-
Alzheimer’s disease is one of the most debilitating neurodegenerative diseases affecting the aging population. One of the neuropathological hallmarks of AD is an aggregation of the tau protein into neurofibrillary tangles (NFTs) in neurons and glial cells of the brain. Tau is a microtubule-associated protein expressed mainly in neurons of the central nervous system [1]. When tau is hyperphosphorylated, it cannot bind to microtubules, resulting in the self-aggregation of tau into NFTs, which prevents axonal transport and normal neuronal function [2]. Alonso Adel et al. [3] showed that tau-S199 and tau-T231 are critical sites for phosphorylation, leading to the transformation of tau into an inhibitory molecule that sequesters normal microtubule-associated proteins. During the early stages of AD, tau may be aberrantly localized to dendrites after specific phosphorylation at S199. Phosphorylation of human tau (hTau) at S199 triggers synaptotoxicity independent of tau aggregation, which is correlated with impaired memory in patients with AD [4]. p‐Tau231 is present in pre‐NFTs before the formation of the overt filament. CSF mid‐p‐tau231 is one of the most promising biomarkers for diagnosing preclinical AD [5]. Glycogen synthase kinase3-β (GSK3-β) is a key kinase involved in tau phosphorylation. GSK-3β is a constitutively active protein kinase that is primarily regulated by activation via phosphorylation at its Tyr216 site [6]. Okadaic acid (OA) is one of the most common and widely distributed marine toxins. OA induces phosphorylation of tau by inhibiting PP2A and activating GSK-3β, thereby promoting the formation of intracellular NFTs[7]. The triterpene ganoderic acid A (GAA) is a compound found in Ganoderma lucidum. Studies have shown that GAA inhibits the release of cellular histamine, enhances the function of various digestive organs, and possesses pharmacological activities, such as depressurization, hepatic protection, anti-hyperlipidemia, and anti-inflammation [8]. GAA may promote the activation of PI3K/AKT and mTOR by enhancing miR-153 in hypoxia-impaired PC12 cells [9]. However, few reports are available on the activity of ganoderic acid B (GAB). GAA and GAB are the best characterized among the dozens of isolated GAs. There are no reports on their neuroprotective effects or their correlative effects on OA-induced neuronal apoptosis. Therefore, this study investigated the protective functions of GAA and GAB in OA-impaired PC12 cells to provide a basis for a novel AD therapeutic strategy.
GAA and GAB (purity ≥ 98%) were purchased from Shanghai Yuanye Biotechnology (Shanghai, China). OA was purchased from Sigma-Aldrich (St. Louis, MO, USA). We established in vitro PC12 cell models using OA. Cell survival, PC12 cell morphology, lactate dehydrogenase (LDH) release, [Ca2+]i, and caspase-3 activity were assessed. Moreover, the expression of Bax, Bad, p-Tau (S199), p-Tau (T231), p-GSK-3β (T216), and GSK-3β were determined to reveal the underlying mechanisms.
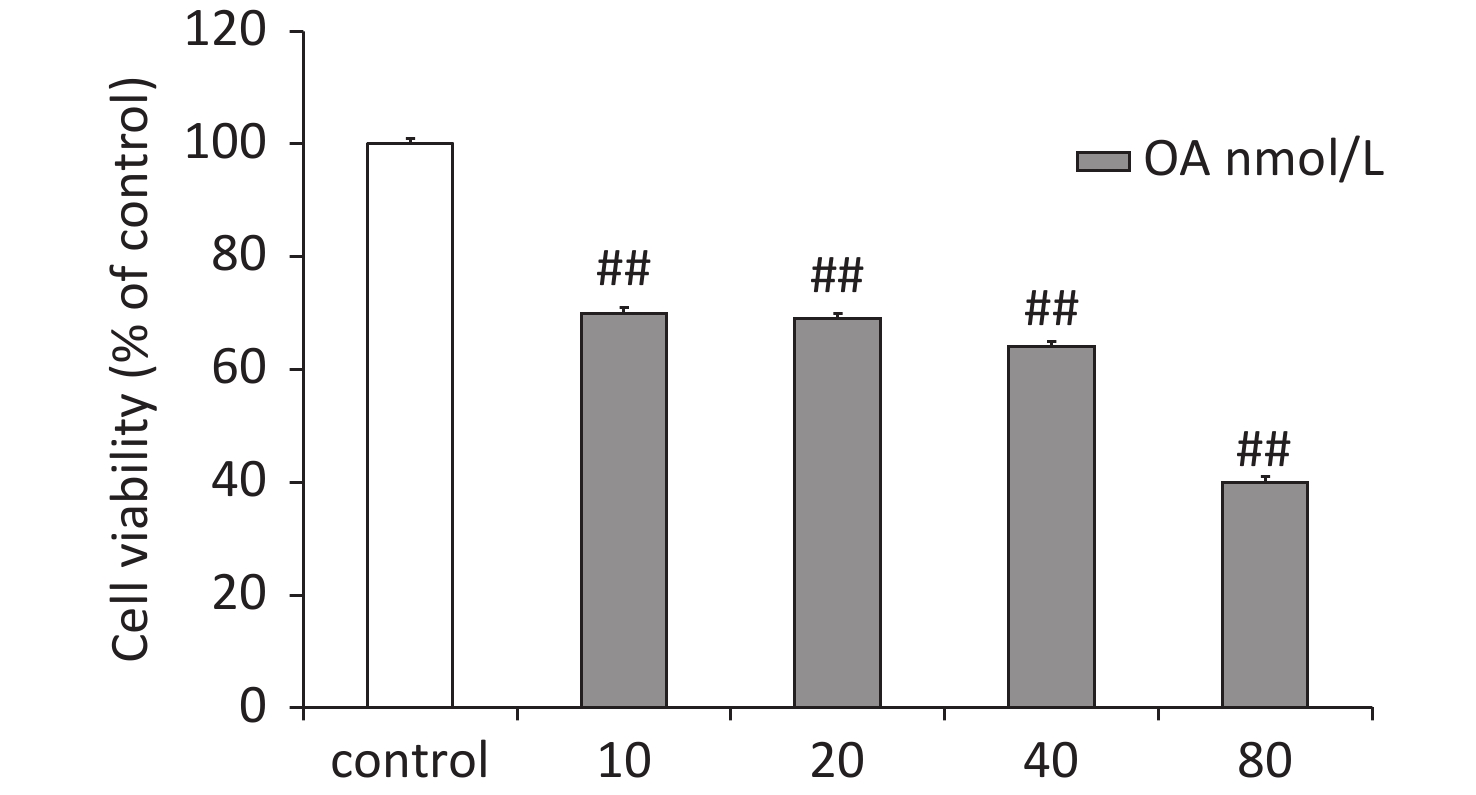
Differentiated rat pheochromocytoma PC12 cells were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). The PC12 cells were grown in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA), 100 U/mL penicillin, and 100 U/mL streptomycin at 37 °C in a humidified 5% CO2 atmosphere. The PC12 cells were treated with different concentrations of OA to determine the optimal damaging concentration (Supplementary Figure S1, available in www.besjournal.com). As results, OA (40 nmol/L) reduced cell viability by 64.0% ± 3.6% in PC12 cells and was selected as the optimal concentration for subsequent experiments. The cells were pretreated with GAA/GAB (12.5, 25, or 50 μg/mL), and then exposed to OA (40 nmol/L) for 24 h.
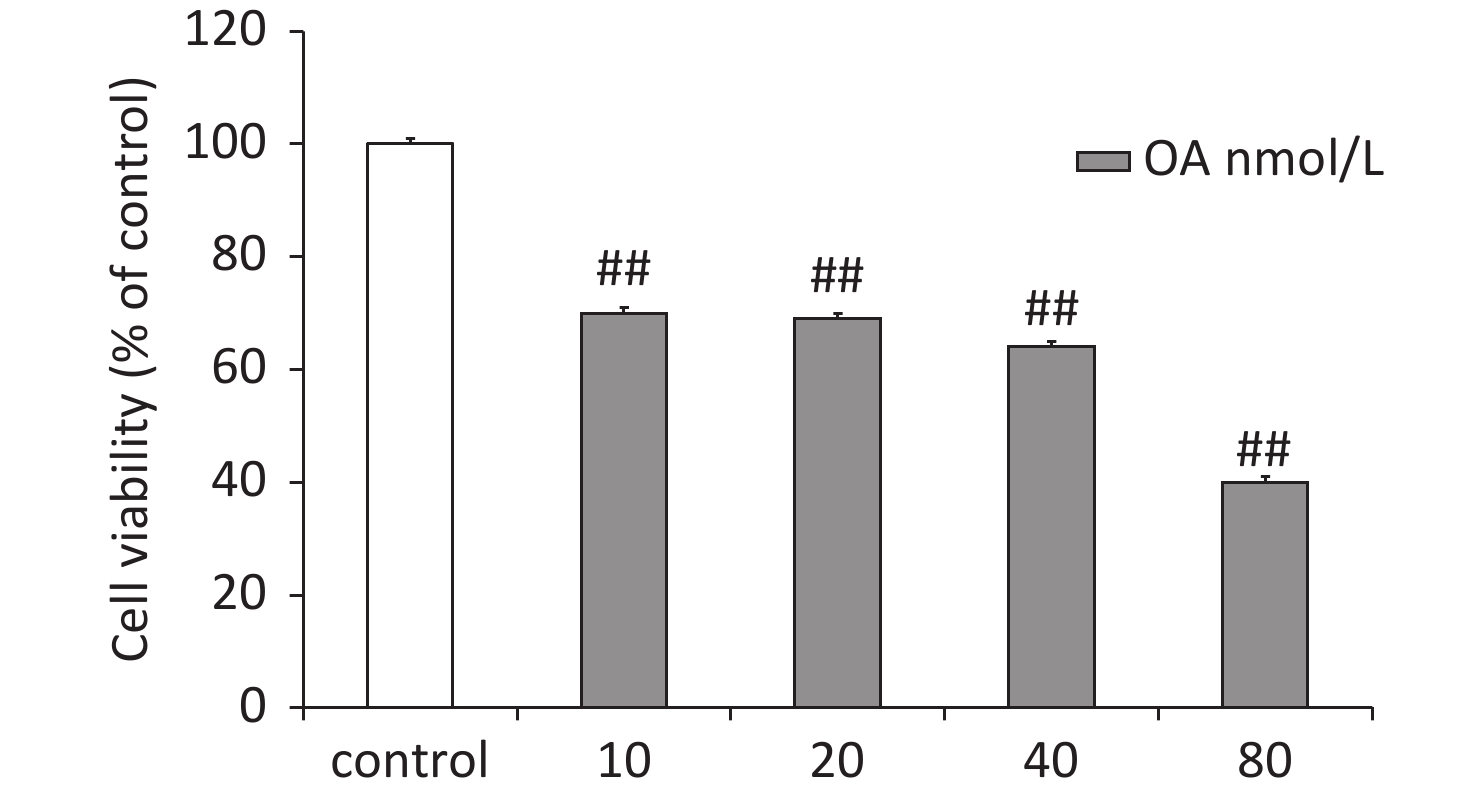
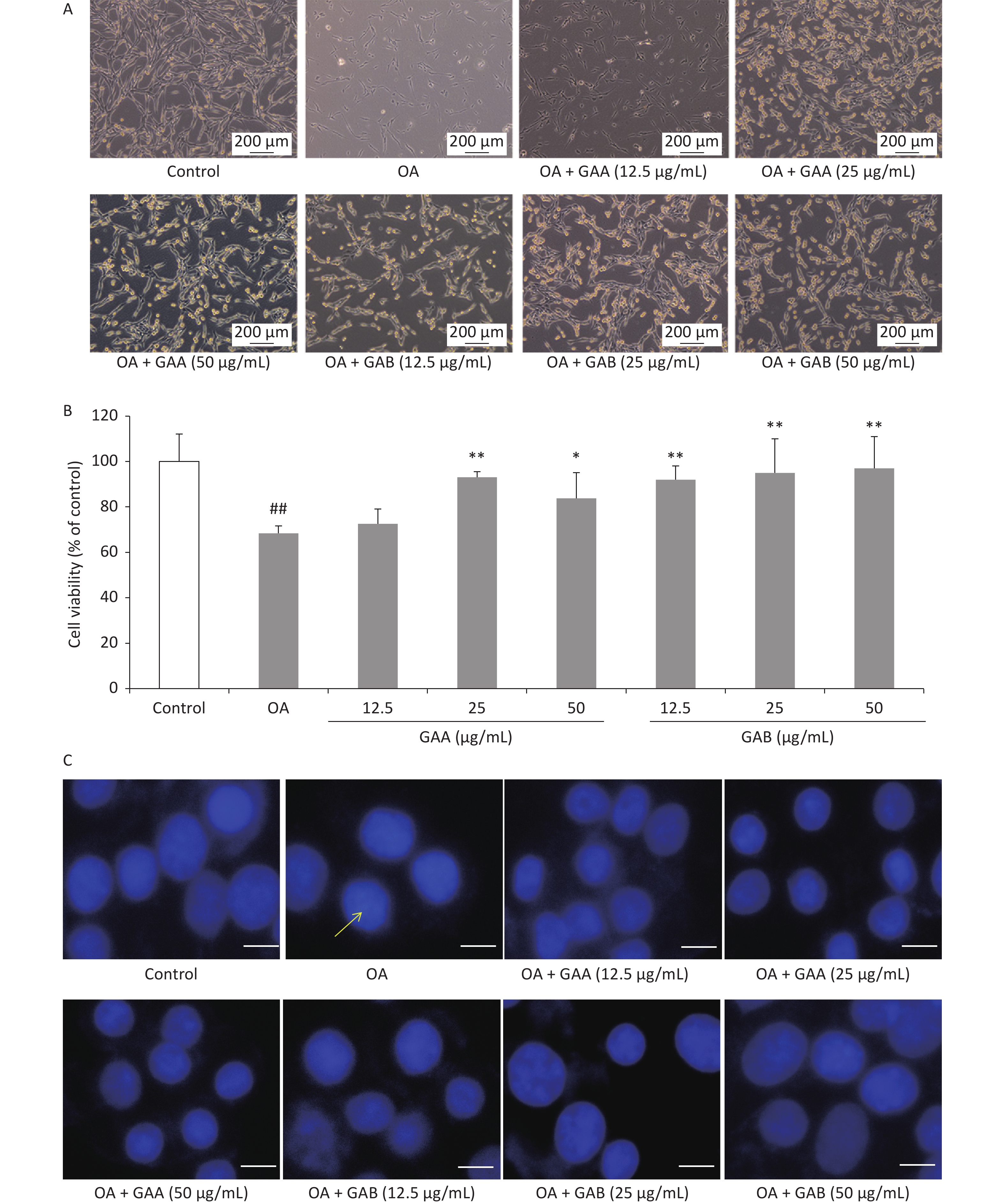
Figure S1. Effects of OA-induced changes in PC12 cell viability according to the MTT assay. Data are mean ± SD (n = 5). ##P < 0.01 compared to the control group.
GAA/GAB-mediated inhibition of OA-induced neurotoxicity was assessed by morphological observations, the MTT assay, and Hoechst33342 staining. Cell morphology was observed and recorded using an inverted phase-contrast microscope (Leica, Wetzlar, Germany). The viability of GAA- and GAB-treated PC12 cells was assessed using the MTT method. Cell viability was expressed as a percentage of untreated control cells. Cell morphology improved in the groups pretreated with 12.5, 25, or 50 μg/mL of GAA/GAB (Figure 1A). The viability of PC12 cells treated with 40 nmol/L OA alone decreased significantly (68.4% ± 3.2% viability of control cells). However, GAA/GAB significantly increased the viability of PC12 cells in a concentration-dependent manner when compared to the OA group (Figure 1B). Nuclear morphology was evaluated using membrane-permeable Hoechst33342. As shown in Figure 1C, PC12 cells treated with 40 nmol/L OA for 24 h exhibited apoptotic characteristics, including chromatin condensation, shrinkage of nuclei, and an increased proportion of DNA-fragmented cells. Fewer DNA-fragmented cells were present after incubating the neurons with GAA/GAB. These results indicate that GAA/GAB may alleviate cellular damage caused by OA and may have neuroprotective effects.
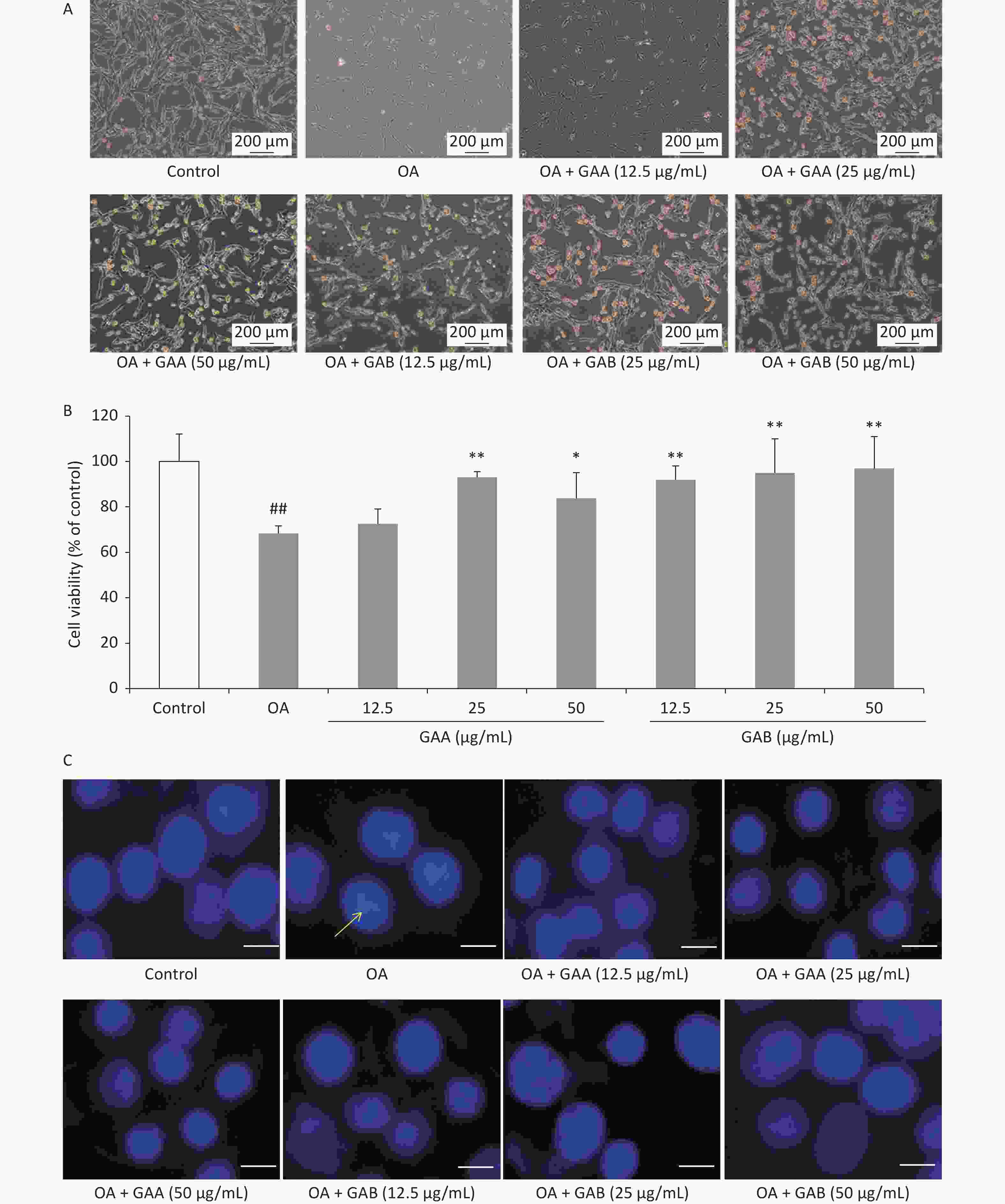
Figure 1. Effects of GAA/GAB on the morphology and viability of PC12 cells treated with 40 nmol/L OA. (A) Representative photomicrographs of cell morphology (400×). (B) Effects of GAA/GAB on OA-induced changes in PC12 cell viability based on the MTT assay. (C) Effects of GAA/GAB on OA-induced changes in PC12 nuclear morphology based on Hoechst33342 staining (Scale = 50 µm). OA, okadaic acid; GAA, ganoderic acid A; GAB, ganoderic acid B. Data are mean ± SD (n = 5). ##P < 0.01 compared to the control group. *P < 0.05 and **P < 0.01 compared to the OA group.
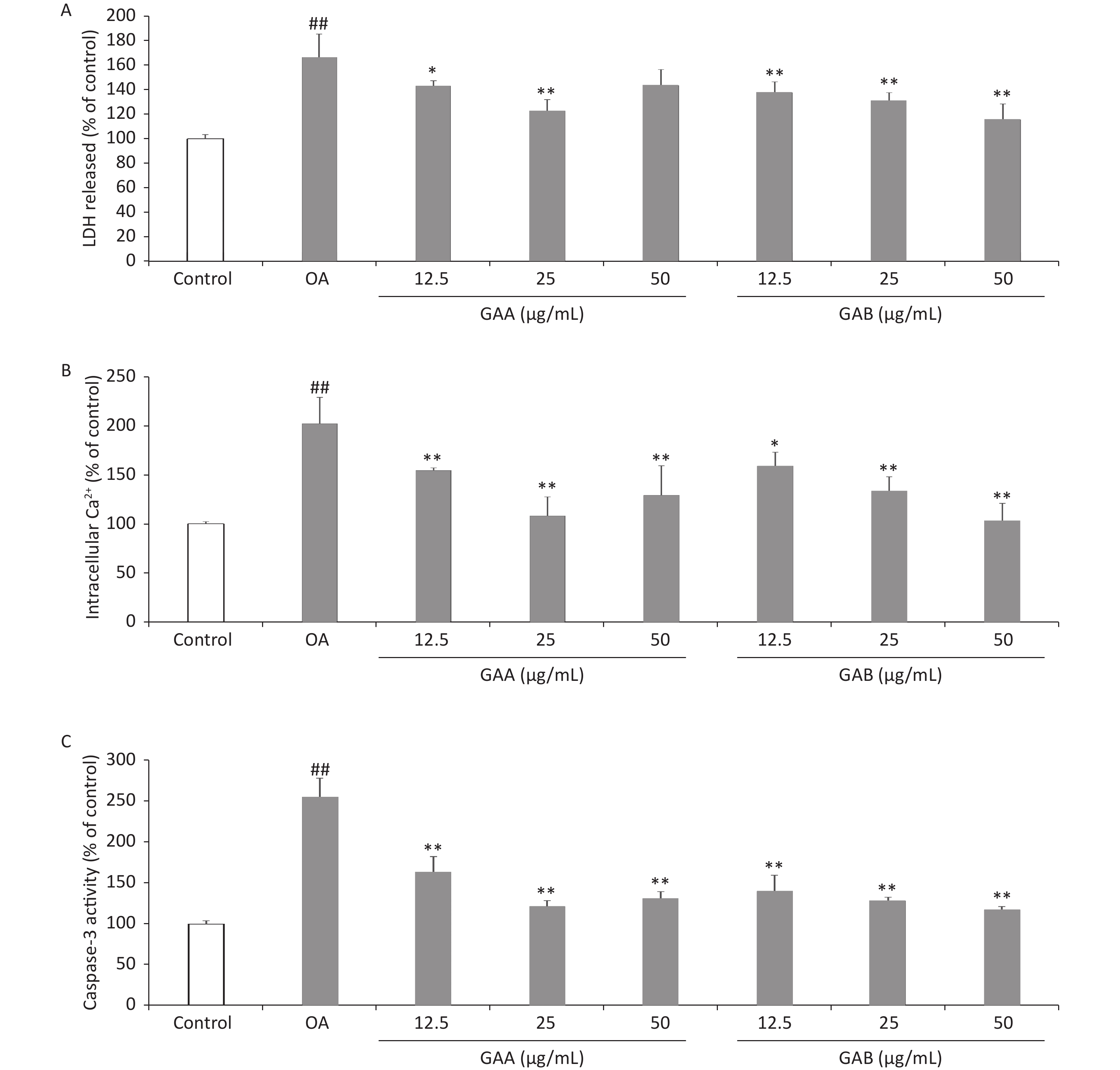
Cytotoxicity was quantitatively assessed by assaying the activity of LDH released from PC12 cells into the culture medium using an LDH cytotoxicity assay kit (Beyotime, Shanghai, China). The Ca2+-sensitive fluorescent Fluo-3/AM probe was used to monitor the changes in [Ca2+]i using spectrofluorometry. Caspase-3 activity was measured using a caspase-3 assay kit (Beyotime) according to the manufacturer’s instructions. Incubating the PC12 cells with 40 nmol/L OA for 24 h increased the release of LDH (166.4% ± 19.1% of the control). GAA significantly attenuated OA-induced toxicity, reducing LDH leakage to 143.0% ± 4.2%, 122.7% ± 9.2%, and 143.6% ± 12.9% at 12.5, 25, and 50 μg/mL, respectively. GAB significantly attenuated OA-induced toxicity and reduced LDH leakage to 137.7% ± 8.7%, 131.2% ± 6.2%, and 115.9% ± 12.4% at 12.5, 25, and 50 μg/mL, respectively (Figure 2A). LDH is widely used as a biomarker to diagnose cellular, tissue, and organ damage in toxicology and clinical chemistry. Our results demonstrate that OA exposure-induced LDH leakage in PC12 cells was attenuated by GAA/GAB. After exposing the PC12 cells to 40 nmol/L OA for 24 h, [Ca2+]i increased to 202.4% ± 26.9% of the control value. Pretreating with GAA attenuated the increase in [Ca2+]i to 154.8% ± 2.6%, 108.4% ± 19.3%, and 129.4% ± 29.8% of the control at 12.5, 25, and 50 μg/mL, respectively. Pretreatment with GAB dose-dependently attenuated the increase in [Ca2+]i to 159.3% ± 14.0%, 133.3% ± 15.1%, and 103.2% ± 17.9% of the control at 12.5, 25, and 50 μg/mL, respectively (Figure 2B). Ca2+ regulates the function of various enzymes and proteins, which play important roles as secondary messengers in signal transduction pathways, including the cell survival, proliferation, differentiation, and apoptosis pathways. Ca2+ triggers Ca2+-activated kinases, which mediate tau phosphorylation, leading to the formation of NFTs in an AD mouse model [10]. GAA/GAB significantly decreased OA-induced Ca2+ levels, indicating that GAA/GAB protects PC12 cells from damage by preventing Ca2+overload. Our findings suggest a new mechanism of action to further clarify the neuroprotective effects of GAA/GAB. Caspase-3 activity in PC12 cells treated with 40 nmol/L OA for 24 h increased significantly (255.2 ± 23.1% of the control value, Figure 2C). However, pretreating the cells with GAA/GAB before the OA treatment significantly attenuated the increased caspase-3 activity. GAA attenuated caspase-3 activity to 163.8% ± 18.4%, 121.9% ± 6.6%, and 131.4% ± 8.1% of the control at 12.5, 25, and 50 μg/mL, respectively. GAB dose-dependently attenuated caspase-3 activity to 140.0% ± 19.5%, 128.6 ± 4.0%, and 117.1% ± 4.0% of the control at 12.5, 25, and 50 μg/mL, respectively.
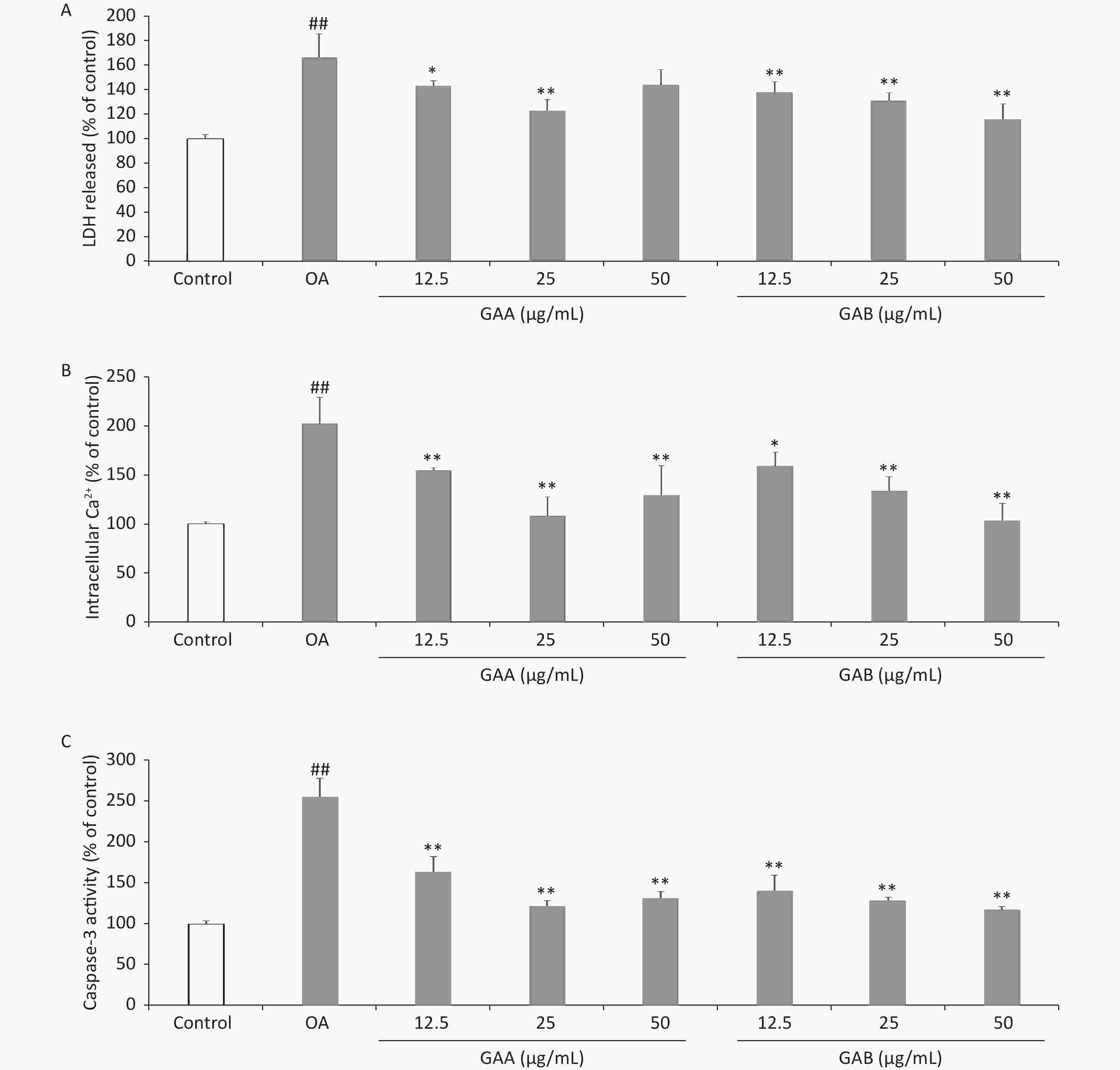
Figure 2. Effects of GAA/GAB on LDH leakage, [Ca2+]i, and caspase-3 activity in OA-treated PC12 cells. The cells were pretreated with increasing concentrations of GAA/GAB for 24 h and then exposed to 40 µmol/L OA for 24 h. The effect of GAA/GAB on (A) LDH release, (B) [Ca2+]i, and (C) caspase-3 activity in OA-treated PC12 cells. OA, okadaic acid; GAA, ganoderic acid A; GAB, ganoderic acid B. LDH, lactate dehydrogenase. Data are mean ± SD (n = 5). #P < 0.05 and ##P < 0.01 compared to the control group. *P < 0.05 and **P < 0.01 compared to the OA group.
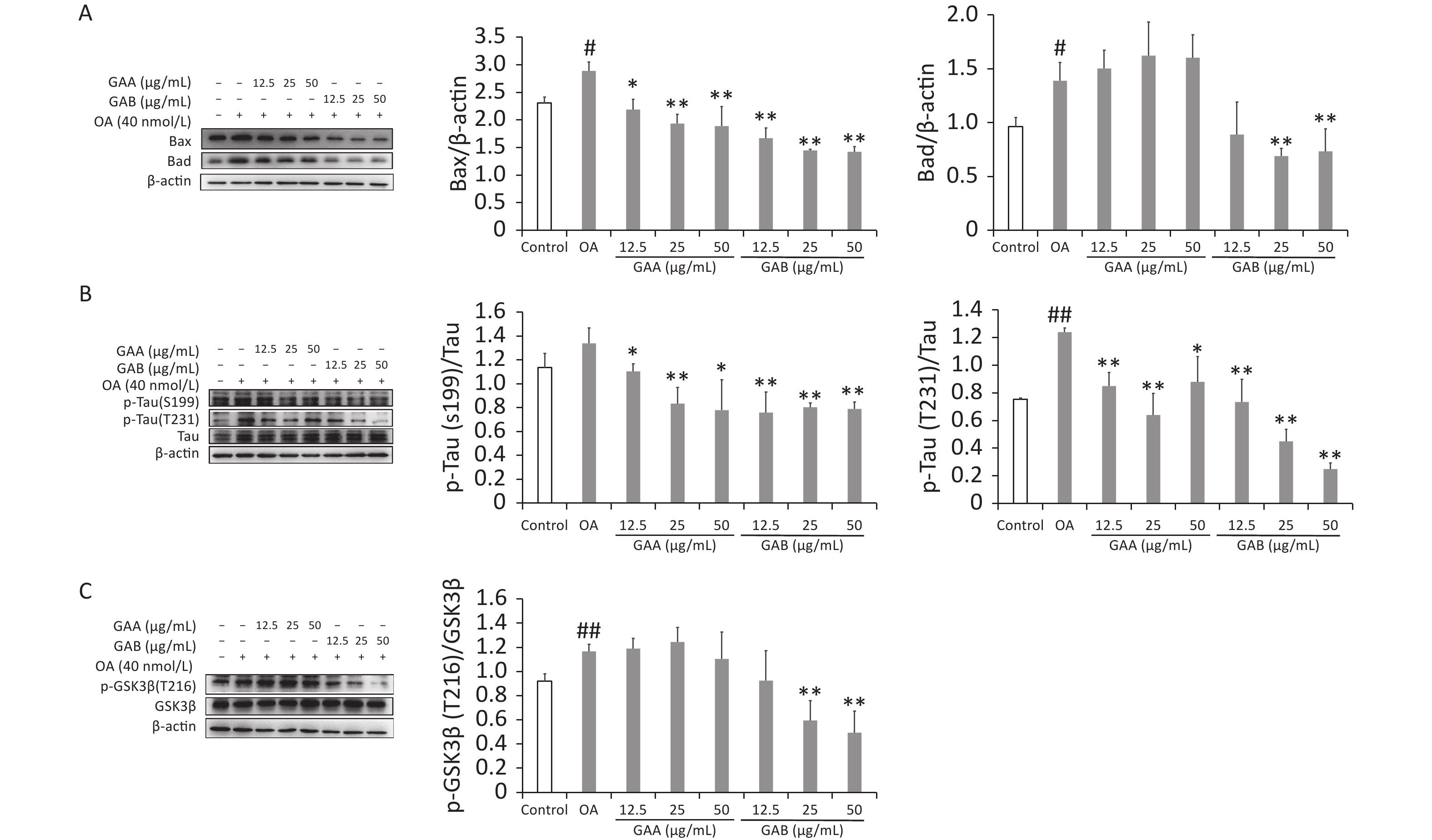
Bax, Bad, tau, p-tau (S199), p-tau (T231), GSK-3β, and p-GSK-3β (T261) (Cambridge, UK) levels were evaluated by western blotting. Apoptosis is a gene-regulated cell death process regulated by the caspase-3 and Bax families. Pro-apoptotic Bcl-2 proteins, such as Bad and Bax, activate caspases and promote cell death by releasing mitochondrial intermembrane space proteins. Tau hyperphosphorylation and neuronal death are induced by OA and are associated with the induction of Bax in rat brains. Bad activity increases significantly in the neurons of AD patients and 5XFAD mice. Our results show that the OA treatment increased caspase-3 activity while upregulating Bax and Bad expression. GAA significantly reduced caspase-3 activity and downregulated Bax expression in the OA-treated groups; however, it did not significantly affect Bad expression. In contrast, GAB reduced caspase-3 activity and downregulated Bax and Bad expression (Figure 3A). Abnormal tau hyperphosphorylation is a key pathological change correlated with neurodegeneration in the brains of patients with AD. Tau has over 40 possible phosphorylation sites, and phosphorylation is regulated by several kinases and phosphatases. GSK-3β is an upstream signaling molecule of tau hyperphosphorylation that is proposed to be a major kinase responsible for tau hyperphosphorylation during the progression of AD. OA treatment significantly increased the phosphorylation of the tau protein at sites S199 and T231 in PC12 cells. The GAA/GAB pretreatment reversed this effect (Figure 3B). Treating PC12 cells with OA significantly increased the phosphorylation of GSK-3β at Tyr216, and the GAB treatment significantly decreased the phosphorylation of GSK-3β at Tyr216 (P < 0.01). However, no differences were observed in the GAA group compared to the OA group (Figure 3C).
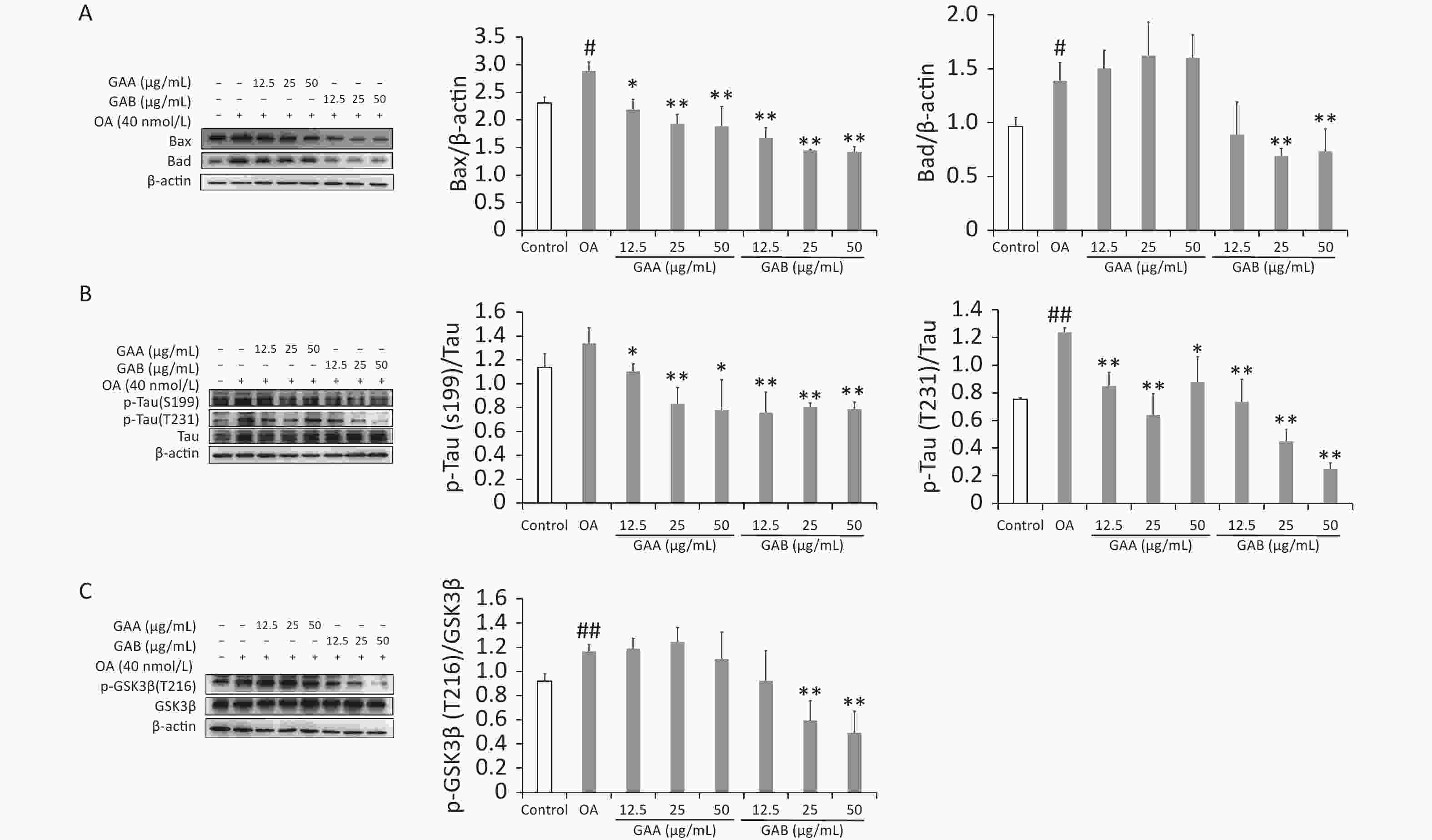
Figure 3. Effects of GAA/GAB on protein expression in OA-treated PC12 cells. (A) Bax and Bad expression. (B) p-tau (S199) and p-tau (T231) expression. (C) p-GSK-3β (T216) and GSK-3β expression. OA, okadaic acid; GAA, ganoderic acid A; GAB, ganoderic acid B. Data are mean ± SD (n = 3). #P < 0.05 compared to the control group. *P < 0.05 and **P < 0.01 compared to the OA group.
In conclusion, this study demonstrates that GAA/GAB protected PC12 cells against OA-induced apoptosis and that the protective effect of GAB was superior to that of GAA. Our results also indicate that GAA significantly inhibited tau phosphorylation at S199 and T231 and markedly inhibited apoptosis of PC12 cells by downregulating Bax; however, the underlying mechanism remains unclear. Unlike GAA, GAB downregulated Bax and Bad. The GAB treatment reduced GSK-3β activity by downregulating the phosphorylation of GSK-3β at T216. Taken together, these results suggest that GAB mainly reduces tau hyperphosphorylation by inhibiting GSK-3β activity. Therefore, the results of this study support GAB as a potential therapeutic compound for treating AD and other tau-related neurodegenerative disorders.
The authors have no conflicts of interest to declare.
CUI Jing performed the experiments and prepared the original draft of the manuscript. MENG Yu Han and WANG Zhan Wei assisted in performing the experiments. WANG Jing drafted the manuscript. SHI Dong Fan and LIU Duo proofread the manuscript and provided technical writing assistance. All authors have approved the final article.
We thank Editage [www.editage.cn] for English language editing.
HTML
 22327Supplementary Materials.pdf
22327Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: