-
Sophageal cancer (EC) ranks as one of the deadliest cancers globally, second only to pancreatic cancer in terms of its case-fatality rate. It is the eleventh most common cancer and the seventh leading cause of cancer-related deaths. In 2022, EC accounted for 2.6% of all new cancer cases and 4.6% of cancer deaths, totaling 510,716 cases and 445,129 deaths[1]. The incidence and mortality rates of EC show significant sex disparities, with approximately 70% of cases occurring in males - rates that are two to three times higher than those observed in females[1]. Geographical variations in EC incidence are also notable. East Asia has the highest incidence, largely because China accounts for over 50% of the global burden[2]. Squamous cell carcinoma remains the predominant histological type worldwide. Despite a global decline in esophageal squamous cell carcinoma (ESCC), esophageal adenocarcinoma (EAC) incidences have been on the rise in Western countries over the past five decades, accounting for approximately two-thirds of EC cases[1,3].
Incidence, mortality, and prevalence are commonly used to describe disease burden, but survival is another important descriptive indicator[4]. Given the high case fatality rate of EC, approximately 94%, understanding its survival rates is crucial to inform better treatment decisions for patients and oncologists[5]. Identifying regional differences in survival rates and exploring underlying mechanisms can improve the assessment of the effectiveness of cancer treatment and prevention in different locations, facilitating the design and implementation of optimal cancer control strategies[6]. However, current research predominantly focuses on global patterns and trends in EC incidence and mortality, with limited attention to its survival patterns and regional or demographic variations.
Population-based cancer registries play a crucial role in national cancer control programs, which aim to provide comprehensive, timely, and accurate data on cancer incidence, mortality, and survival rates[7]. In contrast to survival rates derived from clinical trials and hospital-based follow-up studies, which may be limited by selective sampling, survival rates from population-based registries provide a key measure of the overall effectiveness of health systems in cancer control, offering a broader perspective on cancer prognosis across the entire population. They assess the effectiveness of cancer treatment and prevention strategies and serve as vital indicators of progress in cancer control in specific regions[8].
This study aimed to systematically review all published survival rates of EC patients from global population-based cancer registries since the 1990s to better understand global survival patterns, changes over time, and international comparisons.
-
This study was reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement[9], with the detailed checklist provided in Supplementary Table S1. Survival studies published until 31 December 2023 were identified by searching Chinese databases (SinoMed) and English databases (including PubMed, Embase, Web of Science, and SEER). Key search terms related to ‘esophageal cancer’, ‘survival rate’ ‘population-based’, and ‘cancer registry’ were used, with the comprehensive search strategy detailed in Supplementary Table S2. The SEER Program, developed by the National Cancer Institute, provides comprehensive U.S. cancer statistics derived from population-based cancer registries. In this study, we utilized SEER*Explorer, an interactive online tool within SEER, to access recent EC survival data from 22 SEER registries, covering 47.9% of the U.S. population[10]. EC cancer was defined using the International Classification of Diseases, 10th Revision (ICD-10) codes C15.0–C15.9, and the histopathological tumor type was coded using the International Classification of Diseases-Oncology, third Edition (ICD-O-3).
-
Articles were selected based on the following criteria: 1) survival analysis studies using cancer registries or population-based studies; 2) data on at least one of the following indicators for EC: net survival rate (NSR), overall survival rate (OSR), relative survival rate (RSR), and age-standardized RSR/NSR.
-
Articles were excluded if they met the following criteria: 1) duplicate studies or abstracts without an available full-text version; 2) non-research articles, such as conference abstracts, reviews, etc.; 3) study results on incidence and/or mortality rather than survival; 4) data overlapping with other articles based due to using the same cancer registries; 5) age or stage groups with significant heterogeneity; 6) non-English literature.
-
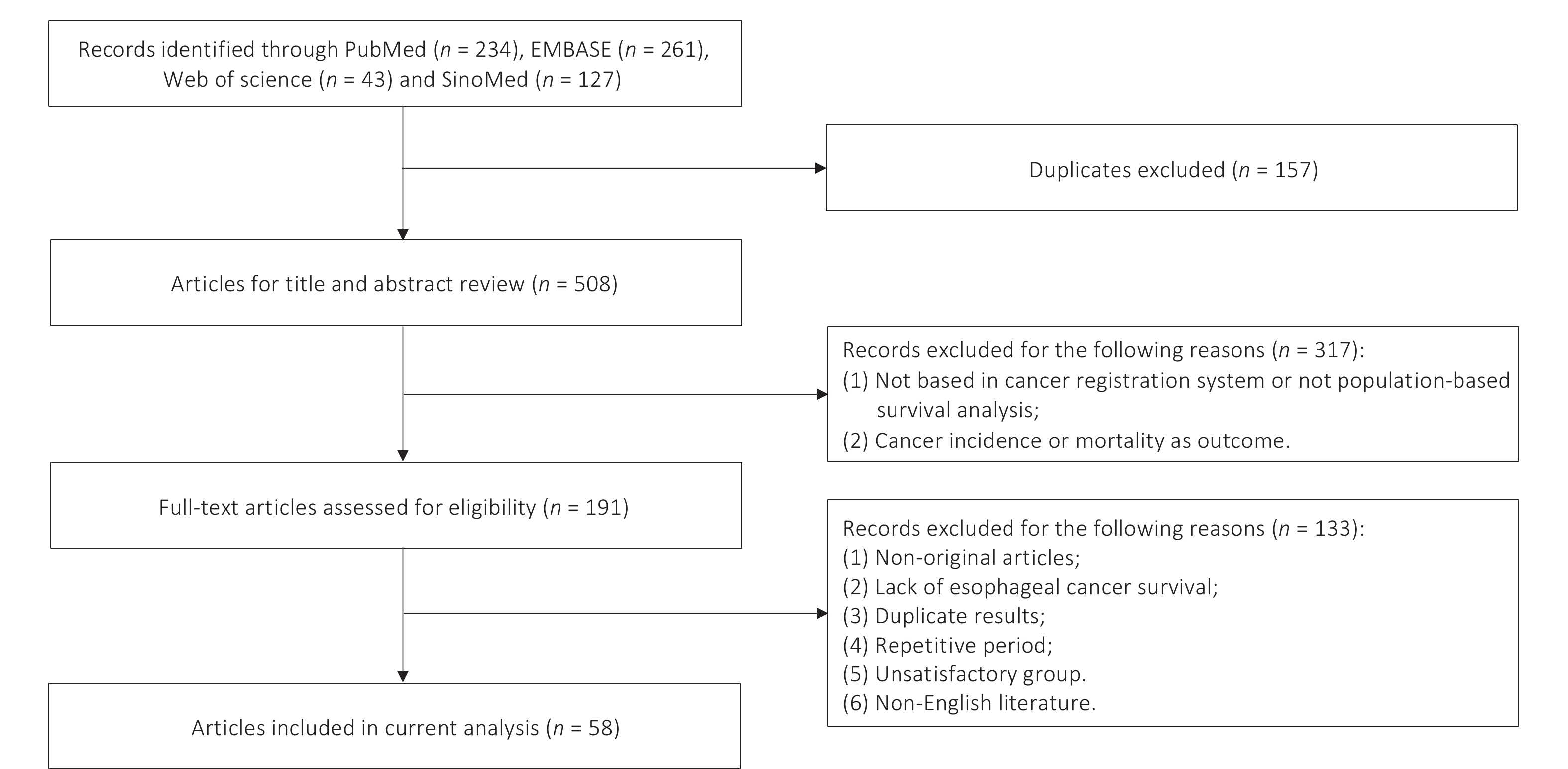
Two researchers (ZJY and DNY) independently assessed studies for inclusion criteria and extracted data. A total of 665 articles were initially selected using the specified search strategy. After removing 157 duplicates, 508 articles underwent screening by titles and abstracts. Subsequent evaluation of 191 full-text articles led to the exclusion of 133 articles due to meeting the exclusion criteria. The final analysis included 58 studies, 19 in Chinese and the remaining 39 in English (Figure 1). Key characteristics of the included studies are summarized in Supplementary Table S3, and reasons for the exclusion of each excluded article are listed in Supplementary Table S4.
-
Estimates of OSR, RSR, and NSR were extracted from each study. OSR estimates the probability of survival for a certain period after diagnosis, using all-cause mortality as the endpoint. NSR refers to the survival probability assuming the cancer of interest is the only possible cause of death. Given the limited reliability of cause of death data from population-based cancer registries, RSR is frequently employed to estimate NSR. RSR calculates the ratio of observed all-cause survival among cancer patients to expected survival in a demographically comparable population (age, race, sex, etc.) and is therefore preferred by population-based surveys and global cancer registries. Both the NSR and RSR methods exclude the influence of other causes of death on survival rates[11-13]. In this study, these two indicators were introduced as RSR.
Furthermore, considering the different mortality risks among cancer patients by age, the temporal variability in the age distribution of cancer patients within a region or country, and the potential differences in the age distribution of patients with the same cancer type between regions or countries[14], we utilized age-standardized RSR or NSR to facilitate international comparisons. Most studies employed the International Cancer Survival Standard, Age Group 1 (ICSS-1), a widely recognized method that adjusts for variations in age distribution across populations, ensuring comparability of EC survival rates[15]. As sex, age at diagnosis, pathology, and clinical stage have been identified as the primary prognostic factors for EC, we further collected and compared survival rates for these factors across different subgroups. In our review, histological types were classified as squamous cell carcinoma, adenocarcinoma, and other specified and unspecified types.
Endnote 20 (Clarivate Analytics, Philadelphia, PA, USA) and Excel 2016 (Microsoft Corporation, Redmond, WA, USA) were used for literature management and data analyses.
-
Table 1 displays the overall 1-, 3-, and 5-year OSRs of EC in China[16-31], India[32], Iran[33], the United States[34,35], and Canada[36]. The data indicated a marked variability in OSRs between different countries and between different regions within China. The highest 1-year OSRs were observed in Lianyungang (2011, China)[20] and Huai’an (2010, China)[18], at 69.2% and 58.0%, respectively. These were significantly higher than those in other regions in China and in other countries during the same period. The most frequently reported 5-year survival rate was 34.6% in Linzhou (2003–2012, China)[26], followed by 25.1% in Jiulongpo (2014-2016, China)[27]. The lowest 5-year OSRs were in Qidong (1992-1996, China)[17] and Sihui (1997–2006, China)[29], at 3.9% and 4.4%. Globally, the United States (2010–2016)[35] had the highest 5-year OSR, at 23.3%, while Dindigul (2003, India)[32] had the lowest, at 6.0%.
Continent Country Region Area Period 1-year 3-year 5-year Asia East China[16] 2003−2005 54.0 25.5 18.4 Jiangsu Qidong[17] 1992−1996 16.1 − 3.9 1997−2001 22.3 − 5.6 2002−2006 25.2 − 7.7 2007−2011 32.7 − 11.0 2012−2016 42.7 − 13.6 Huai’an[18] 2010 58.0 29.8 22.6 Jiangyin[19] 2012−2013 − − 20.3 Lianyungang[20] 2011 69.2 42.3 − Yangzhong[21] 1991 − 25.9 − 2012 − 60.9 − Shanghai Nanhui[22] 2002−2004 16.4 8.4 7.6 Yangpu[23] 2002−2012 45.0 22.3 17.8 Pudong[24] 2002−2006 24.0 14.7 12.1 Henan Linzhou 1990−1994[25] − − 14.5 1995−1999[25] − − 18.6 2000−2004[25] − − 24.9 2003−2012[26] − − 34.6 Chongqing Jiulongpo[27] 2014−2016 − − 25.1 Sichuan Shehong[28] 2016−2020 − − 20.0 Guangzhou Sihui[29] 1997−2006 − − 4.4 2007−2009 − − 13.1 Hebei Cixian[30] 2000−2002 40.6 24.6 17.8 Taiwan[31] 2008−2014 − − 16.8 South India Dindigul[32] 1990−1999 − − 7.0 2003 − − 6.0 Mumbai[37] 1992−1994 32.7 13.6 9.7 West Iran Babol[33] 1990−1991 23.0 15.0 13.0 America North United States 2000−2016[34] − − 19.3 2010−2016[35] − 31.6 23.3 Canada British Columbia[36] 1990−1999 − − 8.8 Note. − no figures or reports in original publications. Table 1. Overall 1-, 3-, and 5-year observed survival rates (%) of esophageal cancer in selected countries and regions
Figure 2 shows the age-standardized 5-year RSRs/NSRs for EC in selected countries and regions from Africa[38], Asia[16,38,39], America[38,40,41], Oceania[38,40], and Europe[38,40,42-45]. Analyzing age-standardized 5-year RSRs/NSRs after 2010, it was noted that Jordan (41.1%, 2010–2014), Algeria (37.3%, 2010–2014), Japan (36.0%, 2010–2014), China (33.4%, 2015–2017), and South Korea (31.3%, 2010–2014) reported rates exceeding 30%[38,46]. Conversely, India (2010–2014) and Estonia (2010–2014) had the lowest rates, of 4.1% and 5.4%[38], respectively, with survival rates almost ten times lower than the highest observed. A detailed summary of overall and age-standardized 1- and 5-year RSRs/NSRs for EC across selected countries and regions is presented in Supplementary Table S5.

Figure 2. Age-standardized 5-year relative/net survival rates (%) for esophageal cancer in selected countries and regions. (A) Africa and Asia; (B) America and Oceania; (C) Eastern Europe; (D) Northern Europe; (E) Western Europe; (F) Southern Europe; (G) Central Europe.
In reviewing temporal trends by diagnostic year, most countries experienced an increase in age-standardized 5-year RSRs/NSRs. Specifically, South Korea experienced the most remarkable rise, with rates increasing by 12.7% from 2000 to 2014[38]. China followed with an increase of 10.5% between 2002 and 2017[16,38]. However, Slovenia, Slovakia, Lithuania, Finland, and India showed only modest improvements of less than 1% each over the same period[38]. A declining trend was noted in South Africa, Thailand, Argentina, Colombia, Uruguay, Costa Rica, and Russia[38]. Despite initial increases in survival rates between 2005 and 2009 in Jordan, Brazil, Latvia, and Estonia[38], a subsequent decline was observed from 2010 to 2014, with rates falling below the levels of 2000–2004. Of these, Costa Rica and Jordan experienced the largest declines, with decreases of 14.8% and 11.5%, between 2000 and 2014[38].
-
Table 2 shows the sex-specific age-standardized 5-year RSRs/NSRs from studies in China[46,47], United States[46], Brazil[48], Australia[49], and some European countries[43,50]. China (2015–2017)[46] reported the highest rates for both sexes (31.6% and 38.8%), while Sao Paulo State (Brazil, 2000–2018)[48] showed the lowest figures (5.1% and 5.8%). Overall, women had a greater survival advantage than men. The sex gap in rates was particularly large in Finland (1995–1999)[50] and China (2012–2015)[47], where the gap reached 10.9% and 9%. Conversely, sex differences were minimal in Portugal (1995-1999)[50], Spain (1995-1999)[50], Sao Paulo State (Brazil, 2000–2018)[48], and Denmark (2010-2019)[51] ranging from 0.4% to 0.7%. However, in Norway (1995–1999)[50], the Netherlands (1995–1999)[50], and the United States (2008–2009)[46], female rates were slightly lower than male rates, with differences ranging from 0.6% and 1.5%. Furthermore, the sex difference in rates showed an upward trend with years of diagnosis in China, the United States, Norway, France, Switzerland, Spain, and Portugal[43,46,47,50,51]. In contrast, a declining trend was observed in Finland, Denmark, Sweden, Belgium and Italy[46,50,51].
Continent Country Region Period Age-standardized rates (%) Male Female Asia East China 2003−2005[47] 19.9 23.6 2006−2008[47] 23.4 29.4 2009−2011[47] 23.8 30.3 2012−2015[47] 27.7 36.7 2015−2017[46] 31.6 38.8 America North United States[46] 2008−2009 17.5 16.0 2010−2011 18.2 22.3 2012−2014 18.4 23.6 2015−2017 19.6 23.9 Central and South Brazil S ̃ao Paulo State[48] 2000−2018 5.1 5.8 Oceania Australia Victoria[49] 1982−2015 15.4 20.9 Europe 1995−1999[50] 10.2 13.4 North Finland 1995−1999[50] 8.3 19.2 2000−2009[51] 11.6 16.7 2010−2019[51] 14.9 17.7 Denmark 1995−1999[50] 10.2 12.6 2000−2009[51] 8.5 11.8 2010−2019[51] 17.2 17.6 Norway 1995−1999[50] 8.3 7.0 2000−2009[51] 9.8 11.5 2010−2019[51] 21.3 27.7 Sweden 1995−1999[50] 11.8 18.2 2000−2009[51] 11.9 13.3 2010−2019[51] 15.0 18.9 West United Kingdom[50] England 1995−1999 8.1 10.1 Wales 1995−1999 10.2 13.4 Scotland 1995−1999 9.9 11.7 Belgium 1995−1999[50] 17.2 20.9 2000−2004[43] 20.0 23.0 The Netherlands[50] 1995−1999 11.6 11.0 France 1995−1999[50] 11.5 15.4 2000−2004[43] 13.0 19.0 Ireland[50] 1995−1999 11.0 16.8 Central Germany[50] 1995−1999 17.1 23.1 Poland[50] 1995−1999 5.5 9.4 Switzerland 1995−1999[50] 11.2 17.5 2000−2004[43] 16.0 24.0 Slovakia[50] 1995−1999 6.8 8.4 Slovenia[50] 1995−1999 3.9 11.6 South Spain 1995−1999[50] 9.8 10.5 2000−2004[43] 9.0 12.0 Italy 1995−1999[50] 10.0 16.2 2000−2004[43] 11.0 14.0 Portugal 1995−1999[50] 13.3 13.7 2000−2004[43] 9.0 17.0 Note. − no figures or reports in original publications. Table 2. Sex-specific age-standardized 5-year relative/net survival rates (%) of esophageal cancer in selected countries and regions
-
Supplementary Table S6 presents the age-specific 5-year RSRs/NSRs for EC in China[17,52,53], India[37], the United States[41,54], Canada[55], the Netherlands[56], and Germany[41,54]. In each age group, survival rates were higher in Germany (1997–2006)[41,54], with the ≥ 75 age group achieving a 5-year RSR/NSR of 14.5%. It was observed that 5-year RSRs/NSRs generally decreased with age, except for Qidong (China, Jiangsu Province, 2001–2017)[53] and Mumbai (India, 1992–1994)[37], where the lowest rates were found in the 65–74 and 55–64 age groups, respectively. This trend became more evident when the age groups were categorized into 15–54, 55–74, 75–84, and < 75, ≥ 75 (as shown in Supplementary Tables S7 and S8). The 5-year RSRs/NSRs for the 15–54 age group were approximately 1–5 times higher than those for the 75–84 age group, with the rate difference between the < 75 and ≥ 75 age groups ranging from 4.6% to 18.4%[40,43].
-
In terms of pathology type (Supplementary Table S9), the overall and age-standardized 5-year RSRs/NSRs for EAC and ESCC were similar, approximately 3.0%, except in Cixian (China, Hebei Province, 2000–2002), where the survival rate for squamous cell carcinoma was 17.9% higher than that for adenocarcinoma. Recent findings from China and South Korea indicate slightly higher rates for ESCC compared to EAC. Conversely, survival rates for EAC have been consistently higher in the United States, Sweden, and the Netherlands between 1990 and 2017[46,57,58]. When examining temporal trends, the improvement in survival rates has been more pronounced for ESCC. Few publications have reported stage-specific overall and age-standardized 5-year RSRs/NSRs for EC. As seen in Supplementary Table S10, which presents these rates from studies in South Korea[59], the United States[60], and Germany[54], patients with localized EC had a better prognosis than other groups.
-
Table 3 displays the overall and age-standardized 5-year RSRs/NSRs for EC patients across several Chinese regions, including the data from the nation[38,46,61], Jiangsu Province (Qidong, Huai’an, Jiangyin)[17-19], Shanghai Municipality (Nanhui and Pudong)[22,24], Guangdong Province (Zhongshan and Guangzhou)[29,62], Hebei Province (Cixian)[30], Henan Province (Linzhou)[25], Zhejiang Province (Haining and Jiashan)[63], Fujian Province[64], Liaoning Province (Dalian)[65], and China’s Taiwan [38].
Country Region Area Period Overall Age-standardized rates (%) Total Male Female Total Male Female China Total 2000−2004[38] − − − 22.9 − − 2003−2005[61] − − − 20.9 19.9 23.6 2005−2009[38] − − − 27.1 − − 2010−2014[38] − − − 29.7 − − 2015−2017[46] − − − 33.4 31.6 38.8 Jiangsu Qidong[17] 1987−1991 6.3 5.3 8.2 6.4 − − 1992−1996 5.4 5.3 5.5 5.3 − − 1997−2001 7.6 7.6 7.6 7.9 − − 2002−2006 10.5 10.5 10.6 10.5 − − 2007−2011 15.2 14.6 16.4 16.7 − − 2012−2016 17.9 17.4 19.2 20.5 − − Huai’an[18] 2010 26.8 25.9 28.8 − − − Jiangyin[19] 2012−2013 42.0 40.4 57.5 − − − Shanghai Nanhui[22] 2002−2004 10.2 9.1 15.2 − − − Pudong[24] 2002−2006 18.2 − − − − − Guangdong Zhongshan[62] 2010−2013 − − − 11.7 − − Guangzhou[29] 2007−2009 15.5 − − − − − Hebei Cixian[30] 2000−2002 21.7 18.8 25.7 − − − Henan Linzhou[25] 1990−1994 28.2 29.9 26.8 − − − 1995−1999 35.2 37.0 33.1 − − − 2000−2004 40.8 38.4 43.7 − − − Zhejiang Haining and Jiashan[63] 2003−2006 15.7 16.3 13.3 17.3 − − 2007−2010 15.4 14.3 18.7 18.5 − − 2011−2014 18.1 16.9 21.8 18.5 − − Fujian[64] 2012−2014 20.5 21.0 18.7 19.0 19.0 21.8 Liaoning Dalian[65] 2015 − − − 11.9 12.1 14.2 Taiwan[38] 2000−2004 − − − 13.0 − − 2005−2009 − − − 13.2 − − 2010−2014 − − − 15.5 − − Note. − no figures or reports in original publication. Table 3. Overall and age-standardized 5-year relative/net survival rates (%) of esophageal cancer in some areas of China
As illustrated in Table 3, a gradual upward trend in survival rates was observed over time. Female survival rates were generally higher than male survival rates, especially in Jiangyin (China, Jiangsu Province) during 2012–2013[19]. It was observed that 5-year RSRs/NSRs increased slightly after age standardization, although the differences were not striking. The highest age-standardized 5-year RSRs/NSRs in China were recorded at 33.4% during 2015–2017[46], with the lowest observed in Qidong (China, Jiangsu Province) during 1992–1996, at merely 5.3%[17]. Supplementary Table S11 shows sex-specific overall 1-, 3-, and 5-year OSRs of EC in some areas of China highlighting a survival advantage for women[16-19,22,23,29-31,66,67]. The highest survival rates for both sexes were observed in Wuhan (Hubei Province), achieving 5-year OSRs of 36.5% and 45.2% in men and women[67].
-
In this systematic review, we collected overall OSRs, overall RSRs/NSRs, and age-standardized RSRs/NSRs for patients with EC from all available global population-based cancer registries since the 1990s. Our study presents global patterns and trends of EC by the characteristics of the diagnostic period, region, sex, age group, pathology, and clinical stage while facilitating international comparisons of survival rates. Furthermore, given the high incidence of EC in China, we detailed the survival features in China. Overall, although there has been notable improvement in EC survival rates in many countries over time, the prognosis remains suboptimal, with significant disparities between countries and regions.
The overall prognosis of EC has improved significantly in most countries and regions. This improvement can be attributed to various factors, including proportional changes in age, histology, and stage distribution, as well as advances in early diagnosis and treatment technologies such as gastrointestinal endoscopic screening, precise staging techniques, surgical methods, and adjuvant therapy[58,68]. The widespread use of gastrointestinal endoscopy has increased the identification of early-stage EC cases, potentially leading to statistical artifacts such as lead time and length bias[69]. Notably, South Korea and China have seen the most significant improvements in survival rates. Since 2002, South Korea has incorporated upper gastrointestinal endoscopy into its national cancer screening program, and uptake has increased substantially in recent years[70]. China, with a high incidence of EC, has been implementing early screening programs in high-risk regions since the 1970s and has rapidly expanded these efforts to a wider area in this century[71]. In these high-incidence ESCC regions, endoscopy-based early diagnosis is cost-effective for broad application. A long-term study in high-risk areas of China showed that endoscopic screening and subsequent interventions significantly reduce esophageal cancer incidence and mortality[72]. This approach succeeds by effectively removing early tumors, minimizing complications, preserving esophageal function, and reducing recovery time by avoiding invasive surgery[73]. This evidence underscores the critical role of endoscopic screening as an early detection strategy in improving EC survival rates in ESCC high-incidence areas, further highlighting its importance in reducing disease burden in these regions.
Nonetheless, these factors vary greatly between countries and regions. Coupled with variations in economic development, population lifestyles and diets, and the quality of survival data collection and reporting, there are significant geographical disparities in EC survival rates, with gaps as large as tenfold. Our study found that in some countries, there have been no improvements in survival rates, and in some cases, the survival rates have worsened[38]. This may be partly due to rising mortality rates associated with aging populations[74]. Still, it may also reflect an actual decline in survival and highlight the serious challenges of EC in these regions. Additionally, many of these countries are middle-income or developing nations, where limited medical resources, equipment, and technology make implementing nationwide EC screening programs challenging. The lack of specific clinical symptoms in early-stage EC further complicates timely detection, resulting in many patients missing the window for early intervention[75]. Despite a recent decline in EC incidence, policymakers and healthcare providers in areas with limited survival improvements should actively implement targeted strategies and interventions.
Asian countries such as Jordan, Japan, China, and South Korea have reported higher age-standardized 5-year RSRs/NSRs for EC compared to relatively affluent Western countries such as Europe and the United States[38,46]. This finding contrasts with the traditional view that higher economic levels typically correlate with better cancer survival outcomes[38]. In these Western regions, where EAC is the most common histological subtype, key risk factors include excessive body weight, gastroesophageal reflux disease, Barrett’s esophagus, and a declining prevalence of chronic helicobacter pylori infections. These risk factors exhibit significant individual variation, making large-scale screening programs, such as those implemented in ESCC high-incidence areas, less feasible[76]. Furthermore, despite being the gold standard for diagnosis, the invasiveness nature and high cost of endoscopy limit its widespread adoption, highlighting the need for alternative methods[77]; however, no widely applicable alternatives are yet available. The effectiveness of early diagnosis for EAC originating from Barrett’s esophagus also remains debated, further impeding the development of screening programs in Western countries[78]. By contrast, ESCC is associated with higher rates of pathological complete response, often leading to better treatment outcomes[79]. Although EAC generally progresses more slowly, it exhibits higher rates of lymph node involvement and recurrence[80,81]. These factors collectively contribute to the lower survival rates observed in Western countries, where EAC predominates. This underscores the importance of tailoring cancer screening and treatment strategies to specific histological subtypes and regional characteristics to maximize their effectiveness and feasibility.
However, there was considerable geographical variation in EC survival rates across Asian countries such as India and Thailand, reporting rates below 10% in the 2010s. Several studies suggest an inverse relationship between socioeconomic status (SES) at the individual or regional level and EC survival rates, particularly in the early stages[82,83]. SES provides information about an individual’s access to and control over social and economic resources, commonly assessed through indicators such as educational attainment, social class, and income[84]. Lower SES is associated with a 61% higher risk of five-year mortality[85], which may be attributed to factors like delayed diagnosis, advanced tumor stage, and limited access to curative treatment options[86,87]. In these regions, low-SES populations often have unhealthy lifestyles, including alcohol consumption, smoking, and frequent intake of spicy or hot foods, alongside prolonged exposure to high-risk environmental and dietary factors, such as food contamination and nitrosamines[88,89]. The tumor stage at diagnosis is the strongest prognostic factor for EC[86], highlighting the importance of early detection and equitable access to treatment to bridge the survival gap. Therefore, continued monitoring of these disparities, understanding their underlying causes, and focusing screening efforts on socially disadvantaged groups are essential to address these challenges.
Based on survival data from cancer registries, we observed that women had a survival advantage in EC, supporting sex as an independent prognostic factor[90,91]. Previous studies focusing on histological types of EC show women with ESCC have better outcomes than men after adjustment for prognostic factors, whereas no sex difference was observed in EAC[92]. This may be related to higher exposures to smoking and drinking in men[90,93], which are major risk factors for ESCC. It may also be explained by male higher predominance in EAC, which generally has a worse prognosis than ESCC, and experiences higher rates of regional recurrence and distant metastasis. In addition, estrogen may offer protective benefits, especially against ESCC in premenopausal women[94]. However, some studies have shown no survival advantage in premenopausal women compared with men[91], and hospital-based studies have sometimes found no sex disparity, possibly due to unrepresentative samples with higher treatment rates[93]. Further population-based research, controlling for prognostic factors, is needed to clarify these sex differences while minimizing confounding bias.
In addition, we observed that survival rates decreased with increasing age at diagnosis, which is consistent with findings in other studies[95]. Previous reports indicate that age was not a significant prognostic factor after esophagectomy, suggesting that any treatment could potentially improve survival in older patients[96,97]. Despite the common presence of comorbidities and chronic diseases in patients over 70 years of age, which may reduce their ability to receive and tolerate pre- and postoperative treatments[96], age should not be considered as a contraindication to oesophagectomy. Given that the peak incidence of EC has shifted to the 70–79 age group[98], and considering the global trend towards an aging population, future research should focus on improving postoperative quality of life and reducing complications in elderly patients, emphasizing the need for personalized treatment plans based on comprehensive assessments.
Survival rates for ESCC and EAC were similar despite their distinct epidemiological and biological characteristics. This systematic review reveals higher ESCC survival in some Asian countries and higher EAC survival in some Western countries. This may be related to Western monitoring practices for gastroesophageal reflux disease and Barrett’s esophagus, which facilitate early detection of EAC[99]. In Asia, the prevalent ESCC benefits from advanced endoscopic screening and standardized treatment of pre-cancerous lesions, reducing its incidence and mortality[100]. Therefore, Asian countries should prioritize healthy weight management and secondary prevention for Barrett’s esophagus and reflux disease. In contrast, Western countries should improve screening techniques and guidelines, particularly targeting obesity, smoking, and diet, to optimize EC prevention and treatment strategies.
Our systematic review summarized global data on overall RSRs/NSRs, and age-standardized RSRs/NSRs of EC from survival analysis of population-based cancer registries since the 1990s, providing comparisons by region, period, sex, age, pathology, and stage. These findings are important for understanding global EC patterns and trends and evaluating the effectiveness of population-based prevention and treatment. However, there were also several limitations. First, cause-specific and conditional survival rates were not included, potentially limiting a complete understanding of prognostic outcomes. Second, they did not adjust for all confounders such as ethnicity and socioeconomic factors. In addition, many studies lacked clarity on the inclusion of death certificates or autopsy-only cases, which may lead to biased survival estimates. These issues should be addressed in future research.
-
This study showed a significant improvement in global EC survival rates, but significant geographical disparities remain. Globally, EC showed better prognosis in women and younger patients; further population-based research is needed to confirm these findings while controlling for confounding factors. Regions with lower survival rates and disadvantaged socioeconomic status should prioritize EC prevention and screening, integrating strategies specifically adapted to regional characteristics and histological subtypes, maximizing their effectiveness and feasibility. Moreover, countries should continue to promote screening and lifestyle modifications like smoking cessation, alcohol moderation, weight management, and dietary improvements. At the same time, future research needs to focus on improving the quality of life and treatment options for elderly people with EC in the context of aging.
HTML
Search Strategy and Selection Criteria
Inclusion Criteria
Exclusion Criteria
Study Selection and Data Extraction
Statistical Analyses
Global Patterns and Trends
Survival by Sex
Survival by Age Group
Survival by Pathology and Stage
Survival in China
 24498+Supplementary Materials.pdf
24498+Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad:
