-
Approximately half of the patients with cancer globally are treated with ionizing radiation (IR) therapy nowadays, either alone or as a complement to other treatments [1-4]. Though IR is used as a standard treatment for a variety of tumors, it is paradoxica that IR has also been reported to enhance tumor metastasis and invasion [5-7]. Accumulating evidence indicates that epithelium-to-mesenchymal transition (EMT) is strongly linked to radiation-induced related metastasis [7]. Therefore, a more thorough understanding of the underlying mechanisms of IR-induced EMT may contribute to the development of measures to inhibit metastasis and subsequently improve the safety and efficacy of tumor radiotherapy.
EMT is a multifaceted process during which stationary epithelial cells lose their junctions while gaining the ability to migrate and invade as motile mesenchymal cells [8-10]. This process is characterized by the loss of epithelial morphology and markers, such as E-cadherin, and by the acquisition of mesenchymal molecular markers, including N-cadherin, vimentin, fibronectin, and β-catenin [9]. Several signaling pathways are involved in the regulation of EMT, and among these, the transforming growth factor-β (TGF-β) signaling has a predominant role [9-14].
TGF-β is a pleiotropic cytokine that plays a key role in controlling embryogenic development, inflammation, and tissue repair, as well as in maintaining adult tissue homeostasis [15, 16]. It is now widely accepted that TGF-β is one of the most common cytokines released in irradiated tissues and is released in a radiation dose-dependent manner [17, 18]. Notably, plenty of studies suggest that IR-induced TGF-β contributes to metastasis and EMT [7, 18-21]. For example, in irradiated colorectal carcinoma SW480 and SW620, breast carcinoma MCF-7 and MDA-MB-231, and lung carcinoma A549 and PC14 cells, the expressions of TGF-β1, which is the most intensively researched TGFβ isoform, and of mesenchymal markers were increased, while expression of epithelial markers was decreased. Concurrently, the migratory and invasive capabilities of these cells were enhanced. Furthermore, cellular events associated with EMT induced by IR in A549 could be reversed through inhibition of TGF-β signaling using the TGF-β1 antibody [18].
microRNAs (miRNAs) are a class of small noncoding RNAs that function as negative regulators of gene expression at the post-transcriptional level [22]. Through direct inhibition of target protein synthesis, miRNAs can modulate a variety of biological processes, with several miRNAs reported to regulate EMT [12]. miR-190 was reported to antagonize TGF-β-induced EMT by targeting SMAD2 in breast cancer [13]. The expression of miR-3622a-3p in colorectal cancer suppressed stemness and EMT by targeting SALL4 [23]. miR-663a was reported to efficiently suppress EMT in glioblastoma cells and papillary thyroid carcinoma cells via inhibition of TGF-β1 [24, 25].
There is a strong interaction between miRNAs and cellular radiation response because miRNAs are critical endogenous gene regulators of DNA damage repair, autophagy, apoptosis, among others [26]. Though numerous miRNAs are expressed ectopically in response to IR [27], only a few of them are found to regulate radiation-induced EMT. For example, γ-ray irradiation-induced miR-21 expression promotes EMT progression in lung epithelial cells [28], and downregulation of miR-155-5p by radiation in alveolar epithelial cells promotes EMT via GSK-3β/NF-κB pathway [29]. Thus, other miRNAs could possibly participate in the regulation of radiation-induced EMT.
Our previous study detected the decrease of miR-663a in X-ray-irradiated 786-O cells and HeLa cells [30, 31] and uncovered the regulatory role of miR-663a in radiation-induced bystander effect via TGF-β1 inhibition [31]. In consideration of the key function of TGF-β during the radiation-induced EMT, we hypothesized that the decrease in miR-663a by IR might participate in radiation-induced EMT via TGF-β1.
In this study, to verify our hypothesis, EMT was induced by treating cells with TGF-β1 or IR to induce EMT, followed by miR-663a transfection. Cell migration and the expression of TGF-β1 and EMT-associated factors were quantified to determine the role of miR-663a in EMT via TGF-β1. We report that miR-663a transfection suppressed enhancement of cell migration and promotion of mesenchymal-like cell morphology changes induced by either TGF-β1 or radiation. Furthermore, the decrease of E-cadherin and increase of β-catenin and fibronectin were also reversed by miR-663a. Lastly, both X-rays and carbon ions irradiation resulted in an upregulation of TGF-β1 and downregulation of miR-663a while the suppression of TGF-β1 by miR-663a reversed EMT after radiation. Our data suggest that the radiation-responsive miRNA, miR-663a, is capable of suppressing EMT via TGF-β1 after exposure to IR.
-
Human non-small cell lung cancer cell lines A549 and NCI-H1299, human small cell lung cancer cell line NCI-H446, and human bronchial epithelium cell line Beas-2B were purchased from the Shanghai Institute for Biological Sciences (Shanghai, China). The A549 cells were cultured in DMEM/F-12 medium (Gibco, 12500-062, USA), H1299 cells in DMEM medium (Gibco, 12800-082), and other cell lines in RPMI-1640 medium (Gibco, 31800-105). All these media were supplemented with 10% fetal bovine serum (Gibco, 10099-141), 100 units/mL penicillin, and 100 mg/mL streptomycin. Cells were maintained at 37 ℃ in a humidified atmosphere of 95% air and 5% CO2.
-
Exactly 2 × 105 cells were seeded in 35 mm dishes and cultured for 24 h prior to transfection with small RNAs including miR-663a mimics (RiboBio, miR10003326-1-5, Guangzhou, China), negative control (RiboBio, miR1N0000001-1-5), TGF-β1 siRNA (RiboBio, siG091112132156-1-5), and its negative control (RiboBio, siN0000001-1-10) at 40%–60% confluence using Lipofectamine TM 2000 (Invitrogen, 11668-019, CA, USA). The final concentration of the miRNA duplex was 30 mmol/L. The medium was replaced with fresh culture medium 6 hours following transfection. After transfection, cells were treated with or without 5 ng/mL recombinant human TGF-β1 (R&D Systems, 240-B-010/CF, Redmond, WA, USA).
-
Carbon ion (12C6+) beam irradiation (energy: 80 MeV/u, LET: 50 KeV/μm) was performed at the biomedical terminal of the Heavy Ion Research Facility in Lanzhou at the Institute of Modern Physics, Chinese Academy of Sciences, Lanzhou, China. The X-rays (energy: 225 KV/13.3 mA) were generated by an X-Rad 225 generator (Precision, North Branford, CT, USA).
-
Total RNA was extracted using TRIzol reagent (Invitrogen, 15596018, CA, USA). Reverse transcription of miRNA or mRNA was conducted with All-in-OneTM miRNA First-Strand cDNA Synthesis kit (GeneCopoeia, QP114, Guangzhou, China) or All-in-OneTM First-Strand cDNA Synthesis kit (GeneCopoeia, AORT-0060). To quantify the mRNA levels of TGF-β1, E-cadherin, β-catenin, and fibronectin and the expression of miR-663a, real time PCR was performed on CFX96 Touch™ Real Time PCR Detection System (Bio-Rad, CA, USA) using All-in-OneTM miRNA qPCR Kit (GeneCopoeia, QP011) or All-in-OneTM qPCR Mix (GeneCopoeia, QP002) based on SYBR-Green. PCR primers for U6 (HmiRQP9001), miR-663a (HmiRQP0774), universal Adaptor PCR Primer (QP029), GAPDH (HQP006940), TGF-β1 (HQP110577), E-cadherin (HQP054891), β-catenin (HQP003539), and fibronectin (HQP118676) were all purchased from GeneCopoeia, Inc. Primer sequences were as follows: miR-663a forward primer 5’-GCGCCGCGGGACCGCAA-3’ (HmiRQP0774) and universal Adaptor PCR Primer was used as a reverse primer; TGF-β1 forward primer 5’-GCACGTGGAGCTGTACCA-3’, reverse primer 5’-AAGATAACCACTCTGGCGAGTC-3’ (HQP110577); E-cadherin forward primer 5’-GAATCCAAAGCCTCAGGTCA-3’, reverse primer 5’-TCAAAATGATAGATTCTTGGGTTG-3’ (HQP054891); β-catenin forward primer 5’-CTCCATCCTGGCCTCGCTGT-3’, reverse primer 5’-TTTGAAGGCAGTCTGTCGTA-3’ (HQP003539); and fibronectin forward primer 5’-ATGGCTTGGAATGAGACTGC-3’, reverse primer 5’-TTCCATCATGGGGTCCATAC-3’ (HQP118676). In each qRT-PCR analysis, snRNA U6 and GAPDH mRNAs were used as controls for normalization. The relative expression was calculated using the 2−ΔΔCt method.
-
Protein lysates were made in RIPA buffer (Beyotime, P0013C, China). Proteins were separated by 12% SDS-PAGE electrophoresis and transferred to a methanol-activated PVDF membrane (Millipore, IPVH00010, USA). The membrane was blocked with 5% milk in PBS containing 0.1% Tween-20 for 1 h and subsequently probed with anti-TGF-β1 antibody (CST, 56E4, MA, USA), anti-E-cadherin antibody (CST, 24E10), anti-β-catenin antibody (CST, 6B3), anti-fibronectin antibody (CST, E5H6X), and anti-GAPDH antibody (Abcam, ab8245, MA, USA) at room temperature for 2 h. After 1 h incubation with goat anti-rabbit HRP-conjugated secondary antibody (Abcam, ab97051) or goat anti-mouse HRP-conjugated secondary antibody (Abcam, ab97023), the protein bands were detected with luminal reagent (Millipore, WBKLS0500, USA).
-
A scratch wound healing assay was carried out to determine cellular migration ability as described previously [32]. A total of 2 × 105 exponentially growing cells were seeded onto a 35-mm dish and incubated at 37 °C. When cells reached 90% confluence, a wound field was made using a 200 μL pipette tip. Dishes were washed twice with PBS and fresh culture medium was added prior to re-incubation for 18 h at 37 °C. Wound closure was observed by Leica DMI6000B inverted microscope (Leica Microsystems GmbH, Wetzlar, Germany).
-
Cells (1 × 105) were seeded into 12-well plates and cultured for 24 h. Following miRNA duplex transfection, cells were exposed by 2 Gy X-rays [18, 33] and treated with 5 ng/mL TGF-β1 [13, 14]. At 0, 24, 48, 72, and 96 h after treatment, cells were counted using a Coulter counter (Beckman Coulter, Inc., Brea, CA, USA), and the experiment was carried out three times for the construction of proliferation curves.
-
Cells were harvested by trypsinization and resuspended in RPMI-1640 medium supplemented with 10% FBS. An appropriate number of cells were plated onto a 60-mm dish to produce 50–100 colonies. Following incubation for 8 days, cells were fixed with 75% ethanol for 5 min and stained with 0.5% crystal violet for 10 min at room temperature. Colonies containing > 50 cells were counted as survivors. At least three parallel dishes were scored for each treatment, and each experiment was conducted at least three times independently. Colony survival (CS) was calculated as follows: CS = PE/PE0. In this calculate, PE represents the plating efficiency of the treated cells, and PE0 represents the plating efficiency of the control cells.
-
All the experiments were repeated at least three times and results are shown as mean ± SD. The statistical significance of the results was determined by Student’s t-test and P < 0.05 was considered as statistically significant.
-
TGF-β1 has been known to induce EMT in vitro [9, 11, 12]. To verify the induction of EMT by TGF-β1, A549, H1299, H446, and Beas-2B cells were treated by 5 ng/mL TGF-β1 for 48 h, after which, cellular morphology, wound closure in the scratch assay, and the expression of EMT-related factors were observed.
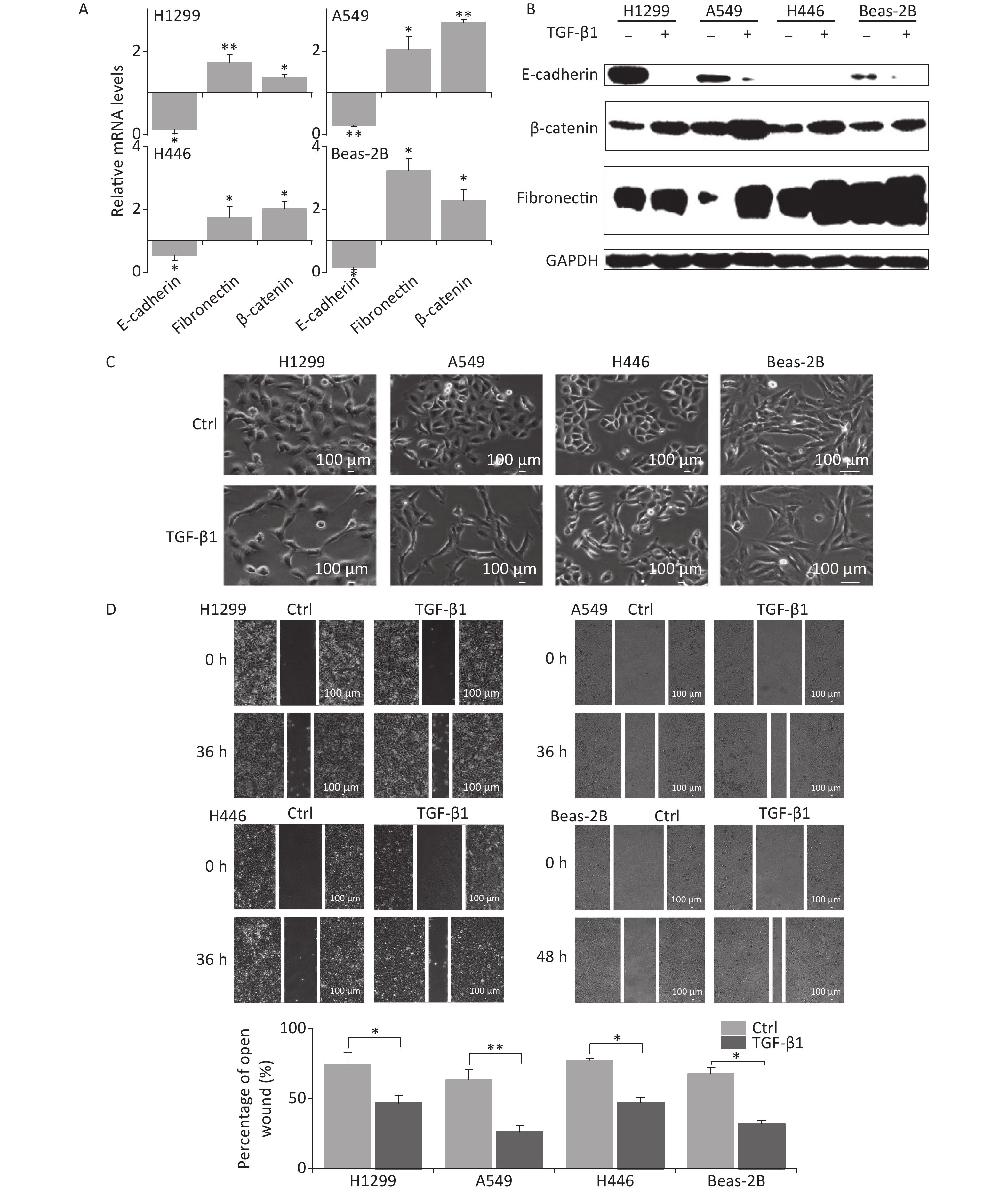
In the presence of TGF-β1, the expression of the EMT epithelial marker E-cadherin was significantly decreased in A549, H1299, H446, and Beas-2B cells at mRNA level (Figure 1A). Meanwhile, the mesenchymal markers fibronectin and β-catenin were remarkably upregulated (Figure 1A). These were consistent with changes in protein levels (Figure 1B). At 48 h following TGF-β1 treatment, the epithelial lung carcinoma cells A549, H1299, and H446 showed changes from a round, cobblestone-like appearance to a more elongated and spindle-shaped mesenchymal-like morphology, typical seen in EMT (Figure 1C). However, the change in cell morphology in Beas-2B cells after TGF-β1 treatment was not obvious (Figure 1C). Next, scratch assays were used to determine the effects of TGF-β1 on the migration ability of cells. The results showed that the wound-healing abilities of all A549, H1299, H446, and Beas-2B cells were enhanced by TGF-β1. Moreover, the rates of wound healing in the three tumor cell lines A549, H1299, and H446 were faster than that of normal bronchial epithelium cell Beas-2B (Figure 1D). These results confirm that TGF-β1 can induce significant EMT in A549, H1299, H446, and Beas-2B cells, as well as changes in expression of EMT markers.
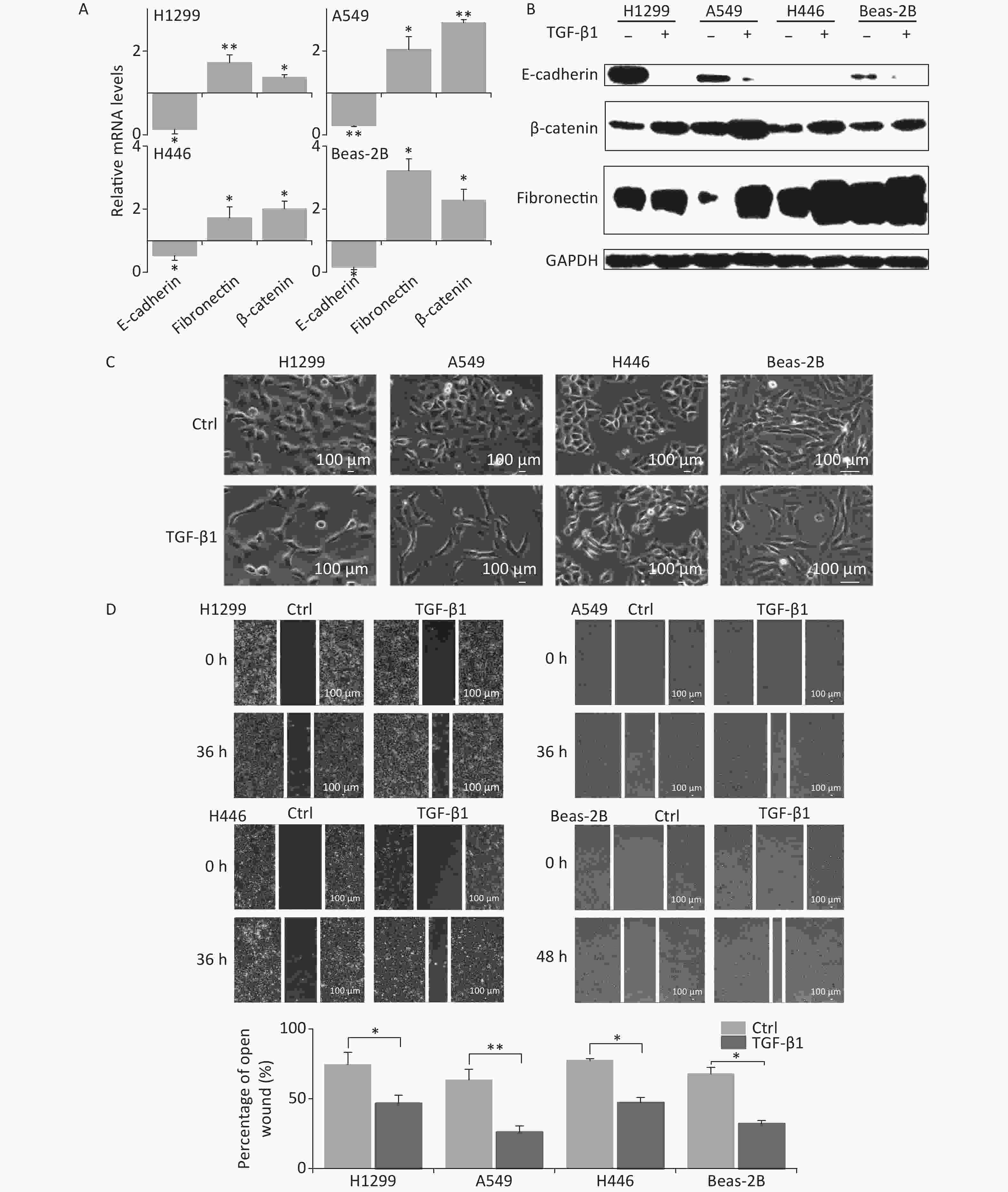
Figure 1. TGF-β1 induced EMT in A549, H1299, H446, and Beas-2B cells. (A) Relative mRNA expression levels of E-cadherin, β-catenin, and fibronectin in H1299, A549, H446, and Beas-2B cells at 48 h after 5 ng/mL TGF-β1 treatment. *P < 0.05 or **P < 0.01 represents statistical significance of comparison against non-treated control. (B) Protein levels of E-cadherin, β-catenin, and fibronectin in H1299, A549, H446, and Beas-2B cells with and without 48 h TGF-β1 treatment. (C) Cell morphology changings of cells after TGF-β1 treatment. (D) Scratch wound healing assay of cells treated with or without TGF-β1. *P < 0.05 or **P < 0.01 represents statistical significance between control and TGF-β1 treated group at 36 h or 48 h after treatment.
Considering the EMT-promoting effect of TGF-β1 in all lung cancer cell lines and in Beas-2B cells, we chose the A549 cell line for subsequent studies because the effect of TGF-β1 was more remarkable in it.
-
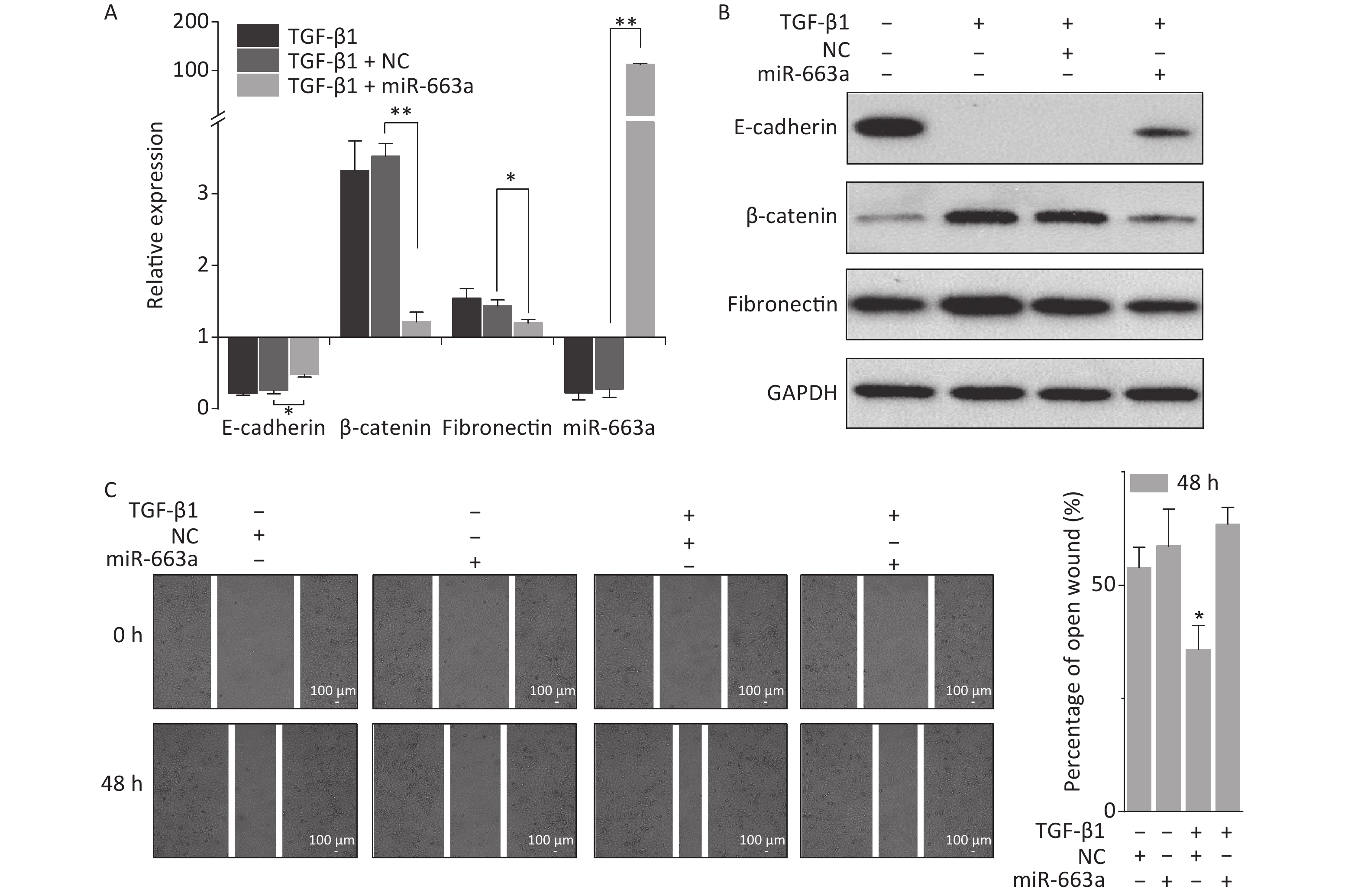
miR-663, a member of the primate-specific miRNA family, is associated with many important biologic processes [24, 25, 31, 34]. Particularly, in glioblastoma cells and pancreatic cancer cells, it was reported to inhibit EMT via targeting TGF-β1 [24, 25]. To determine its regulatory role in EMT in lung carcinoma cells, A549 cells were transfected with miR-663a mimics or negative control RNA sequences (NC) and then treated with 5 ng/mL TGF-β1. The expression levels of EMT-related factors and of miR-663a were quantified.
As shown in Figure 2A, transfection with miR-663a mimics in A549 cells resulted in a more than 100-fold increase in miR-663a levels. TGF-β1 treatment resulted in an 80% suppression of miR-663a expression in NC-transfected cells, and this result was consistent with our previous study, which revealed negative-feedback regulation between miR-663 and TGF-β1 [31]. Meanwhile, a remarkable decrease in the epithelial marker E-cadherin was detected after TGF-β1 treatment, and this decrease was partially reversed by miR-663a (Figure 2A and 2B). Expression levels of both mesenchymal markers, fibronectin, and β-catenin were significantly increased by TGF-β1, and those increases in expression were inhibited by miR-663a transfection. As seen in Figure 2C, in the absence of TGF-β1, the difference in the open wound width between NC- and miR-663-transfected groups at 48 hours after transfection was not obvious. This result indicates that miR-663a transfection did not influence the migration ability of A549 cells when not treated by TGF-β1. On the other hand, in the presence of TGF-β1, the healing of the scratched wound was much slower in miR-663a-transfected A549 cells than that in NC-transfected A549 cells. The data hints that the TGF-β1 enhanced migration ability was suppressed by miR-663a. Taken together, these results indicate that the TGF-β1-induced EMT in A549 cells could be suppressed by miR-663a.
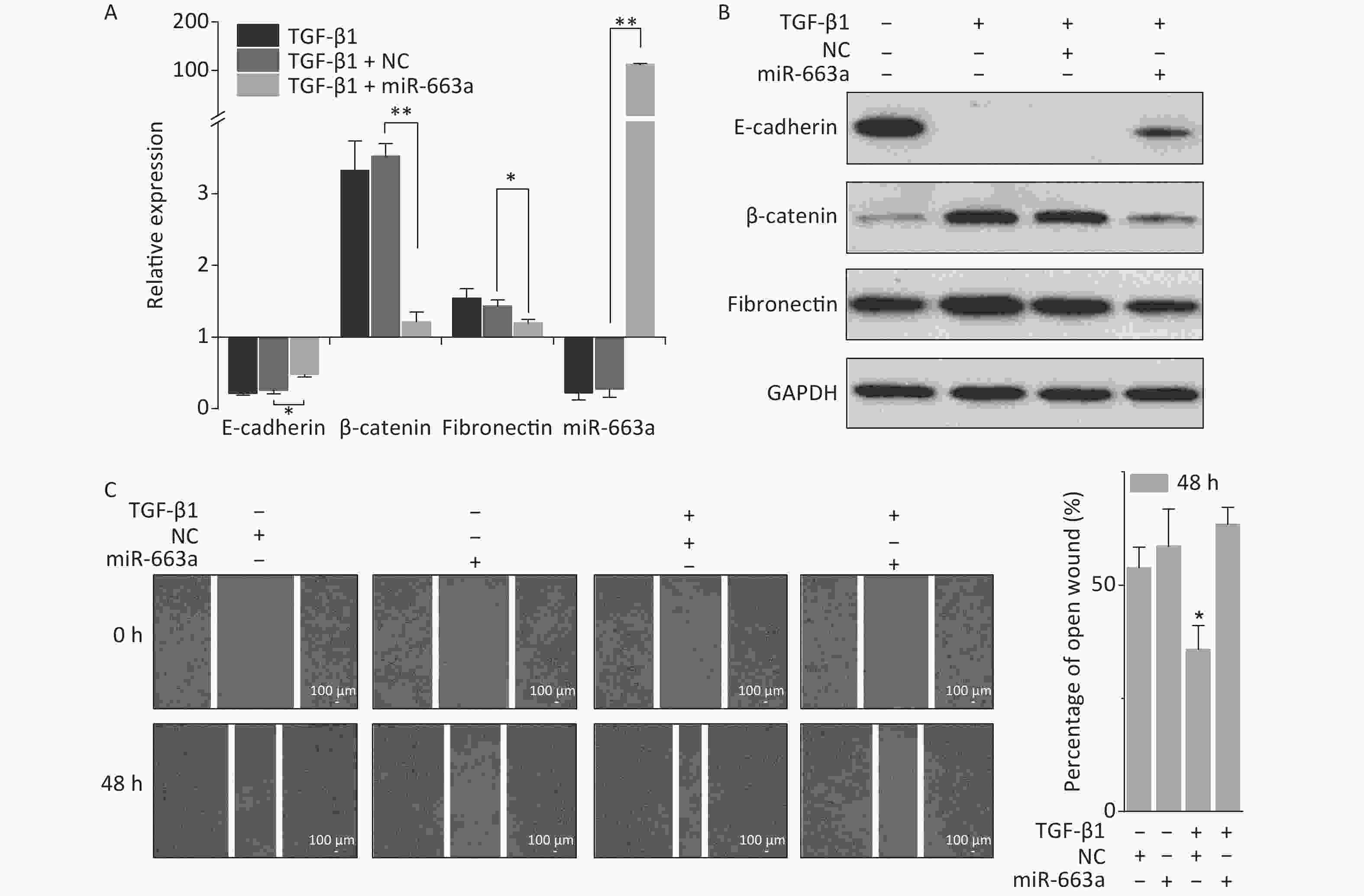
Figure 2. miR-663a transfection reserves TGF-β1 induced EMT in A549 cells. (A) Relative expression of E-cadherin, β-catenin, fibronectin, and miR-663a in A549 cells at 48 h after 5 ng/mL TGF-β1 treatment and miR-663a mimics’ transfection. *P < 0.05 or **P < 0.01 represents statistical significance of comparison. (B) Protein levels of E-cadherin, β-catenin, and fibronectin in A549 cells at 48 h after TGF-β1 treatment and miR-663a transfection. (C) Scratch wound healing assay result of A549 cells after TGF-β1 treatment and miR-663a transfection. *P < 0.05 represents statistical significance of comparison against NC.
-
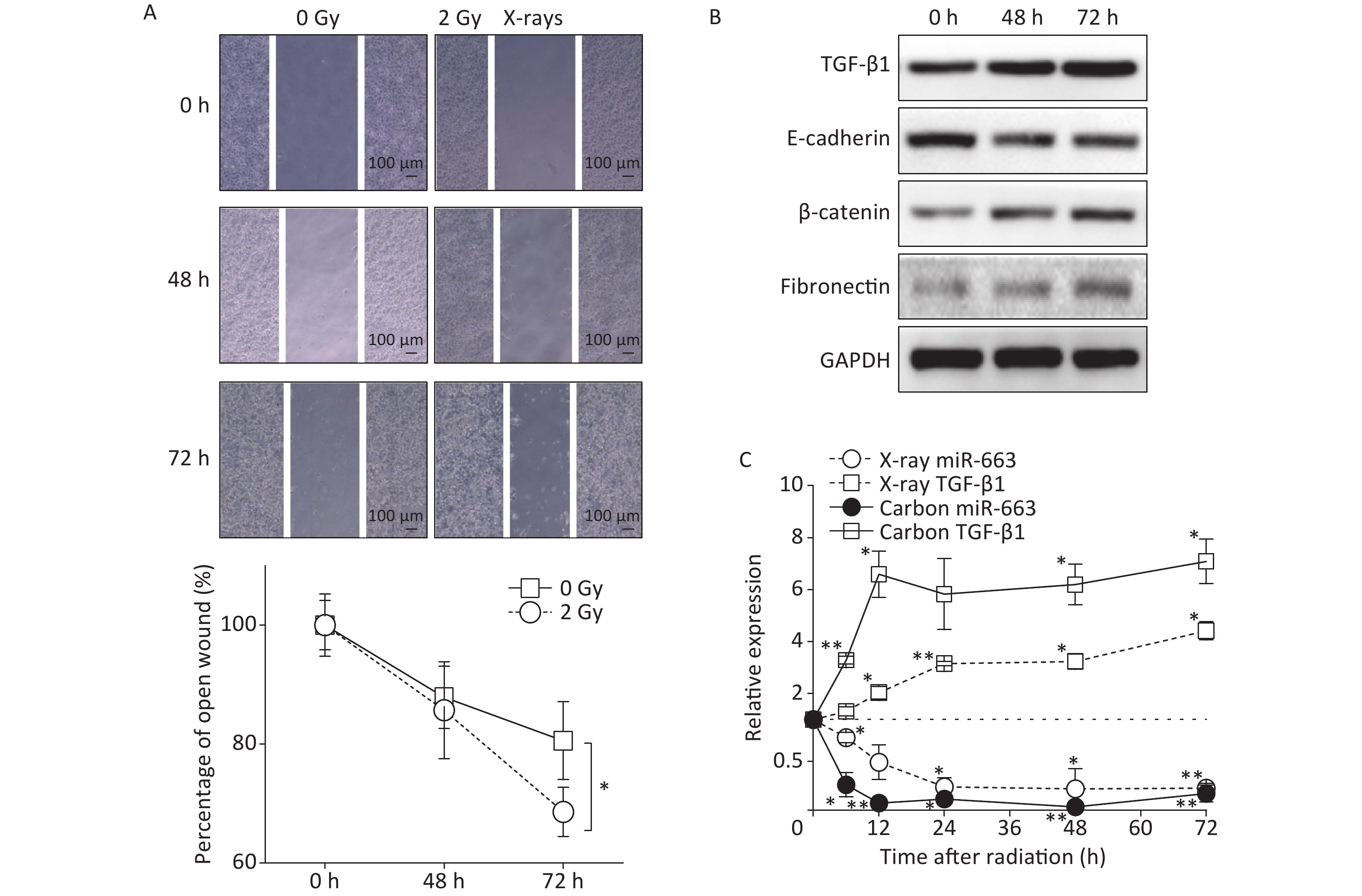
To examine the induction of EMT by IR in lung epithelial carcinoma, A549 cells were irradiated, followed by detection of cell migration using the scratch assay and the expression of EMT-related proteins using western blot analysis.
Results of the scratch assay showed that without radiation, the width of open wound at 72 h was about 80% of baseline, while the open wound width of irradiated samples at 72 h was about 65% of baseline (Figure 3A). The open wound width in the irradiated cells was remarkably narrower than that of the control group at 72 h after radiation, indicating that the speed of wound healing was promoted by X-ray irradiation. At the protein level, the expression of E-cadherin was reduced (Figure 3B). Meanwhile, the expression of fibronectin and β-catenin were enhanced in A549 cells at 48 h and 72 h after 2 Gy irradiation, compared with the levels detected in non-irradiated controls (Figure 3B).
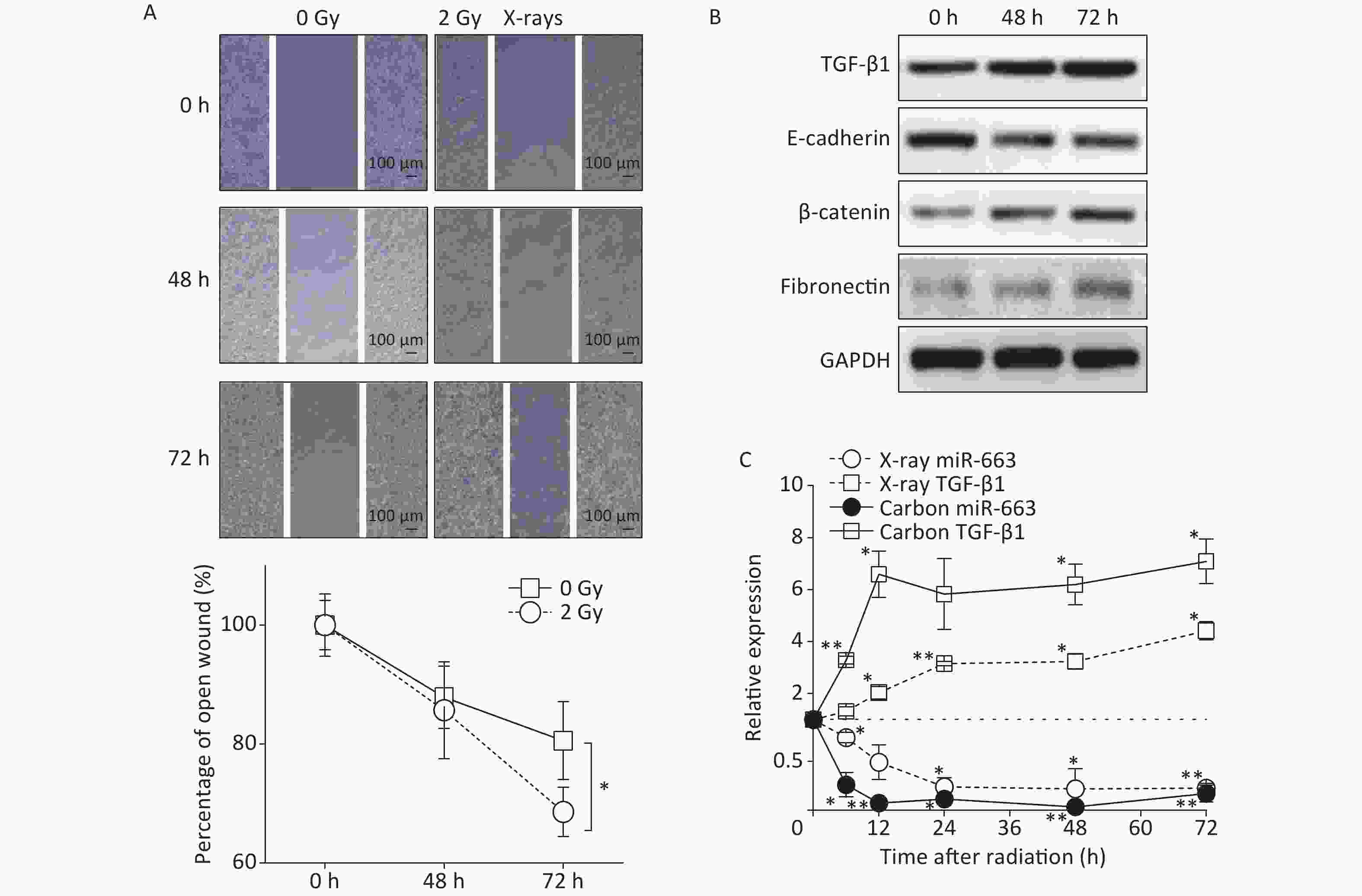
Figure 3. Carbon ions and X-ray irradiation suppresses miR-663a expression and increases TGF-β1 levels. (A) Scratch wound healing assay result of A549 cells with 0 Gy or 2 Gy X-rays irradiation. *P < 0.05 represents statistical significance of comparison at 72 hours after radiation. (B) Protein levels of TGF-β1, E-cadherin, β-catenin, and fibronectin in A549 cells at 48 h and 72 h after X-rays irradiation. (C) Relative expression of TGF-β1 mRNA and miR-663a in A549 cells after X-rays irradiation. *P < 0.05 or **P < 0.01 represents statistical significance of comparison against non-irradiated control.
Furthermore, the expression of miR-663a and the mRNA levels of TGF-β1 were determined at different time points by real time PCR after irradiation with 2 Gy of X-rays and 2 Gy of Carbon ions. The data showed a significant decrease in miR-663a expression after irradiation with either Carbon ions or X-rays (Figure 3C). Moreover, the mRNA levels of TGF-β1 were upregulated, correlating with the protein levels (Figure 3B and 3C). Particularly, the suppression of miR-663a expression and the induction of TGF-β1 expression by carbon ion irradiation were more remarkable than that by X-rays (Figure 3C). These data confirm that radiation induces pulmonary carcinoma epithelial cells to undergo EMT. Additionally, the radiation-induced increase in TGF-β1 and the inverse relationship between TGF-β1 and miR-663a expression after IR suggests a regulatory role of miR-663a in radiation-induced EMT.
-
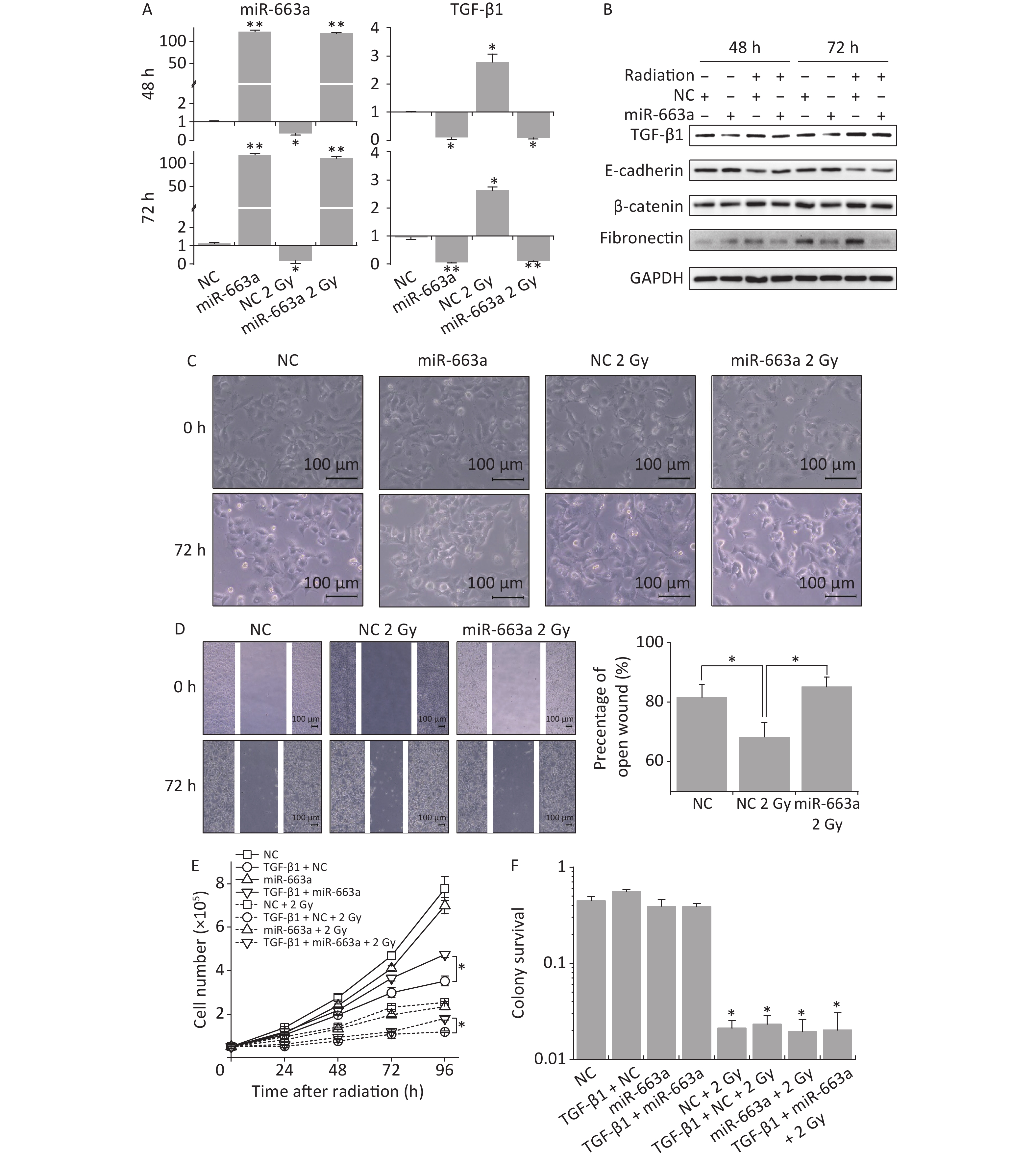
Since miR-663a overexpression was shown to suppress TGF-β1-induced EMT in A549 cells, we explored the potential of upregulated miR-663a in preventing IR-induced EMT. A549 cells were transfected with miR-663a mimics or NC in the presence or absence of 2 Gy X-rays irradiation. Expression of miR-663a, TGF-β1 and EMT-associated factors, cell migration, and cell morphological changes were detected.
According to real time PCR results, X-ray irradiation reduced miR-663a expression by more than 80% after NC transfection compared with non-irradiated control. The miR-663a level was upregulated 120-fold in cells transfected with mimics, even under IR treatment conditions (Figure 4A). Meanwhile, the levels of TGF-β1 mRNA were increased to 2.78 times at 24 h and slightly reduced to 2.63 times at 48 h after irradiation. Additionally, TGF-β1 mRNA levels were inhibited more than 85% after miR-663a transfection, and this inhibition was not reversed by radiation (Figure 4A). Moreover, the changes in TGF-β1 protein levels were consistent with that of mRNA levels after radiation and transfection with miR-663a mimics (Figure 4B).
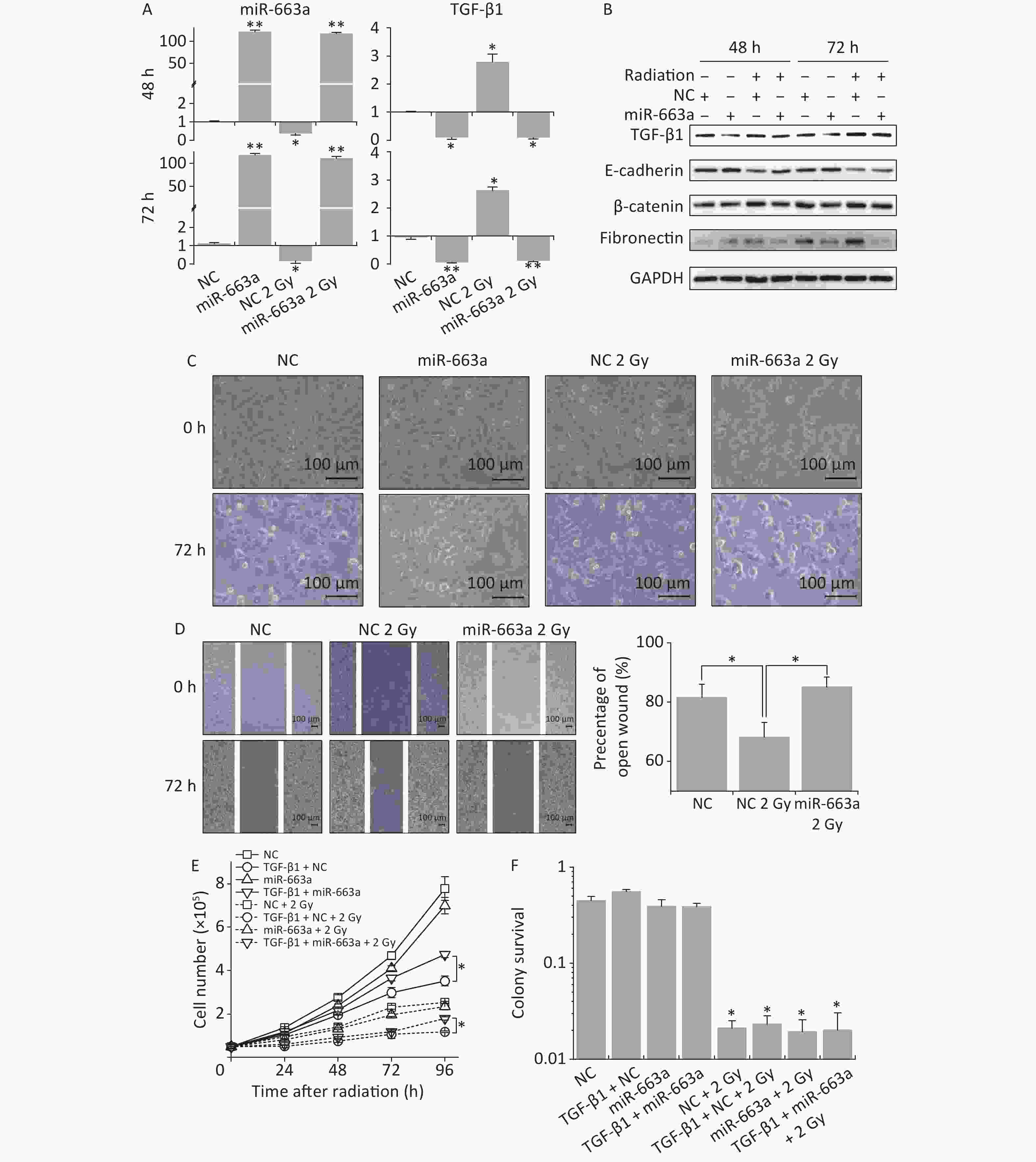
Figure 4. The suppression of TGF-β1 by miR-663a reserves the IR-induced EMT but affects neither survival nor proliferation. (A) Relative expression of TGF-β1 mRNA and miR-663a in A549 cells after X-rays irradiation and miR-663a transfection. *P < 0.05 or **P < 0.01 represents statistical significance of comparison against NC. (B) Protein levels of TGF-β1, E-cadherin, β-catenin, and fibronectin in A549 cells at 48 h after X-rays irradiation and miR-663a transfection. (C) Cell morphology changings of A549 cells treated with X-rays and miR-663a transfection. (D) Scratch wound healing assay result of A549 cells with X-rays irradiation and miR-663a transfection. *P < 0.05 represents statistical significance of comparison. (E) Cell numbers of A549 cells treated with X-rays and miR-663a transfection. *P < 0.05 represents statistical significance of comparison at 96 h after treatments. (F) Colony survival rates of A549 cells treated with X-rays and miR-663a transfection. *P < 0.05 represents statistical significance of comparison against NC.
The expression of EMT-associated proteins was also measured to evaluate the influence on EMT by IR and miR-663a. As showed in Figure 4B, the suppression of TGF-β1 expression by miR-663a resulted in an upregulation of E-cadherin while the radiation-induced increase in TGF-β1 resulted in the downregulation of E-cadherin both at 48 h and 72 h after irradiation. The changes in protein levels of fibronectin and β-catenin paralleled the changes in the TGF-β1 level, which is upregulated by radiation and downregulated by miR-663a (Figure 4B).
Cell morphology was observed at 72 h following irradiation and miR-663a transfection. Compared with the non-irradiated NC group, X-ray-irradiated A549 cells lost their round, cobblestone-like appearance and showed an elongated mesenchymal-like morphology (Figure 4C). However, this morphological change was abolished in the irradiated miR-663a transfected group. Additionally, scratch assays were used to detect the effects of miR-663a on the migration ability of irradiated cells. The width of the open wound at 72 h was about 80%, 68%, and 83% of that at 0 hour in non-irradiated NC-transfected, 2 Gy irradiated NC-transfected, and 2 Gy irradiated miR-663a-transfected groups, respectively (Figure 4D). The speed of wound healing was significantly promoted by X-ray irradiation, while this promotion was attenuated by miR-663a.
Furthermore, cell proliferation and colony survival (CS) assays were also performed to evaluate the effect of miR-663a on the cellular radiation response. As shown in Figure 4E, the cell proliferation rate of miR-663a-transfected A549 cells was slower than that of NC both in the presence and absence of radiation, but the difference is insignificant. On the other hand, at 96 hours, TGF-β1 treatment restrained cell proliferation rate when transfected with NC, while the miR-663a-transfected cells significantly reversed the suppression effect of TGF-β1 when treated or untreated with X-rays (Figure 4E). As expected, after 2 Gy of X-ray irradiation, the proliferation rates of A549 cells in each team were all suppressed (Figure 4E). Moreover, the colony formation rate of IR-treated cells was remarkably less than that of non-irradiated cells. However, neither the TGF-β1 treatment nor the miR-663a transfection induced a significant change when compared with NC both in the presence and absence of IR (Figure 4F).
Taken together, our data revealed that miR-663a transfection is capable of suppressing radiation-induced EMT via TGF-β1 without influencing cell proliferation or CS.
-
Though radiotherapy is one of the main treatment strategies in cancer, accumulating evidence shown that radiation exposure can induce EMT and enhance the invasive and migratory abilities of tumor cells [5-7]. Considering that metastasis is the major cause of cancer-related deaths, the prevention of radiation-induced metastasis is fundamental in improving radiotherapy outcomes. In this study, we found both radiation-specific suppression of miR-663a and stimulation of TGF-β1 contribute to radiation-induced EMT, which indicated by changes in morphology and the expression of EMT marker proteins in A549 cells. Increased miR-663a effectively suppresses IR-induced EMT, as well as the suppression of TGF-β1-induced EMT in A549 cells.
Generally, EMT has been shown to play an important role in the acquisition of stemness and of migratory and invasive properties in cancer cells, enabling the initiation of metastasis, which has been associated with poor prognosis [8, 35]. TGF-β has long been considered as an important factor that induces EMT in developmental processes, cancer, and other pathological conditions [13-16]. In the present study, we chose a dose of 5 ng/mL of TGF-β1 to induce EMT in cancer cells as reported in previous researches [13, 14]. Our results confirm that TGF-β1 stimulation remarkably increases migration, induces mesenchymal-like cell morphology, downregulates the epithelial marker E-cadherin, and upregulates mesenchymal molecular markers, such as β-catenin, and fibronectin, in A549, H1299, H446, and Beas-2B cells (Figure 1).
Upregulation of TGF-β1 by IR was linked with IR-induced EMT in previous studies [19-21]. Here we detected an increase in the expression of TGF-β1 after X-ray irradiation at both transcriptional and translational levels. Meanwhile, our data also showed enhanced cell migration, downregulation of E-cadherin, and upregulation of β-catenin and fibronectin, which herald radiation-induced EMT (Figures 3–4). These findings were in line with previous studies [18-21].
miRNAs are known to be important post-transcriptional regulators of gene expression in a variety of life processes, including EMT and radiation response. In the present study, transfection with miR-663a mimics in A549 cells resulted in upregulation of E-cadherin and downregulation of β-catenin and fibronectin, as well as neutralized the TGF-β1-induced promotion of cell migration (Figure 2). The data confirm the suppression effect of miR-663a on TGF-β1-induced EMT.
Aberrant expression of miRNAs has been robustly detected during cellular radiation responses [27, 36]. Our previous studies uncovered the radiation-responsive expression of several miRNAs, such as miR-663, miR-5094, and miR-300, and identified their functions during radiation response [27, 30, 31, 37].
miR-663, a member of the primate-specific miRNA family, is an important miRNA in tumorigenesis, and its main functions have been specified in the regulation of tumor proliferation, invasion, metastasis, and apoptosis [34]. The expression of miR-663 in glioblastoma, papillary thyroid carcinoma, and pancreatic cancer was lower than that of normal tissues, and miR-663 inhibited EMT via TGF-β1 in cancer cells [24, 25, 34]. Our previous study found that IR inhibits the expression of miR-663a in HeLa cells and 786-O cells [30, 31]. In the present study, downregulation of miR-663a after X-ray and carbon ion irradiation was also detected (Figure 3). In contrast, a previous study reported that an increase in miR-663a has been detected in irradiated human peripheral blood lymphocytes and human lymphoblast AHH-1 cells [38]. This inconsistency between previous research and our current study of miR-663a expression may be due to differences in the cell types used.
Even with X-ray irradiation, the increase in miR-663a by miR-663a mimic transfection inhibited the increase in TGF-β1, recovered E-cadherin expression, suppressed both β-catenin and fibronectin expression, and neutralized the IR-induced changes in cell morphology (Figure 4). These findings reveal that miR-663a transfection is capable of suppressing radiation-induced EMT via TGF-β1. According to data shown in Figure 4E, TGF-β1 is enabling to suppress cell proliferation both in the presence and absence of radiation treatment. This phenomenon is consistent with previous studies, which demonstrated that TGF-β1 induces apoptosis in cancer cells. [14, 39] However, we failed to detect a remarkable downregulation of colony formation by TGF-β1 treatment (Figure 4F), and the underlying mechanism for this is worthy of further study.
Interestingly, at 96 hours after treatment, miR-663a transfection significantly reversed the suppression of cell proliferation by TGF-β1 both in the presence and the absence of radiation (Figure 4E). Furthermore, we noticed that miR-663a transfection slightly suppressed both cell proliferation rate and cell CS rate, but not significantly (Figure 4E and 4F). The data reveals that miR-663a has no influence on cellular radiation sensitivity in A549 cells, although it is capable of suppressing TGF-β1-induced EMT and cellular damages. X-rays are most commonly used in current radiotherapy, and carbon ion radiotherapy is an emerging radiation therapy, which has demonstrated superior clinical advantages over conventional radiation [40]. However, both X-ray and carbon ion irradiation resulted in a downregulation of miR-663a expression. Considering that transfection of miR-663a after IR has no effect on cell proliferation nor cell survival but significantly suppressed EMT (Figure 4), miR-663a can potentially be applied to enhance the efficiency of radiotherapy via metastasis suppression.
In summary, our present study uncovered an EMT-suppressive effect of miR-663a by targeting TGF-β1. Additionally, radiation-specific decrease in miR-663a could emerge as an important regulator of IR-induced EMT via TGF-β1. Although further investigations are necessary to elucidate the detailed mechanisms of EMT suppression by miR-663a during cellular radiation response, here we provide sufficient data to link miR-663a with radiation-induced EMT and to reveal a novel mechanism of miRNA-TGF-β signaling during tumor metastasis.
-
We would like to thank the support of Heavy Ion Research Facility in Lanzhou (HIRFL). We also want to thank the biomedical platform of the Public Technical Service Center, Institute of Modern Physics, Chinese Academy of Sciences for experimental support.
-
The authors have declared that no competing interest exists.
MiR-663a Inhibits Radiation-Induced Epithelium-to-Mesenchymal Transition by Targeting TGF-β1
doi: 10.3967/bes2022.059
- Received Date: 2021-08-19
- Accepted Date: 2021-10-19
-
Key words:
- Epithelium-to-mesenchymal transition (EMT) /
- Ionizing Radiation /
- TGF-β1 /
- microRNA /
- miR-663a
Abstract:
| Citation: | QU Pei, SHAO Zhi Ang, WANG Bing, HE Jin Peng, ZHANG Ya Nan, WEI Wen Jun, HUA Jun Rui, ZHOU Heng, LU Dong, DING Nan, WANG Ju Fang. MiR-663a Inhibits Radiation-Induced Epithelium-to-Mesenchymal Transition by Targeting TGF-β1[J]. Biomedical and Environmental Sciences, 2022, 35(5): 437-447. doi: 10.3967/bes2022.059 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: