HTML
-
Chronic inhalation of crystalline silica (SiO2) is associated with the development of silicosis, which is characterized by inflammation and lung fibrosis[1-2]. A multitude of cell types are known to be involved in the process of pulmonary fibrosis. Extensive evidence has suggested that the fibroblast/myofibroblast is responsible for lung fibrosis, which is recruited to and accumulated at the injured site[3]. The fibroblasts/myofibroblasts that can secrete excessive extracellular matrix (ECM) can be derived from any of the following three potential sources: from the local lung fibroblasts, by the process of epithelial–mesenchymal transition, or by the recruitment and differentiation of circulating mesenchymal precursors known as fibrocytes. Fibrocytes were first defined in 1994 as a distinct population of bloodborne fibroblast-like cells that rapidly enter into the injured sites and contribute to wound repair[4]. Fibrocytes co-express the markers of leukocytes, hematopoietic progenitor cells, and fibroblasts[5]. They also express several chemokine receptors and costimulatory molecules[6]. Fibrocytes participate in both tissue fibrosis and remodeling by producing ECM and matrix metalloproteinases[7]. Fibrocytes are the source of several inflammatory cytokines, chemokines, and growth factors involved in the process of fibrosis[8]. Fibrocytes are potent antigen-presenting cells that are capable of initiating and promoting T-cell immunity[9]. Although a few studies have suggested that fibrocytes play an important role in fibrotic diseases[4, 10], their role in the pathogenesis of lung fibrosis remains unclear, and to our knowledge, no study has yet examined whether fibrocytes participate in silicosis.
In this study, we aimed to investigate the differentiation of fibrocytes that were co-cultured with AMs and exposed to crystalline silica in vitro. In addition, we intended to explore whether fibrocytes participate in silicosis in vivo. We also analyzed whether fibrocytes could amplify the pool of collagen-I-secreting cells in silicosis.
-
A total of 30 6-to 8-week-old Sprague Dawley (SD) rats (SPF degree, male, weight 180-220 g) were purchased from the animal center of Henan province (Henan, China). The rats were housed under controlled temperature (22 ℃ ± 2 ℃) and exposed to a 12-h light-dark cycle. All the rats were provided with free access to standard rat feed and tap water. The entire study was conducted in accordance with the guidelines of the Chinese Association of Laboratory Animal Care following a protocol approved by the relevant provisions of the Experimental Animal Ethics Committee of Zhengzhou University (Zhengzhou, China). The rats were anesthetized using chloral hydrate and then sacrificed by cervical dislocation.
-
Rat peripheral blood was collected into Ethylene Diamine Tetraacetic Acid (EDTA) tubes. The fibrocytes were prepared as previously described[11-12]. Peripheral blood mononuclear cells (PBMCs) were isolated and incubated in dulbecco's modified eagle medium (DMEM) supplemented with 20% fetal bovine serum (FBS), 1% penicillin 10, 000 UI/mL, and streptomycin 10, 000 mg/mL at 37 ℃ in a humidified atmosphere of 5% CO2. The PBMCs were cultured for 3 days in vitro, and then the nonadherent cells were removed and culturing was continued for 12 days. Rat macrophagocytes (Ams) were collected from the SD rats by bronchoalveolar lavage (BAL). Tracheas were cannulated, and BAL was performed with 5 mL of cold PBS. After counting the cell number, the cells were incubated at 37 ℃ for 2 h at 5% CO2 and then refreshed with new medium.
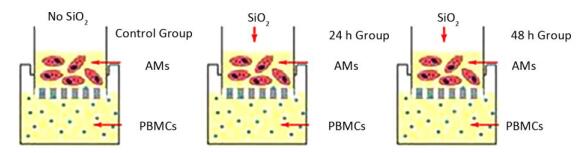
Rat AM/rat fibrocyte coculture: A co-culture system was established by permeable membrane inserts in six-well Transwell microplate supports transwell (Corning, NY, USA). The details of the permeable membrane inserts have been described previously[13] as follows: membrane type: tissue culture treated-polyester; insert diameter: 24 mm; insert membrane growth area: 4.67 cm2; nominal membrane thickness: 10 mm; pore size: 0.4 µm; nominal pore density: 4 × 106 pores/cm2; volume added to the inside of the permeable membrane insert: 1.5 mL; and volume added per well plate: 2.6 mL. PBMCs were seeded into six-well plates at 5 × 106 cells/well containing DMEM supplemented with 20% FBS, penicillin 10, 000 UI/mL, and streptomycin 10, 000 mg/mL and then incubated at 37 ℃ in a humidified atmosphere containing 5% CO2 for 6 days. Then, AMs were seeded into the insert at 1 × 106 cells/mL, and the insert was placed into a six-well dish exposed to 100 µg/mL SiO2 for 0, 24, and 48 h. (Supplemental Figure, available in www.besjournal.com)
-
Circulating fibrocytes were identified by flow cytometry as described earlier[14]. Briefly, the cells or blood or monodisperse cell suspensions of rat lung tissue were labeled with CD45-PECy5 (BD Biosciences, CA, USA). Then, cytofix/cytoperm (BD Biosciences, CA, USA) was used to permeabilize the cells. Finally, the cells were incubated with FITC-conjugated anti-Col I (Rockland, PA, USA). The samples were analyzed by a flow cytometer (Accuri C6; BD Biosciences, CA, USA).
-
A double-color immunofluorescence was performed to identify the fibrocytes in the cells grown on cover slips or in lung tissue sections. Briefly, the cells grown on cover slips or in the sections were first blocked by goat serum for 1 h at room temperature and then incubated with mouse anti-rat CD45 mAb (1:50 dilution; Abcam, MA, USA) and rabbit anti-rat Col I polyclonal Ab (1:400 dilution; Rockland, PA, USA) or appropriate isotype controls overnight at 4 ℃, followed by incubation with Alexa Fluor488-conjugated goat anti-rabbit IgG (1:400 dilution; OriGene Technologies, Inc., Beijing, China) and TRICT-conjugated goat anti-mouse IgG (1:50 dilution; OriGene Technologies, Inc., Beijing, China) at 37 ℃ for 1 h. Then, 4', 6'-diamidino-2-phenylindole dihydrochloride (DAPI) (0.2 µg/mL; Sigma, MO, USA) was used to stain the nuclei. Fixed cells or sections were observed under Nikon Eclipse Ti microscope. For counting the fibrocytes in the lung, two observers assessed the number of double-positive fibrocytes. Ten fields were randomly selected to be examined, and the number of double-positive fibrocytes was counted and calculated as the mean number of positively stained cells per total number of collagen-I-positive cells in lung sections at 200 × magnification.
-
After exposing AMs to 100 μg/mL SiO2 in the coculture system for 0, 24, and 48 h, the total RNA of fibrocytes was extracted using Trizol (TaKaRa, Tokyo, Japan) RNA isolation protocol. The first-strand cDNA was synthesized using PrimeScriptTM RT reagent kit (TaKaRa, Tokyo, Japan) according to the manufacturer's protocol. Collagen I, collagen III, and α-SMA mRNA expression was assessed by Mx3000P QPCR System (Stratagene, California, USA) using the SYBR Premix ExTM Taq (Tli RNaseH Plus) kit (TaKaRa, Tokyo, Japan) and gene-specific primers (Sangon Biotech, Shanghai, China). The primers are listed as follows: collagen I (169 bp) 5'-CGTGGAAACCTGATG TATGCT-3' (forward), 5'-CCTATGACTTCTGCGTCTGG-3' (reverse); collagen III (117 bp) 5'-GATCCTAACCA AGGCTGCAA-3' (forward), 5'-ATCTGTCCACCAGTGC TTCC-3' (reverse); α-SMA (103 bp) 5'-CTTCAATGTCCC TGCCATGT-3' (forward), 5'-AGTCACGCCATCTCCAGA GT-3' (reverse); GAPDH (143 bp) 5'-GGCACAGTCAA GGCTGAGAATG-3' (forward), 5'-ATGGTGGTGAAGAC GCCAGTA-3' (reverse). All values were normalized to the transcription of the housekeeping gene GAPDH.
-
After exposing AMs to 100 μg/mL SiO2 in the coculture system for 0, 24, and 48 h, the levels of collagen I, collagen III, and TGF-β1 in cell culture supernatants were detected by ELISA using a commercially available ELISA kit (USCN Business Co., Ltd, Wuhan, China) according to the manufacturer's instructions.
-
Expression of α-SMA in fibrocytes was determined by immunohistochemical staining. Briefly, the cells grown on cover slips were first blocked by goat serum for 45 min at room temperature and then incubated with mouse anti-rat α-SMA mAb (1:200 dilution, Wuhan Boster Biological Technology, Ltd., Wuhan, China) or appropriate isotype controls overnight at 4 ℃, followed by incubation with biotinylated goat anti-rabbit IgG at a concentration of 1:100 at 37 ℃ for 45 min. Positive IHC staining was reflected as brown staining in cytoplasm and quantified by average optical density (AOD) in 10 high-power vision fields using Image-Pro Plus 6.0 software (AOD = Integrated Optical Density (IOD) SUM/Area SUM).
-
A total of 24 SD rats were randomly divided into model group and control group (n = 12 per group). The rats in the model group were administered a single intratracheal instillation of SiO2 solution (100 mg/0.5 mL/rat), and those in the control group were administered an equal amount of 0.9% saline. All rats were sacrificed for further study at 1 and 9 weeks after silica instillation.
Carboxyfluorescein succinimidyl ester (CFSE)-labeled fibrocytes were injected into another 10 SD rats via the tail vein. The rats were exposed to SiO2 or saline immediately. After 1 week, the rats were sacrificed, and CFSE+ fibrocytes were detected by flow cytometry and immunofluorescence.
-
Briefly, the lung tissues were routinely fixed, embedded in paraffin, and cut into 5-μm sections. The rat lung sections were dewaxed with xylene and rehydrated through graded concentrations of ethanol. HE staining and Masson's trichrome staining were performed to evaluate the histopathology and collagen deposition. As described previously[15-16], the extent of lung fibrosis was evaluated by measurement of area of fibrosis (%) using Image-Pro Plus 6.0 software.
-
Purified fibrocytes from peripheral blood were stained with CFSE following the manufacturer's instructions. Flow cytometry and trypan blue exclusion method were applied to assess the labeling efficiency and cell vitality, and both were > 80%. Then, the CFSE-labeled fibrocytes (4 × 106 cells in 1 mL of PBS) were injected into the 10 rats through the tail vein with. A week later, the rats were sacrificed, and left lung tissue was used to obtain formaldehyde-fixed frozen lung sections to observe the location of labeled fibrocytes under Nikon Eclipse Ti microscope. The right lung tissues were minced and digested to obtain monodisperse cell suspensions as previously described[17], and then the number of labeled fibrocytes in the lung was counted by flow cytometry.
-
Statistical analysis was performed using SPSS 17.0 (IBM, NC, USA). Two-tailed Student's t-test was conducted to compare the differences between the two groups. P < 0.05 was used to define a statistically significant difference.
Animals
Cell Isolation, Cell Culture, and Cell Stimulation
Flow Cytometric Analysis
Double-color Immunofluorescence Analysis
RNA Isolation and Real-time PCR
ELISA
Immunohistochemical Staining
In Vivo Experiments using Animals
Examination of Lung Histological and Morphological Alterations
Tracking the CFSE-labeled Fibrocytes in Rats
Statistical Analysis
-
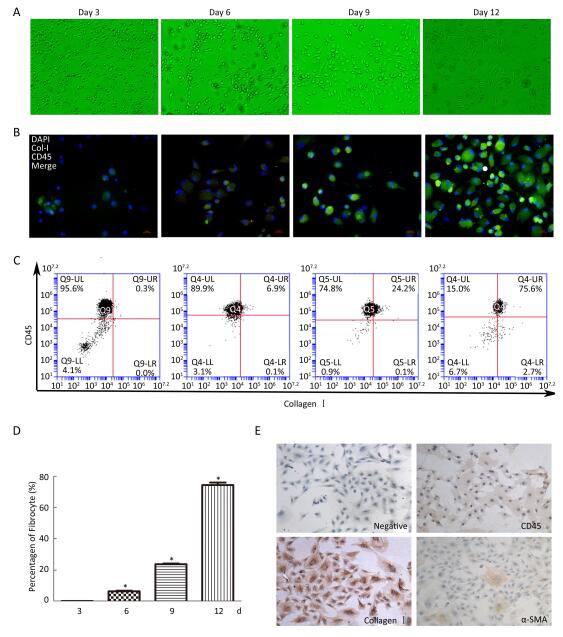
The adherent PBMCs became elongated and spindle-shaped in the cultures (Figure 1A). After 3 days, elongated statistical analysis was performed using SPSS 17.0 (IBM, NC, USA). Two-tailed Student's t-test was conducted to compare the differences between the two groups. A P value of < 0.05 was used to define a statistically significant difference. Immunofluorescence results showed double-positive fibrocytes from day 3 to day 12 (Figure 1B). Furthermore, the number of fibrocytes was increased, and purified fibrocyte population represented up to 75.6% as assessed by flow cytometry after 12 days (Figure 1C and 1D). Purified fibrocytes still expressed collagen I and began to express α-SMA protein at day 20; however, CD45 expression was rarely observed (Figure 1E). These results suggested that PBMCs can differentiate into fibrocytes and further obtain α-SMA phenotype in vitro.

Figure 1. Morphology and identification of fibrocytes from cultured PBMCs in vitro. (A) At day 3, some PBMCs become elongated spindle-shaped. The number of elongated spindle-shaped cells is increased for continue culturing (magnification × 100). (B) Immunofluorescence analysis the expression of CD45 (red), collagen I (green) and DAPI (blue) at different culture period, dual labeled CD45 and collagen I positive fibrocytes are yellow (magnification × 400). (C) Flow cytometric analysis the number of CD45+collagen I+ fibrocytes at different culture period. The number of fibrocytes was increased by a time depengdent manner (C and D). (E) Immunohistochemistry results showing that fibrocyte (20 d) expresses collagenI and α-SMA, but less CD45 (magnification × 100). Data are expressed as mean ± SD. These data are representatives of three separate experiments. *P < 0.05 vs. day 3.
-
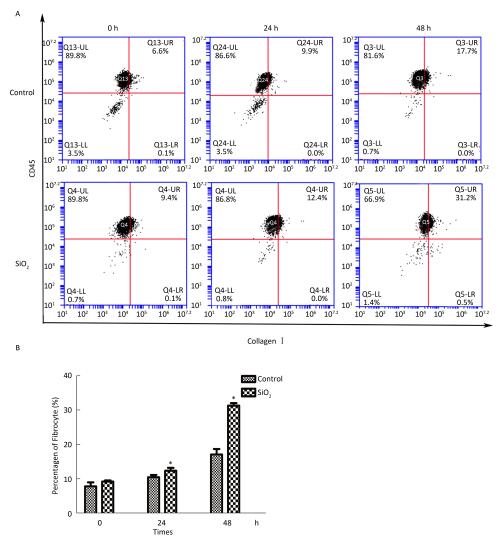
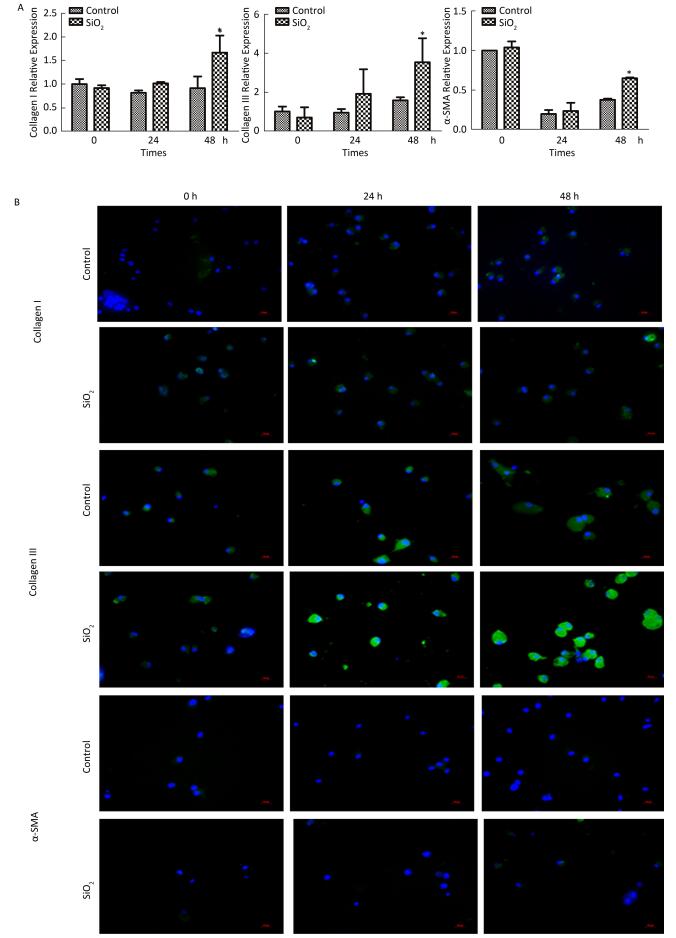
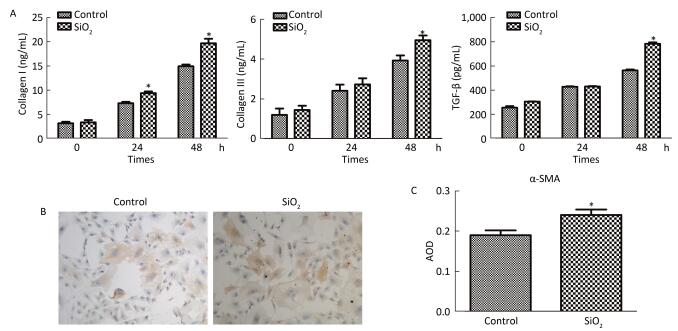
The percentage of fibrocytes after 24 or 48 h in the SiO2 treatment group was significantly increased compared with that in the control group (Figure 2A and 2B). Collagen I, collagen III, and α-SMA mRNA levels of fibrocytes after 48 h in the SiO2 treatment group were significantly elevated than those in the control group (Figure 3A). Furthermore, the immunofluorescence staining showed that all the groups can express collagen I and collagen III proteins, but not α-SMA protein (Figure 3B). The ELISA results depicted in Figure 4A showed that compared with the control group, the expression levels of collagen I, collagen III, and TGF-β1 were increased at 48 h in the SiO2 treatment group. As shown in Figure 4B and 4C, the expression of α-SMA protein in fibrocytes (20 days) was increased after exposure of AMs to 100 μg/mL SiO2 for 48 h in the coculture system. These results indicated that SiO2 stimulation can promote PBMC differentiation into fibrocytes and also promote fibrocyte differentiation into myofibroblasts.
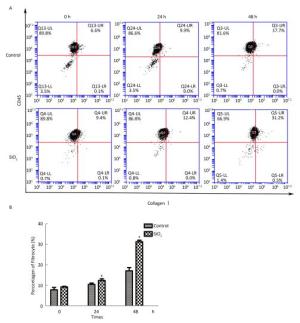
Figure 2. Effects of SiO2 exposure on PBMC differentiate into fibrocyte in co-culture system. The number of fibrocytes was significantly increased in SiO2 exposure co-culture system (A and B, *P < 0.05 vs. control). Data are expressed as mean ± SD. Data shown are representative of three separate experiments.
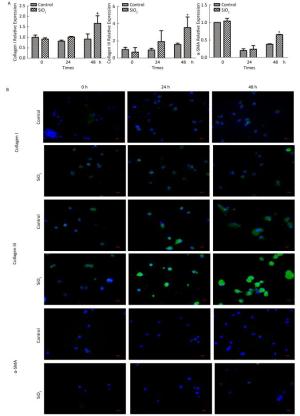
Figure 3. Effects of SiO2 exposure on collagen I, collagen III and α-SMA mRNA and protein expression of fibrocytes in co-culture system. (A) qPCR results showing that collagen I, collagen III and α-SMA mRNA expression were increased at 48 h group. (B) Collagen I and collagen III protein can be observed by immunofluorescence, but no α-SMA protein was found (magnification × 400). Data are expressed as mean ± SD. Data shown are representative of three separate experiments. *P < 0.05 vs. control
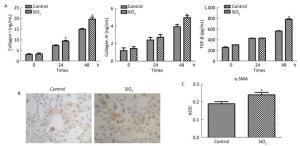
Figure 4. (A) ELISA results showing collagen I, collagen III, and TGF-β1 were increased at 48 h group. (B and C) Immunohistochemistry results showing that α-SMA protein expression of fibrocytes (20 d) were increased (magnification × 100). Data are expressed as mean ± SD. Data shown are representative of three separate experiments. *P < 0.05 vs. control.
-
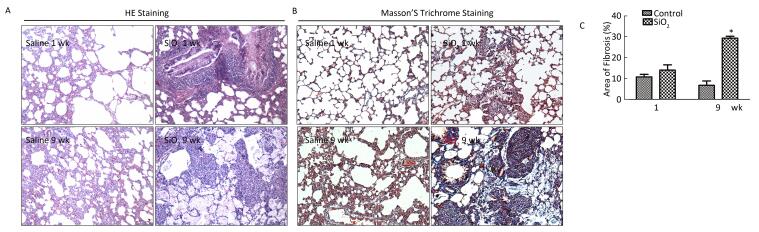
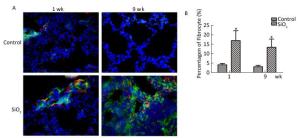
HE staining was performed to examine the pathological changes in the lung. As shown in Figure 5A, compared with the control group, severe inflammatory damage was observed in the lung tissues of SiO2-exposed rats, including infiltration of inflammatory cells, fused alveolar walls, as well as injury and thickening of bronchial epithelial cells at 1 week. After SiO2 exposure for 9 weeks, silicotic nodules were formed in the SiO2-treated rats. Excessive ECM secretion and silicotic nodule formation were indicators of silicosis. Compared with the control group, trichrome staining showed strong collagen deposition in the peribronchial areas and around pulmonary arteries in the model group at 1 or 9 weeks (Figure 5B). The Image-Pro Plus 6.0 software was used to quantify the area of fibrosis (%). As shown in Figure 5C, the area of lung fibrosis (%) (blue staining represents collagen fiber) in SiO2-treated rats had significantly increased compared with that in the control group (P < 0.05). The rats exposed to SiO2 for 9 weeks had already developed silicosis.

Figure 5. The histological changes and collagen deposition in lung tissue. (A) The histopathology of lung was evaluated by hematoxylin and eosin (HE) staining (magnification × 200). (B) Masson trichrome staining was used to determine collagen deposition in lung. Areas of fibrosis (%) were quantified with Image-Pro Plus 6.0 software. The areas of fibrosis was increased at week 9 (B and C, magnification × 200). Data are expressed as mean ± SD. *P < 0.05, vs. control. wk, week
-
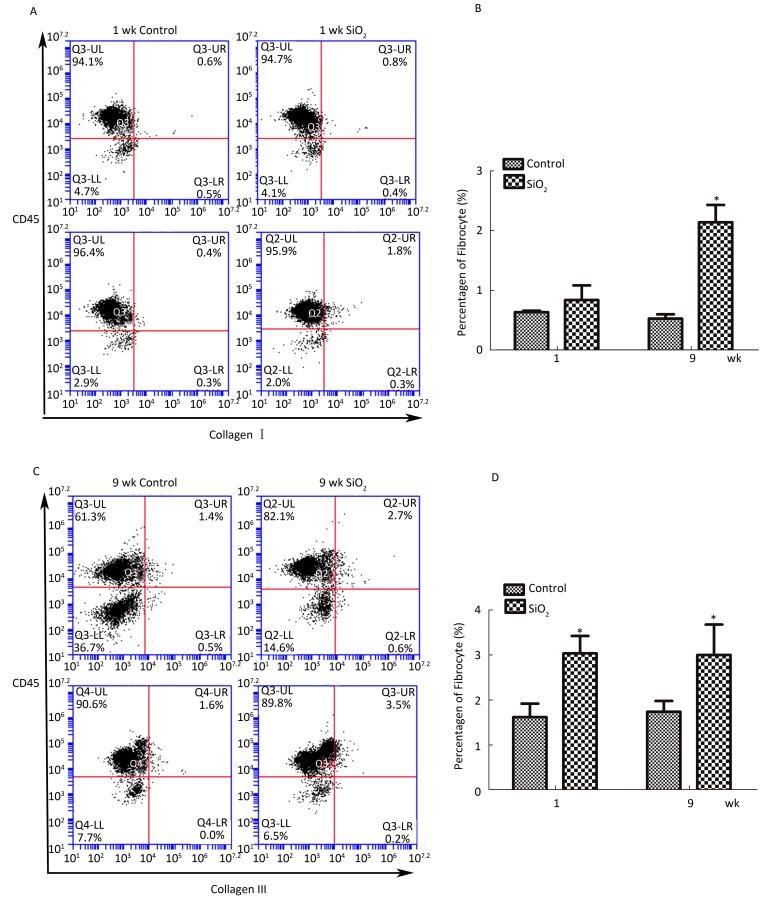
As shown in Figure 6A and 6B, compared with the control group, the number of fibrocytes in blood was significantly increased at week 9. Furthermore, the number of fibrocytes in monodisperse cell suspensions of lung was also increased at 1 and 9 weeks compared with that in the control group.
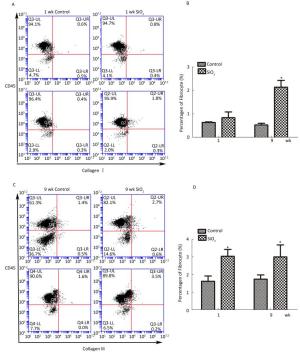
Figure 6. SiO2 exposure increased the number of fibrocytes in peripheral blood and lung tissue.(A) The number of fibrocytes in peripheral blood was increased at week 9, there is not obvious changes at week 1 (A and B). (B) The number of fibrocytes in lung tissue was increased at both week 1 and week 9 (C and D). Data are expressed as mean ± SD. Data shown are representative of three separate experiments. *P < 0.05 vs. control. wk, week
-
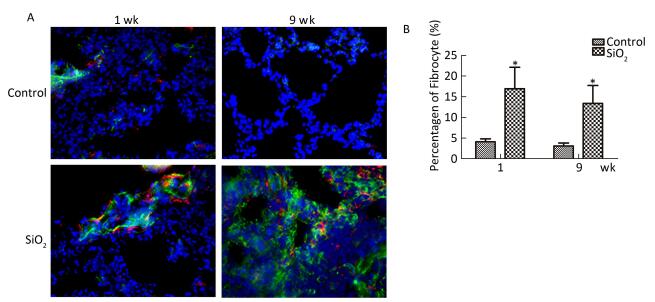
To confirm the existence of fibrocytes, and to evaluate the contribution of fibrocytes to ECM secretion, we further examined the fibrocytes in the lung by double-color immunofluorescence. As shown in Figure 7A and 7B, CD45+collagen I+ fibrocytes primarily located in the bronchia or the pulmonary artery at 1 week. In addition, CD45+collagen I+ fibrocytes were found in the silicotic nodules at 9 weeks. Compared with the control group, the percentage of fibrocytes in collagen-I-secreting cells (9%-22%) in rats with silicosis was increased at 1 and 9 weeks. This result indicated that fibrocytes partici-pate in silicosis by secreting excessive collagen I.
-
To further elucidate whether fibrocytes migrate from peripheral blood to the lung and the potential effects of SiO2 on this process, we tracked CFSE-labeled fibrocytes in rats. As shown in Figure 8A, CFSE-labeled cells can be observed in frozen lung sections in both the SiO2-exposed group at 1 week and the control group. The CFSE-labeled cells primarily accumulated in the vessel. We prepared monodisperse cell suspensions of lung to quantify fibrocyte migration to the lung by flow cytometry. Compared with the control group, there was a significant increase in the number of CFSE-labeled fibrocytes in the lung tissue of rats exposed to SiO2 (Figure 8B).
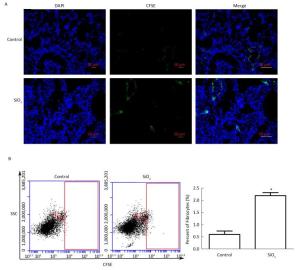
Figure 8. Tracking CFSE-labeled fibrocytes in rats after SiO2-treated for 1 week. (A) The CFSE-labeled fibrocytes were found in the lung tissue by immunofluorescence (magnification × 200). (B) The number of CFSE-labeled fibrocytes was counted by Flow Cytometric. SSC, Side scatter. The number of CFSE-labeled fibrocytes of SiO2-treated rats was increased. Data are expressed as mean ± SD. *P < 0.05, vs. control
Rat PBMCs Can Differentiate into Fibrocytes
SiO2 Potentiates Fibrocyte Differentiation in Vitro
SiO2 Exposure Increases the Inflammatory Reaction and Fibrosis of Lung Tissue
SiO2 Exposure Increases the Number of Fibrocytes in Blood or Lung Tissue
Fibrocytes as the Source of Collagen I in Silicosis
SiO2 Exposure Promotes Fibrocyte Migration to Lung
-
Silicosis is a type of fibrotic disease characterized by silicotic nodule formation and interstitial fibrosis. There are more than 40 different cell types in the lung tissue, and fibroblasts/myofibroblasts present in fibroblast foci that secrete excessive ECM are closely related to the development of pulmonary fibrosis[18-19]. Therefore, exploring the source of fibroblasts and how these fibroblasts are recruited is necessary to further understand the mechanism of silicosis. It has been previously reported that fibrocytes are associated with several fibrotic diseases[20]. Fibrocytes can differentiate into fibroblasts in vitro and in vivo, which may be an important source of myofibroblasts[21-22], suggesting that fibrocytes may participate in the development and progression of silicosis. It may be a potential prognostic biomarker for silicosis.
Fibrocytes comprise 0.1%-1.0% of peripheral blood nucleated cells in healthy hosts[23]. However, the number of fibrocytes is increased in several fibrotic diseases, such as Idiopathic Pulmonary Fibrosis (IPF)[24]. Fibrocytes have been considered as mesenchymal progenitor cells that have the potential to differentiate into other mesenchymal lineage cells[25]. Previous studies have reported that fibrocyte precursor cells in human peripheral blood can obtain a fibrocyte phenotype and migrate to the wound site[17]. Then, the fibrocytes can express α-SMA and secrete ECM (collagen I and collagen III) or various inflammatory mediators (cytokines, chemokines, and growth factors) that promote wound healing and remodeling[6]. Fibrocytes are also associated with excessive ECM deposition in lung fibrosis[26]. In the present study, PBMCs were cultured for 12 days to obtain fibrocytes. Fibroblast-like cells appeared at day 3, which might be fibrocyte precursor cells. At day 6, the number of spindle cells increased and began to proliferate. At day 12, almost all the cells had a spindle-shaped morphology and expressed both CD45 and collagen I. Further analysis showed that the number of fibrocytes was increased from day 3 to day 12. It was observed that the cells began to proliferate at day 6, which is the key time point when PBMCs differentiate into fibrocytes. The cells must be passaged according to their proliferative status, and purified fibrocytes could be obtained at day 12 (75%). The purified fibrocytes were continuously cultured, after which they began to express α-SMA protein at day 20. This result is consistent with others studies that found that fibrocytes can express α-SMA at day 21 in vitro[26-27]. The expression of collagen I protein showed no obvious changes, but that of CD45 was rarely observed. This implies that the number of CD45+collagen I+ fibrocytes was decreased. In our study, co-expression of collagen I and CD45 was used to identify fibrocytes. Hence, it can be considered that the number of fibrocytes was highest at day 12. CD45 expression began to decrease following the culture days, indicating that the fibrocytes differentiated into fibroblasts. The fibrocytes obtained α-SMA phenotype, suggesting that the fibrocytes may further differentiate into myofibroblasts. It has been reported that fibrocytes can secrete TGF-β1[28], and in the present study, we observed that fibrocytes can secrete TGF-β1 from day 3 to day 12, and much larger amounts of TGF-β1 are produced by purified fibrocytes at day 12 (data not shown). This indicated that PBMCs could differentiate into fibroblasts/myofibroblasts that may be due to autocrine or paracrine of TGF-β1 in vitro.
AM is regarded as the first barrier to defend SiO2 in the lung. It may secrete various mediators that can affect fibrocyte differentiation in peripheral blood during SiO2 exposure. In addition, fibrocytes and AMs coexist in the lung when AMs are recruited to the lung. To better simulate the silicosis induced by SiO2 in vivo, transwell was used to establish a coculture model of AMs and PBMCs. The SiO2 exposure time was chosen according to the studies of Herseth et al.[29-30]. The CCK8 assay results showed that the IC50 of AMs exposed to SiO2 is 104.84 μg/mL, and hence, we chose 100 μg/mL as our exposure concentration that can induce toxicity in AMs (data not shown).
Compared with the control group, there was a significant increase in the number of fibrocytes, collagen I and collagen III mRNA expression, and protein expression at 48 h in the SiO2-exposed group. This result suggested that SiO2 exposure may enhance the ability of PBMCs to differentiate into fibrocytes. In addition, no α-SMA protein expression was observed in fibrocytes in this study. This may be due to the short culture time in vitro, and fibrocytes did not begin expressing α-SMA protein (6-day PBMCs were used to establish the coculture system). We found that 48 h of SiO2 exposure can promote PBMC differentiation into fibrocytes. To understand its effects on myofibroblast differentiation, AMs and purified fibrocytes (20 days) were used to establish a new coculture system. The expression of α-SMA protein was increased after AMs were exposed to 100 μg/mL SiO2 for 48 h. All these results suggested that SiO2 exposure can promote PBMC differentiation into fibrocytes and further enhance the ability of fibrocyte differentiation into fibroblasts/myofibroblasts.
We have already observed that PBMCs can differentiate into fibrocytes and SiO2 exposure may promote this process in vitro. To further understand whether fibrocytes participate in silicosis in vivo, we established a rat silicosis model. Inflammatory and structural changes in the lung tissue were observed after SiO2 exposure for 1 week. Typical silicotic nodules were formed, and collagen deposition was significantly increased at week 9. The increased number of fibrocytes in peripheral blood or monodisperse cell suspensions of lung in rats with silicosis indicated that SiO2 exposure can enhance fibrocyte differentiation in vivo. Double-color immunofluorescence analysis results further showed that fibrocytes really exist in the lung tissue and account for 9%-22% of collagen-I-secreting cells. Fibrocytes can participate in silicosis and play an important role in ECM deposition.
To investigate whether these fibrocytes that migrated to the lung tissue were really derived from peripheral blood, we tracked CFSE-labeled fibrocytes in the peripheral blood in SiO2-or saline-exposed rats at 1 week. SiO2-exposed rats showed higher numbers of CFSE-labeled fibrocytes in the lung tissue than those in the control group. The CFSE-labeled fibrocytes were obviously located at the peribronchiolar area or around new blood vessels. Taken together, these results suggested that the labeled fibrocytes were recruited from the circulation into the lung as a result of SiO2 exposure. SiO2 exposure seemed to promote more labeled fibrocyte migration to the lung and played the same role as that of fibroblasts. These results further confirmed that circulating fibrocytes may be involved in the pathogenesis of silicosis. However, the exact mechanism of how fibrocytes migrate to the lung is not clear. Previous studies have shown that the CXCR4/CXCL12 axis may play an important role in this process, and we intend to focus on this aspect in further research.
The present study provided implications for future studies on silicosis. Fibrocytes exist in peripheral blood, and a further study is needed to determine whether they can be used as a biomarker to assess fibrotic diseases. In addition, the effects of fibrocytes in silicosis prompt further investigation. A potential limitation exists in the present study as we did not assess how fibrocytes migrate to the lung, which limits the further mechanism study on fibrocytes. However, the results of this study can provide us some clues about fibrocyte differentiation. Identifying a mechanism to block the migration of fibrocytes may be a novel therapeutic target in silicosis.
In summary, the present study confirmed that fibrocyte differentiation was enhanced by SiO2 exposure in vitro and fibrocytes may participate and play an important role in silicosis. In addition, the present study results may provide a new method to prevent and control silicosis. Fibrocytes may represent a novel target of therapeutic intervention in silicosis.
-
The authors of the present study are grateful to the staff of the Department of Public Health of the Zhengzhou University.
-
No conflict of interest to declare.
This study was supported by grants from the National Natural Science Foundation of China 81472954








 Quick Links
Quick Links
 DownLoad:
DownLoad: