-
Ocular dominance (OD) is referred to the superiority of one eye over the other when the visual sense is produced [1]. This condition can be considered as a preference for the particular laterality of the eye during a mono-visual task or the relative contributions of visual signal transduction between eyes [2]. Empirically, OD tendencies are exhibited in habitual and subconscious behavior. Although active OD practices in daily life are not that wide and definite, their significance in clinical practice has gradually been found [3]. In 2003, Waheed found that eye dominance may be an important determinant of the visual handicap suffered by patients with unilateral macular holes (MH) [4]. Affected dominant eyes were more inclined to undergo surgery. The possible reason may be that the historically dominant eye was more symptomatically aware of binocular interference than the nondominant eye. Up to now, there is a lack of studies that compare the macular anatomic structure in the background of maculopathy between dominant and nondominant eyes; thus, we were interested in the possible differences. In this article, we included consecutive patients with MH or epiretinal membrane (ERM) eligible for operation and focused on the clinical characteristics in dominant versus nondominant eyes.
This research was reviewed by an independent ethical review board and adhered to the applicable principles and guidelines for protecting human subjects in biomedical research. This cross-sectional study was performed at the Department of Ophthalmology, Eye & ENT Hospital of Fudan University, Shanghai, China. Consecutive patients afflicted with idiopathic MH or ERM requiring pars plana vitrectomy surgery from January 2021 to September 2021 were recruited in this study. This study was conducted following the tenets of the Declaration of Helsinki. The patient inclusion criteria were 1) symptoms of visual impairment, loss of central visual acuity, or metamorphopsia (micropsia or macropsia); 2) unilateral MH or ERM manifestations confirmed by optical coherence tomography (OCT); and 3) laterality of OD tested by “hole-in-card” test. The exclusion criteria were 1) any other maculopathy, such as choroidal neovascularization, pathologic myopia, or inherited maculopathy; 2) secondary MH or ERM caused by uveitis, tractional retinopathy, or retinal vasculopathy; 3) the history of ocular trauma or surgeries, except cataract surgery; and 4) the history of retinal photocoagulation or cryocoagulation. The basic demographics were reviewed for all recruited patients, including name, sex, age, and duration of symptoms. The duration of the symptom was the time beginning from the moment of the patients’ first awareness of visual impairment. The clinical records included best-corrected visual acuity (BCVA) in logarithms of the minimum angle of resolution (logMAR), OCT measurements, disease laterality, and OD. The OCT was performed using the Spectralis® HRA + OCT system (Heidelberg Engineering, Heidelberg, Germany). The MH measurements included the minimum diameter at the nearest ends of the broken retinal tissue and the base diameter between the ends of the retinal tissue at the upper bound of the retinal pigment epithelial layer. The central macular thickness (CMT) in ERM was assessed by manually measuring the distance between the internal limiting membrane and the retinal pigment epithelium in the central fovea. Both horizontal and vertical scans across the central fovea were performed to obtain the average OCT measurement values. The sighting dominance was determined by the “hole-in-card” test [3]. The subject holds a rectangular card with a round hole in the center at the length of the arm. Then, the subject tries to view a single small letter on a visual chart through the round hole at a 6 m distance. The examiner covers either eye of the subject, and the dominant eye continues to see the letter. The OD laterality was established by three successive consistent trials with the same outcomes. All data analyses were performed using GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA, USA). The data fit the normal distribution displayed by average ± standard deviation. When the data does not follow a normal distribution, the data were displayed by a median and 25th percentile value (P25) to 75th percentile value (P75), the nonparametric Wilcoxon signed-rank test was used to compare differences, and the Spearman correlation analysis was used to evaluate the underlying relations. A P < 0.05 was considered to indicate statistical significance.
Ninety-two patients (28 men and 64 women) with unilateral MH and with a median age of 64 years (range 37–77) were included. The median value of symptoms of duration was 5.5 months (range, 0.6–72). The median logMAR BCVA was 1.00 (range, 0.3–3.00). For 50 eyes (54.3%), the right side was affected, and for 42 eyes (45.7%), the left side was affected. Among these, the number of dominant eyes was 24 eyes (26.1%) (Table 1). The right eye was dominant for 50 eyes (54.3%), whereas the left eye was dominant for 42 cases (45.7%). One hundred twenty-eight patients (34 men and 94 women) with unilateral ERM were included, with a median age of 65 years (range 39–82). The median value of symptoms of duration was 12 months (range, 0.5–120). The median logMAR BCVA was 0.70 (range, 0.22–2.00). For 60 eyes (46.9%), the right side was affected, and for 68 (53.1%) eyes, the left side was affected. Among these, the number of dominant eyes was 30 eyes (23.4%) (Table 1). For 70 eyes (54.7%), the right eye was identified as the dominant one, whereas the left eye was identified as dominant for 58 (45.3%) eyes.
Item Macular hole (n = 92) Epiretinal membrane (n = 128) Dominant eye
(n = 24, 26.1%)Non-dominant eye
(n = 68, 73.9%)Dominant eye
(n = 30, 23.4%)Non-dominant eye
(n = 98, 76.6%)Age (years) 61.5 (40−74) 66 (37−77) 65.5 (63−73) 64.5 (39−82) Gender Male 4/Female 20 Male 24/Female 44 Male 14/Female 14 Male 24/Female 76 Disease laterality Right 16/Left 8 Right 34/Left 34 Right 16/Left 14 Right 44/Left 54 Note. The age did not follow a normal distribution, the data was displayed by a median and 25th percentile value (P25) to 75th percentile value (P75). Table 1. Maculopathy disease laterality and ocular dominance
One striking finding was that the disease laterality in MH and ERM happens to the dominant eyes less frequently. Our results showed that 24 (26.1%) eyes were affected with MH, and 28 (23.4%) were affected with ERM, which was the dominant side. Wheed et al. reported the historical eye dominance in patients presenting with MH and found that 56% of the affected eyes were the dominant side [4]. The proportion was significantly higher in our work. The OD tests, where the OD was mainly determined by a designed questionnaire focusing on the eye laterality the candidates had previously used for a one-eyed task, may be among the possible causes of the difference. The outcomes may be more influenced by subjective mood and daily environment. In addition, the historical eye dominance represented the eye that used to be the dominant side before the course of the disease rather than the currently affected dominant side tested in our study. The affected OD laterality may experience a switch from one to another. In addition, we found the proportion of right-eye sighting dominance in our study to be lower than that in the normal cohort. In our study, the right eye was dominant for 50 eyes (54.3%) in MH, whereas in ERM, the right eye was identified as the dominant one for 70 eyes (54.7%). Previous studies reported that right-eye sighting dominance occurs in 2/3 of normal individuals [5]. Perhaps, some of the patients switched eye dominance from the right to the left side.
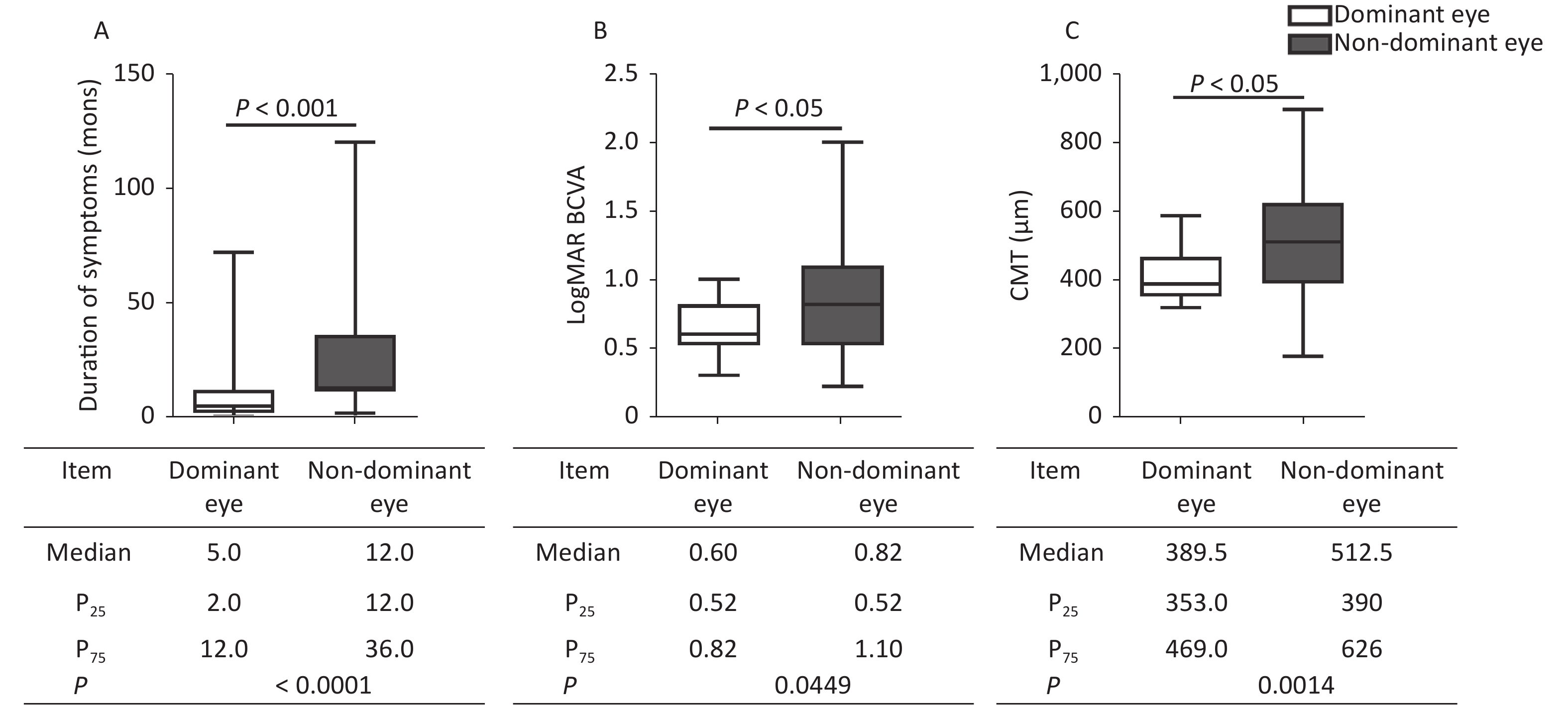
The duration of symptoms, logMAR BCVA, and anatomic measurements on OCT were compared between the dominant and nondominant eyes. In MH, when compared with nondominant eyes (Figure 1), the dominant eyes had a shorter duration of symptoms (P < 0.0001), better logMAR BCVA (P < 0.0001), and smaller hole diameter in MH (P = 0.0389, P = 0.0094). In ERM, besides the shorter duration (P < 0.0001) and better logMAR BCVA (P = 0.0449) in the dominant eyes, the CMT was thinner (P = 0.0014) than in the nondominant eyes (Figure 2). The dominant eyes showed less impaired macula than the nondominant eyes on OCT (Supplementary Figure S1, available in www.besjournal.com). The correlation analysis shows a statistically significant correlation in the duration of symptoms and logMAR BCVA in the dominant and nondominant eyes. The dominant eyes in MH and ERM had a more powerful correlation than the nondominant eyes. Supplementary Figure S2 (available in www.besjournal.com) depicts the linear fit curves. The macular structure and visual acuity were less impaired in the dominant eyes. The central visual function reflects the anatomical condition, as previously verified [6,7]. The duration of symptoms perceived by the patients was far shorter in the dominant eyes. In macular disease, the course duration is associated with the progression of the disease, which implies that a shorter duration is accompanied by less damage and better visual function. The duration of the symptom was subjective statements, beginning from the moment patients’ first awareness of the visible abnormality. The shorter duration in the dominant eyes suggested an earlier perception of ocular symptoms. Only the macular anatomic injury accurately described the genuine course of the disease, whereas the duration of symptoms merely represented the subjective feelings of the sufferers. Hence, the affected dominant eyes appeared to be more congruent in the subjective and objective course of the disease. This may come from an anatomical superiority of OD columns in eye-specific segregation in the primary visual cortex, or the differences in the molecular level signaling pathways that participated in OD formation and plasticity [8-10]. The OD contributed relatively more during visual perception production, leading to greater sensitivity to visual impairment.
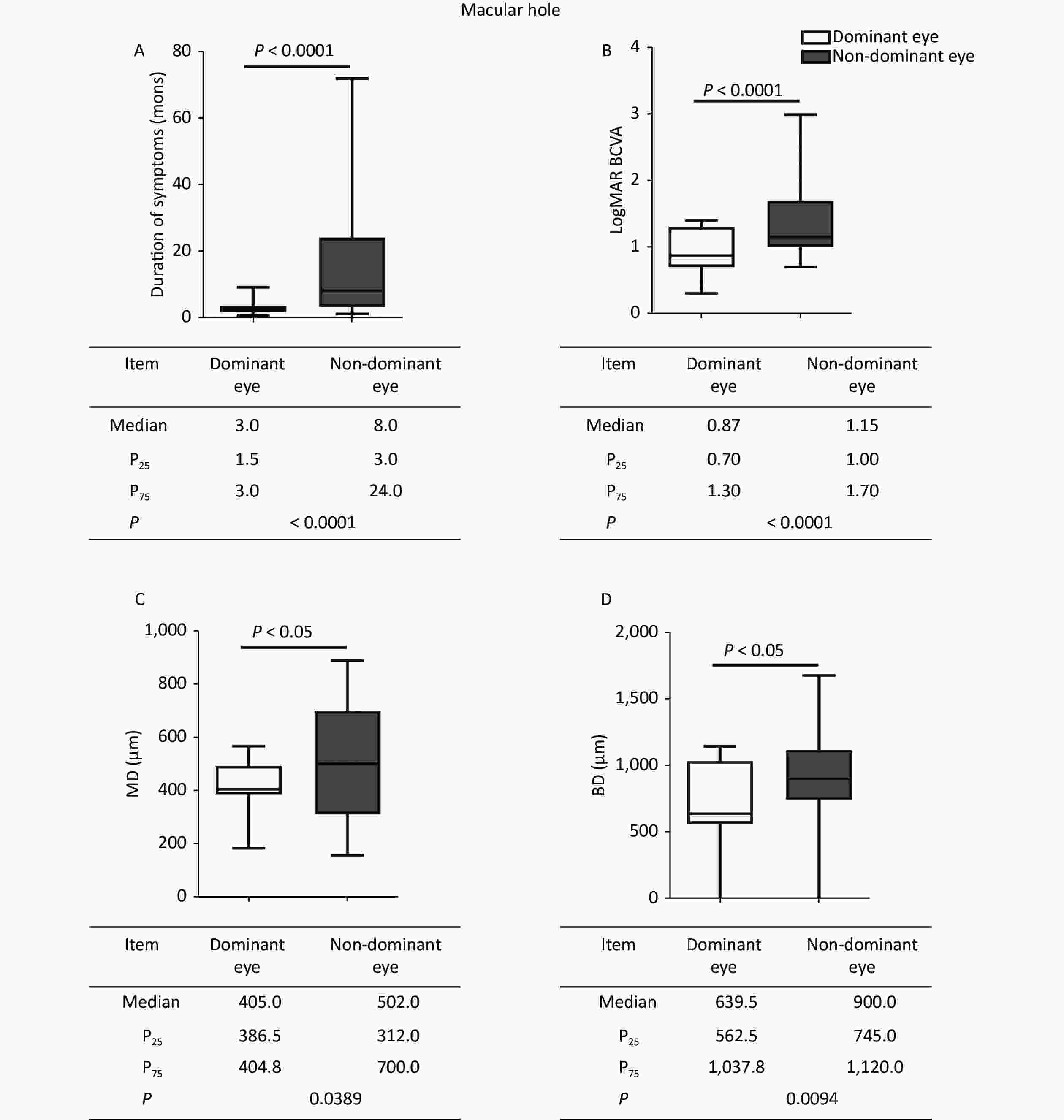
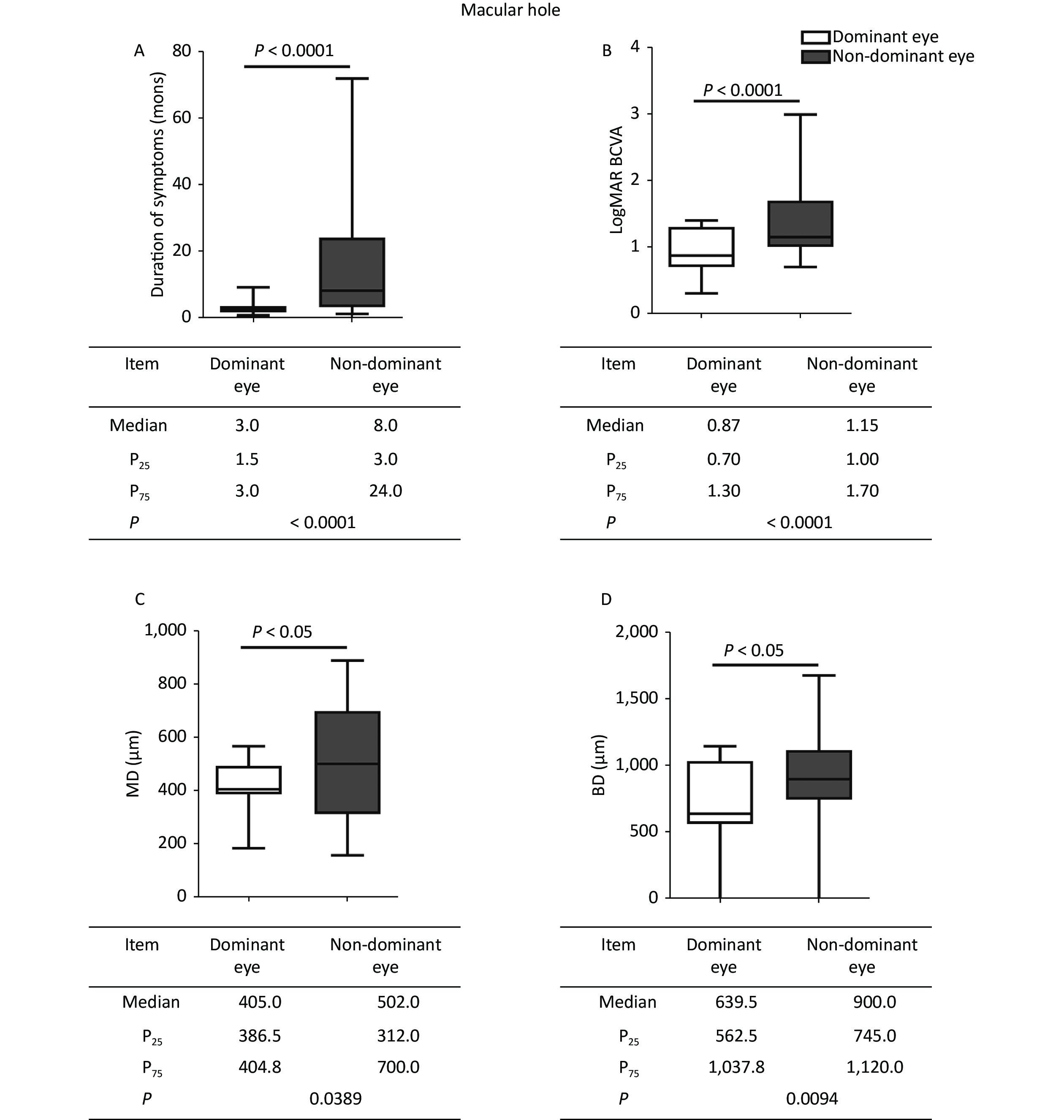
Figure 1. Comparisons of clinical characteristics between the dominant and nondominant eyes in MH. The dominant eyes were found to have (A) shorter durations, (B) better logMAR BCVA, and (C, D) smaller hole diameters than the nondominant eyes. The statistical analysis showed a significant difference. MH, macular hole; P25, 25th percentile value; P75, 75th percentile value; MD, minimum diameter; BD, base diameter.
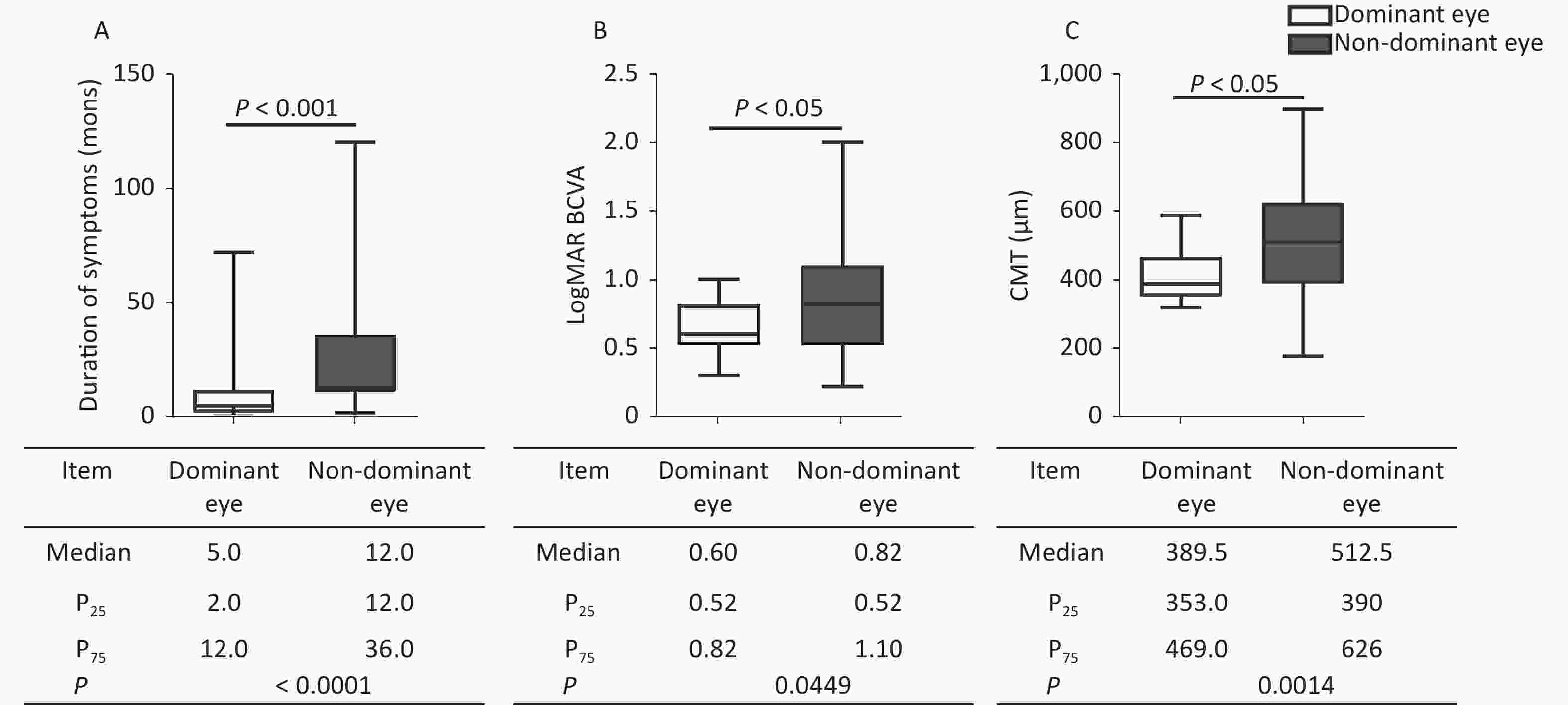
Figure 2. Comparison of clinical characteristics between the dominant and nondominant eyes in ERM. The dominant eyes were found to have (A) shorter durations, (B) better logMAR BCVA, and (C) thinner CMT compared with the nondominant eyes. The statistical analysis showed a significant difference. ERM, epiretinal membrane; P25, 25th percentile value; P75, 75th percentile value; CMT, central macular thickness.
This study has several limitations. Its inherent cross-sectional nature made it difficult to observe dynamic changes in OD during the procedure of visual deterioration. In addition, we have not designed to explore the effect of OD on disease prognosis after the treatment. Moreover, the Amsler grid testing the central visual impairment was not used. Although the “hole-in-card” is relatively widely accepted in OD determination, more accurate tests may help OD qualitative evaluation. We are willing to improve further study in the future.
In this study, we evaluated the OD in patients who suffered from MH and ERM and found 24 (26.1%) eyes affected with MH and 28 (23.4%) with ERM, which was the dominant side. The dominant eyes suffering from maculopathy had better visual acuity, a shorter duration of symptoms, and less impaired macular structure. When it happens to the dominant eyes, it is noticed earlier and at a less severe stage.
Data Availability All data generated or analyzed during this study are included in this article. Further datasets are available from the corresponding author upon request.
Competing Interests The authors have no conflicts of interest to declare.
Authors’ Contributions LIU Yang, WANG Xin, and ZHU Min participated in the collection of clinical information. LIU Yang and WANG Xin reviewed the literature and drafted the manuscript. XU Ge Zhi designed the study and critically revised the article. All authors gave final approval of the version to be published.
-
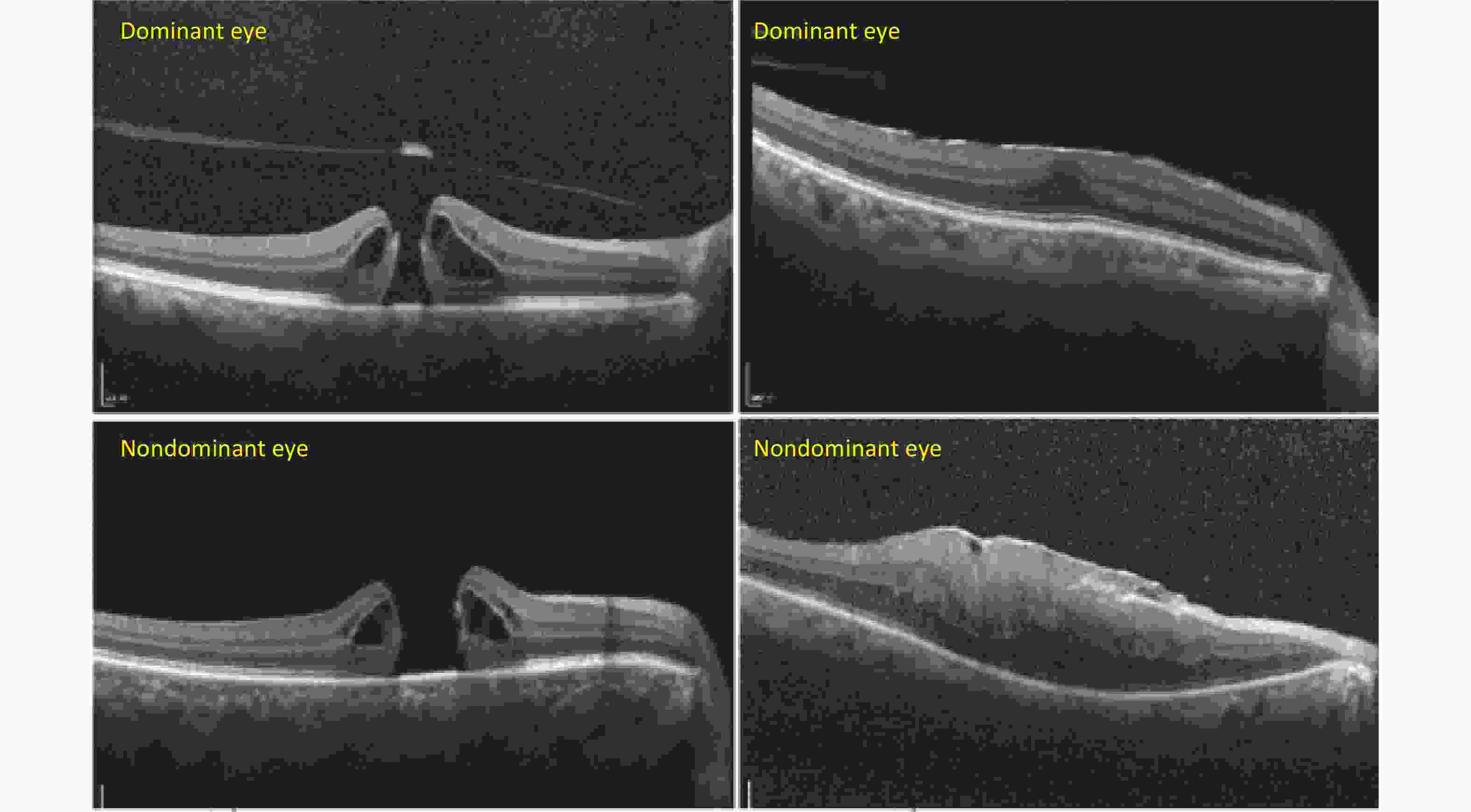
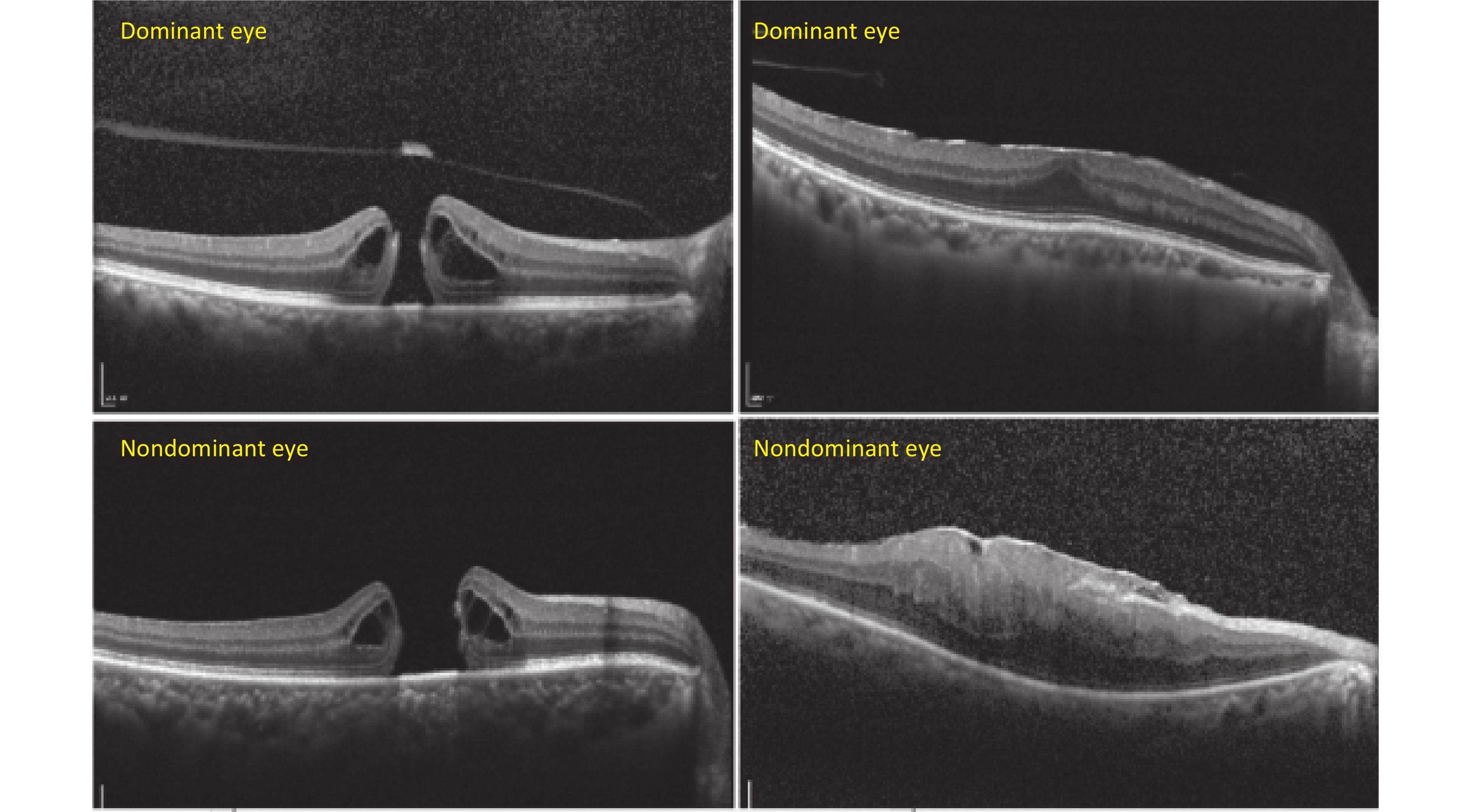
Figure S1. Representative cases of macular anatomic changes on OCT between the dominant and nondominant eyes in MH and ERM. The dominant eyes were shown to have smaller hole diameters and thinner central macular thickness, as well as a less damaged macula compared with the nondominant eyes on OCT.
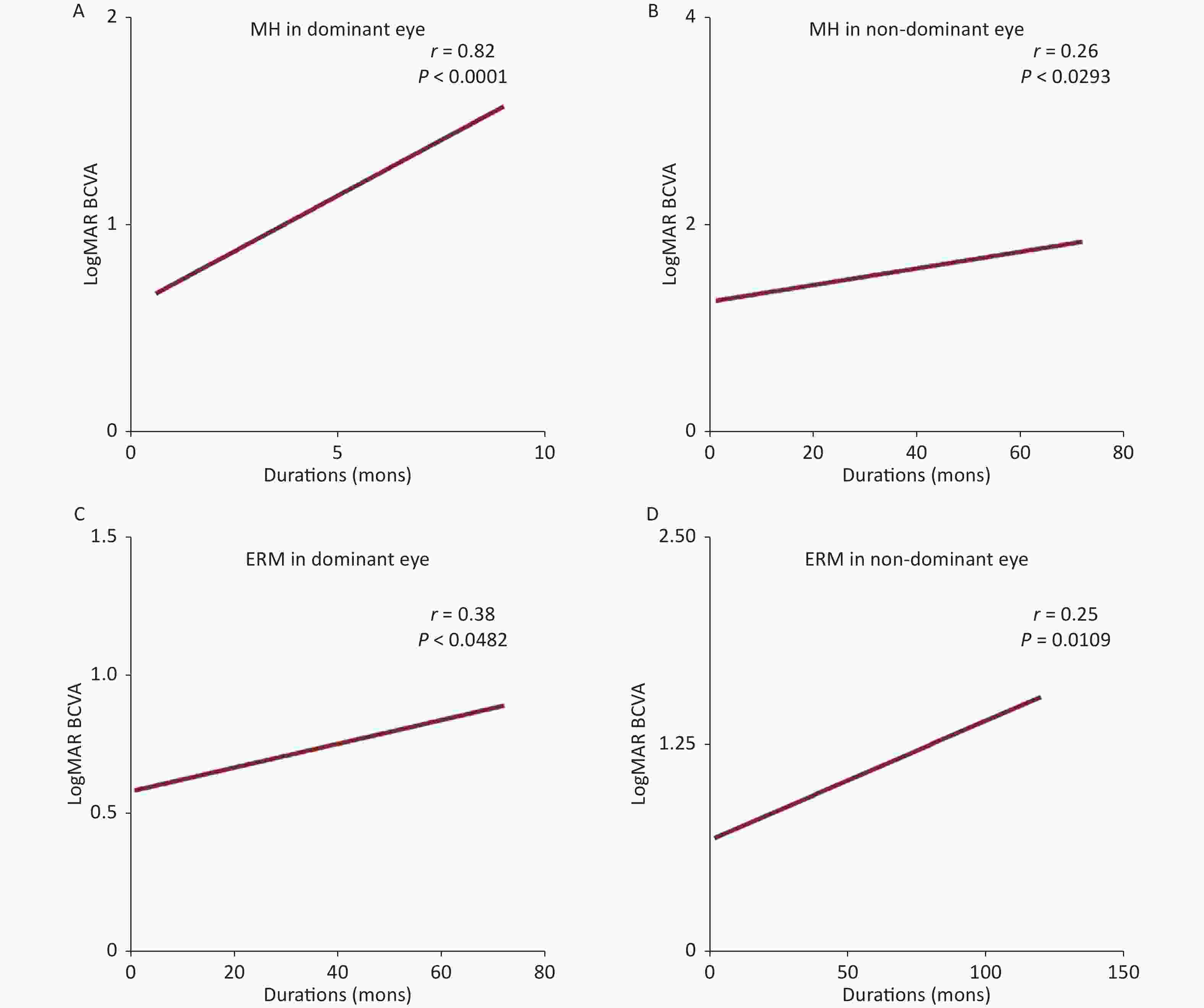
Figure S2. Correlation analysis between dominant and nondominant eyes in MH and ERM. A significant correlation between the duration of symptoms and logMAR BCVA in dominant eyes. A more powerful correlation was found in the dominant eyes of MH and ERM. MH, macular hole; ERM, epiretinal membrane; BCVA, best corrected visual acuity.
HTML
Reference
 22249Supplementary Materials.pdf
22249Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: