologies of the mitochondria and nuclei of HBL-100 cells exposed to 0, 5, and 50 mg/L HBCD for 24 h, respectively.
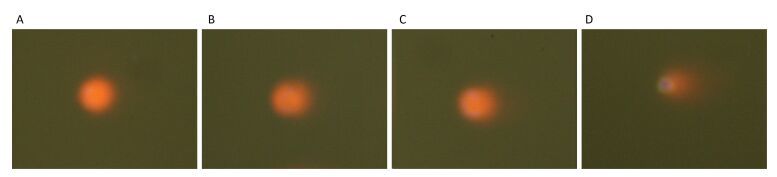
Comet assay was used to quantify the detriment on DNA, which found the DNA tail was prolonged with HBCD increase (Figure S2, available in www.besjournal.com), thereby indicating a potential genotoxicity of HBCD on HBL-100 cells. CASP1.2.2 was used to analyze Tail-DNA%, which showed a dose-effect changing manner (Figure 1D). Tail-DNA% of cells exposed to 5, 10, and 50 mg/L HBCD were promoted by 2.7, 3.2, and 6 times compared with that of the control, respectively, but the significant difference was only observed at 50 mg/L HBCD. It demonstrated that DNA was a major target of HBCD induced oxidative stress. Oxidative base damage is highly mutagenic and, if unrepaired, might increase the likelihood of cancer development. Therefore, DNA damage response plays an important role in the maintenance of genome integrity[15].

Figure Figure S2. Genotoxicity of HBCD determined by the comet assay. (A), (B), (C), and (D) DNA damages to HBL-100 cells exposed to HBCD with concentrations of 0, 5, 10, 50 mg/L for 24 h, respectively.
This research investigated the expression levels of four related genes and attempted to further reveal the regulation and repair mechanisms of HBL-100 cells in response to HBCD. The relative expression levels of the target genes were shown in Table 1. The expression of ATM was significantly promoted by HBCD with a dose-dependent manner. The expression of BRCA1 changed in a similar trend with hOGG1 and hMTH1, as up-regulated at lower dose of HBCD but down-regulated at higher dose.
Genes qRT-PCR Analysis Results control 5 mg/L 10 mg/L 50 mg/L NM_007295 (homo-BRCA1) 1 2.896 ± 0.176 4.108 ± 0.791** 0.796 ± 0.126 NM_000051.3 (homo-ATM) 1 2.272 ± 0.207 2.659 ± 0.097** 3.738 ± 0.217** NM_002542.5 (homo-hOGG1) 1 0.749 ± 0.043 1.290 ± 0.088** 0.472 ± 0.021** NM_002452 (homo-hMTH1) 1 0.786 ± 0.020 1.097 ± 0.039 0.214 ± 0.021* Note.*and **represent significant (P < 0.05 and P < 0.01) differences compared with the control value. Table 1. Relative expression levels of DNA damage repair genes by qRT-PCR (n = 3)
ATM kinase is a critical regulator in initiating DNA damage response and activating DNA repair. The activated ATM could down-regulate BRCA1 and improved DNA repair genes expression. BRCA1 is a protein involved in the predisposition to breast cancer and required for DNA breaks repair, protein ubiquitination, regulation of transcription, and regulation of cell cycle[9]. In this study, BRCA1 expression was promoted with HBCD increase but reduced only at 50 mg/L HBCD, which exhibited a prognostic of breast cancer to HBL-100 cells. Even if the damaged DNA could be repaired in many pathways, defects of specific DNA repair pathway may arise during tumor development[5]. qRT-PCR results in our study showed that the expression of hOGG1 and hMTH1 mRNA were decreased significantly after exposure to 50 mg/L HBCD (P < 0.01), whichgenetic materials of HBL-100 were in a tremendous risk.
To explain the regulation mechanisms of HBL-100 cells under HBCD exposure, a correlation analysis among ROS, DNA break level and repair genes was conducted to determine their relationship and identify the possible response and regulation pathways in HBL-100 cells.
Table 2 showed that DNA break level had a positive correlation with ROS and ATM (P < 0.01), but had a negative correlation with hOGG1 and hMTH1 (P < 0.05), which indicated ROS was the direct effecting factor for DNA break, with ATM as prognostic factor. Meanwhile, damaged DNA was inhibited by hOGG1 and hMTH1, which was confirmed to be a DNA repair pathway in HBL-100 cells. Moreover, hOGG1 and hMTH1 presented very significant positive correlation, which demonstrated their synergies in DNA repair process.
Item ROS ATM BRCA1 hOGG1 hMTH1 DNA Break ROS Pearson correlation - 0.707 -0.046 -0.421 -0.532 0.911 Significance (both side) 0.010** 0.886 0.173 0.075 0.000** ATM Pearson correlation 0.707 - -0.070 -0.467 -0.571 0.785 Significance (both side) 0.010** 0.830 0.126 0.052 0.002** BRCA1 Pearson correlation -0.046 -0.070 - 0.708 0.564 -0.155 Significance (both side) 0.886 0.830 0.010** 0.056 0.631 hOGG1 Pearson correlation -0.421 -0.467 0.708 - 0.927 -0.607 Significance (both side) 0.173 0.126 0.010** 0.000** 0.036* hMTH1 Pearson correlation -0.532 -0.571 0.564 0.927 - -0.669 Significance (both side) 0.075 0.052 0.056 0.000** 0.017* DNA Break Pearson correlation 0.911 0.785 -0.155 -0.607 -0.669 - Significance (both side) 0.000** 0.002** 0.631 0.036* 0.017* Note.*Represent significant correlation (bilateral) at the P < 0.05 level. **Represent significant correlation (bilateral) at the P < 0.01 level. Table 2. Results of the Correlation Analysis
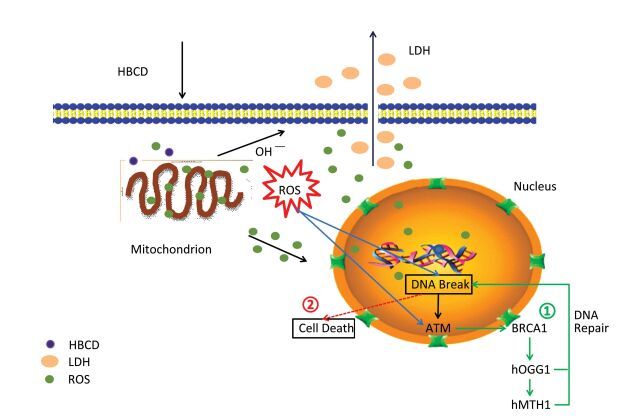
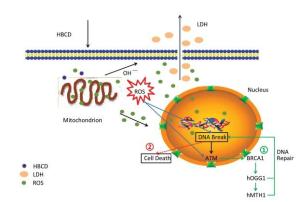
As many regulation pathways existed in cells, the genotoxicity mechanism of HBCD was complicated as well. Usually, two cellular strategies were initiated to response to DNA damage: (1) DNA damage was repaired or tolerated; (2) Cells that harbor DNA damage were removed from the population by death. Non-repaired DNA damage often has harmful consequences that manifest as chromosomal changes, gene mutations, and malignant transformation[10]. To identify the genotoxicity mechanism of HBCD in HBL-100 cells, we present a diagrammatic sketch of the regulation pathways for these factors in response to HBCD. As shown in Figure S3 (available in www.besjournal.com), HBCD induced oxidative stress in two pathways: (1) DNA was broken by the ROS from mitochondrion; the activated ATM down-regulated downstream gene BRCA1 and promoted the expression of hOGG1 and hMTH1. Damaged DNA was repaired or removed. (2) Cells with non-repaired DNA can result to death, that's may attribute to inhibited expression of hOGG1 and hMTH1.
Furthermore, the triggers for cell death and inhibiting hOGG1 and hMTH1 expression were still unknown. With 50 mg/L HBCD exposure, ROS were significantly increased and hOGG1 and hMTH1 decreased sharply, however, there was no obvious correlation among them (P > 0.05). It was indicated that oxidative stress was not the incentive for DNA repair defect. Future research should clarify the mechanisms of DNA repair cease and cell death. Moreover, ROS and the repair genes expression were both correlated to DNA break significantly (P < 0.05). Thus, DNA is such a sensitive factor that can be detected in damage or repair pathways to predict the genotoxicity of environmental pollutants, which can facilitate genotoxins control and disease prevention. This research was expected to reveal the genotoxic mechanism of HBCD on HBL-100. Further studies were needed to put it into environmental pollutants control.
Hexabromocyclododecane-induced Genotoxicity in Cultured Human Breast Cells through DNA Damage
doi: 10.3967/bes2017.039
This research was supported by the National Natural Science Foundation of China No.41406088
The open fund of Key Laboratory for Ecological Environment in Coastal Areas, State Oceanic Administration 201506
- Received Date: 2016-11-03
- Accepted Date: 2017-03-30
| Citation: | LI RuiJing, GAO Hui, NA GuangShui, LU ZiHao, YAO Yao, YANG Fan. Hexabromocyclododecane-induced Genotoxicity in Cultured Human Breast Cells through DNA Damage[J]. Biomedical and Environmental Sciences, 2017, 30(4): 296-300. doi: 10.3967/bes2017.039 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: