-
Patients with type 2 diabetes (T2DM) constitute the largest proportion (> 85%) of all patients with diabetes[1]. In 2035, the number of patients with T2DM is expected to increase to 592 million[2], which has aroused widespread concern about related prevention and treatment.
Weight management, which is a key step in T2DM prevention and treatment, has a great impact on blood glucose control[3]. Failed weight management can lead to poor glycemic control. In addition, metabolic syndrome, a widely recognized risk factor for cardiovascular diseases, is more common among patients with obesity and T2DM than among those with T2DM only[4, 5].
Currently, a new class of incretin-based antidiabetic drugs, including glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and dipeptidyl-peptidase IV inhibitors (DPP-4Is), have been introduced into clinical practice. These incretin-based drugs can effectively lower blood glucose without raising the risk of hypoglycemia. Moreover, they can help control body weight, reduce blood pressure, and alleviate inflammation[6-8]. Glucagon-like peptide-1 (GLP-1) can reduce postprandial blood glucose by stimulating insulin secretion and suppressing glucagon secretion in a glucose-dependent manner[9]. However, GLP-1 in blood will be rapidly inactivated by dipeptidyl peptidase-4 (DPP-4) or cleared by kidney, resulting in the short half-life of GLP-1[10]. Thus, GLP-1 RAs and DPP-4Is were manufactured to solve this problem[11].
To date, several randomized controlled trials (RCTs) have been conducted to explore the effects of incretin-based therapies on weight, body mass index (BMI), and waist circumference (WC) among patients with T2DM[12-15], including the well-known Helping Evaluate Exenatide in Patients with Diabetes Compared with Long-acting Insulin (HEELA) study[16]. The HEELA study reported that exenatide causes less weight gain in patients with overweight and T2DM, compared with long-acting insulin with similar glycemic control efficacy. Rosenstock, et al.[17] discovered that an additional alogliptin treatment in consistent insulin therapy with or without metformin could help achieve better glycemic control in patients with T2DM, while not increasing weight gain. However, almost no RCTs were specially designed for evaluating BMI[18-20] and WC[21-23]. In fact, these parameters were provided as supplementary results in previous studies. Although most of existing RCTs reported that incretin-based agents can lower weight, BMI, or WC, there are still several RCTs with opposite results. Thus, a meta-analysis on these topics is essential. Most of related meta-analyses[24-29] were standard pairwise meta-analyses, although there are several network meta-analyses (NMAs) as well. One study[30] tried to evaluate the effect of antidiabetic drugs added to metformin on body weight, and the original studies included were published before December 2011. Therefore, the results need to be updated, considering the growing number of trials. In 2015, our team published two NMAs in this area[31, 32]. However, the two previous studies only focused on GLP-1 RAs. Additionally, most of previous meta-analyses focused on the effects of GLP-1 RAs on weight. There have been few meta-analyses investigating DPP-4Is as well as outcomes including BMI and WC. Furthermore, the effects of GLP-1 RAs and DPP-4Is on weight-related indicators are expected to be clarified.
This study adopted NMA to overcome the drawbacks of previous studies. Unlike in a standard pairwise meta-analysis, multiple treatments can be compared in a single NMA by combing direct and indirect evidence. The indirect evidence is formed by common comparators [33]. This study also included body weight, BMI, and WC as outcomes to allow an overall analysis of the effects of incretin-based therapies on indicators of overweightness and obesity, thereby providing more evidence and references for clinical decision-making.
-
This study was registered on the International Prospective Register of Systematic Reviews (PROSPERO), number CRD42018115756. This NMA was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) for NMA, and the specific items are provided in Supplementary Files available in www.besjournal.com.
-
Medline, Embase, the Cochrane Library, and clinicaltrials.gov (www.clinicaltrials.gov) were searched from inception to June 23, 2017. RCTs associated with GLP-1 RAs and DPP-4Is were retrieved. The specific search strategy is provided in Supplementary Files.
-
Only RCTs with complete results on the effects of incretin-based therapies (GLP-1 RAs and DPP-4Is) on weight, BMI, or WC compared with other hypoglycemics or placebo were included. We excluded ongoing, unfinished, or suspended trials. Four reviewers (SSW, JY, LG, and FS) assessed the studies in duplicate.
-
The Aggregate Data Drug Information System (ADDIS) v1.16.5 was adopted to collect information regarding trial (author, publication year, sample size, trial duration, and types of intervention and control), population characteristics (age, diabetes duration, background therapy, gender, fasting plasma glucose, and baseline level of HbA1c), reported outcomes (changes in weight, BMI, and WC in each treatment group), and methodology.
We used the risk of bias (ROB) tool recommended by the Cochrane handbook to evaluate the quality of included studies. The items considered are as follows: 1) random sequence generation; 2) allocation concealment; 3) blinding of participants and personnel; 4) blinding of outcome assessment; 5) complete outcome data; 6) selective reporting; 7) company funding. The possible answers to items 1-5 are ‘yes’ (representing low risk), ‘no’ (representing high risk), or ‘unclear’ (representing unclear risk). For item 6, ‘yes’ represented high risk, ‘no’ represented low risk, and ‘unclear’ represented unclear risk. Furthermore, grading of recommendation assessment, development, and evaluation (GRADE), which includes five aspects (study limitation, indirectness, inconsistency, imprecision, and publication bias), was utilized to evaluate the quality of evidence contributing to each comparison and the overall ranking of treatment[34].
Data extraction was conducted by four investigators (SSW, JY, LG, and FS) in duplicate.
-
The DerSimonian-Laird random effects model was utilized to carry out standard pairwise meta-analysis. Weighted mean differences (WMDs) of the three outcomes with 95% confidence intervals (CIs) were computed for measuring effects. I2 statistic reflects the proportion of between-study heterogeneity in the overall variation.
-
A random-effects NMA within a frequentist framework[35] was performed to achieve the combined results in the form of WMDs with 95% CIs. To obtain a treatment hierarchy, we used surface under the cumulative ranking curve (SUCRA)[36] and mean ranks. SUCRA is a percentage indicating the probability of a treatment’s effectiveness ranking first without uncertainty. In this study, it is equivalent to 1 if the treatment is certain to be the best and 0 if it is certain to be the worst. The larger the SUCRA is, the lower the rank is. Subgroup analysis (grouping by age, years of T2DM, hemoglobin A1c level (HbA1c%), trial duration, sample size, and sponsorship), sensitivity analysis (by excluding studies with no allocation concealment or studies with a sample size of less than 50), as well as univariate and multivariate meta-regressions (by age, HbA1c%, and years of T2DM) were carried out. In NMA, sensitivity analysis is used to test the robustness of results by excluding studies that may bring inconsistency[33], which is different from traditional sensitivity analysis (leaving one study out at each time). Given that NMA involves multiple treatment comparison, traditional sensitivity analysis is not applicable in NMA, but it can be conducted in standard pairwise meta-analysis. In univariate meta-regressions, every variate is added to the model separately each time, whereas in multivariate meta-regression, all variates are added to the model at one time. We conducted NMA on the condition that direct and indirect comparisons were sufficiently similar. We detected the existence of inconsistency locally in all triangular or quadratic loops in the NMA model by the loop-specific approach[37-39]. Discrepancy between the two types of evidence and their 95% CIs were used to detect inconsistency in all loops. We defined inconsistency as a difference between direct and indirect evidence with a 95% CI excluding 0. Additionally, we adopted the node-splitting model[40] to detect a potential inconsistency between direct and indirect evidence. I2 statistic was used to assess the extent of heterogeneity of all direct comparisons in different studies. We analyzed global heterogeneity (using I2 statistic) and global inconsistency (using Q statistic) by using the R 3.5.0 ‘netmeta’ package[41]. Predictive interval plots were also used to evaluate global heterogeneity.
In addition, we made comparison-adjusted funnel plots[42] to assess potential publication bias. If there were no publication biases, scatters of the same color should be distributed symmetrically on both sides of the longitudinal axis.
A simple linear regression line was attached to the funnel plot to make it easier to visually distinguish publication bias between small and large studies.
All statistical analyses were conducted by STATA 14.0 (pairwise meta-analysis, NMA, I2 calculations, estimation of inconsistency, SUCRA graphs, funnel plots, model fit and meta-regressions) and R 3.5.0 (global heterogeneity and global inconsistency).
-
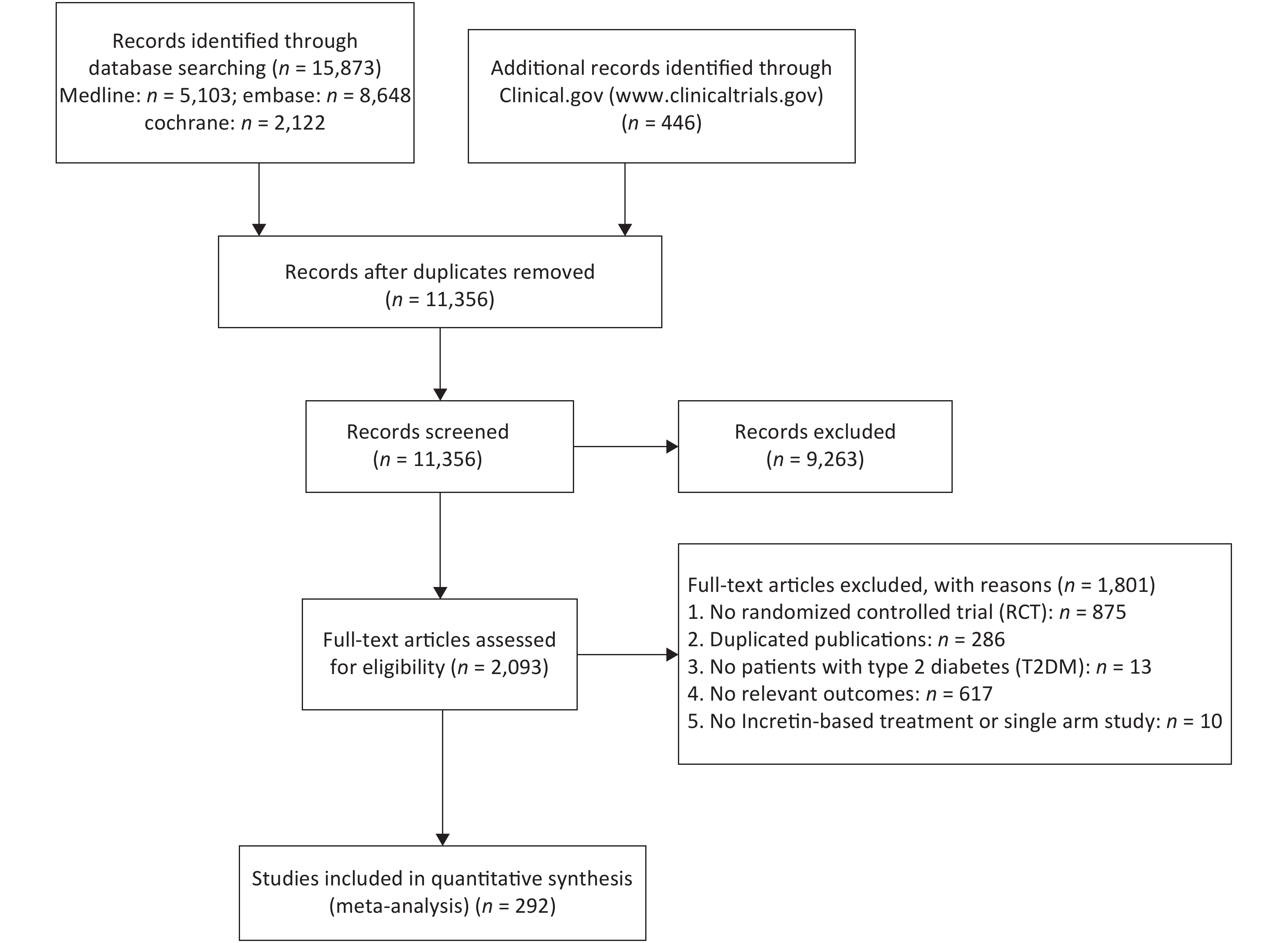
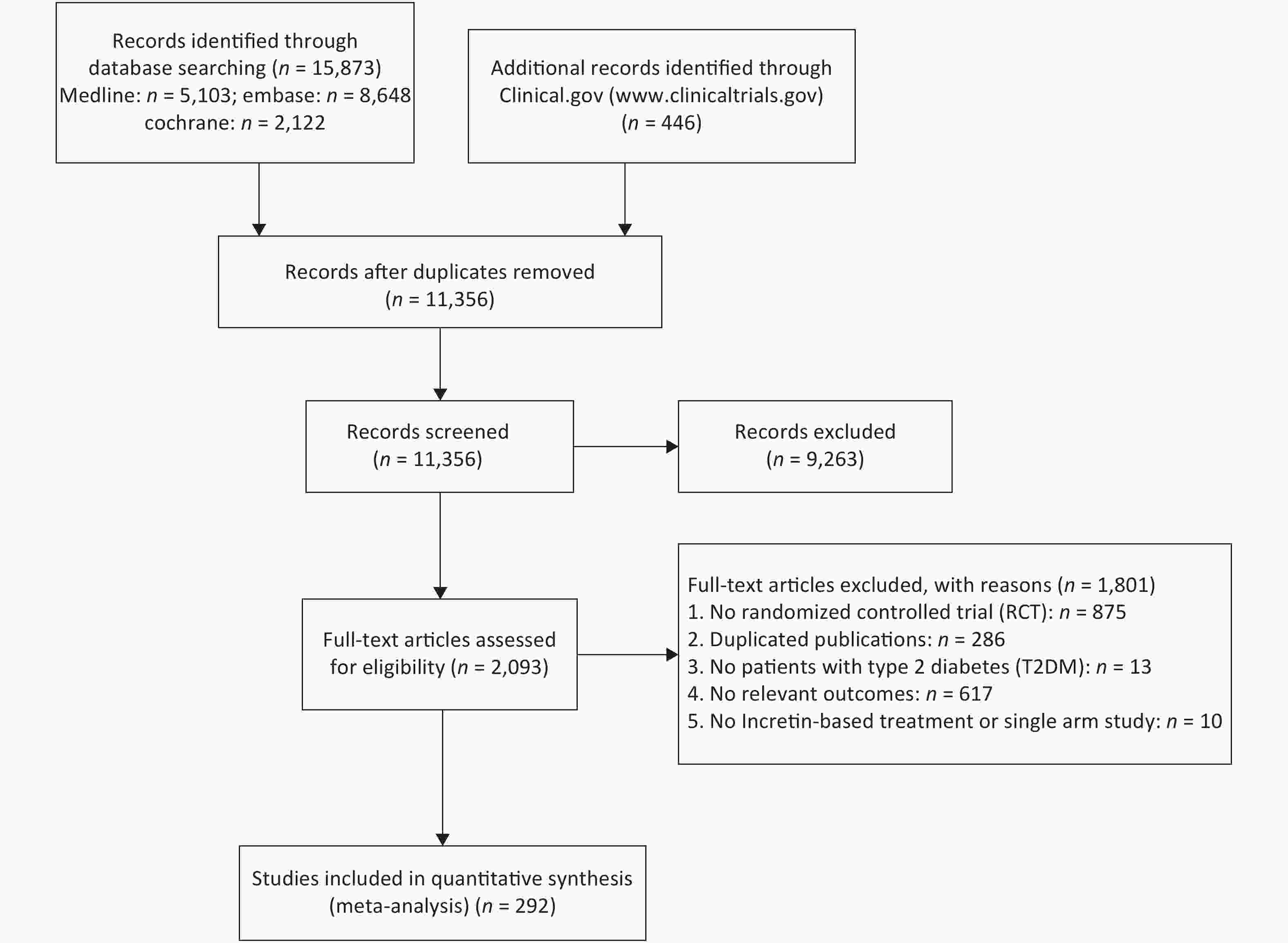
A total of 292 RCTs were selected for this study, 262, 91, and 56 of which were related to weight, BMI, and WC, respectively. The whole inclusion and exclusion processes are shown in Figure 1. All trial durations were longer than 4 weeks except for one study that lasted for 2.4 weeks, with 24 weeks being the longest duration. The mean ages of patients in these trials were between 28.9 and 74.2 years old. The boxplots in Supplementary Files show the distribution of baseline characteristics in these trials.
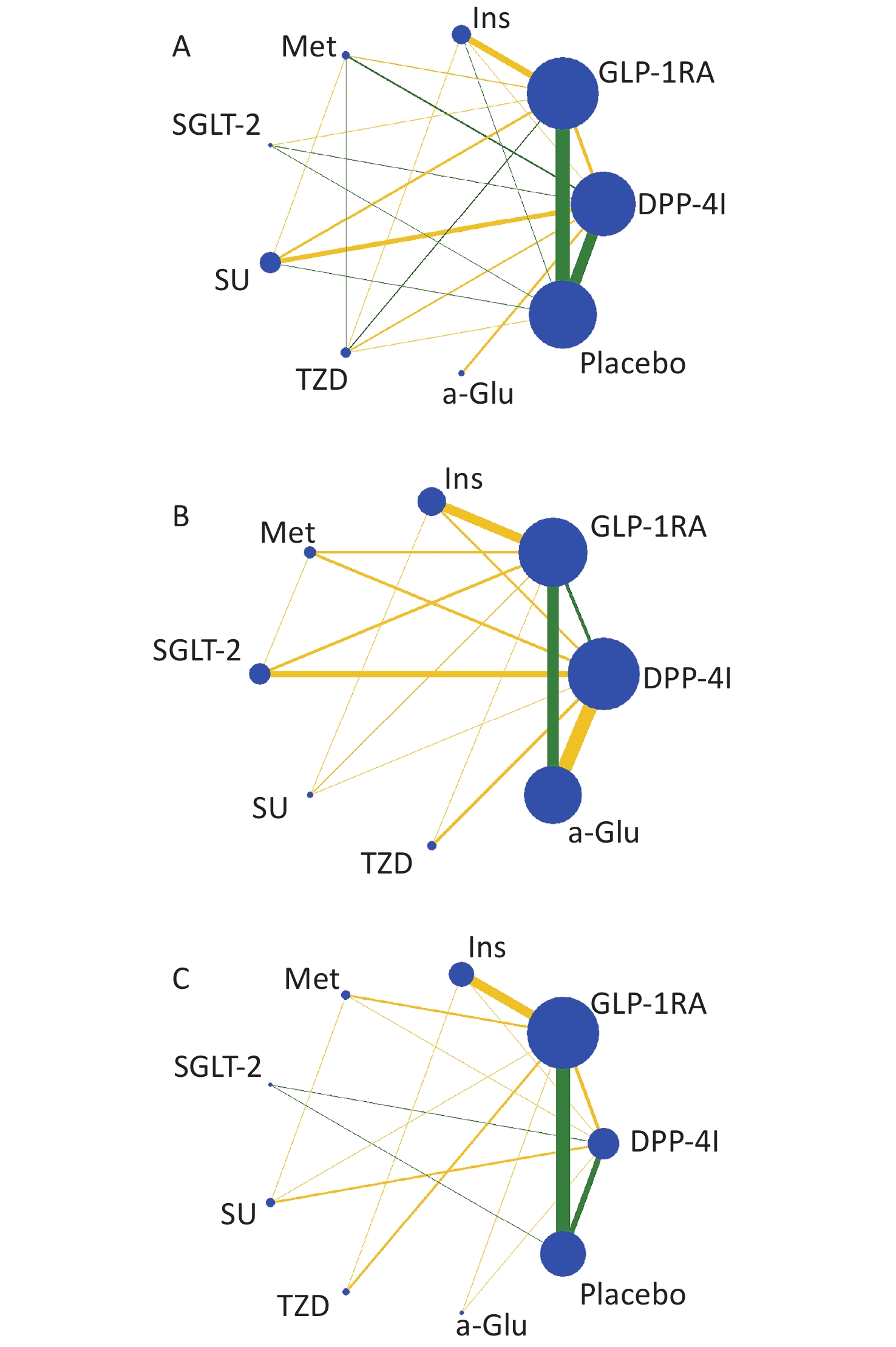
Nine treatments were involved in this study, including GLP-1 RAs, DPP-4Is, insulin, metformin (Met), sodium-dependent glucose transporters 2 (SGLT-2), sulfonylurea (SU), thiazolidinedione (TZD), α-glycosidase inhibitor (a-Glu), and placebo. Most of the studies were two-arm (n = 276), and the others were three-arm (n = 13) and four-arm (n = 2). The plots of evidence structures are provided in Figure 2. According to the contribution plots (Supplementary Files), DPP-4Is versus placebo and GLP-1 RAs versus placebo were the two most contributing direct comparisons in the entire network.
Figure 2. Evidence structure of eligible comparisons for network meta-analysis: weight (A), body mass index (B), and waist circumference (C). Lines connect head-to-head (direct) comparisons in the eligible randomized controlled studies. The width of the lines represents the number of RCTs for each pairwise comparison, and the size of each node is proportional to the number of randomized participants (sample size). The yellow lines represent trials with unclear or high risk of allocation concealment, and the green lines represent low risk.
-
Regarding random sequence generation, 227 studies were at low risk and there was no study at high risk. Regarding allocation concealment, there were 102, 118, and 71 studies at high, low, and unclear risk, respectively. Regarding blinding of participants and personnel, double-blind trials and open-label trials accounted for 46.39% and 35.05% of all studies, respectively. As for blinding of outcome assessment, 102 trials were at high risk and 183 trials were at low risk. A total of 269 trials were at low risk in terms of complete outcome data. In addition, 260 trials were at low risk in terms of selective reporting. Among all trials, 68.38% were sponsored by companies.
-
The results of standard pairwise meta-analysis on weight are shown in Figure 3. Compared with placebo, GLP-1 RAs and SGLT-2 reduced weight by −1.04 kg (95% CI: −1.14, −0.95) and −2.23 kg (95% CI: −2.56, −1.89), respectively. Compared with placebo, traditional hypoglycemic drugs, including insulin, SU, and TZD, increased weight by 2.02 kg (95% CI: 1.02, 3.02), 2.44 kg (95% CI: 1.81, 3.08), and 2.46 kg (95% CI: 1.81, 3.11), respectively. Compared with Met, SGLT-2, and a-Glu, DPP-4Is caused weight gain by 2.68 kg (95% CI: 2.59, 2.76), 2.61 kg (95% CI: 2.30, 2.91), and 0.91 kg (95% CI: 0.71, 1.12), respectively. Compared with other traditional hypoglycemic drugs, including Insulin, SU, and TZD, DPP-4Is significantly decreased weight by −1.61 kg (95% CI: −2.18, −1.04) to −1.44 kg (95% CI: −1.69, −1.19). GLP-1 RAs were observed to reduce weight significantly versus insulin (−3.35 kg, 95% CI: −3.47, −3.24), SU (−3.88 kg, 95% CI: −3.93, −3.84), and TZD (−3.35 kg, 95% CI: −3.63, −3.06). Compared with GLP-1 RAs, DPP-4Is increased weight by 1.72 kg (95% CI: 1.53, 1.92).
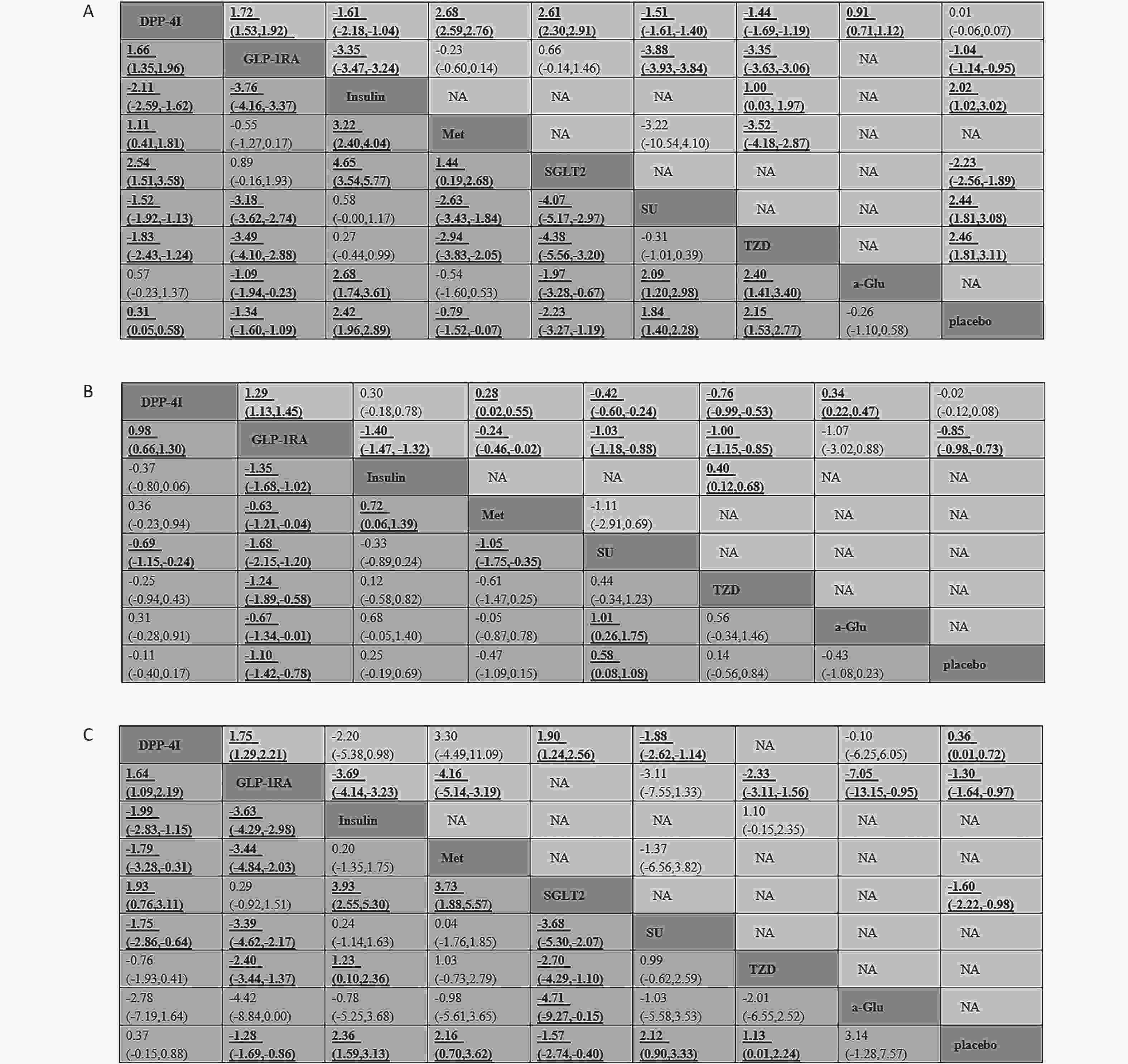
Figure 3. Weighted mean difference with 95% CI of network meta-analysis for weight (A), body mass index (B), and waist circumference (C). Treatments were reported in an alphabetical order. Results of direct comparisons are listed in the upper triangle, and the estimation was calculated as the row-defining treatment compared with the column-defining treatment. Results of network meta-analysis are listed in the lower triangle; the estimation was calculated as the column-defining treatment compared with the row-defining treatment. NA: not available.
-
GLP-1 RAs decreased BMI by –0.85 kg/m2 (95% CI: −0.98, −0.73) compared with placebo (Figure 3). DPP-4Is increased BMI significantly versus Met (0.28 kg/m2, 95% CI: 0.02, 0.55) and a-Glu (0.34 kg/m2, 95% CI: 0.22, 0.47). DPP-4Is reduced BMI, compared with SU (−0.42 kg/m2, 95% CI: −0.60, −0.24) and TZD (−0.76 kg/m2, 95% CI: −0.99, −0.53). Compared with traditional hypoglycemic drugs except a-Glu, GLP-1 RAs effectively reduced BMI by −1.40 kg/m2 (95% CI: −1.47, −1.32) to −0.24 kg/m2 (95% CI: −0.46, −0.02). Compared with GLP-1 RAs, DPP-4Is increased BMI by 1.29 kg/m2 (95% CI: 1.13, 1.45).
-
Compared with placebo, DPP-4Is increased WC by 0.36 cm (95% CI: 0.01, 0.72), whereas GLP-1 RAs and SGLT-2 decreased WC by −1.30 cm (95% CI: −1.64, −0.97) and −1.60 cm (95% CI: −2.22, −0.98), respectively (Figure 3). DPP-4Is increased WC by 1.90 cm (95% CI: 1.24, 2.56) compared with SGLT-2, and decreased WC by −1.88 cm (95% CI: −2.62, −1.14) compared with SU. GLP-1 RAs reduced WC to a greater extent than insulin, Met, TZD, and a-Glu, by −7.05 cm (95% CI: −13.15, −0.95) to −2.33 cm (95% CI: −3.11, −1.56). Compared with GLP-1 RAs, DPP-4Is increased WC by 1.75 cm (95% CI: 1.29, 2.21).
-
Compared with placebo, DPP-4Is increased weight slightly by 0.31 kg (95% CI: 0.05, 0.58) and GLP-1 RAs decreased weight by −1.34 kg (95% CI: −1.60, −1.09) (Figure 3). Compared with placebo, insulin, SU, and TZD induced weight gain of 2.42 kg (95% CI: 1.96, 2.89), 1.84 kg (95% CI: 1.40, 2.28), and 2.15 kg (95% CI: 1.53, 2.77), respectively. Met and SGLT-2 led to weight loss of −0.79 kg (95% CI: −1.52, −0.07) and −2.23 kg (95% CI: −3.27, −1.19), respectively, versus placebo. Compared with insulin, SU, and TZD, DPP-4Is decreased weight by −2.11 kg (95% CI: −2.59, −1.62), −1.52 kg (95% CI: −1.92, −1.13), and −1.83 kg (95% CI: −2.43, −1.24), respectively. Compared with Met and SGLT-2, DPP-4Is increased weight by 1.11 kg (95% CI: 0.41, 1.81) and 2.54 kg (95% CI: 1.51, 3.58), respectively. Compared with traditional hypoglycemic drugs (including insulin, SU, TZD, and a-Glu), GLP-1 RAs resulted in weight loss of -3.76 kg (95% CI: −4.16, −3.37) to −1.09 kg (95% CI: −1.94, −0.23). A statistically significant weight gain was observed after treatment with DPP-4Is compared with that after treatment with GLP-1 RAs, with a mean difference of 1.66 kg (95% CI: 1.35, 1.96).
-
GLP-1 RAs decreased BMI by −1.10 kg/m2 (95% CI: −1.42, −0.78) compared with placebo (Figure 3). Compared with placebo, SU increased BMI by 0.58 kg/m2 (95% CI: 0.08, 1.08). DPP-4Is decreased BMI by −0.69 kg/m2 (95% CI: −1.15, −0.24), compared with SU. Compared with all other traditional hypoglycemic drugs, GLP-1 RAs decreased BMI by −1.68 kg/m2 (95% CI: −2.15, −1.20) to −0.63 kg/m2 (95% CI: −1.21, −0.04). Compared with GLP-1 RAs, DPP-4Is increased BMI by 0.98 kg/m2 (95% CI: 0.66, 1.30).
-
Compared with placebo, GLP-1 RAs and SGLT-2 decreased WC by −1.28 cm (95% CI: −1.69, −0.86) and −1.57 cm (95% CI: −2.74, −0.40), respectively (Figure 3). Insulin, Met, SU, and TZD increased WC by 2.36 cm (95% CI: 1.59, 3.13), 2.16 cm (95% CI: 0.70, 3.62), 2.12 cm (95% CI: 0.90, 3.33), and 1.13 cm (95% CI: 0.01, 2.24), respectively, compared with placebo. Compared with insulin, Met, and SU, DPP-4Is decreased WC by −1.99 cm (95% CI: −2.83, −1.15), −1.79 cm (95% CI: −3.28, −0.31), and −1.75 cm (95% CI: −2.86, −0.64), respectively. DPP-4Is increased WC by 1.93 cm (95% CI: 0.76, 3.11), compared to SGLT-2. Compared with insulin, Met, SU, and TZD, GLP-1 RAs decreased WC more effectively by −3.63 cm (95% CI: −4.29, −2.98) to −2.40 cm (95% CI: −3.44, −1.37). In terms of decreasing WC, DPP-4Is were inferior to GLP-1 RAs, with a mean difference in WC of 1.64 cm (95% CI: 1.09, 2.19).
-
Subgroup analysis showed that DPP-4Is, compared with placebo, did not significantly reduce weight, BMI, and WC in all subgroups, but GLP-1 RAs lowered weight, BMI, and WC compared with placebo in every subgroup. The specific results of subgroup analysis are provided in Supplementary Files. According to the sensitivity analysis, the main results of this NMA were robust, as there were no large differences between the results before and after excluding certain RCTs (Supplementary Files). Based on the univariate meta-regression, it was found that DPP-4Is increased weight by 0.52 kg per 1% HbA1c rise, and that GLP-1RA caused weight loss of 0.08 kg per 1-year change in diabetes duration, compared with placebo. Multivariate meta-regression did not show similar results, but it indicated that, compared with placebo, GLP-1RA increased weight by 0.70 kg per 10-year increase in age. Limited by the number of studies, multivariate meta-regression could not be performed for BMI and WC. Supplementary Files shows all meta-regression results.
-
Table 1 shows that GLP-1 RAs ranked second and DPP-4Is ranked sixth in terms of inducing weight loss. According to Table 1, GLP-1 RAs had the highest efficacy in decreasing BMI and DPP-4Is ranked fourth in terms of efficacy in decreasing BMI. In reducing WC, the efficacy of GLP-1 RAs and DPP-4Is ranked second and fourth, respectively. Ranking results on weight after meta-regression are presented in Supplementary Files.
Treatment Weight Body mass index Waist circumference SUCRA (%) Rank SUCRA (%) Rank SUCRA (%) Rank DPP-4I 38.6 6 53.9 4 60.8 4 GLP-1RAs 87.2 2 99.4 1 91.2 2 Insulin 3.2 9 21.1 7 17.8 9 Met 73.8 3 75.4 2 23.5 7 SGLT-2 99.2 1 / / 95.7 1 SU 22.3 7 4.1 8 24.3 6 TZD 11.9 8 32 6 45.7 5 a-Glu 60.2 4 72.9 3 18.4 8 Placebo 53.6 5 41.2 5 72.6 3 Note. DPP-4I: dipeptidyl-peptidase IV inhibitors; GLP-1RAs: glucagon-like peptide-1 receptor agonists; Met: metformin; SGLT-2: sodium-glucose co-transporter; SU: sulphanylureas; TZD: thiazolidinediones; a-Glu: alpha-glucosidase. SUCRA; surface under the cumulative ranking curve. Table 1. Ranking probability of the effectiveness of different treatments on weight, body mass index, and waist circumference
-
We judged inconsistency by using the data in Supplementary Files. Regarding the studies on weight, the inconsistency test showed that 21 loops from all the 22 loops (including 1 quadratic loop and 21 triangular loops) were consistent (P > 0.05 with 95% CIs including 0). For the studies on BMI, 8 loops from all the 10 triangular loops were consistent. As for WC, 5 loops from all the 9 triangular loops were consistent. The results suggested that, for the three indicators (weight, BMI, and WC), direct estimates of the summary effects were not different from the indirect estimates. The node-splitting model revealed that there were 6, 0, and 2 comparisons with significant inconsistency on weight, BMI, and WC, respectively (Supplementary Files). The results of global inconsistency suggested that the consistency model was no different from the inconsistency model for all three indicators (weight: Q = 27.75, P = 0.479; BMI: Q = 5.26, P = 0.949; WC: Q = 3.79, P = 0.925). According to the predictive interval plots (Supplementary Files) and I2 statistic, global heterogeneity existed in weight (I2 statistic = 91.4%) and BMI (I2 statistic = 84.5%), but not in WC (I2 statistic = 36.7%).
-
In all three comparison-adjusted funnel plots (Supplementary Files), scatters of the same color were almost symmetrical visually, which meant that publication bias was relatively low for weight, BMI, and WC.
-
In light of the GRADE framework (Supplementary Files), the evidence quality ranking of treatments was low, very low, and moderate for weight, BMI, and WC, respectively. For each comparison, the evidence quality rank varied from very low to high, with low- and moderate-quality evidence showing larger proportions.
-
Obesity is a risk factor of diabetes that can incur insulin resistance[43] and cardiovascular diseases[44]. BMI was reported to have a direct relationship with diabetes. Abdominal obesity, which can be measured by WC, is associated with dyslipidemia and hypertension[45]. Patients with obesity and diabetes are prone to worse outcomes; thus, losing weight is considered an effective way to treat diabetes[46]. Body weight, BMI, and WC are all indicators of overweightness or obesity.
Several genres of traditional antidiabetic drugs (including insulin, TZD, and SU) may cause weight gain, which may be caused by ‘defensive snacking’ to deal with hypoglycemia risk[5]. Although, compared with these traditional drugs, incretin-based drugs can achieve similar results in reducing blood glucose, they may have fewer side effects, such as weight gain, by slowing down gastric emptying and inhibiting food intake[47]. Therefore, this study aimed to deeply explore the effects of incretin-based drugs on weight, BMI, and WC.
Albeit there have been several evidence-based studies[24-32] on this topic, most of them were in the form of standard pairwise meta-analysis. Moreover, they mainly discussed body weight.
An NMA published in 2012[30] aimed to evaluate the effects of antidiabetic drugs in combination with metformin on glycemic control, hypoglycemia risk, and body weight. That study found that, compared with placebo, GLP-1 RAs reduced weight by −1.66 kg (95% CI: −2.26, −1.09) and that DPP-4Is had no significant effect on weight loss (0.23 kg, 95% CI: −0.13, 0.60). All the above results were consistent with our results, except that we discovered modest weight gain by 0.31 kg (95% CI: 0.05, 0.58) in DPP-4Is versus placebo. However, that study[30] by Liu et al. was somewhat different from our work. For starters, the study in 2012 only considered the agents in combination with metformin; thus, the study did not involve SGLT-2, but include glinides; moreover, the inclusion criterion of patients was different from ours. Our team also published an NMA on the effects of GLP-1 RAs on body weight in 2015[31]. A total of 51 trials were included in the final analysis in that study; however, 262 trials on weight were included in this study. The GLP-1 RAs concerned in that study were exenatide and liraglutide at varying dosages (exenatide: 5 µg twice daily, 10 µg twice daily, and 2 mg once weekly; liraglutide: 0.6 mg once daily, 0.9 mg once daily, 1.2 mg once daily, and 1.8 mg once daily). Compared with placebo, only exenatide (10 µg twice daily) and liraglutide (1.8 mg once daily) decreased weight by −1.92 kg (95% CI: −2.61, −1.24) and −0.98 kg (95% CI: −1.94, −0.02), respectively.
A previous NMA focusing on how GLP-1 RAs influence WC was also conducted by our team[32]. That study only included 17 RCTs, whereas the present study included 56 RCTs on WC. In the previous study, the GLP-1 RAs studied included exenatide (5 µg twice daily, 10 µg twice daily, and 2 mg once weekly), liraglutide (0.6 mg once daily, 1.2 mg once daily, and 1.8 mg once daily), taspoglutide (10 mg once weekly and 20 mg once weekly), and sitagliptin. The results of the previous study showed that, compared with placebo, exenatide (10 µg twice daily), liraglutide (1.2 mg once daily), liraglutide (1.8 mg once daily), and sitagliptin significantly decreased WC.
Regarding BMI, no meta-analysis on how incretin-based therapies affect BMI had been published. Thus, our study filled this gap.
In this study, comparison of incretin-based therapies with six other traditional hypoglycemic drugs revealed that GLP-1 RAs were not less effective than any other traditional hypoglycemic drug in decreasing weight, BMI, and WC. However, DPP-4Is were not less effective than other traditional hypoglycemic drugs only in decreasing BMI. In inducing weight loss, DPP-4Is were less effective than Met and SGLT2, but more effective than insulin, SU, and TZD. In terms of lowering WC, DPP-4Is were less effective than SGLT2, but more effective than insulin, Met, and SU.
Our study found that GLP-1 RAs were also more effective than DPP-4Is in decreasing all three indicators. Notably, a study reported that liraglutide, a type of GLP-1 RAs, primarily reduces fat mass (especially visceral fat and intrahepatic fat)[48], rather than lean tissue[49] mass, such as skeletal muscles[48]. It was reported that liraglutide reduces more visceral fat tissues than subcutaneous fat tissues[50-53]. Visceral adipose tissue is considered the source of inflammation and promoter of atherosclerosis[54], and it promotes the development of type 2 diabetes[55]. Furthermore, elderly people deserve more attention as they are prone to type 2 diabetes[56]. GLP-1 RAs can help improve cognitive performance in the elderly[57] and protect them from sarcopenia[58]. Thus, GLP-1 RAs can bring great benefits to patients with type 2 diabetes. Our study had several advantages. Firstly, a large number of trials related to incretin-based therapies on weight, BMI, and WC were included, making the evaluation reliable and accurate. Secondly, instead of adopting standard pairwise meta-analysis, we carried out NMA. Because standard pairwise meta-analysis can only combine the results of head-to-head comparisons, it can cause a waste of information in the studies without the direct comparisons that we wanted. However, NMA can achieve indirect comparisons between multiple treatments. In addition, through NMA, multiple treatments can be ranked. There is no doubt that the ranking results can assist clinicians in selecting appropriate treatments in their work.
Nonetheless, there were still several drawbacks in this study. First, the documents included in this study were all published in English; thus, publication bias might exist. Second, most included studies were not especially designed to evaluate the effects of incretin-based therapies on weight, BMI, or WC, raising the potential of inaccurate measurement of the three indicators. Third, we did not consider the variations in products and dosages. Fourth, the placebos varied in the different studies, but this is common in NMA using wide inclusion criteria to gain generalized results[59]. This study reported the effectiveness of incretin-based therapies on weight, BMI, and WC, compared with other traditional therapies and placebo, which will be helpful for future clinical practice. Still, more high-caliber RCTs that emphasize blinding and allocation concealment are being expected to improve the current evidence quality.
-
We are grateful to all cooperating organizations and their staff who have promoted the completion of the whole study. We also sincerely acknowledge all authors of the original studies who provided the materials we needed.
-
The authors declared no conflict of interest.
-
SUN Feng, ZHAN Si Yan, and XU Lu were involved in study conceptualization and design. HUANG Yi and YANG Zhi Rong were involved in the methodology. XU Lu, GAO Le, and YU Shu Qing were responsible for the formal analysis. XU Lu and YU Shu Qing were responsible for writing and preparing the original draft. All authors participated in the writing, review, and editing of the manuscript.
HTML
Search Strategy
Inclusion and Exclusion Criteria
Data Extraction and Quality Evaluation
Statistical Analysis
Standard Pairwise Meta-analysis
Network Meta-analysis
Study Characteristics and Evidence Network
Quality Evaluation
Results of Standard Pairwise Meta-analysis
Weight
BMI
WC
Results of Network Meta-analysis
Weight
BMI
WC
Results of Subgroup Analysis, Sensitivity Analysis, and Meta-regression
Results of Ranking Hierarchy
Results of Inconsistency and Heterogeneity Tests
Detection of Publication Bias
Results of GRADE
 Supplementary_Files.pdf
Supplementary_Files.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: