-
Periodontitis is a chronic inflammatory disease resulting in the loss of periodontal tissues and ultimately teeth, thus posing a substantial public-health burden worldwide[1]. With the widespread increase in mesenchymal stem cell (MSC) therapy and the discovery of MSCs in periodontal ligaments[2], periodontal ligament stem cells (PDLSCs) have been used to reconstruct periodontal tissue and repair bone defects[2,3]. Furthermore, PDLSCs have low immunogenicity and extensive immunomodulatory functions, and allogeneic PDLSCs have shown great promise in repairing periodontal bone defects in miniature pig models[3]. Because osteogenic PDLSCs are considered good candidates for periodontal and alveolar bone regeneration, understanding the osteogenic differentiation of PDLSCs and related influencing factors may facilitate the development of regenerative therapies for periodontitis[4]. Smoking is considered an important factor in the development and progression of periodontitis. Nicotine, an important component of cigarette smoke, is thought to accelerate the loss of periodontal tissues[5]. Thus, we assessed the effects of nicotine on the proliferation, osteogenic differentiation and particularly the immunomodulatory characteristics of PDLSCs in this study.
Normal human impacted third molars were collected from ten non-smokers 18–25 years of age in Weifang, Shandong Province, who provided informed consent to participate in the study. PDLSCs were isolated from periodontal ligament tissues attached to the middle third of the tooth root, as described previously [2]. PDLSCs at passages three to five were used in the following experiments. To characterize PDLSCs, we analyzed the expression profiles of STRO-1 (R&D systems, Minneapolis, USA), CD14, CD34, CD45, CD29, CD90, and CD146 (Abcam, Cambridge, UK) with FACSCalibur flow cytometry (BD Immunocytometry Systems, San Jose, CA, USA) as previously reported [2,3].
For osteogenic and adipogenic differentiation, PDLSCs were seeded at 6 × 104 per well in 12-well plates in α-modified Eagle’s medium (α-MEM). When PDLSCs attained 80% confluence, osteoinductive medium, i.e., α-MEM containing 10 mmol/L β-glycerophosphate (Sigma-Aldrich, St. Louis, MO, USA), 50 mg/L vitamin C (WAKO) and 10 nmol/L dexamethasone (Sigma-Aldrich), and adipogenic induction medium, i.e., α-MEM containing 10 μmol/L dexamethasone (Sigma-Aldrich), 2 mmol/L indomethacin (Sigma-Aldrich), 10 mg/L insulin (Sigma-Aldrich) and 0.5 mmol/L 3-isobutyl-1-methylxanthine (Sigma-Aldrich), were administered for 21 days. The inductive medium was changed every 3 days. Three weeks later, calcification of the extracellular matrix was verified via alizarin red staining or von Kossa staining, and oil red O staining was used to identify lipid-laden fat cells.
Peripheral blood mononuclear cells (PBMCs) from 12 healthy donors in Weifang, Shandong Province, were obtained through density gradient centrifugation with Ficoll (1.077 g/mL; Dingguo, Beijing, China) and resuspended in Roswell Park Memorial Institute-1640 medium[3].
PDLSCs (5.0 × 103) were co-cultured with different concentrations of nicotine (Sigma-Aldrich; 10−4 mol/L, 10−5 mol/L, or 10−6 mol/L) for 5 days [6,7]. The proliferation profiles of PDLSCs were measured every day during the 5-day incubation with Cell Counting Kit-8 kits (CCK-8; Dojindo Laboratories, Japan). The absorbance at 450 nm was measured with an enzyme-linked immunosorbent assay reader (Molecules Devices, Sunnyvale, CA, USA). Proliferation of PDLSCs (5.0 × 103) alone was assessed as a control.
To assess osteogenic differentiation ability, we seeded PDLSCs (5.0 × 104) in 6-well plates and maintained them in osteogenic medium (α-MEM containing 10 mmol/L β-glycerophosphate, 50 mg/L vitamin C and 10 nmol/L dexamethasone). Different concentrations of nicotine (10−4 mol/L, 10−5 mol/L, and 10−6 mol/L) were added to the medium. Osteogenic differentiation of PDLSCs (5 × 104) alone was used as a control. The alkaline phosphatase (ALP) activity of PDLSCs on days 1 and 7 was measured [8]. To detect osteogenic differentiation 3 weeks after induction, we assessed mineralized nodule formation with von Kossa staining [3].
PDLSCs were adjusted to 1.0 × 104, 5.0 × 104, or 2.5 × 105 and seeded, and allogeneic PBMCs (5.0 × 104 in 2 mL medium per well) were stimulated with 0.5 μg/mL phytohemagglutinin (PHA; Sigma-Aldrich). Nicotine was added into the experimental group (10−4 mol/L). T cells were harvested on day 3, and the proliferation was measured with CCK-8 kits [3,8].
To study the effects of PDLSCs in a two-way mixed lymphocyte reaction (MLR) [3], we seeded PDLSCs from a third person in 24-well plates, and the cell concentration was adjusted to 1.0 × 104, 5.0 × 104, or 2.5 × 105. Two hours later, PBMCs (5.0 × 104) in 2 mL medium per well from two other individuals were incubated with PDLSCs. Nicotine was added in the experimental group (10−4 mol/L). The proliferation of T cells was assessed after 3 days, as described above.
Enzyme-linked immunosorbent assays were used to quantify the prostaglandin E2 (PGE2) (R&D systems) in collected supernatants from 3-day-cultures of PDLSCs alone (5.0 × 104), PDLSCs (5.0 × 104) + PBMCs (5.0 × 104) + PHA (0.5 μg/mL), or PDLSCs (5.0 × 104) + PBMCs (5.0 × 104) + PHA (0.5 μg/mL) + nicotine (10−4 mol/L). For the PGE2 inhibition experiments, the PGE2 inhibitor indomethacine (5 μmol/L; Sigma-Aldrich) was added. The measurement was performed according to the manufacturer’s protocols [3,8].
All data are expressed as mean ± standard deviation. The stimulation index values were calculated as previously reported [3,8]. Statistical significance was assessed with Student’s t-test or analysis of variance, and a P value less than 0.05 was considered statistically significant. For multiple comparisons, the Bonferroni method was used to adjust the probability level.
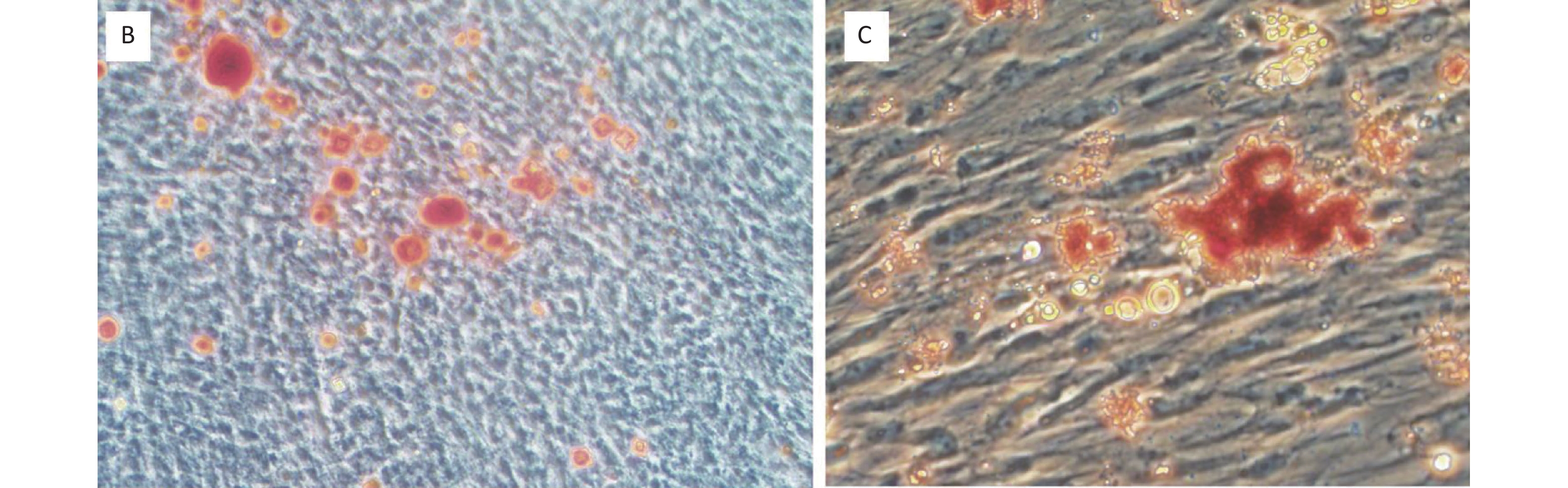
In this study, the PDLSCs were positive for HLA-І and markers of culture expanded MSCs, including STRO-1, CD90, and CD146 (Figure 1A). However, the PDLSCs were negative for CD34 and HLA-ПDR (Figure 1A). In addition, the PDLSCs underwent osteogenic (Figure 1B) and adipogenic (Figure 1C) differentiation under inductive medium.
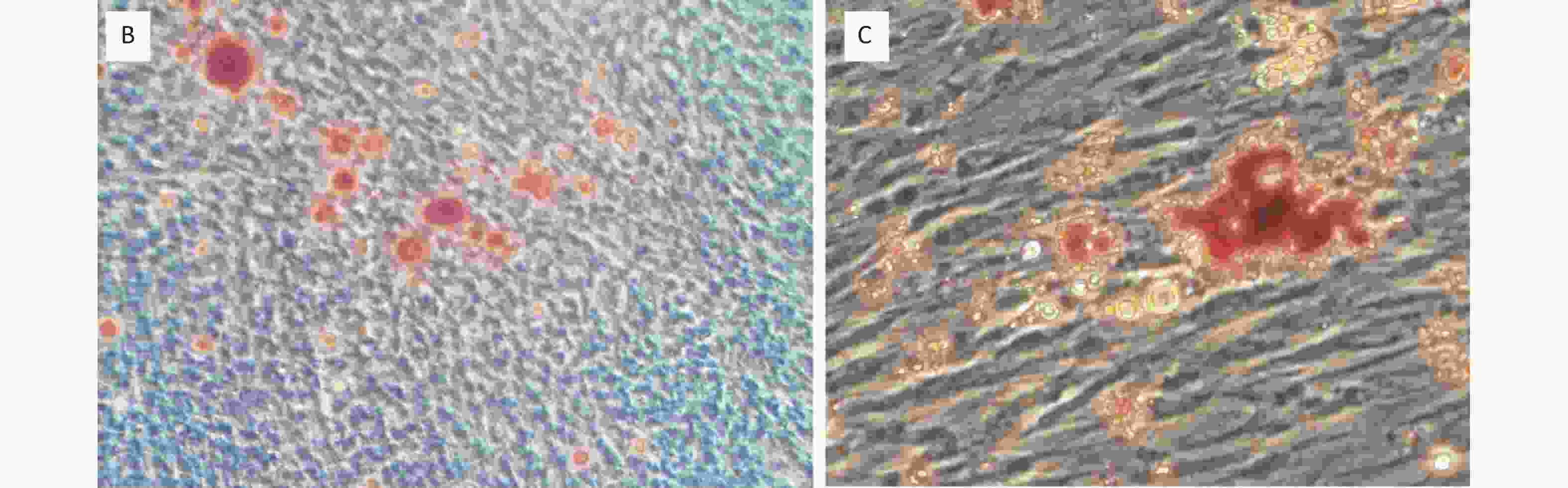
Figure 1. Characterization of PDLSCs. (A) PDLSCs were positive for STRO-1, CD90, CD146 and HLA-І, but negative for CD34 and HLA-П DR. PDLSCs underwent osteogenic (B) and adipogenic (C) differentiation under inductive medium. Magnification in (B) and (C): 100× and 400×, respectively. The experiments were repeated three times.
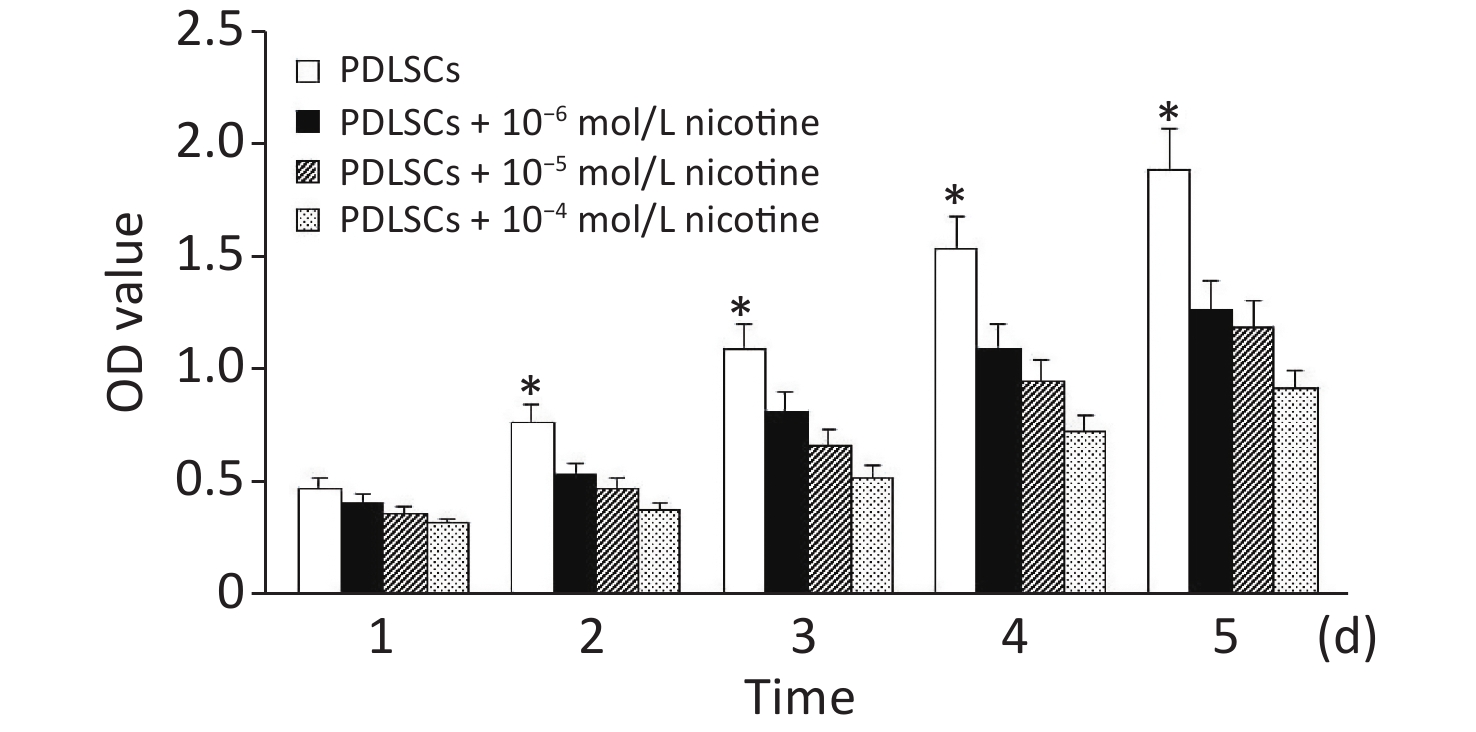
PDLSCs were co-cultured with different concentrations of nicotine for 5 days. According to the optical density values, nicotine at three concentrations had no significant effects on the proliferation of PDLSCs on the first day of co-culture. However, the proliferation of PDLSCs was significantly inhibited by different concentrations of nicotine at days 2–5 (Supplementary Figure S1, available in www.besjournal.com).
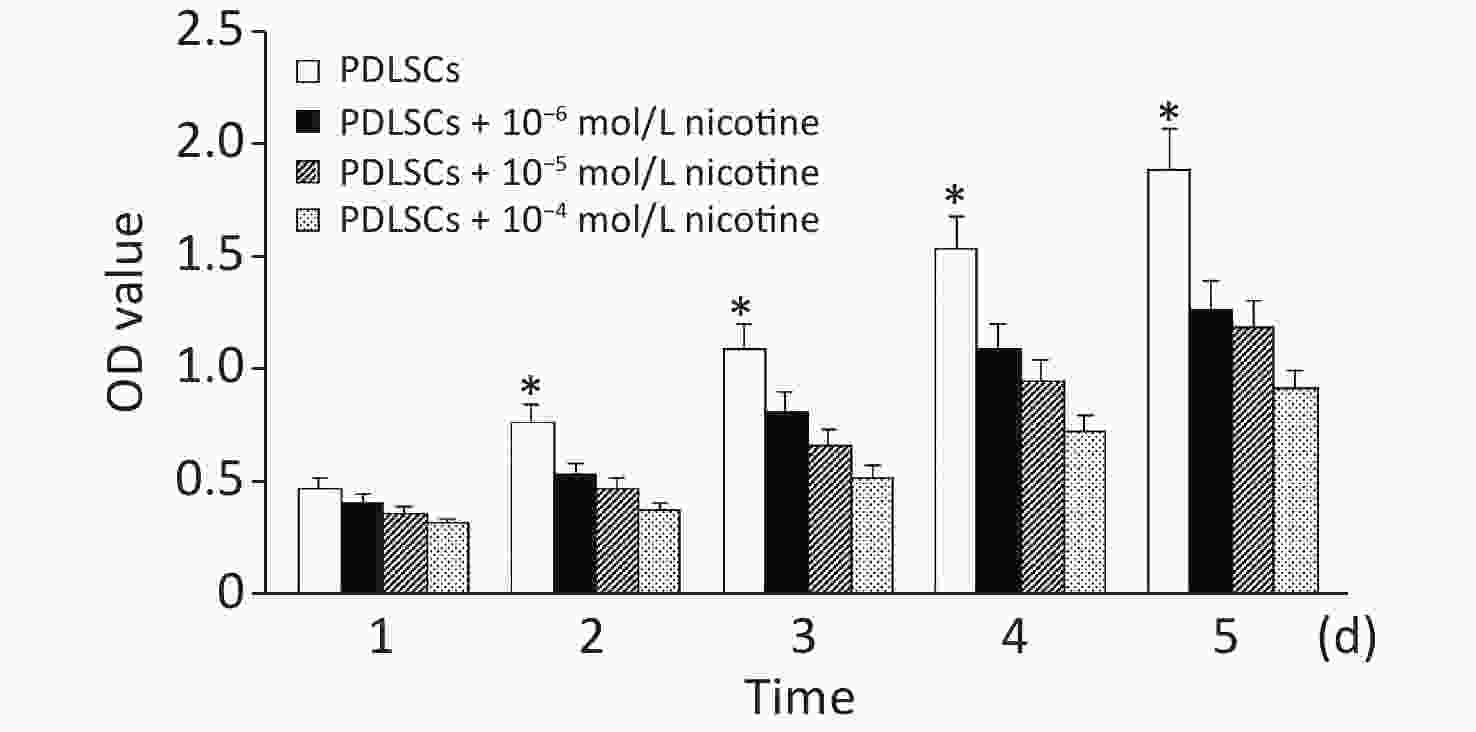
Figure S1. The effect of nicotine on PDLSC proliferation. On the first day, nicotine had no effects on PDLSC proliferation. However, three different concentrations of nicotine significantly inhibited PDLSC proliferation at days 2–5. Data represent the mean ± SD. *Indicates a statistically significant difference with respect to other groups on the same day. The experiments were repeated three times. PDLSCs, periodontal ligament stem cells.
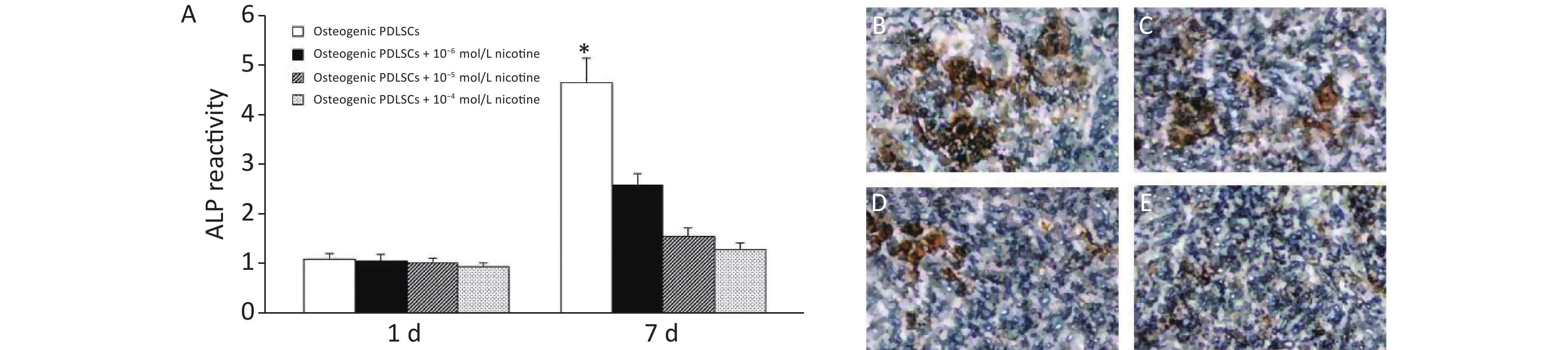
We then tested how nicotine affected the osteogenic potential of PDLSCs. No significant difference in ALP activity was observed in PDLSCs without and with different concentrations of nicotine 1 day after induction. However, the ALP activity was significantly inhibited by nicotine at day 7 (Figure 2A). Von Kossa staining revealed significantly impaired formation of nodular structures that stained black in PDLSCs, indicating calcium accumulation in vitro, in co-cultures with different concentrations of nicotine 3 weeks after induction (Figure 2B, 2C, 2D, 2E).
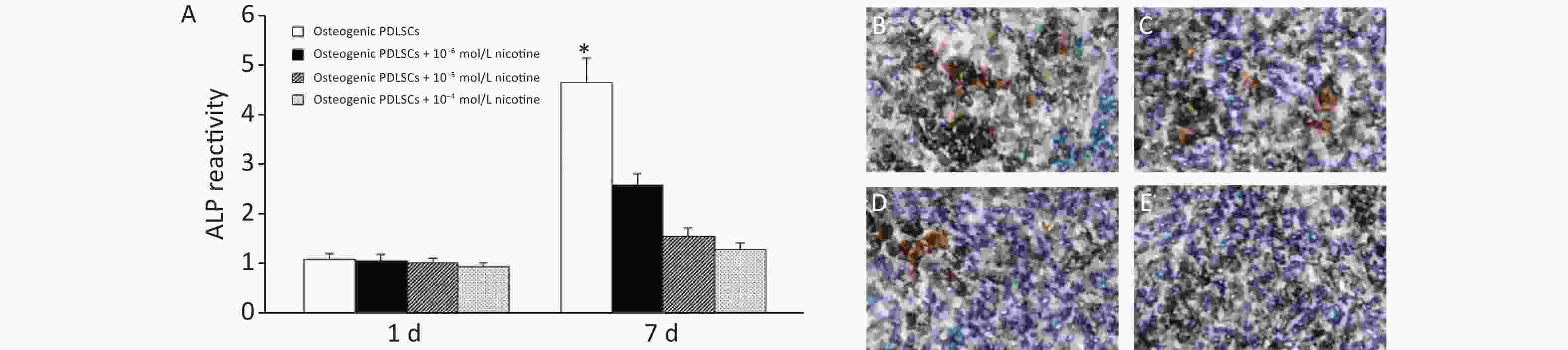
Figure 2. Effects of nicotine on the osteogenic differentiation of PDLSCs. (A) On the first day of osteogenic induction of PDLSCs, nicotine had no effects on ALP activity. On day 7 of osteogenic induction of PDLSCs, nicotine inhibited the ALP activity. Nicotine suppressed the osteogenic differentiation of PDLSCs, as characterized by the formation of mineralized nodules that appeared black with von Kossa staining in the groups with PDLSCs alone (B), PDLSCs + 10−6 mol/L nicotine (C), PDLSCs + 10−5 mol/L nicotine (D) and PDLSCs + 10−4 mol/L nicotine (E). Data represent the mean ± SD in (A). *Indicates a statistically significant difference with respect to other groups on the same day. Magnification, ×400. The experiments were repeated three times. ALP, alkaline phosphatase; PDLSCs, periodontal ligament stem cells.
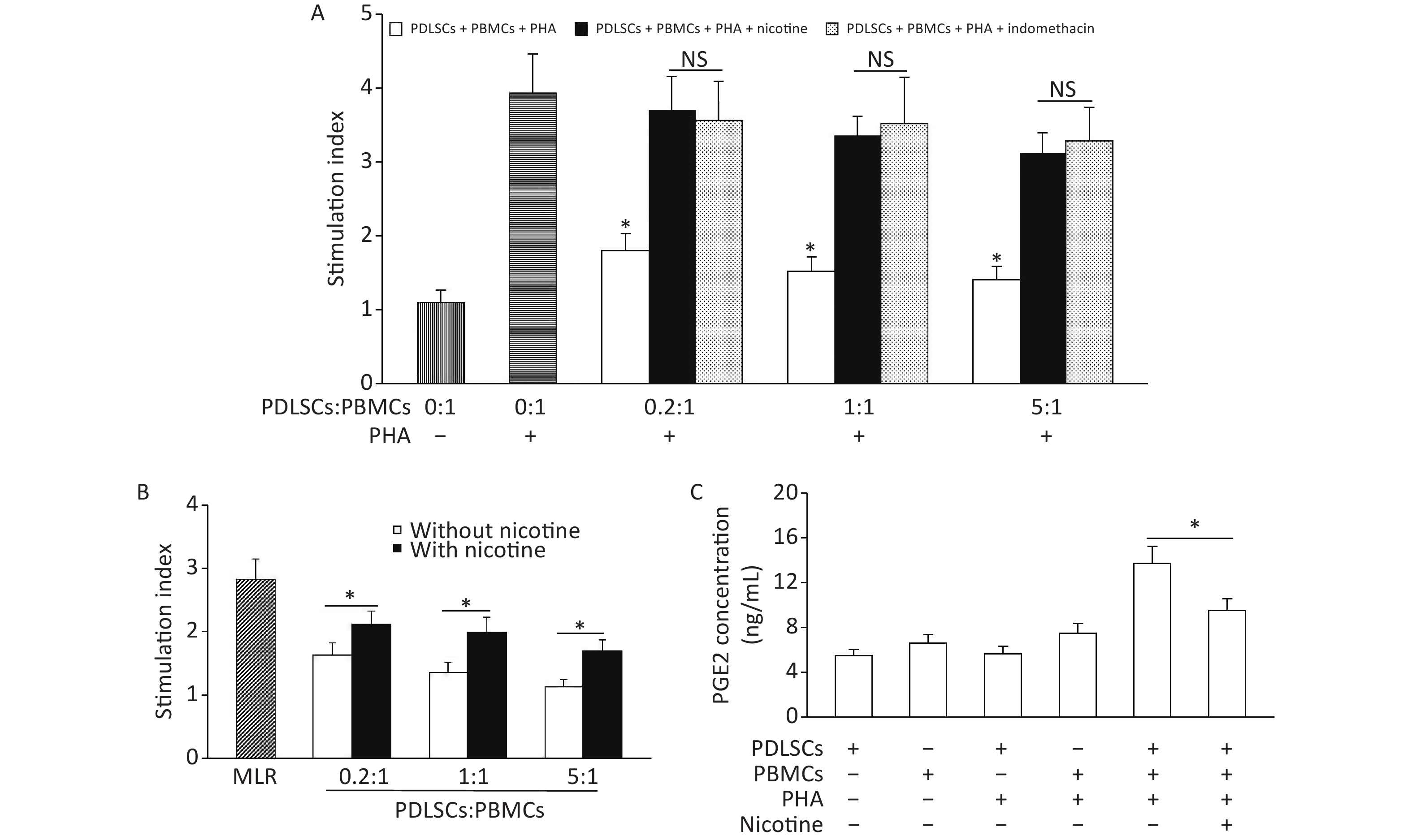
When PDLSCs were co-cultured with PHA-stimulated PBMCs, the proliferation of PBMCs was clearly suppressed. However, this decline in PBMC proliferation was diminished by the addition of nicotine, to a similar extent as that after application of the PGE2 inhibitor indomethacine (Figure 3A). Similar results were obtained in the MLR experiments, in which PBMC proliferation induced by allogeneic PBMCs was inhibited by PDLSCs; this inhibition was also decreased by nicotine (Figure 3B). The concentration of PGE2 in the supernatants of PDLSCs cultured alone for 3 days was low. Co-culture of PBMCs + PHA + PDLSCs resulted in a significant increase in the concentration of PGE2; however, the addition of nicotine downregulated the concentration of PGE2 (Figure 3C).
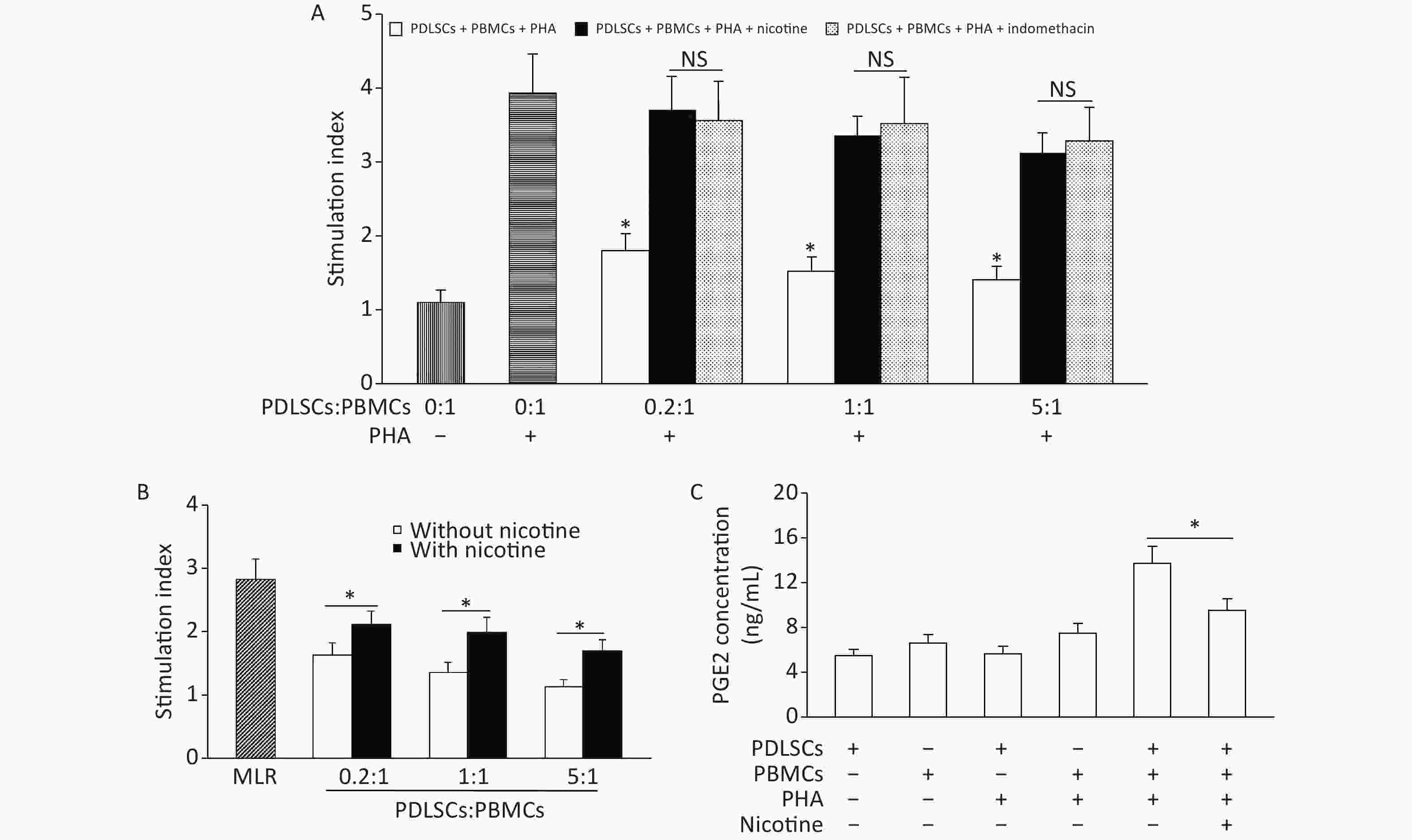
Figure 3. Nicotine attenuated PDLSC-mediated proliferation inhibition of PBMCs via downregulation of PGE2. (A) PDLSCs suppressed the PBMC proliferation induced by PHA, but nicotine attenuated the PDLSC-mediated proliferation inhibition of PBMCs, to a similar extent as the PGE2 inhibitor indomethacine. (B) PDLSCs suppressed the PBMC proliferation in MLR, whereas nicotine attenuated the PDLSC-mediated PBMC proliferation inhibition. (C) The PGE2 concentration, which was low in PDLSC supernatants, increased with the co-culture of PBMCs + PHA + PDLSCs. Nicotine downregulated the PGE2 concentration in PBMCs + PHA + PDLSC co-culture. Data represent the mean ± SD. *Indicates statistically significant. NS, not significant. The experiments were repeated six times. PDLSCs, periodontal ligament stem cells; PBMCs, peripheral blood mononuclear cells; PHA, phytohemagglutinin; MLR, mixed lymphocyte reaction
Smoking is a key factor leading to chronic periodontitis. Nicotine, an important component of tobacco smoke, plays a clear role in the development of periodontitis. Previous studies have shown that the curative effects of periodontitis in non-smokers are clearly better than those in smokers, and smoking aggravates the attachment loss of periodontal tissue and the destruction of alveolar bone in patients with periodontitis. The authors collected PDLSCs from smoking/non-smoking patients, and found stronger stem cell activity, migration and osteogenesis in the cells from non-smoking participants. Co-culture of PDLSCs with nicotine has been found to significantly decrease the migration ability, osteogenic differentiation ability and paracrine effects [6,7,9]. Our data demonstrated nicotine-mediated periodontal injury at the cellular level in PDLSCs, thus providing a theoretical basis for the etiology, treatment and prognostication of chronic periodontitis.
Because of their immunomodulatory ability, MSCs have been widely used in immune diseases, such as systemic lupus erythematosus and graft-versus-host disease. The immunoregulation of MSCs is attributable to their regulation of immune cells, including T cells and B cells, owing to the secretion of soluble factors, such as PGE2. We investigated the immunoregulation capabilities of PDLSCs for potential applications in allogeneic tissue regeneration, and we found that nicotine attenuated the PDLSC-mediated proliferation inhibition of PBMCs stimulated with PHA or MLR. PDLSCs have been reported to inhibit the proliferation of T cells through PGE2[3, 8], and T cell-mediated immunity is considered a key factor in the pathogenesis of periodontitis. In our experiments, we found that nicotine weakened the immunoregulation ability of PDLSCs, and the PGE2 content decreased during the co-culture of PBMCs + PHA + PDLSCs + nicotine, as compared with PBMCs + PHA + PDLSCs. Thus, we hypothesized that PGE2 may be a key mediator of nicotine’s effects in altering the immunoregulation of PDLSCs in vitro.
A previous study has demonstrated that the osteogenesis of MSCs was closely associated with PGE2 secretion, whereas treatment with a selective inhibitor of cyclooxygenase-2, a major enzyme involved in the biosynthesis of PGE2, significantly decreased bone mineralization; therefore, PGE2 appeared to play an important role in the osteogenic activity of MSCs[10]. Our data showed that nicotine decreased the osteogenic differentiation of PDLSCs and the secretion of PGE2, thereby indicating that PGE2 may participate in the osteogenic activity of PDLSCs.
Competing Interests The authors declare that they have no competing interests.
HTML
 22003Supplementary Materials.pdf.pdf
22003Supplementary Materials.pdf.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: