-
Invasive candidiasis (IC) refers to infections by Candida spp. in the bloodstream (a.k.a. candidemia) or other deep sites of the human body, including the abdominal cavity, bone, brain, eye, heart, kidney, lung, liver and spleen[1]. IC is the most prevalent invasive fungal infection[2] and a leading cause of mycosis-associated mortality with an all-cause mortality rate of 46%–75% and an attributable mortality rate of 10%–49%[1-7]. Several hospital-based studies have reported an increase in IC incidence since the 2000s, indicating clinicians must be alert[8-10].
Currently, a proven diagnosis of IC still relies on routine fungal cultures, which have low sensitivity (ranging from 21% to 71%) and may take 2–3 days on average to detect the infection[1]. However, early diagnosis and antifungal therapy are essential as empirical treatments within 24 hours of septic shock due to candidemia could effectively reduce the mortality rate from 97.6% to 52.8%[11]. As there are no specific clinical symptoms or signs of IC, in order to timely identify the vulnerable population and apply appropriate empirical treatment, a comprehensive grasp of IC is important. However, previous studies mainly focused on either mycological aspects or clinical risk factors of candidemia. Few studies have combined the clinical and mycological aspects and comprehensively summarized both candidemia and deep-seated candidiasis. Furthermore, from a mycological perspective, the distribution of pathogens responsible for IC is ever-changing, as well as the distribution of drug resistance. Though Candida albicans remains to be the most common species worldwide, accounting for approximately 37.2%–61.0% of IC cases[5, 8, 9, 12-14], IC caused by C. non-albicans has become increasingly common in recent decades[2, 5, 15], resulting in increased resistance to azoles. Thus, there is a need to comprehensively summarize the characteristics of IC and continuously monitor the mycological characteristics of IC.
This study aimed to combine the latest mycological and clinical data of IC cases in a tertiary hospital in Beijing, to comprehensively investigate the incidence, population distribution, risk factors and the status of diagnosis and treatment of IC in China.
-
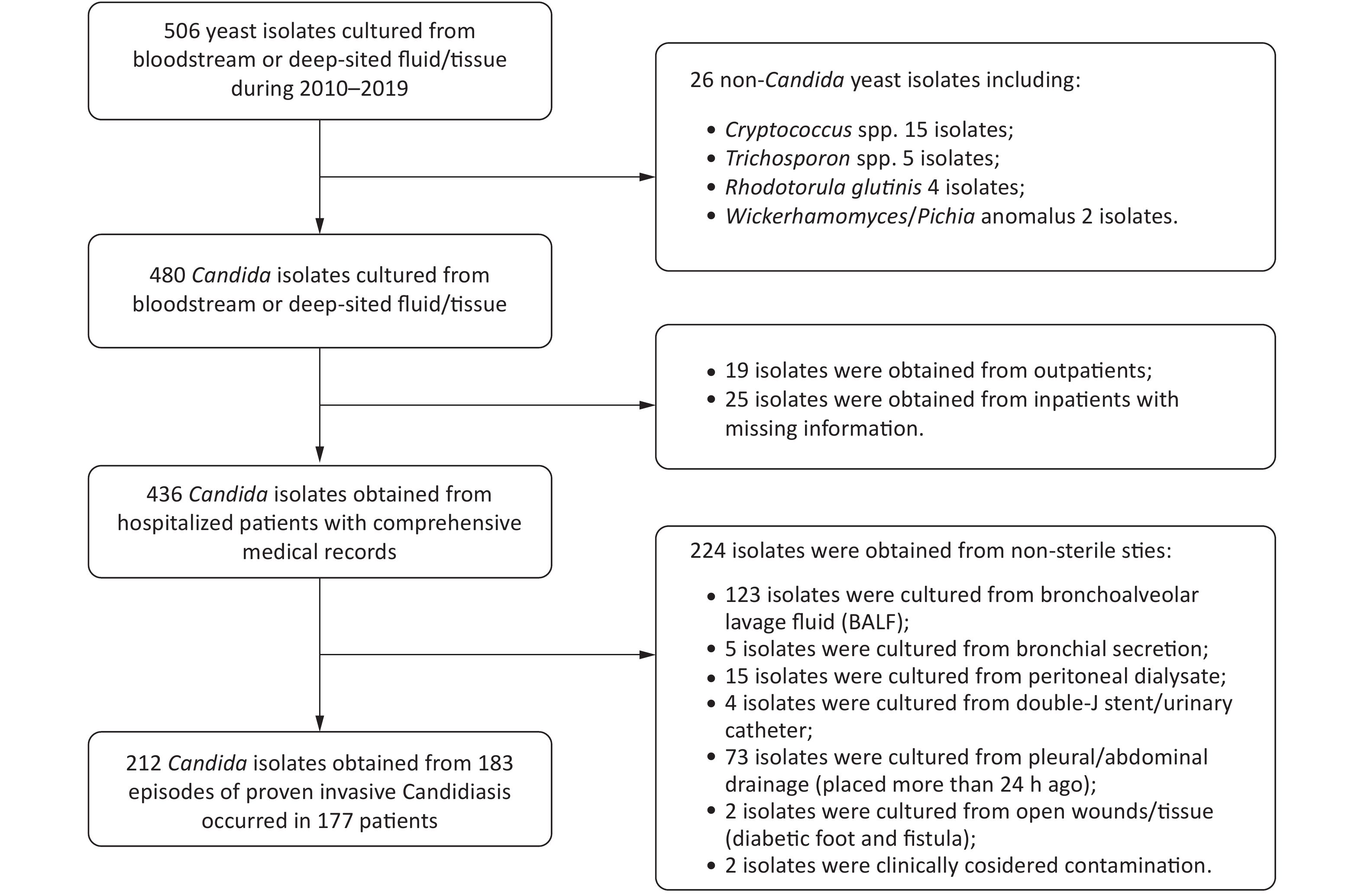
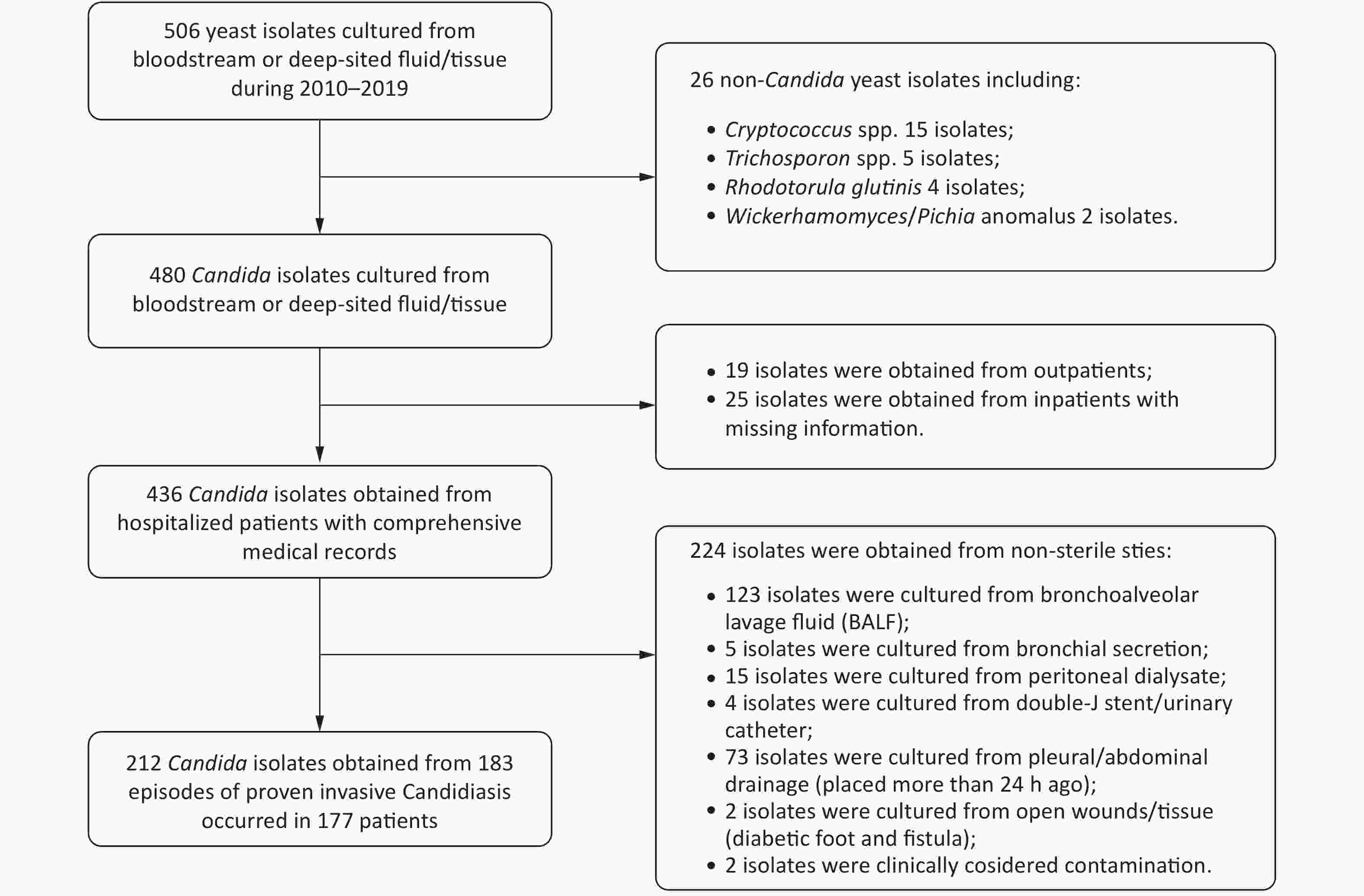
This retrospective study was conducted at Peking University First Hospital, which is a “rank A tertiary” hospital with 64 wards and more than 90,000 admissions per year. All yeast isolates (506 isolates in total) extracted from bloodstream and deep viscera between January 2010 and December 2019 were preserved at the Research Center for Medical Mycology of this hospital. For each corresponding patient of these isolates, medical records were extracted. Cases were included if the diagnosis of IC could be confirmed according to the revised and updated consensus definitions of invasive fungal disease by yeasts published by the European Organization for Research and Treatment of Cancer and the Mycoses Study Group and Research Consortium (EORTC/MSG) in 2019[16]. Specifically, patients were included if they were inpatients with comprehensive medical records who were clinically suspected to have IC and Candida spp. were successfully recovered from normally sterile materials, including blood, central venous catheter (CVC) tips, cerebrospinal fluid (CSF), hydrothorax, ascites, synovial fluid, sterile tissue (e.g., bone) and freshly placed (within 24 hours) drains, which included abdominal drainage fluid, pleural drainage fluid, abscess drainage and bile. In contrast, patients were excluded if they were outpatients with missing records, if they were infected with non-Candida yeast or if Candida spp. were recovered from normally non-sterile materials, such as bronchoalveolar lavage fluid (BALF) and peritoneal dialysate.
Two positive culture results by an identical Candida species within one month or by different Candida species within two weeks were considered as one episode of infection.
-
For each proven IC episode, patient data were collected, including demographic information, hospitalized wards, underlying diseases, other potential risk factors for IC, time from admission to IC onset, time from Candida colonization to onset, infected sites, Candida species, (1,3)-β-D-glucan (BDG) results, antifungal resistance, antifungal therapies and time from onset to death.
-
All potential risk factors for IC existing within 30 days before onset were recorded. Prolonged use of corticosteroids was defined as a mean minimum dose of 0.3 mg/(kg·day) of prednisone equivalent for at least 3 weeks. Broad-spectrum antibiotics were defined as antibiotics covering both Gram-positive and Gram-negative bacteria, mainly including the third- and fourth-generation cephalosporins, carbapenems and quinolones. Exposure to broad-spectrum antibiotics was defined as the use of these drugs within 2 weeks preceding IC onset, while long-term exposure was defined as exposure to these antibiotics for more than 3 weeks continuously. Neutropenia was defined as an absolute neutrophil count lower than 500 cells/μL. Candida colonization was defined as the isolation of a Candida species from at least one unsterile site, including sputum, BALF or tracheal secretions, gastric fluid, urine, stool, swabs from the oropharynx, wound, perineum or catheter insertion sites, and drained fluids collected from catheters placed more than 24 hours[17]. As colonization was only monitored in patients with clinical symptoms, colonization would be considered as none if the test was not conducted. “Candida Score” was defined as the sum of severe sepsis (2 points), surgery (1 point), total parenteral nutrition (TPN, 1 point) and multifocal colonization (i.e., colonization at more than one site, 1 point). Patients with a Candida score of ≥ 3 are prone to IC[18, 19].
-
All the included isolates were re-identified at the species level by MALDI-TOF-MS using MALDI Biotyper RTC 4.0 software (Bruker Daltonik). Antifungal susceptibility tests were performed using an SYO YO10 panel tray (Thermo Scientific, Cleveland, OH, United States). Minimum inhibitory concentration (MIC) data were determined using the Clinical & Laboratory Standards Institute (CLSI)-approved standard M27-S4, and isolate susceptibility was interpreted mainly according to the updated species-specific CLSI clinical breakpoints or epidemiological cutoff values, as described in the study of Song et al.[20].
-
A descriptive analysis of baseline characteristics was conducted. Categorical baseline characteristics of the patients were presented by number (percentage) and compared using chi-squared tests. Normality of the continuous variables was checked using the Shapiro–Wilk test. Non-normally distributed continuous baseline characteristics were presented by median values (interquartile range, IQR) and compared using Mann–Whitney U tests, while normally distributed continuous characteristics were presented by mean values (± standard deviation) and compared using t-tests. All tests of significance were two-tailed.
Univariable and multivariable logistic regression models were used when determining the risk factors influencing 90-day all-cause mortality. A complete-case analysis was employed as the clinical outcomes of 24 episodes were missing. The multivariable logistic regression models adjusted for variables that were both clinically considered as confounding factors and showed possible association with 90-day mortality in the univariable regression (P < 0.1).
All data analysis was conducted using STATA/IC 15[21]. A P-value lower than 0.05 was considered statistically significant.
-
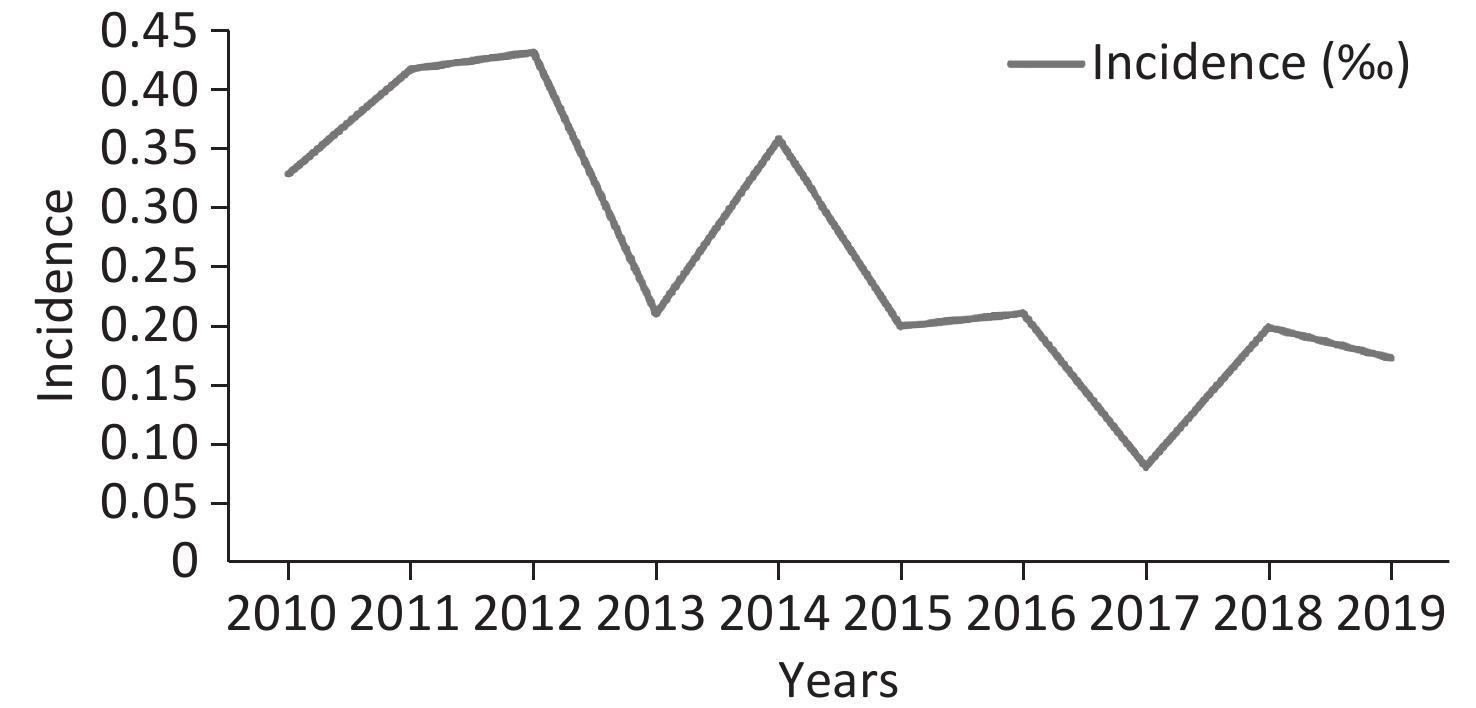
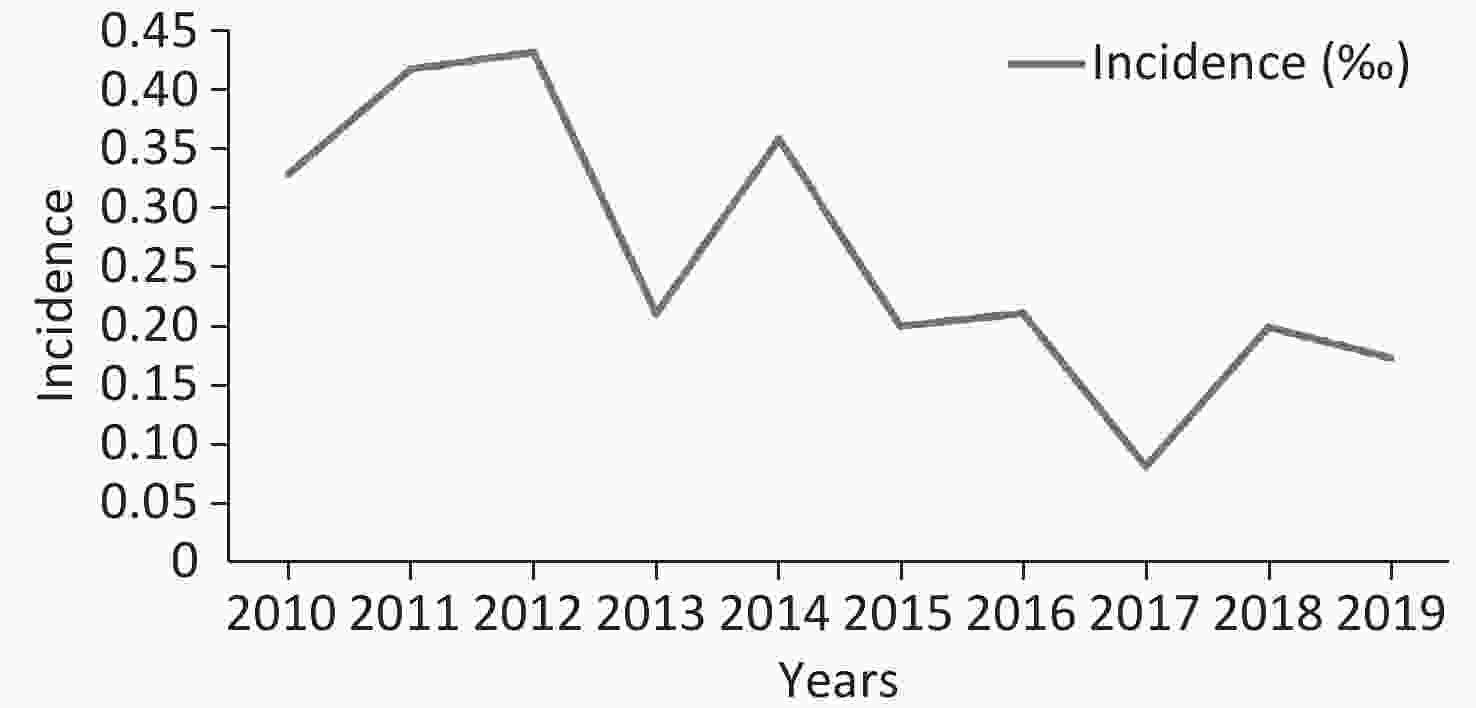
In total, 212 isolates from 183 IC episodes with confirmed diagnosis and comprehensive hospitalized medical records were included in this study (Figure 1). The overall IC incidence in this hospital from 2010–2019 was 0.261 episodes per 1,000 discharges or 0.032 episodes per 1,000 patient days. The incidence of IC ranged from 0.081 per 1,000 discharges (in 2017) to 0.432 per 1,000 discharges (in 2012). The incidence decreased in the recent five years compared to the first five years (P = 0.006, Figure 2).
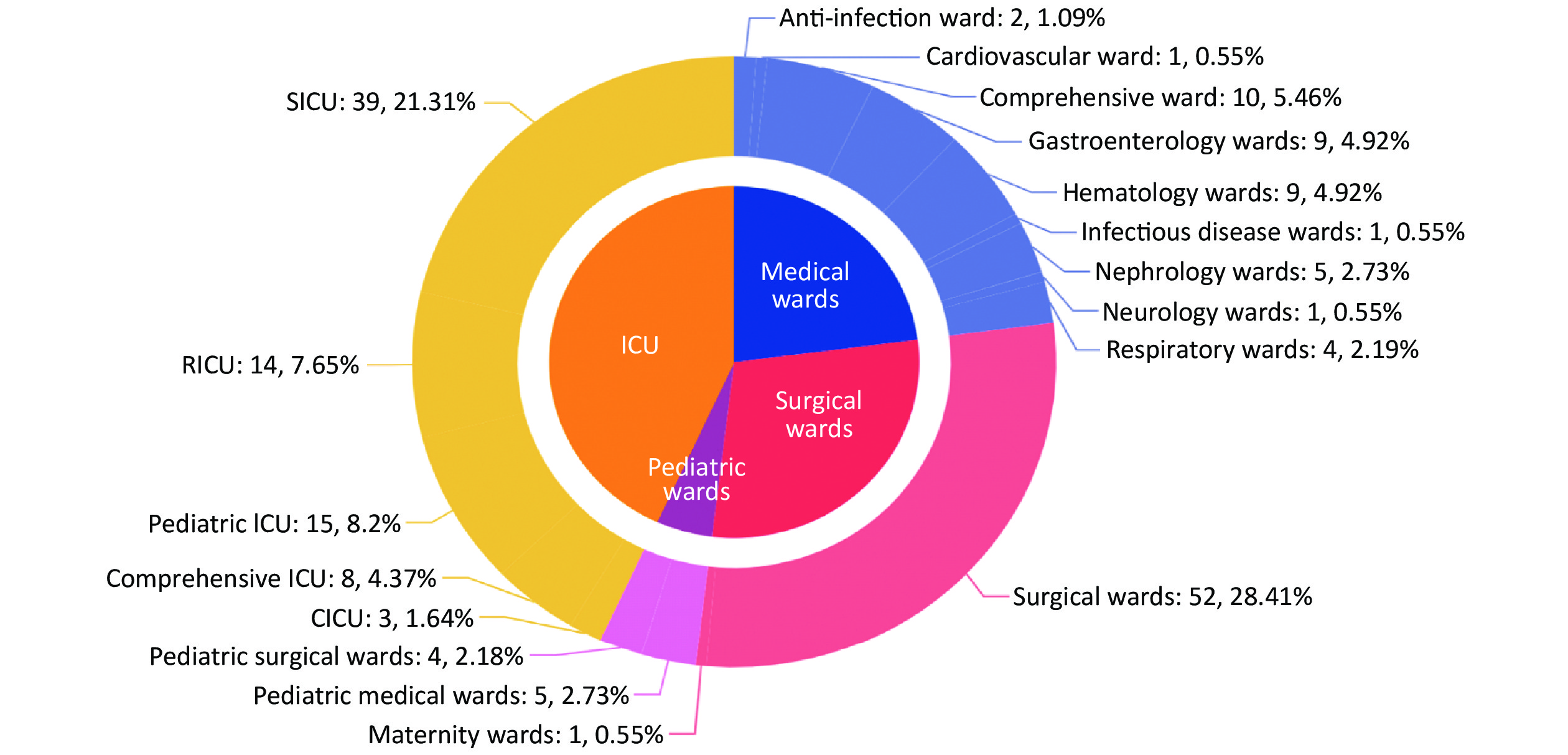
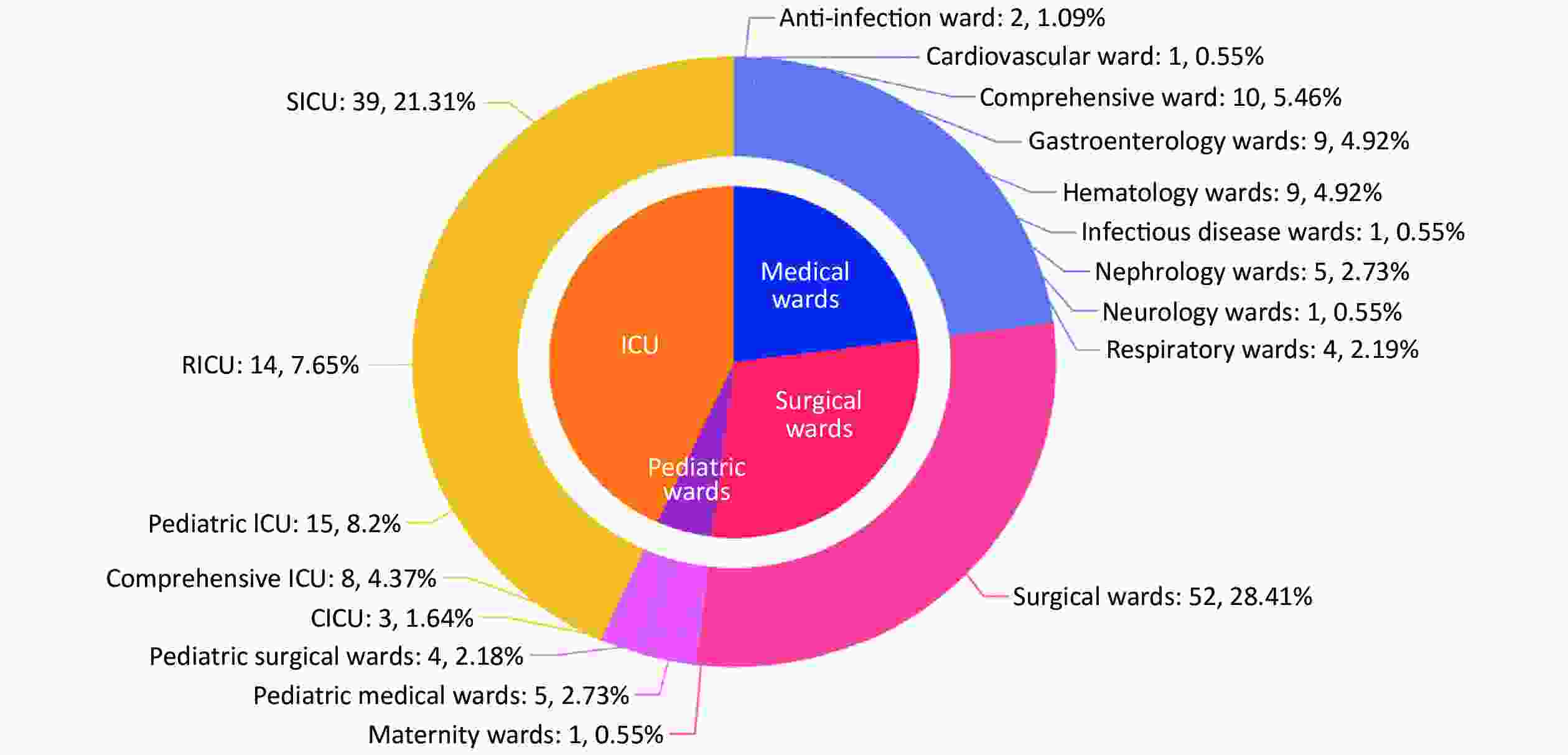
Among the included 183 episodes of IC, 67.8% (124/183) were male and 32.2% (59/183) were female. The median age was 66 years (IQR: 40–78 years, range: 0 days to 96 years). Most patients were ≥ 65 years old (54.1%, 99/183) while 22 patients (12.0%) were ≤ 1 year old. In total, 130 episodes were diagnosed as candidemia, 50 were non-candidemic IC, and three were multi-seated infection with candidemia (Table 1). The infected sites varied among different age groups (Table 1). Blood/CVC was the dominant site of infection for all the age groups. Central nervous system candidiasis mainly affected infants (1 month to 1 year old, P < 0.001) while infection in other fluids (except for CSF) and drains mainly occurred in elderly adults (Table 1). The mortality rate was higher in patients aged ≥ 65 years (37.4%, P = 0.080). Of the IC episodes, 43.2% (79/183) occurred in intensive care units (ICUs), 29.0% (53/183) in surgical wards, 23.0% (42/183) in medical wards and 4.9% (9/183) in pediatric wards (Figure 3). In addition, surgical ICU was the most affected ward among all the ICUs, followed by respiratory ICU. The comprehensive ward, where most patients had a long period of hospitalization, was the most affected ward by IC among all the medical wards.
Site Frequency, n (%)
(n = 183)90-day all-cause mortality
(mortality
rate, %)aAge distribution, n (%) Neonates
(< 1 month,
n = 9)Infants
(1 month to
1 year,
n = 13)Children
(2–17
yeas,
n = 3)Adults
(18–64
years,
n = 59)Senior adults
(> 65
years,
n = 99)P-value Bloodstream related (candidemia) 130 (71.0) 42 (36.2) 9 (100.0) 7 (53.8) 2 (66.7) 41 (69.5) 71 (71.7) 0.226 Blood 88 (48.1) 32 (41.6) CVC tips 4 (2.2) 2 (50.0) Blood+CVC 38 (20.8) 8 (22.9) Newly placed drainsb 29 (15.8) 8 (36.4) 0 (0.0) 0 (0.0) 0 (0.0) 9 (15.3) 20 (20.2) 0.190 Drained abdominal fluids 18 (9.8) 5 (41.6) Drained pleural fluids 2 (1.1) 0 (0.0) Drained bile 4 (2.2) 1 (25.0) Abscess 5 (2.7) 2 (40.0) Puncture fluid from a normally sterile site 19 (10.4) 2 (12.5) 0 (0.0) 6 (46.2) 1 (33.3) 6 (10.2) 6 (6.1) < 0.001*** CSF 8 (4.4) 0 (0.0) 0 (0.0) 6 (46.2) 0 (0.0) 1 (1.7) 1 (1.0) < 0.001*** Ascites 5 (2.7) 1 (50.0) 0 (0.0) 0 (0.0) 1 (33.3) 5 (8.5) 5 (5.1) 0.187 Hydrothorax 4 (2.2) 1 (25.0) Synovial fluids 2 (1.1) 0 (0.0) Bone 2 (1.1) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 1 (1.7) 1 (1.0) 0.975 Multi-seated infection 3 (1.6) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 2 (3.4) 1 (1.0) 0.776 Blood+ascites 1 (0.5) 0 (0.0) Blood+drained abdominal fluids 1 (0.5) 0 (0.0) Blood+synovial fluids 1 (0.5) 0 (0.0) 90-day all-cause mortality (%) − 52 (32.7) 1 (12.5) 2 (16.7) 0 (0.0) 12 (25.0) 37 (37.4) 0.080 Note. Variables are presented as number (percentage, %). aThe total number in this column is 159, which excludes patients with missing mortality data. bAccording to EORTC/MSG, freshly placed (< 24 hours) drains are also regarded as sterile materials. In this study, fresh drains included drained abdominal fluids, pleural fluids, bile and abscess. Abbreviations: CVC: central venous catheter; CSF: cerebrospinal fluid; ***P < 0.001. Table 1. Infected sites, mortality rates and age distributions of the invasive candidiasis episodes in this study
-
Common host factors of IC patients included central venous catheterization (62.1%, 108/174), age ≥ 65 years (56.9%, 99/174), organ failure (56.9%, 99/174), other deep-seated bacterial infection (48.3%, 84/174), major surgery (46.6%, 81/174), long-term use of broad-spectrum antibiotics (42.0%, 73/174), TPN (41.4%, 72/174) and ICU hospitalization (40.2%, 70/174) (Table 2).
Risk factors Candidemia
(n = 124)aNon-candidemic invasive
candidiasis (n = 50)P-value Total (n = 174)b Male 88 (71.0) 30 (60.0) 0.161 118 (67.8) Age 68 (53–80) 66 (42–77) 0.298 67 (51–79) Age ≥ 65 years 72 (58.1) 27 (54.0) 0.624 99 (56.9) Hemoglobin (g/L) 94.2 ± 21.0 103.8 ± 25.5 0.011* 96.9 ± 23.2 Hemoglobin < 80 g/L 29 (23.4) 10 (20.0) 0.628 39 (22.4) Albumin (g/L) 31 (28–35) 31 (28–36) 0.678 31 (28–35) Albumin < 25 g/L 11 (8.9) 3 (6.0) 0.529 14 (8.0) Diabetes mellitus 39 (31.5) 16 (32.0) 0.944 55 (31.6) Diabetic foot 3 (2.4) 1 (2.0) 0.867 4 (2.3) Solid organ malignancies 40 (32.3) 12 (24.0) 0.282 52 (30.0) Lower digestive tract malignancies 13 (10.5) 3 (6.0) 0.354 16 (9.2) Upper digestive tract malignancies 9 (7.3) 2 (4.0) 0.424 11 (6.3) Lung malignancies 5 (4.0) 2 (4.0) 0.992 7 (4.0) Genital system malignancies 4 (3.2) 1 (2.0) 0.661 5 (2.9) Urinary system malignancies 3 (2.4) 0 (0.0) 0.267 3 (1.7) Liver malignancies 2 (1.6) 2 (4.0) 0.342 4 (2.3) Bile malignancies 2 (1.6) 1 (2.0) 0.859 3 (1.7) Pancreas malignancies 2 (1.6) 1 (2.3) 0.859 3 (1.7) Sarcoma malignancies 1 (0.8) 1 (2.3) 0.504 2 (1.1) Bone malignancies 1 (0.8) 0 (0.0) 0.524 1 (0.6) Hematologic malignancies 10 (8.1) 0 (0.0) 0.045* 10 (5.7) ALL 5 (4.0) 0 (0.0) 0.150 5 (2.9) AML 3 (2.4) 0 (0.0) 0.267 3 (1.7) Lymphoma 2 (1.6) 0 (0.0) 0.366 2 (1.1) Sezary syndrome 1 (0.8) 0 (0.0) 0.524 1 (0.6) Bone marrow transplantation 4 (3.2) 0 (0.0) 0.199 4 (2.3) Neutropeniac 12 (9.7) 0 (0.0) 0.023* 12 (6.9) Organ failure 76 (61.3) 23 (46.0) 0.065 99 (56.9) Heart failure 35 (28.2) 15 (30.0) 0.815 50 (28.7) Respiratory failure 53 (42.7) 7 (14.0) < 0.001*** 60 (34.5) Renal failure 38 (30.6) 9 (18.0) 0.089 47 (27.0) Hepatic failure 6 (4.8) 5 (10.0) 0.206 11 (6.3) Infection Other deep-seated bacterial infection 67 (54.0) 17 (34.0) 0.017* 84 (48.3) Mold infection 1 (0.8) 3 (6.0) 0.039* 4 (2.3) Pancreatitis 5 (4.0) 4 (8.0) 0.285 9 (5.2) Cholecystitis 0 (0.0) 2 (4.0) 0.025* 2 (1.1) Ileus 0 (0.0) 2 (4.0) 0.025* 2 (1.1) Digestive tract perforation 1 (0.8) 16 (32.0) < 0.001*** 17 (9.8) Iatrogenic factors Hospitalization (days) 24.5 (17.0–113.5) 3.0 (1.0–20.0) < 0.001*** 19.5 (5.0–72.0) Long-term hospitalization (≥ 90 days) 36 (29.0) 1 (2.0) < 0.001*** 37 (21.3) ICU hospitalization 52 (41.9) 18 (36.0) 0.470 70 (40.2) Hemodialysis 14 (11.3) 0 (0.0) 0.013* 14 (8.0) Use of broad-spectrum antibiotics 113 (91.1) 28 (56.0) < 0.001*** 141 (81.0) Long-term use of broad-spectrum antibiotics (> 3 weeks) 63 (50.8) 10 (20.0) < 0.001*** 73 (42.0) Corticosteroids/immunosuppressants 16 (12.9) 4 (8.0) 0.359 20 (11.5) CVC 93 (75.0) 15 (30.0) < 0.001*** 108 (62.1) Mechanical ventilation 47 (37.9) 6 (12.0) 0.001** 53 (30.5) Candida Score 1.6 ± 1.2 1.0 ± 0.8 < 0.001*** 1.4 ± 1.1 Sepsis 37 (29.8) 3 (6.0) 0.001** 40 (23.0) Surgery 52 (41.9) 29 (58.0) 0.055 81 (46.6) Gastrointestinal surgery 37 (29.8) 23 (46.0) 0.042* 60 (34.5) Pancreatic fistula 1 (0.8) 1 (2.0) 0.504 2 (1.1) Biliary fistula 1 (0.8) 0 (0.0) 0.524 1 (0.6) Intestinal fistula 4 (3.2) 0 (0.0) 0.199 4 (2.3) TPN 60 (48.4) 12 (24.0) 0.003** 72 (41.4) Multifocal colonization 17 (13.7) 2 (4.0) 0.063 19 (10.9) Note. Normally distributed variables are presented as the mean ± standard deviation and compared using two-tailed Student t-tests; non-normally distributed variables are presented as median (interquartile range) and compared using Mann–Whitney U tests; categorical variables are presented as the patient number (percentage, %) and compared using chi-squared tests. aThis group contains three patients who had infection in multiple sites including blood. bCharacteristics of nine neonate patients are summarized in Supplementary Table S1. cNeutropenia could be caused by myelosuppression after chemotherapy, hematological malignancies and benign hematological disease such as aplastic anemia. Abbreviations: ALL: acute lymphocytic leukemia; AML: acute myelocytic leukemia; CVC: central venous catheter; TPN: total parenteral nutrition; *P < 0.05; **P < 0.01; ***P < 0.001. Table 2. Potential risk factors for non-neonate patients to acquire invasive candidiasis in this study
Specifically, the hemoglobin level was 9.6 g/L lower, the hospitalization was 21.5 days longer and the average Candida Score was 0.6 points higher on average in the candidemia group compared to the non-candidemic IC group (P = 0.011, P < 0.001 and P < 0.001, respectively). In the candidemia group, there were significantly more patients with hematologic malignancies (P = 0.045), neutropenia (P = 0.023), respiratory failure (P < 0.001), other deep-seated bacterial infection (P = 0.017), hemodialysis (P = 0.013), long-term use of broad-spectrum antibiotics (P < 0.001), CVC (P < 0.001), mechanical ventilation (P = 0.001), sepsis (P = 0.001) and TPN (P = 0.003). In contrast, there were significantly more patients with mold infection (P = 0.039), cholecystitis (P = 0.025), ileus (P = 0.025), digestive tract perforation (P < 0.001) and gastrointestinal surgery history (P = 0.042) in the non-candidemic IC group.
Differently, for neonates, the most common host factors were low birth weight (100%), maternal late pregnancy (100%), premature birth (88.9%, 8/9), administration of CVC (100%) and mechanical ventilation (88.9%, 8/9) (Supplementary Table S1, available in www.besjournal.com).
Risk factors Neonates (n = 9) Male 6 (66.7) Age (days) 14.4 ± 9.6 (range: 0.0–28.0) Premature birth 8 (88.89) Perinatal conditions Gestational age (weeks) 31.5 ± 2.4 (range: 29.0–37.0) Birth weight (g) 1528.3 ± 281.9 (range: 1090.0–1920.0) Maternal age (years) 34.7 ± 2.1 (range: 32.0–38.0) Maternal disease Gestational diabetes mellitus 1 (11.1) Infections 1 (11.1) Fetal intrauterine distress 3 (33.3) Premature rapture of fetal membrane 3 (33.3) Hemoglobin (g/L) 131.1 ± 17.1 (range: 107–166) Albumin (g/L) 29.0 ± 4.8 (range: 21–37) Neonatal respiratory distress syndrome 3 (33.3) Hydrocephaly 1 (11.1) Iatrogenic factors Corticosteroids/immunosuppressant 0 (0.0) Use of CVC 9 (100.0) Mechanical ventilation 8 (88.9) Candida Score 1.2 ± 1.2 Sepsis 2 (22.2) Surgery 1 (11.1) TPN 6 (66.7) Multi-focal colonization 0 (0.0) Note. Normally distributed variables are presented with mean ± standard deviation while categorical variables are presented with the patient number (percentage, %). Abbreviations: CVC: Central venous catheter; TPN: Total parenteral nutrition. Table S1. Common risk factors for neonates to acquire candidemia
Candida colonization was another common risk factor for acquiring IC; 74/183 patients had tested for Candida colonization before developing IC, and 70.3% (52/74) of these patients tested positive for colonization. With respect to the number of infection sites, 45.9% (34/74) of the patients had Candida spp. colonized at one unsterile site and 24.3% (18/74) of the patients had multifocal colonization (14 at 2 sites, 3 at 3 sites and 1 at 4 sites). Among the 52 patients with Candida colonization before IC, the median time to develop an invasive infection after colonization was 13.5 (IQR: 4.5–37.0, range: 1.0–255.0) days. Sputum/BALF/tracheal secretions was the most frequent site with Candida colonization (Supplementary Table S2, available in www.besjournal.com). Candidemia was more often found in patients with colonization in urine (P = 0.018), while infection in drains was more often found in patients with colonization in sputum (P = 0.032). Notably, 17 patients acquired IC by a Candida species different from the colonized ones. Among them, five patients who were colonized with C. albicans developed C. glabrata invasive infection afterwards. In addition, five patients had colonization with multiple Candida species before the onset of IC by a single Candida species.
Colonization sites Frequency Sputum/BALF/tracheal secretions 28 Urine 20 Stool 18 Pharynx 4 Skin 1 Drained abdominal fluid (tubes placed for over 24 h) 3 Note. BALF: Bronchoalveolar lavage fluid. Table S2. The unsterile sites detected with Candida colonization
-
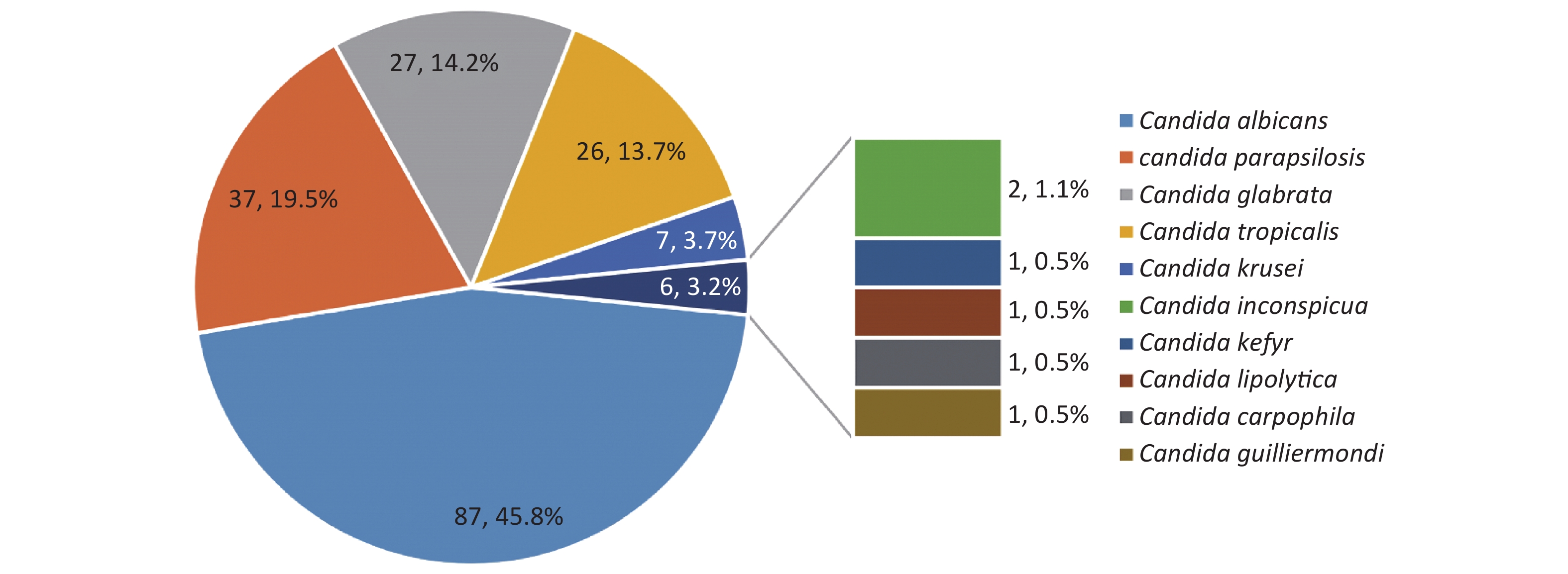
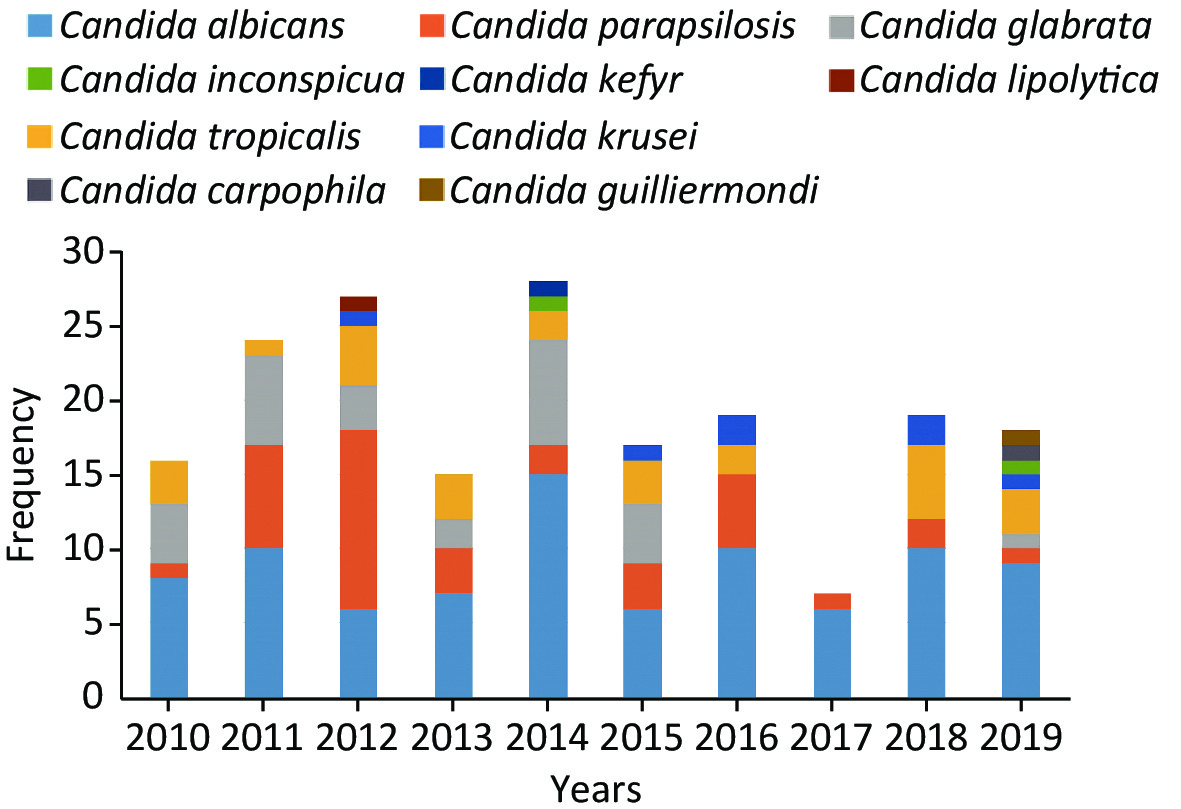
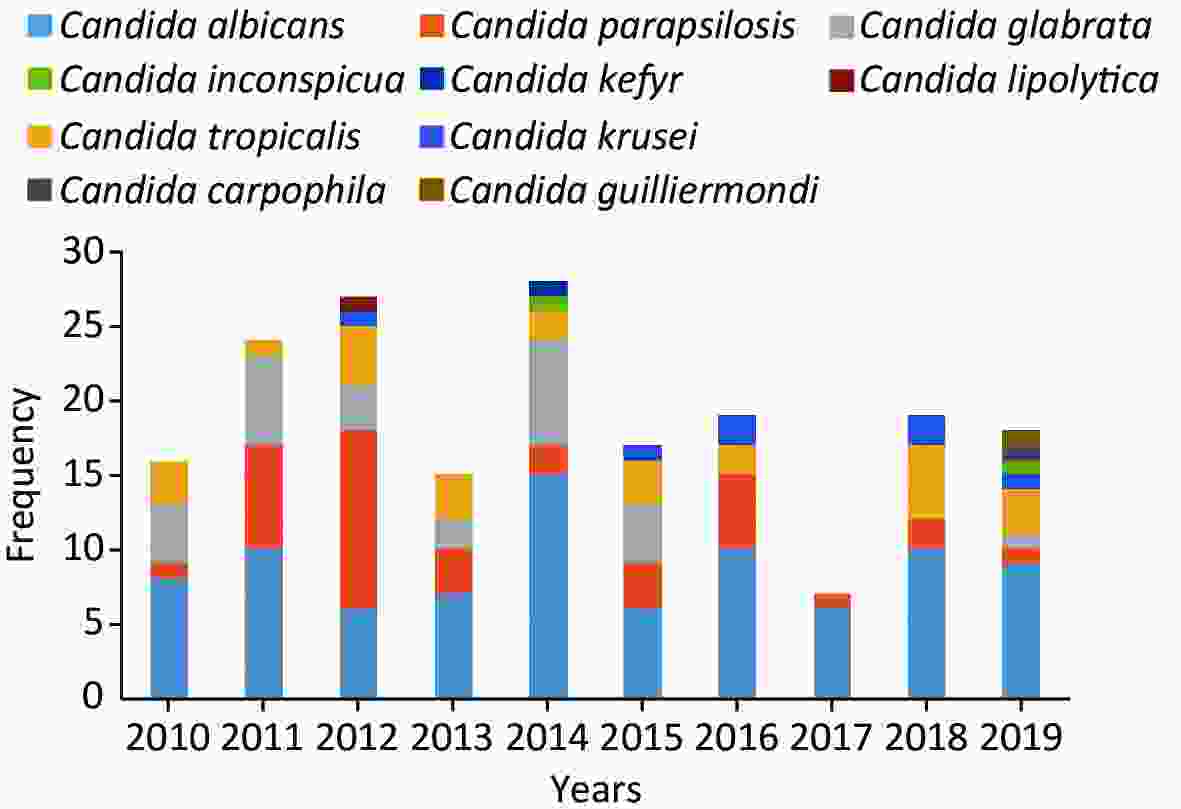
C. albicans was the most prevalent species, contributing to 45.8% of the cases, followed by C. parapsilosis (19.5%), C. glabrata (14.2%), C. tropicalis (13.7%) and C. krusei (3.7%) (Figure 4). Overall, the number of cases caused by C. albicans was similar to the number of cases caused by C. non-albicans except for the years 2012 and 2017. However, the variety of the infected species increased in 2019. C. krusei also became more frequent in the recent five years (Figure 5).
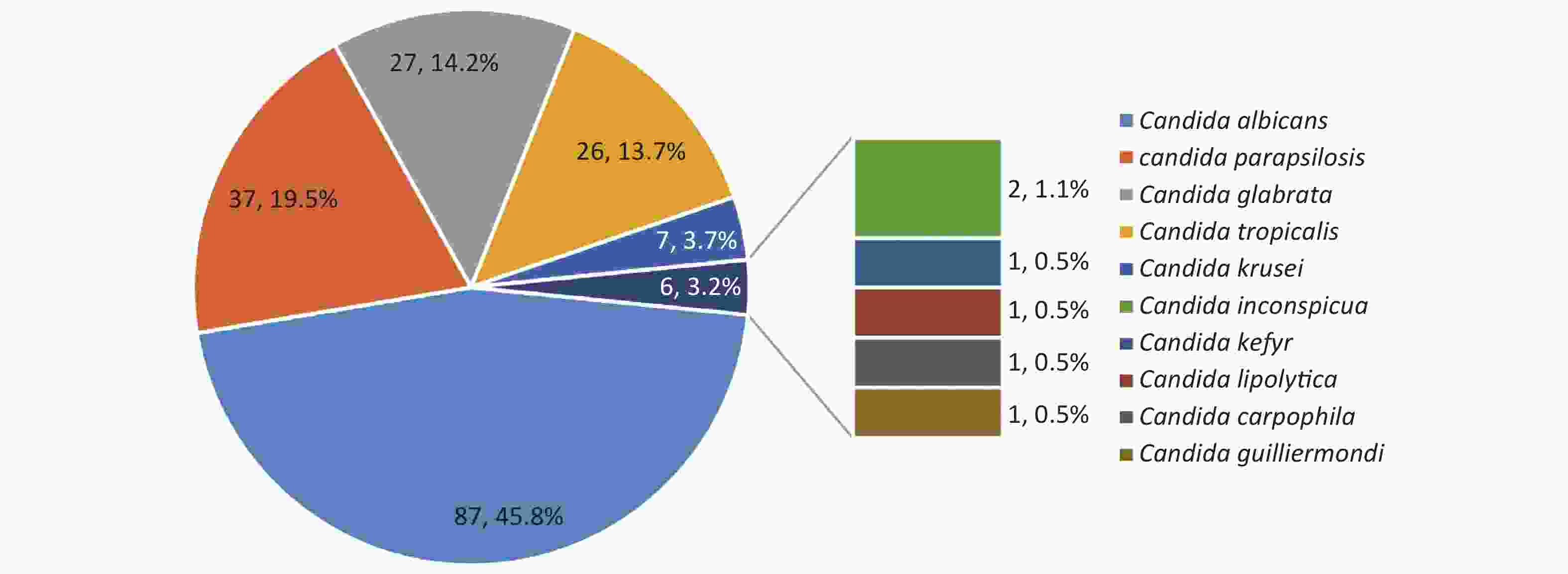
Figure 4. Species distributions of the included isolates. The total number of the included isolates was 190, since seven episodes were infected by mixed species. Four of them were infected by C. albicans + C. glabrata, one by C. albicans + C. tropicalis, one by C. carpophila + C. guilliermondi and one by C. krusei + C. tropicalis.
When comparing cases caused by C. albicans and C. non-albicans, C. non-albicans was more likely to cause candidemia (P = 0.002) while C. albicans was more likely to infect CSF (P = 0.002) and drains (P = 0.022). Patients with C. non-albicans IC had a hemoglobin level that was 6.4 g/L lower on average (P = 0.034) and had stayed in the hospital for a median of 8 days longer (P = 0.011) than those with C. albicans IC. In addition, C. non-albicans IC had a preference in patients with severe anemia (P = 0.018), long-term hospitalization (P = 0.015), hematologic malignancies (P = 0.002), continuous administration of broad-spectrum antibiotics (P < 0.001), mechanical ventilation (P = 0.012) and previous exposure to fluconazole (P < 0.001) and voriconazole (P = 0.001). In contrast, C. albicans tended to infect patients with solid-organ malignancies (P = 0.001) and those undergoing surgery (P = 0.003). No significant difference was observed between the two groups regarding other risk factors listed in Table 3. Differences in basic medical conditions among the five common Candida spp.-caused invasive infections are listed in Supplementary Table S3, available in www.besjournal.com.
Risk factors C. albicans (n = 87) C. non-albicans (n = 96) P-value Male 58 (66.7) 66 (68.8) 0.763 Age 67 (49–81) 65 (38–77) 0.331 Age > 75 years 29 (33.3) 26 (27.1) 0.357 Age < 1 month 8 (9.2) 11 (11.5) 0.616 Hemoglobin (g/L) 102.0 ± 21.9 95.6 ± 24.8 0.034* Hemoglobin < 80 g/L 12 (13.8) 27 (28.1) 0.018* Albumin (g/L) 30 (28–35) 31 (28–35) 0.507 Albumin < 25 g/L 6 (6.9) 9 (9.4) 0.542 Diabetes mellitus 25 (28.7) 30 (31.3) 0.711 Solid organ malignancies 35 (40.2) 17 (17.7) 0.001** Hematologic malignancies 0 (0.0) 10 (10.4) 0.002** Organ failure 47 (54.0) 57 (59.4) 0.465 Heart failure 27 (31.0) 23 (24.0) 0.283 Respiratory failure 25 (28.7) 40 (41.7) 0.068 Renal failure 21 (24.1) 26 (27.1) 0.649 Hepatic failure 6 (6.9) 5 (5.2) 0.631 Other deep-seated bacterial infection 38 (43.7) 49 (51.0) 0.319 Pancreatitis 4 (4.6) 5 (5.2) 0.849 Iatrogenic factors Long-term hospitalization (≥ 90 days) 11 (12.6) 26 (37.5) 0.015* ICU hospitalization 39 (44.8) 40 (41.7) 0.666 Hemodialysis 5 (5.7) 9 (9.4) 0.356 Long-term use of broad-spectrum antibiotics 24 (27.6) 52 (54.2) < 0.001*** Corticosteroids/immunosuppressant 7 (8.0) 13 (13.5) 0.234 CVC 51 (58.6) 66 (68.8) 0.154 Mechanical ventilation 21 (24.1) 40 (41.7) 0.012* Candida Score 1.4 ± 1.2 1.4 ± 1.0 0.283 Sepsis 17 (19.5) 25 (26.0) 0.296 Surgery 49 (56.3) 33 (34.4) 0.003** TPN 40 (46.0) 38 (39.6) 0.382 Multifocal colonization 6 (6.9) 13 (13.5) 0.141 Antifungal drug exposure Fluconazole 3 (3.4) 27 (28.1) < 0.001*** Voriconazole 1 (1.1) 15 (15.6) 0.001** Amphotericin B 1 (1.1) 2 (2.1) 0.619 Echinocandins 2 (2.3) 8 (8.3) 0.073 Sites Blood/CVC 54 (62.1) 79 (82.3) 0.002** Drainage 20 (23.0) 10 (10.4) 0.022* CSF 8 (9.2) 0 (0.0) 0.002** Other puncture fluids 5 (5.7) 8 (8.3) 0.496 Bone 2 (2.3) 0 (0.0) 0.135 90-day all-cause mortalitya 22 (28.9) 31 (37.3) 0.334 Note. Normally distributed variables are presented as the mean ± standard deviation and compared using two-tailed Student t-tests; non-normally distributed variables are presented as the median (interquartile range) and compared using Mann–Whitney U tests; categorical variables are presented as the patient number (percentage, %) and compared using chi-squared tests. Mixed infection with two Candida species was analyzed in both Candida species groups. aMortality data were available in 159 episodes including 76 episodes of C. albicans infection and 83 episodes of C. non-albicans infection. Abbreviations: CVC: central venous catheter; CSF: cerebrospinal fluid; TPN: total parenteral nutrition; *P < 0.05; **P < 0.01; ***P < 0.001. Table 3. Risk factors by common infected Candida species
Risk factors Candida species C. albicans
(n = 87)C. parapsilosis
(n = 37)C. glabrata
(n = 27)C. tropicalis
(n = 26)C. krusei
(n = 7)Others
(n = 6)P-value Male 58 (66.7) 29 (78.4) 15 (55.6) 18 (69.2) 6 (85.7) 4 (66.7) 0.431 Age 67 (49–81) 65 (32–73) 73 (51–88) 64 (39–78) 66 (28–78) 65 (0–70) 0.663 Age > 75 years 29 (33.3) 6 (16.2) 13 (48.1) 8 (30.8) 2 (28.6) 1 (16.7) 0.140 Age < 1 month 8 (9.2) 6 (16.2) 4 (14.8) 0 (0.0) 0 (0.0) 2 (33.3) 0.103 Hemoglobin (g/L) 102.0 ± 21.9 101.3 ± 21.5 94.5 ± 28.6 93.6 ± 21.1 81.4 ± 31.9 86.2 ± 19.2 0.080 Hemoglobin < 80 g/L 12 (13.8) 5 (13.5) 12 (44.4) 9 (34.6) 4 (57.1) 3 (50.0) 0.007** Albumin (g/L) 30 (28–35) 30 (28–35) 30 (27–36) 32 (30–35) 31 (28–35) 28 (21–31) 0.379 Albumin < 25 g/L 6 (6.9) 3 (8.1) 1 (3.7) 3 (11.5) 1 (6.9) 2 (33.3) 0.259 Diabetes mellitus 25 (28.7) 8 (21.6) 15 (55.6) 8 (30.8) 2 (28.6) 0 (0.0) 0.033* Solid organ malignancies 35 (40.2) 7 (18.9) 6 (22.2) 5 (19.2) 1 (14.3) 0 (0.0) 0.031* Hematologic malignancies 0 (0.0) 0 (0.0) 0 (0.0) 7 (26.9) 1 (14.3) 2 (33.3) < 0.001*** Neutropenia 1 (1.1) 0 (0.0) 0 (0.0) 6 (23.1) 3 (4.3) 2 (33.3) < 0.001*** Organ failure 47 (54.0) 23 (62.2) 18 (66.7) 13 (50.0) 7 (100.0) 1 (16.7) 0.042* Heart failure 27 (31.0) 6 (16.2) 10 (37.0) 7 (26.9) 3 (42.9) 1 (16.7) 0.399 Respiratory failure 25 (28.7) 17 (45.9) 16 (59.3) 7 (26.9) 1 (14.3) 1 (16.7) 0.020* Renal failure 21 (24.1) 10 (27.0) 8 (29.6) 6 (23.1) 6 (85.7) 0 (0.0) 0.010* Hepatic failure 6 (6.9) 2 (5.4) 0 (0.0) 2 (7.7) 1 (14.3) 0 (0.0) 0.649 Other deep-seated bacterial infection 38 (43.7) 20 (54.1) 18 (66.7) 13 (50.0) 2 (28.6) 1 (16.7) 0.135 Pancreatitis 4 (4.6) 0 (0.0) 2 (7.4) 2 (7.7) 1 (14.3) 0 (0.0) 0.482 Iatrogenic factors Long-term hospitalization (≥ 90 days) 11 (12.6) 12 (32.44) 9 (33.3) 7 (26.9) 0 (0.0) 0 (0.0) 0.021* ICU hospitalization 39 (44.8) 20 (54.1) 17 (63.0) 3 (11.5) 2 (28.6) 2 (33.3) 0.004** Hematodialysis 5 (5.7) 5 (13.5) 2 (7.4) 4 (15.4) 0 (0.0) 0 (0.0) 0.426 Long-term use of broad spectrum antibiotics 24 (27.6) 22 (59.5) 16 (59.3) 12 (46.2) 2 (28.6) 3 (50.0) 0.006** Corticosteroids/immunosuppressant 7 (8.0) 2 (5.4) 1 (3.7) 7 (26.9) 3 (42.9) 1 (16.7) 0.004** CVC 51 (58.6) 27 (73.0) 18 (66.7) 19 (73.1) 2 (28.6) 5 (83.3) 0.145 Mechanical ventilation 21 (24.1) 17 (45.9) 15 (55.6) 8 (30.8) 0 (0.0) 3 (50.0) 0.006** Candida Score 1.0 (1.0–2.0) 1.5 (0.0–2.0) 1.0 (0.5–2.0) 1.0 (0.0–2.5) 1.0 (0.0–1.0) 0.0 (0.0–3.0) 0.816 Sepsis 17 (19.5) 10 (27.0) 7 (25.9) 9 (34.6) 1 (14.3) 2 (33.3) 0.636 Surgery 49 (56.3) 15 (40.5) 7 (25.9) 9 (34.6) 3 (42.9) 0 (0.0) 0.011* TPN 40 (46.0) 15 (40.5) 15 (55.6) 8 (30.8) 2 (28.6) 2 (33.3) 0.474 Multifocal colonization 6 (6.9) 5 (13.5) 6 (22.2) 2 (7.7) 0 (0.0) 0 (0.0) 0.185 Antifungal-drug exposure Fluconazole 3 (3.4) 10 (27.0) 9 (33.3) 7 (26.9) 0 (0.0) 3 (50.0) < 0.001*** Voriconazole 1 (1.1) 5 (13.5) 3 (11.1) 5 (19.2) 1 (14.3) 1 (16.7) 0.032* Echinocandins 2 (2.3) 3 (8.1) 3 (11.1) 1 (3.8) 1 (14.3) 0 (0.0) 0.351 Amphotericin B 1 (1.1) 0 (0.0) 1 (3.7) 1 (3.8) 0 (0.0) 0 (0.0) 0.769 Sites Blood/CVC 54 (62.1) 34 (92.0) 23 (85.2) 19 (73.1) 4 (57.1) 4 (66.7) 0.011* Drainage 20 (23.0) 1 (2.7) 2 (7.4) 5 (19.2) 3 (42.9) 1 (16.7) 0.025* CSF 8 (9.2) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0.078 Other puncture fluids 5 (5.7) 2 (5.4) 2 (7.4) 4 (15.4) 1 (14.3) 1 (16.7) 0.562 Bone 2 (2.3) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0.793 90-day all-cause mortalitya 22 (28.9) 9 (25.7) 11 (45.8) 9 (40.9) 1 (25.0) 1 (20.0) 0.507 Note. Normally distributed variables are presented with mean±standard deviation and compared using two-tailed student t-tests; Non-normally distributed variables are presented with median (interquartile range) and compared using one-way ANOVA; Categorical variables are presented with the patient number (percentage, %) and compared using chi-squared tests. Other Candida species in this table include Candida inconspicua, Candida kefyr, Candida lipolytica, Candida carpophila, Candida guilliermondii. Mixed infection with two Candida species were analyzed in both Candida species groups, thus the total number of isolates in this table was 190. aMortality data were available in 159 episodes including 76 episodes of C.albicans infection and 83 episodes of C.non-albicans infection. Abbreviations: CVC: Central venous catheter; CSF: Cerebrospinal fluid; TPN: Total parenteral nutrition. *P < 0.05; **P < 0.01; ***P < 0.001. Table S3. Risk factors by common infected Candida species
-
All patients included in the present study were diagnosed by culture according to the study design. The time used to detect fungal colony growth was reported to be 28.0 (IQR: 14.5–38.5) hours in 68 blood samples.
The BDG test was performed in 110 episodes of IC within seven days before or after the date when diagnosis was confirmed by culture. Only 33 (30%) tested positive for BDG. The cutoff value for a positive result in this center was 100 pg/mL. However, the median value of BDG within seven days of confirmed diagnosis was 30.5 (IQR 5.0–197.0) pg/mL.
Among those who tested positive for the BDG test, the median time for BDG to become positive after confirmed diagnosis according to culture was 3 (IQR: 0–12) days. A probable diagnosis was made in ten episodes according to a positive BDG result before successful recovery of a yeast.
-
Drug resistance of the included 212 isolates is summarized in Supplementary Table S4, available in www.besjournal.com. In vitro resistance testing showed that 11.0% (22/200) of C. non-krusei isolates were resistant/non-wild type (non-WT) to fluconazole, 9.6% (20/208) were resistant/non-WT to voriconazole, 3.8% (8/208) were resistant to micafungin, 2.9% (6/208) were resistant to caspofungin, while all of the Candida isolates were WT to Amphotericin B. Fluconazole resistance was significantly more prevalent in C. tropicalis (37.0%, P < 0.001) than other species, and the voriconazole non-WT phenotype was more prevalent in C. glabrata (58.6%, P < 0.001).
Candida spp. (n = 208)a Drug resistance Non-wild type FCZ (%) VCZ (%) AMB (%) MICA (%) CAS (%) FCZ (%) VCZ (%) AMB (%) MICA (%) CAS (%) C. albicans (n = 98) 4 (4.1) 3 (3.1) − 3 (3.1) 1 (1.0) − − 0 (0.0) − − C. parapsilosis (n = 44) 1 (2.3) 0 (0.0) − 0 (0.0) 0 (0.0) − − 0 (0.0) − − C. glabrata (n = 29) 7 (24.1) − − 3 (10.3) 3 (10.3) − 17 (58.6) 0 (0.0) − − C. tropicalis (n = 27) 10 (37.0) 0 (0.0) − 2 (7.4) 2 (7.4) − − 0 (0.0) − − C. krusei (n = 8) IR 0 (0.0) − 0 (0.0) 0 (0.0) IR − 0 (0.0) − − C. guilliermondii (n = 1) − − − 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) − − C. kefyr (n = 1) − − − − − 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) Note. Variables are presented as number (percentage). aFour other isolates are not shown in this table including Candida lipolytica, Candida inconspicua and Candida carpophila, since the reference cut-off value to determine resistance has not been established due to its rarity. Abbreviations: AMB: Amphotericin B; CAS: Caspofungin; FCZ: Fluconazole; IR: Intrinsic resistance; MICA: Micafungin; VCZ: Voriconazole. Table S4. In vitro resistance of the 212 yeast isolates to five antifungal agents in this study
In total, 59 isolates belonged to patients who had previously been exposed to fluconazole with a median of 12 (IQR: 6–26) days before isolation. For the 200 C. non-krusei isolates, the fluconazole-resistant isolates had been exposed to fluconazole for a longer duration than the non-fluconazole-resistant isolates (median/10%–90% percentile range: 0/0–85 days vs. 0/0–16 days, P = 0.002). Furthermore, resistance to voriconazole was significantly associated with longer previous exposure to fluconazole (P = 0.002), and resistance to fluconazole was significantly associated with longer previous exposure to voriconazole as well (P < 0.001). Similarly, resistance to micafungin might also be associated with longer previous exposure to caspofungin (P = 0.053). Further tests confirmed that resistance to fluconazole and voriconazole were mutually associated (P < 0.001), and resistance to micafungin and caspofungin were also mutually associated (P < 0.001).
-
The median time at which systematic antifungal drugs were administered was 1 day (IQR: day −1 to day 3) after confirmed diagnosis. Prophylaxis/empirical/preemptive antifungal drugs were given to 38 patients (20.8%) and targeted antifungal drugs were given to 152 patients (83.1%). The most commonly used drug to initiate antifungal therapy both before and after confirmed diagnosis was fluconazole (52.6% and 54.6%, respectively). Though the main antifungal therapy changed (including the dosage and type of drugs) from the initial ones in 71 cases, the main antifungal drug used during the disease course was still fluconazole 42.1% (64/152) (Table 4). Compared with micafungin, initiating antifungal therapy with fluconazole was associated with better prognosis (P = 0.031, Supplementary Table S5, available in www.besjournal.com). Patients who were singly treated with fluconazole during the disease course also had better prognosis than those who received combination treatment with Amphotericin B and azoles (Supplementary Table S5). Of note, 31 patients did not receive any antifungal drugs and 64.5% (20/31) of them survived after removing the catheters, effective drainage or surgery (details in Supplementary Table S6, available in www.besjournal.com). In this study, neither prophylaxis antifungal treatment in hematologic malignancies nor empirical/preemptive antifungal treatment in the whole population was associated with better survival (P = 0.090 and 0.170, respectively).
Treatment Sites Total
(n = 183)Blood related
(n = 130)Drains
(n = 29)CSF
(n = 8)Other fluids
(n = 11)Bone
(n = 2)Mixed
(n = 3)Prophylaxis therapy 15 (11.5) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 15 (8.2) Empirical therapy 21 (16.2) 4 (13.7) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 25 (13.7) Preemptive therapy 4 (3.1) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 4 (2.2) Initial prophylaxis/empirical treatment 34 (26.2) 4 (13.7) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 38 (20.8) FCZ 20 (58.8) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 20 (52.6) VCZ 9 (26.5) 1 (25.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 10 (26.3) CAS 3 (8.8) 1 (25.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 4 (10.5) MICA 2 (5.9) 2 (50.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 4 (10.5) Targeted therapy 115 (88.5) 19 (65.5) 7 (87.5) 6 (54.5) 2 (100.0) 3 (100.0) 152 (83.1) Initial targeted therapy 115 (88.5) 19 (65.5) 7 (87.5) 6 (54.5) 2 (100.0) 3 (100.0) 152 (83.1) FCZ 67 (58.3) 2 (10.5) 5 (71.4) 5 (83.3) 2 (100.0) 2 (66.7) 83 (54.6) VCZ 10 (8.7) 2 (10.5) 0 (0.0) 1 (16.7) 0 (0.0) 0 (0.0) 13 (8.6) CAS 14 (12.2) 9 (47.4) 0 (0.0) 0 (0.0) 0 (0.0) 1 (33.3) 24 (15.8) MICA 14 (12.2) 5 (26.3) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 19 (12.5) AMB 0 (0.0) 0 (0.0) 2 (28.6) 0 (0.0) 0 (0.0) 0 (0.0) 2 (1.3) LAMB 4 (3.5) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 4 (2.6) CAS+FCZ 0 (0.0) 1 (5.3) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 1 (0.7) CAS+VCZ 3 (2.6) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 3 (2.0) CAS+AMB 1 (0.9) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 1 (0.7) MICA+FCZ 1 (0.9) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 1 (0.7) POS+VCZ+AMB 1 (0.9) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 1 (0.7) Main therapy (71 changes) 115 (88.5) 19 (65.5) 7 (87.5) 6 (54.5) 2 (100.0) 3 (100.0) 152 (83.1) FCZ 51 (44.3) 2 (10.5) 3 (42.9) 5 (83.3) 2 (100.0) 1 (33.3) 64 (42.1) VCZ 15 (13.0) 2 (10.5) 0 (0.0) 1 (16.7) 0 (0.0) 0 (0.0) 18 (11.8) POS 1 (0.9) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 1 (0.7) CAS 16 (13.9) 9 (47.4) 0 (0.0) 0 (0.0) 0 (0.0) 1 (33.3) 26 (17.1) MICA 10 (8.7) 4 (21.1) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 14 (9.2) AMB 0 (0.0) 0 (0.0) 2 (28.6) 0 (0.0) 0 (0.0) 1 (33.3) 3 (2.0) LAMB 7 (6.1) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 7 (4.6) VCZ+FCZ 1 (0.9) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 1 (0.7) POS+VCZ+AMB 1 (0.9) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 1 (0.7) CAS+FCZ 2 (1.7) 1 (5.3) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 3 (2.0) CAS+VCZ 3 (2.6) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 3 (2.0) CAS+AMB 1 (0.9) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 1 (0.7) CAS+LAMB 1 (0.9) 1 (5.3) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 1 (0.7) MICA+FCZ 2 (1.7) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 2 (1.3) MICA+VCZ 4 (3.5) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 4 (2.6) AMB+FCZ 0 (0.0) 0 (0.0) 2 (28.6) 0 (0.0) 0 (0.0) 0 (0.0) 2 (1.3) Note. Variables are presented as frequency (percentage, %). Abbreviations: AMB: Amphotericin B; CAS: caspofungin; FCZ: fluconazole; MICA: micafungin; LAMB: liposomal Amphotericin B; POS: posaconazole; VCZ: voriconazole. Table 4. Prophylaxis/empirical/preemptive and targeted antifungal drugs administered
Risk factors Univariate analysis Multivariate analysis OR (95% CI) P-value OR (95% CI) P-value Male 0.84 (0.41–1.72) 0.632 0.79 (0.26–2.39) 0.674 Age 1.02 (1.01–1.04) 0.001** 1.01 (0.99–1.03) 0.347 Hemoglobin < 80 g/L 0.92 (0.40–2.12) 0.844 0.78 (0.20–3.08) 0.720 Albumin < 25 g/L 1.32 (0.41–4.24) 0.645 1.12 (0.21–5.85) 0.893 Diabetes mellitus 2.00 (0.99–4.06) 0.053 1.76 (0.64–4.81) 0.273 Solid organ malignancies 1.25 (0.60–2.60) 0.543 2.07 (0.62–6.93) 0.239 Hematologic malignancies 1.25 (0.29–5.44) 0.767 44.29 (2.38–825.37) 0.011* Neutropenia 1.03 (0.25–4.29) 0.967 38.31 (2.07–709.75) 0.014* Organ failure 8.86 (3.66–21.44) < 0.001*** 2.62 (0.48–14.27) 0.264 Heart failure 4.63 (2.21–9.67) < 0.001*** 3.62 (1.18–11.10) 0.025* Respiratory failure 6.90 (3.31–14.38) < 0.001*** 7.13 (1.94–26.21) 0.003** Renal failure 4.54 (2.14–9.63) < 0.001*** 2.40 (0.79–7.30) 0.122 Hepatic failure 0.87 (0.22–3.53) 0.851 1.01 (0.15–6.64) 0.994 Other deep-seated bacterial infection 2.13 (1.08–4.22) 0.009** 0.31 (0.09–1.02) 0.054 Pancreatitis 0.68 (0.07–6.70) 0.741 0.14 (0.01–2.70) 0.191 Digestive tract perforation 1.52 (0.46–5.04) 0.494 13.02 (1.46–116.29) 0.022* Iatrogenic factors Long-term hospitalization (≥ 90 days) 1.66 (0.79–3.57) 0.195 0.12 (0.03–0.58) 0.006** ICU 2.02 (1.04–3.97) 0.039* 1.28 (0.44–3.72) 0.656 Hematodialysis 1.37 (0.77–2.42) 0.287 0.48 (0.17–1.18) 0.104 Long-term use of broad-spectrum antibiotics 3.43 (1.71–6.85) < 0.001*** 5.30 (1.55–18.08) 0.008** FCZ exposure 2.49 (1.10–5.60) 0.028* 0.99 (0.23–4.17) 0.989 Corticosteroids/ immunosuppressant 0.91 (0.27–3.10) 0.877 1.76 (0.29–10.83) 0.543 Central venous catheter 0.88 (0.44–1.76) 0.719 0.12 (0.03–0.55) 0.006** Mechanical ventilation 3.05 (1.52–6.10) 0.002** 0.71 (0.19–2.59) 0.602 Candida Score 1.44 (1.08–1.92) 0.013* 1.43 (0.95–2.13) 0.084 Sepsis 2.67 (1.26–5.65) 0.011* 1.25 (0.39–4.00) 0.709 Surgery 0.58 (0.29–1.15) 0.121 1.16 (0.35–3.87) 0.811 Gastrointestinal surgery 0.76 (0.36–1.58) 0.459 1.70 (0.40–7.20) 0.470 TPN 1.89 (0.96–3.71) 0.064 3.30 (1.18–9.22) 0.023* Multifocal colonization 2.28 (0.85–6.14) 0.103 0.77 (0.17–3.40) 0.728 Initial treatment FCZ 1 (reference) 1 (reference) VCZ 0.80 (0.20–3.20) 0.757 1.04 (0.12–9.34) 0.970 CAS 2.98 (1.07–8.31) 0.037* 3.02 (0.47–19.38) 0.243 MICA 3.45 (1.14–10.38) 0.028* 7.36 (1.20–45.02) 0.031* Main treatment FCZ 1 (reference) 1 (reference) VCZ 3.83 (1.11–13.30) 0.034* 2.05 (0.25–17.02) 0.382 AMB 1.10 (0.20–6.05) 0.917 0.52 (0.05–5.20) 0.576 CAS 3.29 (1.09–9.88) 0.034* 0.83 (0.12–5.85) 0.852 MICA 2.46 (0.73–8.31) 0.146 0.52 (0.06–4.70) 0.561 Echinocandins + Azoles 2.74 (0.72–10.34) 0.137 1.60 (0.14–18.35) 0.382 Amphotericin B + Azoles 1.53 (0.13–18.13) 0.734 25.10 (1.47–429.67) 0.026* Note. 159 episodes with mortality data were included in the analysis. Abbreviations: AMB: Amphotericin B; CAS: Caspofungin; FCZ: Fluconazole; ICU: Intensive care units; MICA: Micafungin; OR: Odds ratio; TPN: Total parenteral nutrition; VCZ: Voriconazole; *P < 0.05; **P < 0.01; ***P < 0.001. Table S5. Association between risk factors and 90-day all-cause mortality
Infected sites Survived (n = 20) Died (n = 8) Missing outcome
(n = 3)n comments n comments Blood related
(n = 15)10 4 1 Blood (n = 10) 6 1. One patient acquired candidemia after surgery and recovered after changing a CVC.
2–4. Three patients acquired candidemia after surgery (two of the three received toe amputation for diabetic foot). However, no clinical abnormality was recorded in relation to fungal sepsis.
5. One patient re-admitted for renal abscess 20 days later, but culture for the abscess was negative for Candida species.
6. One patient was considered as acute abdomen induced candidemia and recovered after surgery.3 1–2. Two patients gave up and both died four days later;
3. One patient died on the day when the culture result was obtained one day later.1 CVC (n = 1) 1 1. The infection was hematodialysis-related and was cured by extubation. 0 0 Blood+CVC (n = 4) 3 1–3. Three patients acquired candidemia after surgery and recovered after extubation. 1 1. One patient gave up and died 18 days later; 0 Drains (n = 10) 6 3 1 Drainage after
surgery (n = 5)4 1–2. No clinical abnormality was recorded in these two patients regarding the intra-abdominal IC.
3–4. Two patients with acute abdomen (one perforation and one ileus) recovered after surgery.0 1 Abscess (n = 3) 1 1. One patient with an abdominal abscess recovered after effective drainage. 2 1. One patient with a subcutaneous abscess recovered from antibiotics (Metronidazole) but died from STEMI afterwards.
2. One patient with an abdominal abscess died 12 days later.0 Bile (n = 2) 1 1. The patient was diagnosed as acute cholecystitis and recovered after surgery and drainage. 1 1. The patient died before the culture results came back. 0 CSF (n = 1) 1 1. The original culture of CSF in this patient was negative while enrichment culture was positive for Candida species. Thus, the patient was not considered IC clinically. 0 0 Other fluids (n = 5) 3 1. One patient with infection in synovial fluid recovered after TKA surgery.
2. One patient with encapsulated pleural effusion recovered after effective drainage.
3. One patient with a tracheo-esophageal fistula recovered after drainage.1 1. The patient did not add antifungal agents due to his hepatic failure, and died nine days later. 1 Note. There were 31 patients in this studies who did not receive any systematic antifungal drugs and were not included when comparing the drug effectiveness. The details for these patients, including their infected sites and other local treatment are listed below. Abbreviations: CSF: Cerebrospinal fluid; CVC: Central venous catheter; STEMI: ST-segment elevation myocardial infarction; TKA: Total knee arthroplasty. Table S6. Information for patients who did not receive systematic antifungal drugs
-
In total, 24 patients requested a discharge despite a poor prognosis. Among the 159 left infection episodes, there were 52 deaths (32.7%) within 90 days of diagnosis and 38 deaths (23.9%) were due to IC. The 7-day and 30-day all-cause mortality rates were 10.1% (16/159) and 24.5% (39/159), respectively, and 7-day and 30-day attributable mortality rates were 8.2% (13/159) and 18.9% (30/159), respectively. The median time to attributable mortality after proven diagnosis was 14.0 (IQR: 4.5–34.0, range: 1.0–143.0) days.
More deaths were found in patients infected with C. glabrata (45.8%, P = 0.507, Supplementary Table S3). Univariable logistic regression analysis revealed that more deaths occurred in patients with increased ages [odds ratio (OR) 1.02, P < 0.001], organ failure (OR 8.86, P < 0.001), especially heart failure (OR 4.63, P < 0.001), renal failure (OR 4.54, P < 0.001) and respiratory failure (OR 6.90, P < 0.001), bacterial infection in other sites (OR 2.13, P = 0.009), ICU hospitalization (OR 2.02, P = 0.039), continuous administration of broad-spectrum antibiotics (OR 3.43, P < 0.001), mechanical ventilation (OR 3.05, P = 0.002), previous exposure to fluconazole (OR 2.49, P = 0.028) and higher Candida Scores (OR 1.44, P = 0.013), especially with sepsis (OR 2.67, P = 0.011). After adjusting for confounding factors, risk factors that could significantly increase the risk of 90-day all-cause mortality included hematologic malignancies (OR 44.29, P = 0.011), neutropenia (OR 38.31, P = 0.014), heart failure (OR 3.62, P = 0.025), respiratory failure (OR 7.13, P = 0.003), digestive tract perforation (OR 13.02, P = 0.022), continuous administration of broad-spectrum antibiotics (OR 5.30, P = 0.008) and TPN (OR 3.30, P = 0.023). In contrast, protective factors against mortality included long-term hospitalization (OR 0.12, P = 0.006) and CVC-induced IC (OR 0.12, P = 0.006) (Supplementary Table S5).
-
This was a comprehensive retrospective study including 183 IC cases according to the latest diagnostic guideline by EORTC/MSG[16]. Descriptive analysis, chi-squared tests, Mann–Whitney U tests and t-tests were used to analyze the characteristics of the included patients and Candida species, antifungal susceptibility, status of diagnostic tests, treatment and prognosis. Logistic regression models were employed to investigate the association between risk factors, treatment and prognosis.
The incidence of IC in this study (0.26‰) was low compared to the incidence in previous studies based on hospital settings, which was reported to be 1.9‰–2.4‰ in the US, 1.18‰ in Latin America, 0.92‰–1.19‰ in Europe and 0.13‰–1.22‰ in Asia[8, 13]. In addition, though the incidence of IC was increasing in several centers owing to the increasingly intensive care such as long-term administration of broad-spectrum antibiotics, the ten-year period in this study witnessed an overall decrease in IC incidence. This might be due to the increasing awareness of this disease and the effective prevention of nosocomial infection. Several studies in Asia also reported a decrease in healthcare-associated infection including nosocomial candidemia owing to sequential multifaceted interventions such as hand hygiene, care bundles and antimicrobial stewardships in recent years[7, 14, 22]. In addition, 43.2% (79/183) of the episodes in this study occurred in ICU settings, similar to the global statistics, which was approximately 44.5%–50.0%[1, 23-25].
Since the diagnosis of IC based on culture takes a median of 2–3 days and has low sensitivity (21%–71%)[1], timely empirical therapy is often required to reduce mortality. In order to predict the possible infected Candida species and timely prescribe empirical antifungal drugs targeting the suspected species, grasping the geographical distribution and clinical characteristics of each Candida species is essential. In this study, C. albicans was the most common species (45.8%) of the isolates, followed by C. parapsilosis (19.5%). This is consistent with many studies in China, including the multicenter Prospective China Survey of Candidiasis (China-SCAN) study and the National China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study, which all reported C. parapsilosis (19.8%–27.8%) to be the second common species, following C. albicans[9, 12, 14, 26, 27]. There were also single-center studies in China reporting C. tropicalis (18.0%) and C. glabrata (43.0%) as the most popular C. non-albicans[7, 8]. Grasping the local prevalence is essential as the Candida spp. distribution varies from center to center. In terms of clinical prevalence, this study found that C. non-albicans more often caused bloodstream infection than infections in other deep sites, which was consistent with previous findings[26, 28]. Specifically, the present study and several previous studies all found that C. parapsilosis had a preference in younger patients without neutropenia or immunosuppressed conditions, while C. glabrata and C. krusei had a preference in aged patients with poorer conditions such as severe anemia, renal disease and ventilator dependence[5, 28]. C. tropicalis and C. krusei were additionally found to be associated with hematologic malignancies and immunosuppression in this study and the study of Horn et al.[28]. Previous exposure to antifungal drugs may also influence the infected species. Prior fluconazole therapy was found to be a risk factor for infection with non-fluconazole-susceptible C. non-albicans[8]. This study further found prior exposure to voriconazole was more common in C. tropicalis infection, and prior exposure to fluconazole was more common in C. glabrata infection. C. krusei candidemia was also reported to be associated with prior use of antifungal agents[28]. All this information, including local Candida spp. prevalence distribution, the infected sites and medical conditions of the patients, is essential, since it can provide clues for the infected Candida species and help clinicians to give targeted prescriptions timely.
In terms of risk factors, abnormal colonization is regarded as the prerequisite of the subsequent IC; 70.3% of the patients in this study had Candida colonization before IC onset. The median time to develop IC after colonization was 13.5 days in this study and was reported to be 7–25 days in non-neutropenic ICU patients[17, 29]. Thus, additional attention should be paid to those with abnormal Candida colonization, especially to those with colonization in more than two sites, heavy colonization in more than one site and colonization in the urine, as these patients have a significantly higher risk of IC[29]. Interestingly, in this study, 23.0% of the patients developed IC by a Candida species different from the colonized Candida species and 6.8% (5/74) were colonized with multiple Candida species. This might be due to the fact that colonization with different Candida species contributes to the total burden of fungi, which eventually causes symptomatic infections[17]. Thus, when choosing antifungal drugs for the suspected infected species in the empirical treatment, the clinicians should be aware that the colonized Candida species may be different from the ones causing invasive infection. In addition, as Candida is a common symbiotic yeast, the positive predictive value of colonization for IC is low (2%–4%), whereas the negative predictive value is high (99%–100%)[29]. Dynamic monitoring of colonization can be labor-intensive and significantly increase the economic burden of patients[30]. Under these circumstances, the Candida Score is an alternative to predict the risk of IC development, as patients with a Candida Score of ≥ 3 are prone to IC (sensitivity 81%, specificity 74%) and would benefit from empirical treatment[18, 19]. This study also found that patients with higher Candida Scores had an increased risk of mortality, and should thus receive more attention. Apart from the Candida Score, hematologic malignancies were also found to be a dominant underlying disease among patients with invasive fungal diseases[2], and this study further found that hematologic malignancies were associated with candidemia. Long hospital stay is another risk factor. Patients in this study averagely developed IC after hospitalization for 19.5 (5.0–72.0) days, in line with the results from another study (22 days)[5].
Culture from blood or sterile sites is currently the golden standard for IC diagnosis. Though a successful recovery of Candida spp. might take 2–3 days[1], detecting fungal growth in blood sample cultures just took a median of 28 hours in this study. Still, empirical treatment should be delivered when waiting for the results of culture if IC is suspected, since timely antifungal therapy within 24 hours can significantly reduce the mortality of candidemia[11].
The serum BDG test is another widely used test to facilitate diagnosis. BDG is a pan-marker for various fungal infections. Previous studies have demonstrated its high sensitivity (64.8%–89.0% at a threshold of 70–80 pg/mL) yet low specificity (56.7%–60.0% at a threshold of 80 pg/mL) in IC diagnosis[31-33]. Though its sensitivity and specificity may vary due to the difference in the threshold chosen and the performance of the diagnostic kits, the results in this study was still disappointing. At the threshold of 100 pg/mL, only 30% of the cases tested positive for BDG within seven days of disease onset. Further investigation during follow-up showed 29.7% of the false negative results were due to an insufficient increase compared to the threshold, and 14.8% were due to lag in the increase. Some studies previously suggested to use the negative results of the BDG test to exclude IC diagnosis and allow discontinuation of the empirical therapy[31, 34, 35]. However, this might delay the preemptive therapy as BDG in this study reacted slowly (averagely 3 days later) and rose insufficiently, which might cause false negative results. Using the decrease in BDG levels to evaluate the treatment response, as suggested in previous studies[36], is also unreliable, since many factors can cause false positive results in the BDG test, such as albumin administration[32]. Thus, the results in this study remind clinicians that the result of the BDG test, either positive or negative, should not be overly relied on, and the diagnostic culture should be combined to determine the diagnosis. Despite these limitations, ten patients in this study received preemptive therapy according to BDG results, indicating BDG could still respond rapidly and guide timely preemptive therapy in certain circumstances. To further improve its diagnostic value, many other markers have been suggested to be combined with BDG, including traditional inflammatory markers (e.g., hsCRP and platelet count)[37] and other fungal antigen (mannan or anti-mannan immunoglobulin M)[33].
In this study, resistant/non-WT phenotypes were mainly observed for fluconazole (11.0%) and voriconazole (9.6%), similar to the worldwide data, in which fluconazole resistance in all Candida spp. ranged from 8.1% to 39.3%, followed by voriconazole ranging from 5.4% to 14.0%, Echinocandins ranging from 1.4% to 2.1% and Amphotericin B ranging from 0% to 1%[7, 8, 12, 26, 27]. Specifically, fluconazole resistance was observed in C. tropicalis (37.0%) and C. glabrata (24.1%) in this study, indicating a higher prevalence than those reported in previous Chinese studies (3.1%–11.6% for C. tropicalis and 12.0%–18.5% for C. glabrata, respectively)[7, 12, 14, 15, 26]. Voriconazole non-WT C. glabrata isolates in this study (58.6%) were also more common compared to previous studies (0%–17.8%)[3, 5, 12, 15, 26, 38, 39]. This might indicate a rise in the resistance to azoles in C. glabrata and C. tropicalis in recent years, which was also observed in previous studies[15, 26]. These findings indicate that clinicians should be cautious to use fluconazole/voriconazole when C. glabrata and C. tropicalis are suspected to be the causative agents. Though overall resistance to Echinocandins is uncommon, C. glabrata (10.3%) and C. tropicalis (7.4%) could develop resistance to Echinocandins in this study. Furthermore, this study found that previous exposure to fluconazole/voriconazole could mutually increase the risk of developing resistance to voriconazole/fluconazole. Exposure to caspofungin could also increase the risk of micafungin resistance. Further tests also confirmed the existence of cross-resistance between fluconazole and voriconazole and between caspofungin and micafungin. All of these indicate the need to perform drug susceptibility tests irrespective of the type of antifungal drug, azoles or Echinocandins, especially when the patient has previous antifungal drug exposure. Amphotericin B is an alternative when multi-resistance is evident, since this study together with most previous studies reported non-resistance to Amphotericin B in all five common Candida species[7, 12, 15, 27].
In terms of treatment, apart from the systematic antifungal therapy, there are two essential interventions contributing to effective infection control: source control and timely administration of systematic antifungal therapy. In the present study, 15 candidemia patients did not receive any systematic antifungal treatment. Five of them recovered after changing CVC or hemodialysis tubes. One acute abdomen induced candidemic patient recovered after surgery. Seven non-candidemic IC patients recovered after surgery or drainage without systematic antifungal therapy. All of these cases demonstrated the importance of effective source control. Previous studies also demonstrated that failure or delay in source control significantly increased the mortality risk (OR 6.78–77.40)[11, 27]. Timely systematic antifungal drug administration, which can significantly reduce mortality, is also essential[11]. Empirical treatment was strongly recommended in ICU hospitalized patients suspected with IC[40]. In this study, more than half of the patients received antifungal treatment within 24 hours, which might contribute to the lower mortality rates. However, we failed to detect any significant association between prophylaxis/empirical treatment and better survival. This might be due to insufficient dosage administered in the prophylaxis/empirical treatment groups. Of those patients who received prophylaxis, 53.5% (8/15) initiated with ≤ 200 mg/d fluconazole/voriconazole, while the recommended dosage is 400 mg/d[40]. Also, 61.9% (13/21) received empirical therapy with fluconazole/voriconazole at a lower dosage than the recommended 400 mg/d.
Currently, Echinocandins are recommended as the first-line therapy in both empirical and targeted therapy of IC, and fluconazole is an alternative only recommended in patients with hemodynamic stability and without fluconazole resistance[1, 40]. However, in this study, the majority of patients received fluconazole as initial therapy and main therapy, even though 27.8% (59/212) of the isolates had exposure to fluconazole before isolation. After adjustment in the multivariable regression models, patients singly treated with fluconazole during the disease course had a better prognosis than those receiving treatment combining Amphotericin B and azoles. This might be due to the fact that patients receiving multiple antifungal drugs usually had more deteriorated conditions and suffered from more drug–drug interactions. In addition, initiating antifungal therapy with fluconazole also led to a better survival outcome compared to micafungin. This might be due to the small proportion of C. krusei and C. glabrata infections included in this study and the relatively low prevalence of resistant/non-WT phenotypes in the isolates. This indicated that fluconazole could still be a cost-effective choice as long as resistance is not evident.
The attributable mortality rate of IC could exceed 70%, while the majority reported it to be 10%–38%[1, 4-7]. Regardless of the evidence of statistical significance, most studies, including the present study, reported lower mortality rates in C. parapsilosis[1, 28] and higher mortality rates in C. glabrata[7, 14, 28]. Particularly, one C. parapsilosis-infected patient enrolled in this study presented with three episodes of IC with the longest duration of 143 days, indicating the relatively weak virulence of C. parapsilosis. In terms of baseline medical condition, the majority of studies reported advanced age, neutropenia, severe sepsis, respiratory and renal failure and iatrogenic factors including ICU hospitalization, mechanical ventilation and dialysis were associated with increased mortality[4, 9, 14, 27, 39], while appropriate empiric antifungal therapy administered within five days and proven catheter-related candidemia were found to be protective factors against early mortality[9]. In this study, we also confirmed the timely removal of contaminated CVC to be a protective factor, and we further found hematologic malignancies, digestive tract perforation, heart failure, long-term administration of broad-spectrum antibiotics and TPN to be risk factors for increased mortality. Due to these differences in the baseline conditions of the included population, the specific mortality in each study differed a lot. The crude and attributable 30-day mortality rates in this study were 24.5% and 18.9%, respectively, lower than those reported in most previous studies. This might be due to the large proportion of C. parapsilosis infections, the high proportion of child patients included and timely systematic antifungal drug administration in this study. In addition, the median time to death after diagnosis was reported to be 14–15 days[4, 14], which was consistent with this study.
There were several limitations in this study. First, as a retrospective study, many tests investigated were not conducted or monitored regularly, including colonization, BDG and culture re-examination, which might influence the determination of infective conditions and cause bias. Particularly, for most patients, the BDG test was not performed on the same day of the disease onset. Thus, the results of the BDG tests performed within the 7 days before and after disease onset were used to calculate its sensitivity, and this might lead to underestimation of its sensitivity. In addition, the mortality outcomes in 13.1% (24/183) of the patients were missing, which might lead to underestimation of the mortality rate, as many patients with missing outcomes requested a discharge regardless of a poor prognosis. Second, due to limited data availability, only IC patients were included. The lack of a control group without IC with similar demographic characteristics limited the ability of this study to explore the true risk factors of developing IC. Third, as a single-center study, the collected data were limited even though ten-year data were used. The small sample size might reduce the power of this study to detect statistical significance. Thus, a large-scale multi-center prospective study is required to further investigate the characteristics of IC.
-
In conclusion, the incidence of IC has declined in this center in the recent five years due to the intensified preventive surveillance. Senior people were the major affected population, and colonization was a prevalent risk factor. C. albicans was still the most common species, while C. non-albicans caused 54.2% of the IC episodes in this study center. Among these, C. glabrata infection was associated with the highest mortality rates. Culture was still the major method for diagnosis. Fluconazole-resistant/non-WT phenotypes were relatively common in this study and were associated with previous exposure to fluconazole or voriconazole. Fluconazole was still the most commonly used drug throughout the disease course in the recent ten years. The prognosis of IC was poor with 90-day all-cause mortality rate exceeding 30%. To reduce the limitations due to missing data and the small sample size, a multi-center prospective study is needed to further investigate the characteristics of IC.
-
All authors were involved in the study design. YANG Zhi Hui collected the clinical data from patients, conducted statistical analyses, drafted the initial manuscript and revised the manuscript according to the suggestions of the other authors. SONG Ying Gai was involved in the identification and drug susceptibility test of the isolates. LI Ruo Yu helped revise the manuscript and supervised the overall project. All authors read and approved the final manuscript.
-
The authors declare that they have no conflicts of interest.
-
This study was approved by the ethics committee of Peking University First Hospital. The need for informed consent was waived by the Clinical Research Ethics Committee as this was a retrospective study.
HTML
Hospital Setting and Study Population Selection
Data Collection
Definitions
Species Identification and Antifungal Susceptibility Testing
Statistical Analysis
Incidence and Patient Demographics
Risk Factors
The Fungal Distribution
Diagnostic Methods
Antifungal Resistance
Treatment
Prognosis
 21279Supplementary Materials.pdf
21279Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: