-
Prion diseases (PrDs) are fatal neurodegenerative conditions that affect humans and other mammals, which consist of sporadic, genetic, and acquired forms in human PrDs[1]. The genetic form accounts for approximately 5%–15% of all human PrDs. Human genetic prion diseases (gPrDs) are further classified as genetic Creutzfeldt-Jakob disease (gCJD), Gerstmann-Sträussler syndrome (GSS), and fatal familial insomnia (FFI), according to the differences in genetic variants, clinical symptoms, and neuropathology[2]. To date, more than 60 PRNP mutants have been reported worldwide, most of which are pathological[3]. The clinical and histopathological characteristics of gPrDs vary greatly depending on the different PRNP mutations. The proportion of PrDs caused by PRNP mutations differs notably among countries and/or geographic regions, which is closely linked to racial diversity.
Since 2006, the China National Surveillance for Creutzfeldt-Jacob disease has identified 20 gPrDs-associated mutations in PRNP (Shi et al., in preparation). The top five types of mutations in the Chinese population were T188K, D178N, E200K, E196A, and P102L. T188K gCJD is the most commonly identified gPrDs in Chinese but very rare in other countries, including the neighboring countries (Japan and South Korea)[4]. D178N FFI, one of the most frequent gPrDs worldwide, is commonly observed in Chinese, whose frequency seems to be higher than that in Japanese and Korean. The E200K gCJD is the most popular gCJD in Europe, while it is also relatively common among the Chinese. E196A gCJD is extremely rare globally, but more than 10 cases have been reported in China. The clinical manifestations of T188K, E200K, and E196A gCJD are comparable to those of sCJD, which is usually difficult to differentiate without gene sequencing[5]. Similar to many countries, P102L GSS is also the predominant subtype of GSS among the Chinese.
Real-time quaking-induced conversion (RT-QuIC) is a technique that can detect small amounts of PrPSc in various tissues, including cerebrospinal fluid (CSF)[6]. The implementation and significance of CSF RT-QuIC in patients with sCJD have been described[7,8]; however, its application to gPrDs, particularly in Chinese gPrDs, is not well documented. Meanwhile, the difference in the reactivity of CSF RT-QuIC in various gPrDs remains unsolved. To address these questions, a total of 117 CSF samples from Chinese gPrDs cases with five major types of mutations were enrolled in this study and their RT-QuIC reactivities, focusing on the positive rate, conversion time, and peak of relative ThT fluorescence units (rfu) were compared with other main clinical, epidemiological, and laboratory factors.
The stored intravitally collected CSF samples from 117 patients with gPrDs confirmed by PRNP sequencing were subjected to the established RT-QuIC assay. We included 10 cases of P102L GSS, 37 cases of D178N FFI, 35 cases of T188K gCJD, 10 cases of E196A gCJD, and 25 cases of E200K gCJD. In total, 36 out of 117 (30.8%) CSF samples were positive in RT-QuIC, showing remarkable various kinetic patterns in the lag phase time and the maximum rfu reached. The potential general relationship of CSF RT-QuIC reactivity in gPrDs cases was analyzed with some major factors. Only periodic sharp wave complexes (PSWC) in electroencephalogram (EEG) showed a significant association with RT-QuIC reactivity (positive rate, P = 0.006; conversion time, P = 0.005; peak of rfu, P = 0.009), whereas the other factors were not significant (Supplementary Table S1, available in www.besjournal.com).
Factors Grouped Positive rate (%) Positive time post-reaction (h) Peak ThT fluorescence units (rfu) Positive no./
grouped no.P-value Median
(min, max)P-value Median
(min, max)P-value Age
(years)< 50 13/35 (37.1) 0.376a 8.64 (2.88, 53.40) 0.369b 141,434
(30,882, 260,000)0.455b 50–70 22/74 (29.7) 7.56 (2.16, 21.60) 127,168
(52,188, 250,144)> 70 1/8 (12.5) 20.16 62,818 Gender Male 18/58 (31.0) 0.951a 7.96 (2.88, 44.70) 0.254c 116,813
(51,161, 245,791)0.569c Female 18/59 (30.5) 5.76 (2.16, 53.40) 143,248
(30,882, 260,000)Tau ELISA
(pg/mL)< 1,400 16/58 (27.6) 0.460a 6.12 (2.16, 44.70) 0.425c 118,388
(51,161, 250,144)0.340c ≥ 1,400 20/59 (33.9) 8.00 (2.16, 53.40) 132,739
(30,882, 260,000)14-3-3Western
blotPositive 22/71 (31.0) 0.950a 5.76 (2.16, 44.70) 0.172c 126,029
(51,161, 260,000)0.559c Negative 14/46 (30.4) 9.04 (2.16, 53.40) 127,168
(30,882, 223,636)EEG Positive 16/32 (50.0) 0.006a 4.32 (2.16, 12.24) 0.005c 151,876
(87,548, 260,000)0.009c Negative 20/85 (23.5) 10.80 (2.16, 53.40) 91,822
(30,882, 242,450)MRI Positive 25/77 (32.5) 0.581a 7.92 (2.16, 53.40) 0.503c 118,884
(30,882, 260,000)0.850c Negative 11/40 (27.5) 8.00 (2.88, 44.70) 132,304
(51,161, 242,450)Disease duration
(months)≤ 8 16/44 (36.4) 0.433a 7.96 (2.16, 34.00) 0.797c 113,832
(52,188, 242,450)0.268c > 8 15/52 (28.8) 7.92 (2.16, 53.40) 146,947
(30,882, 260,000)Note. aChi-Square test was used for comparing the positive rate of each factor groups. bKruskal-Wallis H test was used for comparing the positive time post-reaction and the peak ThT fluorescence units of each factor that more than two groups. cMann-Whitney U test was used for comparing the positive time post-reaction and the peak ThT fluorescence units of each factor between two groups. Table S1. Correlation of reactivity of CSF RT-QuIC with factors of genetic prion diseases
Under our experimental conditions, the positive rates were 60.0% in P102L GSS, 16.2% in D178N FFI, 25.7% in T188K gCJD, 40.0% in E196A gCJD, and 44.0% in E200K gCJD (Table 1), with a statistically significant difference (P = 0.032). The real-time rfu data of every positive sample were collected, and the individual averaged RT-QuIC reactive curves of gPrDs were generated. As shown in Figure 1 (various controls in each reaction are illustrated in Supplementary Figure S1, available in www.besjournal.com), E200K-gCJD showed the shortest conversion time and the highest average peak of rfu (> 150,000 rfu), whose averaged curve reached the maximal rfu approximately 10 h post-reaction. T188K-gCJD and E196A-gCJD revealed similar reactive patterns, with relatively long lag times and moderate levels of the averaged peak of rfu (around 100,000 rfu). The lag time of P102L-GSS was similar to that of E196A-gCJD, but the maximal rfu was slightly higher. D178N-FFI showed the longest lag time and the lowest average peak of rfu, which was approximately 65000. Statistical analysis revealed a significant difference in the positive conversion time of RT-QuIC (P = 0.026) among the five types of gPrDs, but not in the rfu at peak (P = 0.211) (Table 1). This indicates a close association between the genotypes of gPrDs and their CSF RT-QuIC reactivity.
Mutants Positive rate (%) Positive time post-reaction (h) Peak ThT fluorescence units (rfu) Positive no./grouped no. P-value Median (min, max) P-value Median (min, max) P-value P102L 6/10 (60.0) 0.032a 9.04 (3.72, 18.00) 0.026b 148,689 (46,332, 184,010) 0.211b D178N 6/37 (16.2) 27.80 (2.88, 53.40) 66,156 (30,881, 223,636) T188K 9/35 (25.7) 8.00 (5.76, 19.44) 112,449 (52,188, 242,450) E196A 4/10 (40.0) 11.52 (5.04, 20.16) 90,851 (56,926, 250,143) E200K 11/25 (44.0) 3.60 (2.16, 10.80) 153,321 (87,548, 260,000) Note. aChi-Square test was used to compare the positive rates among the five types of gPrDs. bKruskal-Wallis H test was used to compare the positive time post-reaction and the peak ThT fluorescence units among the five types of gPrDs. Table 1. Correlation of reactivity of CSF RT-QuIC with PrP mutants
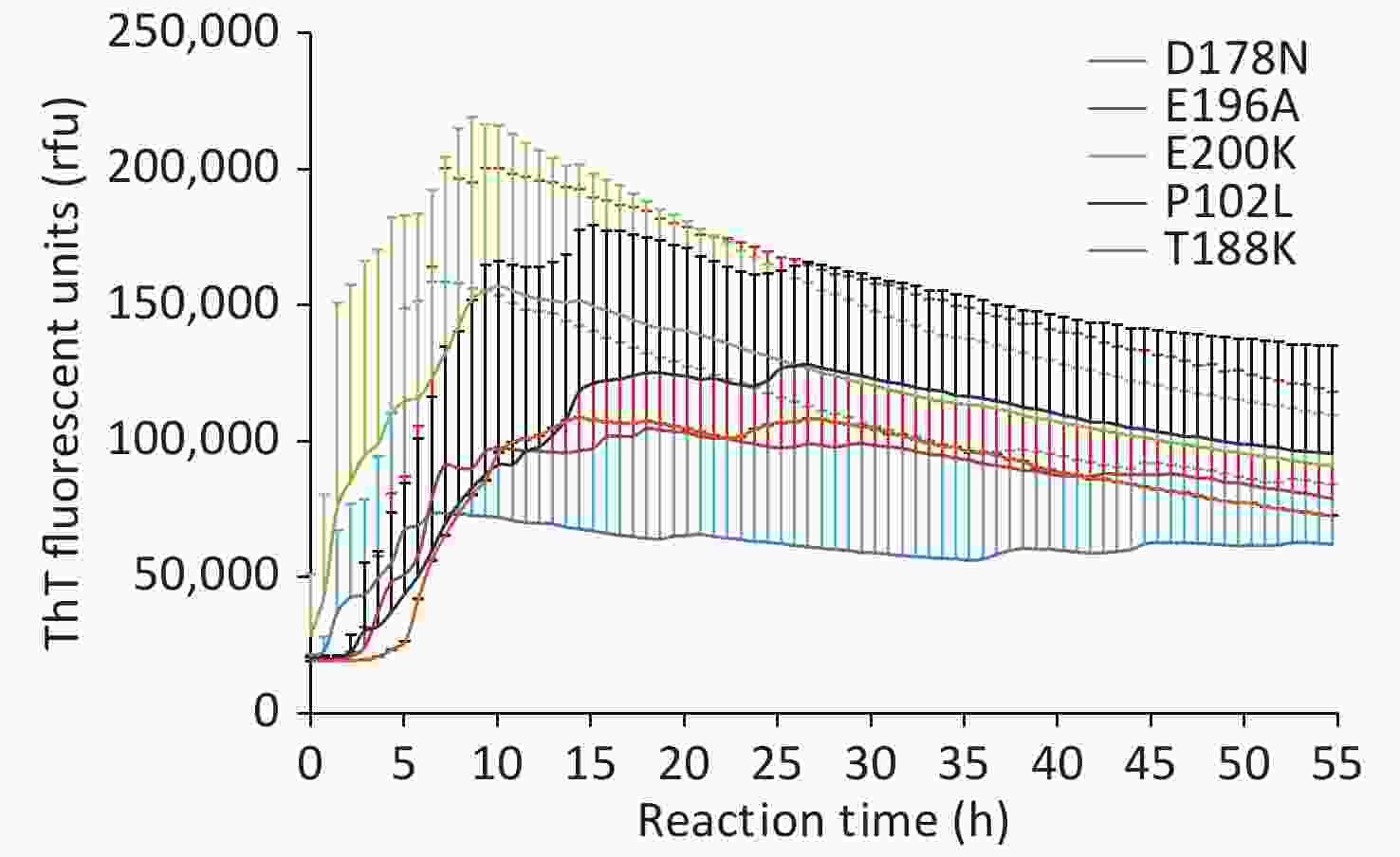
Figure 1. Averaged reactive curves of CSF RT-QuIC of the five gPrDs. The data are obtained from the positive samples, including nine cases of T188K-gCJD, four cases of E196A-gCJD, eleven cases of E200K-gCJD, six cases of D178N-FFI, and seven cases of P102L-GSS. The averaged data of each type of gPrDs were calculated and presented as mean + SD. X-axis, reaction time; Y-axis, ThT fluorescence units.
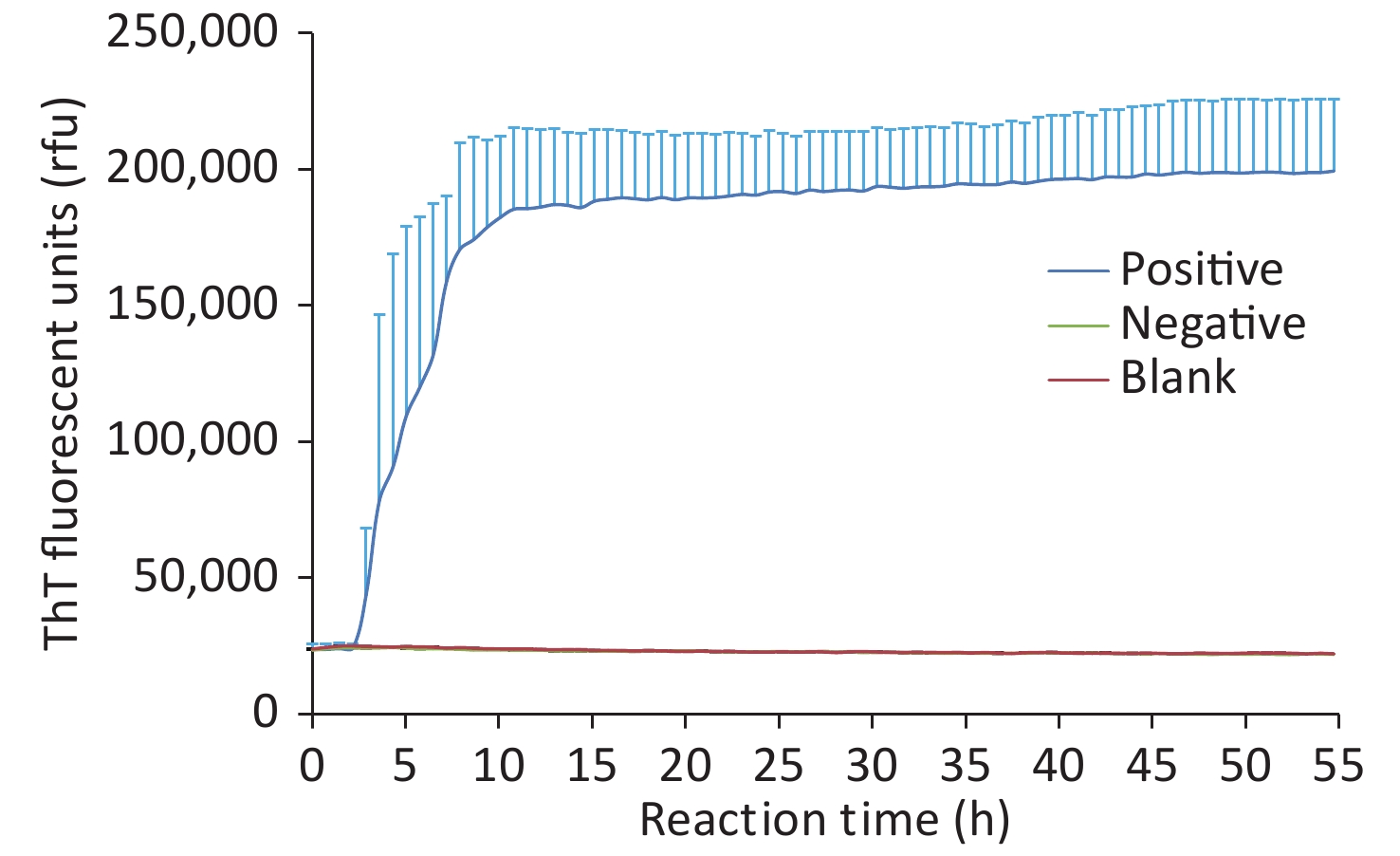
Figure S1. Various control curves of CSF RT-QuIC. The averaged data of each control were calculated and presented as mean + SD. X-axis, reaction time; Y-axis, ThT fluorescence units.
The reactivities of CSF RT-QuIC were also different among the clinical phenotypes of gCJD, FFI, and GSS. Taken together, 70 gCJD cases with the mutants T188K, E196A, and E200K, 24 (34.3%), were positive for RT-QuIC. The median positive conversion time was 7.71 h (2.16−20.16), and the median peak of rfu was 118,873 (52,188−260,000). Statistical assays revealed significant differences between the positive rates of FFI and gCJD (P = 0.048) and between FFI and GSS (P = 0.005), but not between gCJD and GSS (P = 0.116). No statistical difference in the conversion time and peak of rfu was observed among the three phenotypes (Table 1).
The possible association of the reactivity of CSF RT-QuIC for each genotype with the main CJD-related parameters was also evaluated individually (Supplementary Table S2, available in www.besjournal.com). Only in the group of E200K-gCJD, statistical significance in the relation of MRI abnormality with positive rate and in that of disease duration with the peak of rfu were observed. Other tested parameters did not reveal any statistical association with RT-QuIC reactivity in any of the five types of gPrDs. This implies that the reactivity of CSF RT-QuIC of gPrDs is almost unrelated to the main CJD-associated clinical and laboratory parameters.
Grouped Mutants Positive rate (%) Positive time post-reaction (h) Peak ThT fluorescence units (rfu) Positive no./grouped no. P-value Median (min, max) P-value Median (min, max) P-value FFI D178N 6/37 (16.2) 0.048a 27.80 (2.88, 53.40) 0.091b 66,156 (30,881, 223,636) 0.087b gCJD T188K 24/70 (34.3) 7.71 (2.16, 20.16) 118,873 (52,188, 260,000) E196A E200K GSS P102L 6/10 (60.0) 0.116a 9.04 (3.72, 18.00) 0.364b 148,689 (46,332, 184,010) 0.876b gCJD T188K 24/70 (34.3) 7.71 (2.16, 20.16) 118,873 (52,188, 260,000) E196A E200K GSS P102L 6/10 (60.0) 0.005a 9.04 (3.72, 18.00) 0.337b 148,689 (46,332, 184,010) 0.310b FFI D178N 6/37 (16.2) 27.8 (2.88, 53.40) 66,156 (30,881, 223,636) Note. aChi-Square test was used for comparing the positive rate between two groups of gPrDs. bMann-Whitney U test was used for comparing the positive time post-reaction and the peak ThT fluorescence units between two groups of gPrDs. Table S2. Difference between gCJD (T188K, E196A, E200K), FFI (D178N) and GSS (P102L) in RT-QuIC
Our data here display significantly different reactive patterns of CSF RT-QuIC in the five types of Chinese gPrDs cases. It is well known that there are clear differences in the clinical, neuropathological, and laboratory characteristics of gCJD, GSS, and FFI[9]. Even in gCJD displaying similar features as sCJD, there are still slight differences among various genotypes. For example, our surveillance data of T188K, E196A, and E200K gCJD patients showed lower ratios of mutism, CSF 14-3-3 positivity, and increased CSF tau levels (Shi et al., in preparation). Diversity in the reactivity of CSF RT-QuIC among various gPrDs is also likely a reflection of the different neuropathogenesis.
RT-QuIC reactivity is closely related to the amount of PrPSc in the testing specimens[10]. The presence of PrPSc in the CNS is a hallmark of prion disease. GSS is characterized by the presence of large PrPSc plaques in the brain, particularly in the cerebellum tissue[9]. Amounts of PrPSc are also observable in the brain tissues of most gCJD cases, which are usually indistinguishable from sCJD. On the contrary, the brain tissues of FFI patients usually contain much less PrPSc, even undetectable by routine methods, such as proteinase K-digested western blot. Such differences in brain PrPSc are also reflected in the reactivity of CSF RT-QuIC. We also noticed higher positive rates of CSF RT-QuIC in the E200K and E196A gCJD groups than in the T188K gCJD group. The characteristics of brain PrPSc in E200K gCJD are well addressed as one of the most common genotypes of gPrDs worldwide. Most T188K gCJD and all E196A gCJD have been identified in China. Unfortunately, only a few T188K Caucasian cases and none of the E196A cases have documented neuropathological feature[4]. The exact association between brain PrPSc and CSF RT-QuIC reactivity among the three genotypes of gCJD is still unclear.
We noticed that the RT-QuIC reactivity showed an obvious individual appearance. According to our analysis, the majority of the indexes seem to be unrelated to the RT-QuIC reactivity, except for PSWC on EEG. However, such an association was observed in the analysis of the five gPrDs, but not in those of individual genotypes. More cases of D178N FFI, which were all negative in PSWC on EEG and low positive rate in RT-QuIC, created such a phenomenon. Individual differences in the same gPrDs genotype are also frequently observed in other CSF tests, such as CSF 14-3-3 and tau. Besides the amount of brain PrPSc, there are probably other unknown elements influencing the release of brain factors into the CSF.
We have to admit that the positive rates of RT-QuIC in this study, regardless of the type of gPrDs, are relatively low, probably due to some reasons. However, the major objective of this study was to determine the possible differences in the various gPrDs in RT-QuIC rather than to prove the feasibility of RT-QuIC in the diagnosis of gPrDs. Therefore, the relatively less sensitive experimental condition here is somehow more suitable for identifying the slight differences that might be hidden in the higher sensitivity assay among various genotypes of gPrDs.
CH and CC designed the study, drafted the manuscript, and performed the RT-QuIC assay. QS and KX performed data analysis. JC, WZ, YX, WY, and LW carried out data sorting and statistical analysis. XPD conceived the study, participated in its design, and drafted the manuscript. All authors read and approved the final manuscript.
All authors declare that they have no conflict of interest.
HTML
Reference
 20519Supplementary Materials.pdf
20519Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: