-
The HIV/AIDS epidemic in China is spreading rapidly, with the number of people living with HIV (PLHIV) increasing nearly 1.5 times from 0.44 million in 2011 to 1.05 million in 2020 [1,2]. Antiretroviral therapy (ART) is the main AIDS treatment and can effectively reduce HIV transmission, morbidity, and mortality among PLHIV [3, 4]. Highly active antiretroviral therapy (HAART) is provided to confirmed patients with HIV/AIDS in China to expand the coverage and improve treatment effects. CD4+ T cell count recovery is widely used to evaluate ART’s effectiveness and select appropriate ART regimens in clinical practice. In China, a regular free CD4+ T cell count measurement service was provided to patients with HIV/AIDS on HAART treatment at least yearly as recommended by the Chinese Center for Disease Control and Prevention. This strategy enables researchers to monitor HAART’s effectiveness and explore factors affecting CD4+ T cell level recovery during routine diagnosis and treatment.
This ambispective cohort study was undertaken in Jiulongpo District, Chongqing city, China, from January 1, 2018, to November 30, 2020. The participants were enrolled by the Second People’s Hospital of Jiulongpo District, designated as an HIV/AIDS treatment hospital after the patients were confirmed to be infected in Chongqing city. Also, it provided free CD4+ T cell count measurements for patients with HIV/AIDS yearly. The hospital began to record patients’ treatments and related test results (i.e., CD4+ T cell counts) in an independent electronic medical system in 2018.
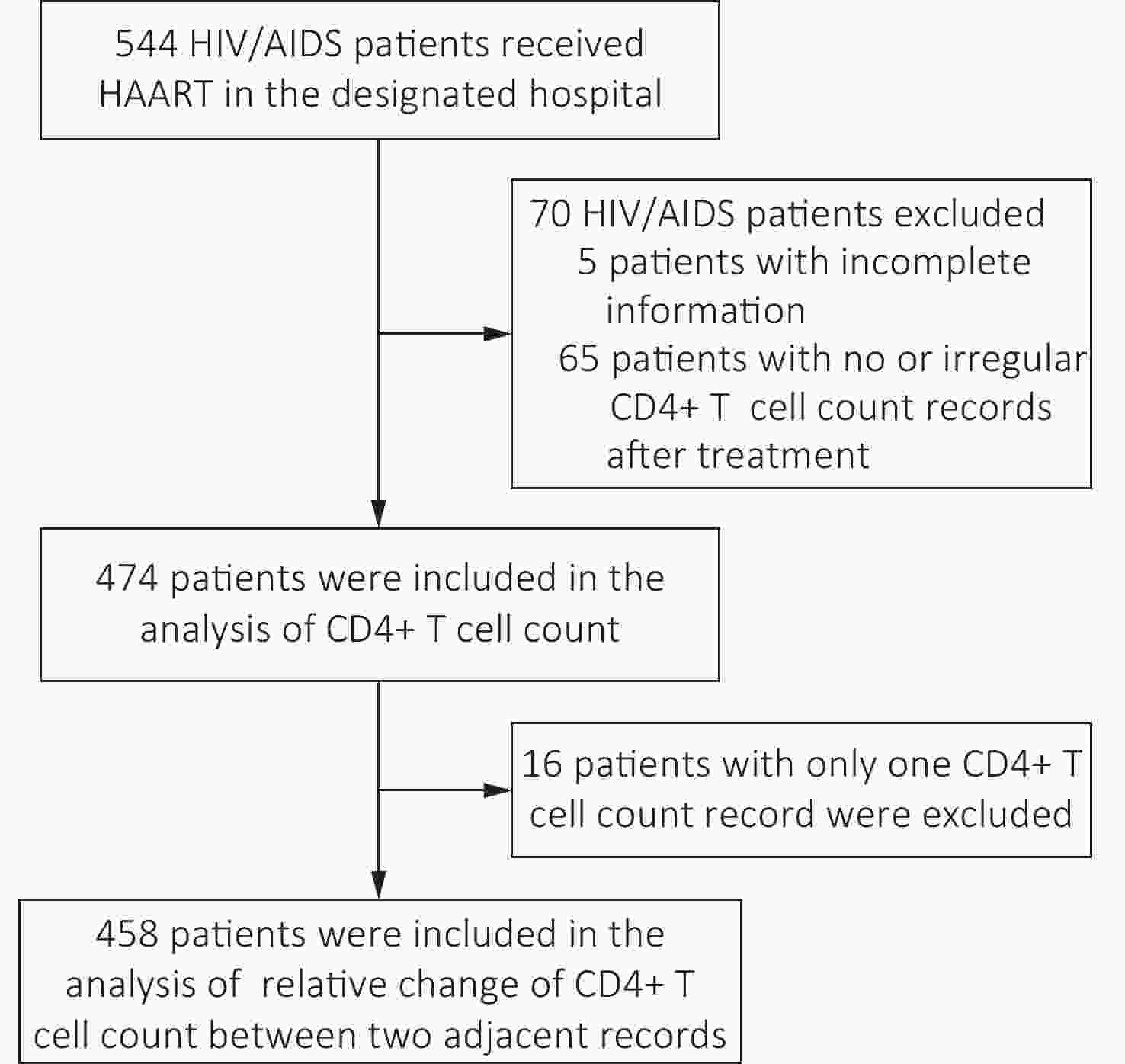
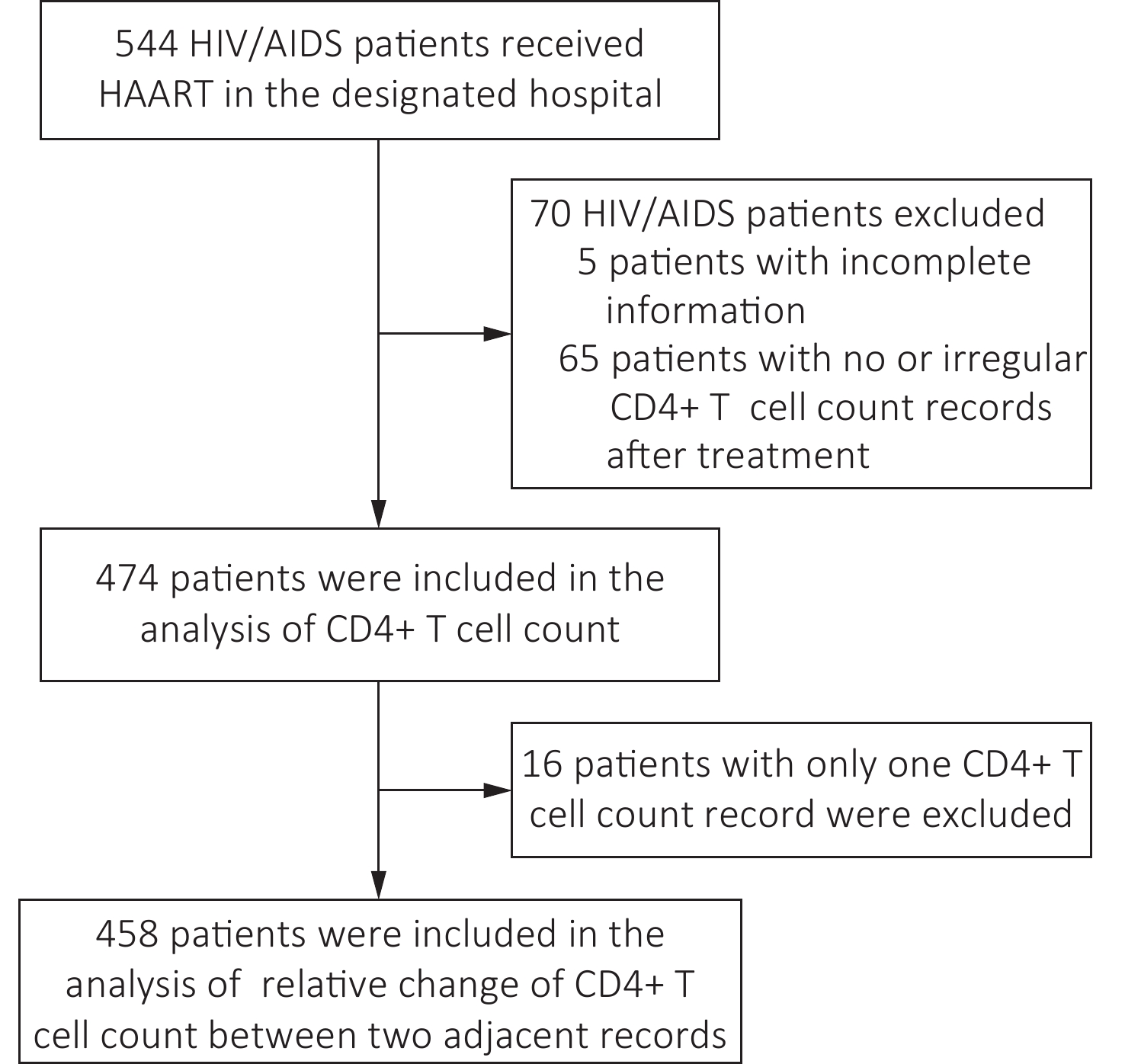
The enrollment of the cohort began on January 1, 2020. Participants were recruited during their visit to the hospital. The patients with HIV/AIDS who met the following inclusion criteria were included in the cohort: (1) 18 years or older; (2) adherence to their HAART treatment regimens and good treatment compliance; (3) continuously received HAART treatment and CD4+ T cell count measurement every year at the designated hospital; (4) did not have severe physical disabilities, immune system and hematological diseases, cancers, or mental diseases; (5) willing to participate in this study and complete the informed consent document. Participants were asked whether they were willing to provide their current and former treatments and CD4+ T cell count results in 2020 and the previous results in 2018 and 2019. Patients without CD4+ T cell count records in any year during the study period after their first record were excluded.
Patients’ demographics (sex, age), HAART treatment (treatment regimens, treatment duration), and CD4+ T cell count data were collected from the electronic medical system by two trained physicians. Patients’ first test records after treatment in the system were defined as the baseline records of the cohort, and the later records were the follow-up records, with a maximum of two follow-ups. Ethics approval for this study was obtained from the Institutional Review Board of the Second People’s Hospital of Jiulongpo District, Chongqing city, China.
We considered that a relatively higher CD4+ T cell count reflected a better immune status and treatment effect. We divided the relative change in CD4+ T cell count between every two tests of one patient into two categories: “Better” and “Worse”. “Better” meant that the later CD4+ T cell count was higher than the former. “Worse” meant that the later CD4+ T cell count was lower than the former.
One-way analysis of variance was used to compare CD4+ T cell counts among the three time points. Student–Newman–Keuls method was used to compare CD4+ T cell counts between every two time points. Univariable and multivariable Generalized Estimating Equation (GEE) models were used to compare the two outcomes by characteristics, respectively. The CD4+ T cell count was a continuous variable, so the unadjusted and adjusted β values with 95% confidence intervals (95% CIs) were reported. The change in CD4+ T cell count was a binary variable (0 = “Worse,” 1 = “Better”), so the unadjusted and adjusted odds ratios (ORs and aORs, respectively) with 95% CIs were reported. A two-sided P value of 0.05 or less was regarded as significant. Statistical analyses were performed with SPSS version 21.0 (IBM Corp).
From January 1, 2018, to November 30, 2020, 544 patients with HIV/AIDS were enrolled in the cohort. After excluding 70 patients, 474 patients were included in the analysis. Of these, 458 had more than two CD4+ T cell count results that could be included in the analysis of the relative change in CD4+ T cell counts (Figure 1). Among the 474 patients, 352 (74.3%) were male. The average age of patients was 50.3 ± 0.7 years. Most (394/474, 83.1%) used the first-line treatment regimen (Table 1). The baseline CD4+ T cell count of these patients was 343.55 ± 195.59 cells/μL, the CD4+ T cell count in their first-year follow-up (396.81 ± 213.97 cells/μL) and second-year follow-up (401.54 ± 183.80 cells/μL) were significantly higher than that at baseline (P < 0.05). (Table 1).
Table 1. CD4+ T cell count level of patients and comparison of CD4+ T cell count by characteristics
Characteristic n (%) CD4+ T cell count level Comparison of CD4+ T cell count by characteristics Baseline
(N = 474)
mean ± SD
(cells/μL)First follow-up
(N = 458)
mean ± SD
(cells/μL)Second follow-up
(N = 346)
mean ± SD
(cells/μL)F P value β (95% CI) P value adjusted β
(95% CI)eP value Total 474 343.55 ± 195.59 396.81 ± 213.97c 401.54 ± 183.80d 11.528 < 0.001 − − − − Sex Male 352 (74.3) 340.92 ± 197.88 395.76 ± 221.69c 393.14 ± 179.32d 7.825 < 0.001 −0.04
(−0.13 to 0.06)0.438 −0.05
(−0.13 to 0.04)0.310 Female 122 (25.7) 351.13 ± 189.40 399.90 ± 190.54 425.05 ± 194.91d 4.195 0.016 Ref Ref Ref Ref Age (years) ≤ 44 172 (36.3) 380.78 ± 193.91 458.43 ± 229.22c 461.41 ± 180.91d 8.219 < 0.001 0.36
(0.23 to 0.48)< 0.001 0.32
(0.21 to 0.44)< 0.001 45–64 208 (43.9) 337.55 ± 199.73 385.88 ± 204.50c 388.24 ± 177.86d 4.143 0.016 0.20
(0.08 to 0.33)0.002 0.18
(0.06 to 0.29)0.003 ≥ 65 94 (19.8) 288.68 ± 175.89 311.78 ± 170.54 305.63 ± 157.30 0.460 0.632 Ref Ref Ref Ref Treatment regimen First line
(EFV+TDF+3TC)a394 (83.1) 335.64 ± 197.91 397.22 ± 215.07c 400.56 ± 184.99d 12.178 < 0.001 −0.05
(−0.16 to 0.06)0.389 0.06
(−0.07 to 0.19)0.336 Second lineb 80 (16.9) 382.49 ± 179.86 394.68 ± 209.54 405.85 ± 179.82 0.270 0.764 Ref Ref Ref Ref Treatment duration
(years)< 1 80 (16.9) 205.88 ± 122.52 295.02 ± 148.90c − 15.831 < 0.001 −0.53
(−0.67 to −0.39)< 0.001 −0.53
(−0.68 to −0.37)< 0.001 1− 156 (32.9) 317.53 ± 170.83 367.87 ± 204.46c 360.02 ± 171.26d 3.345 0.036 −0.20
(−0.31 to −0.09)0.001 −0.23
(−0.36 to −0.10)< 0.001 3− 106 (22.4) 404.74 ± 229.51 465.79 ± 227.17 436.90 ± 198.37 2.006 0.136 0.03
(−0.10 to 0.15)0.684 −0.01
(−0.15 to 0.14)0.943 5− 59 (12.4) 405.32 ± 185.19 422.98 ± 237.73 435.04 ± 206.10 0.280 0.756 −0.01
(−0.16 to 0.14)0.904 −0.07
(−0.22 to 0.09)0.402 > 7 73 (15.4) 411.25 ± 178.21 449.64 ± 204.60 411.40 ± 151.43 1.044 0.354 Ref Ref Ref Ref Note. aEFV+TDF+3TC: Efavirenz + Tenofovir + Lamivudine. bSecond-line drugs included Abacavir (ABC), Lopinavir/r (LPV/r), Dolutegravir (DTG), and compound preparation of Zidovudine and Lamivudine. cCD4+ T cell counts at first follow-up were significantly higher than that at baseline. dCD4+ T cell counts at second follow-up were significantly higher than that at baseline. eSex, age, drug, and treatment durations were included in the GEE model. Compared with patients who were older than 65 years, patients under 44 years (adjusted β: 0.32, 95% CI: 0.21–0.44, P < 0.001), and 45–54 years (adjusted β: 0.18, 95% CI: 0.06–0.29, P < 0.01) presented significantly higher CD4+ T cell counts. Patients with treatment duration of under three years had significantly lower CD4+ T cell counts compared with those longer than seven years (adjusted β≤ 1 year: −0.53, 95% CI: −0.68 to −0.37, P < 0.001; adjusted β1–3 years: −0.23, 95% CI: −0.15 to −0.10, P < 0.001). (Table 1).
For the relative change, male patients were less likely to have better CD4+ T cell count changes than female patients (aOR: 0.71, 95% CI: 0.54–0.92, P < 0.05). CD4+ T cell counts among patients under 44 years were more likely to change better (aOR: 1.61, 95% CI: 1.16–2.25, P < 0.01). CD4+ T cell counts were more likely to change better when patients had a treatment duration of less than one year (aOR: 3.21, 95% CI: 1.79–5.76, P < 0.001). (Table 2).
Table 2. Comparison of CD4+ T cell count change by characteristics
Characteristic n (%)
(N = 458)OR (95% CI)a P value aOR (95% CI)b P value Sex Male 341 (74.5) 0.74 (0.58−0.96) 0.024 0.71 (0.54−0.92) 0.010 Female 117 (25.5) Ref Ref Ref Ref Age (years) ≤ 44 164 (35.8) 1.44 (1.04−1.99) 0.027 1.61 (1.16−2.25) 0.004 45−64 201 (43.9) 1.20 (0.88−1.65) 0.249 1.26 (0.91−1.74) 0.164 ≥ 65 93 (20.3) Ref Ref Ref Ref Treatment regimen First line (EFV+TDF+3TC)c 385 (84.1) 1.04 (0.77−1.39) 0.817 0.94 (0.69−1.28) 0.690 Second lined 73 (15.9) Ref Ref Ref Ref Treatment duration (years) < 1 77 (16.8) 2.83 (1.59−5.03) < 0.001 3.21 (1.79−5.76) < 0.001 1− 151 (33.0) 1.13 (0.83−1.53) 0.437 1.18 (0.86−1.62) 0.315 3− 101 (22.1) 0.94 (0.68−1.30) 0.698 0.98 (0.70−1.37) 0.898 5− 59 (12.9) 0.98 (0.67−1.43) 0.913 0.98 (0.66−1.47) 0.933 > 7 70 (15.3) Ref Ref Ref Ref Note. aOR: odds ratio. baOR: adjusted odds ratio; Sex, age, drug, and treatment duration were included in the GEE model. cEFV+TDF+3TC: Efavirenz + Tenofovir + Lamivudine. dSecond-line drugs included Abacavir (ABC), Lopinavir/r (LPV/r), Dolutegravir (DTG), and compound preparation of Zidovudine and Lamivudine. The CD4+ T cell recovery was associated with patients’ biological characteristics. Females’ physiological CD4+ T cell levels were relatively higher than males, and the thymic function might be affected by male hormones [5]. Pharmacogenetics studies showed immune recovery during HAART is a complex phenotype, and variants in genes could also influence the HAART-induced CD4+ T-cell gains in HIV/AIDS patients [6]. Consistent with our findings, a retrospective study of patients with HIV/AIDS in Zhejiang, China, also found that aging was a risk factor for decreasing the change in CD4+ T cells and resulted in incomplete CD4+ T cell recovery [7]. In China, the epidemic of HIV/AIDS in older people has been rising rapidly in recent years. The number of HIV-infected people older than 60 years increased more than four times from 2012 to 37 thousand cases in 2019 [8]. The rising epidemic interwoven with the poor therapeutic effect among older patients with HIV/AIDS poses a crisis for AIDS control among older individuals. Therefore, it is essential to conduct early detection and early HAART treatment for older patients with HIV/AIDS to rebuild better immunological function.
The duration of HAART treatment also affected CD4+ T cell recovery. The first year was the critical treatment period. Previous studies also showed that CD4+ T cell count increased rapidly in the first year after treatment. The reason is that the redistribution of existing CD4+ T cells from lymphoid organs is quick in the first few months following ART initiation but slows down when new CD4+ T cells are made due to thymic activation [9,10]. Zhou, et al’s study [11] showed that early and immediate ART initiation was also associated with mortality reduction. The quick recovery in the first year after treatment did not mean that it was unimportant to adhere to subsequent treatment. Our findings showed that patients with shorter than three years of treatment presented relatively lower CD4+ T cell levels than patients with longer treatment duration (adjusted β≤ 1 year: −0.53, 95% CI: −0.68 to −0.37, P < 0.001; adjusted β1–3 years: −0.23, 95% CI: −0.15 to −0.10, P < 0.001). Therefore, grasping the critical period of the first year of treatment and adhering to treatment is essential for successful immune reconstruction among patients with HIV/AIDS.
This study has some limitations. First, the study was conducted only at a single site in Chongqing city, China. Second, relatively fewer patients used second-line regimens than first-line treatment, and the combination of various second-line regimens compared with the first-line regimen might cause bias. Third, the characteristics we included in the analysis were somewhat limited. However, this study provided novel evidence on CD4+ T cell recovery among patients with HIV/AIDS receiving HAART regularly in primary care services. In future studies, researchers should include more patients using different HAART regimens at multiple centers to explore associations further, and more sociodemographic, behavioral, and treatment characteristics need to be included. Additional attention should be paid to HIV/AIDS treatment effectiveness in older patients when delivering HIV/AIDS treatment services. It is necessary to encourage patients to grasp the critical period of the first year of treatment and adhere to treatment to achieve successful immune reconstruction.
Acknowledgments We thank the doctors and nurses of the Second People's Hospital of Jiulongpo District for their contributions to participant recruitment and data collection.
Author contributions ZHANG Peng and YAN Xiang Yu were responsible for the study design; ZHANG Peng, YAN Xiang Yu, and LI Yong Jie contributed to writing the report; ZHANG Peng, YAN Xiang Yu, LI Yong Jie, and ZHANG Bo contributed to data analysis; WANG Jing contributed to data collection and data cleaning. All authors have reviewed and approved the final manuscript.
Conflict of interest All authors declare no conflict of interest.
doi: 10.3967/bes2022.035
CD4+ T Cell Recovery among Patients with HIV/AIDS who Received Highly Active Antiretroviral Therapy Regularly in Chongqing, China: An Ambispective Cohort Study
-
&These authors contributed equally to this work.
注释: -
Table 1. CD4+ T cell count level of patients and comparison of CD4+ T cell count by characteristics
Characteristic n (%) CD4+ T cell count level Comparison of CD4+ T cell count by characteristics Baseline
(N = 474)
mean ± SD
(cells/μL)First follow-up
(N = 458)
mean ± SD
(cells/μL)Second follow-up
(N = 346)
mean ± SD
(cells/μL)F P value β (95% CI) P value adjusted β
(95% CI)eP value Total 474 343.55 ± 195.59 396.81 ± 213.97c 401.54 ± 183.80d 11.528 < 0.001 − − − − Sex Male 352 (74.3) 340.92 ± 197.88 395.76 ± 221.69c 393.14 ± 179.32d 7.825 < 0.001 −0.04
(−0.13 to 0.06)0.438 −0.05
(−0.13 to 0.04)0.310 Female 122 (25.7) 351.13 ± 189.40 399.90 ± 190.54 425.05 ± 194.91d 4.195 0.016 Ref Ref Ref Ref Age (years) ≤ 44 172 (36.3) 380.78 ± 193.91 458.43 ± 229.22c 461.41 ± 180.91d 8.219 < 0.001 0.36
(0.23 to 0.48)< 0.001 0.32
(0.21 to 0.44)< 0.001 45–64 208 (43.9) 337.55 ± 199.73 385.88 ± 204.50c 388.24 ± 177.86d 4.143 0.016 0.20
(0.08 to 0.33)0.002 0.18
(0.06 to 0.29)0.003 ≥ 65 94 (19.8) 288.68 ± 175.89 311.78 ± 170.54 305.63 ± 157.30 0.460 0.632 Ref Ref Ref Ref Treatment regimen First line
(EFV+TDF+3TC)a394 (83.1) 335.64 ± 197.91 397.22 ± 215.07c 400.56 ± 184.99d 12.178 < 0.001 −0.05
(−0.16 to 0.06)0.389 0.06
(−0.07 to 0.19)0.336 Second lineb 80 (16.9) 382.49 ± 179.86 394.68 ± 209.54 405.85 ± 179.82 0.270 0.764 Ref Ref Ref Ref Treatment duration
(years)< 1 80 (16.9) 205.88 ± 122.52 295.02 ± 148.90c − 15.831 < 0.001 −0.53
(−0.67 to −0.39)< 0.001 −0.53
(−0.68 to −0.37)< 0.001 1− 156 (32.9) 317.53 ± 170.83 367.87 ± 204.46c 360.02 ± 171.26d 3.345 0.036 −0.20
(−0.31 to −0.09)0.001 −0.23
(−0.36 to −0.10)< 0.001 3− 106 (22.4) 404.74 ± 229.51 465.79 ± 227.17 436.90 ± 198.37 2.006 0.136 0.03
(−0.10 to 0.15)0.684 −0.01
(−0.15 to 0.14)0.943 5− 59 (12.4) 405.32 ± 185.19 422.98 ± 237.73 435.04 ± 206.10 0.280 0.756 −0.01
(−0.16 to 0.14)0.904 −0.07
(−0.22 to 0.09)0.402 > 7 73 (15.4) 411.25 ± 178.21 449.64 ± 204.60 411.40 ± 151.43 1.044 0.354 Ref Ref Ref Ref Note. aEFV+TDF+3TC: Efavirenz + Tenofovir + Lamivudine. bSecond-line drugs included Abacavir (ABC), Lopinavir/r (LPV/r), Dolutegravir (DTG), and compound preparation of Zidovudine and Lamivudine. cCD4+ T cell counts at first follow-up were significantly higher than that at baseline. dCD4+ T cell counts at second follow-up were significantly higher than that at baseline. eSex, age, drug, and treatment durations were included in the GEE model. Table 2. Comparison of CD4+ T cell count change by characteristics
Characteristic n (%)
(N = 458)OR (95% CI)a P value aOR (95% CI)b P value Sex Male 341 (74.5) 0.74 (0.58−0.96) 0.024 0.71 (0.54−0.92) 0.010 Female 117 (25.5) Ref Ref Ref Ref Age (years) ≤ 44 164 (35.8) 1.44 (1.04−1.99) 0.027 1.61 (1.16−2.25) 0.004 45−64 201 (43.9) 1.20 (0.88−1.65) 0.249 1.26 (0.91−1.74) 0.164 ≥ 65 93 (20.3) Ref Ref Ref Ref Treatment regimen First line (EFV+TDF+3TC)c 385 (84.1) 1.04 (0.77−1.39) 0.817 0.94 (0.69−1.28) 0.690 Second lined 73 (15.9) Ref Ref Ref Ref Treatment duration (years) < 1 77 (16.8) 2.83 (1.59−5.03) < 0.001 3.21 (1.79−5.76) < 0.001 1− 151 (33.0) 1.13 (0.83−1.53) 0.437 1.18 (0.86−1.62) 0.315 3− 101 (22.1) 0.94 (0.68−1.30) 0.698 0.98 (0.70−1.37) 0.898 5− 59 (12.9) 0.98 (0.67−1.43) 0.913 0.98 (0.66−1.47) 0.933 > 7 70 (15.3) Ref Ref Ref Ref Note. aOR: odds ratio. baOR: adjusted odds ratio; Sex, age, drug, and treatment duration were included in the GEE model. cEFV+TDF+3TC: Efavirenz + Tenofovir + Lamivudine. dSecond-line drugs included Abacavir (ABC), Lopinavir/r (LPV/r), Dolutegravir (DTG), and compound preparation of Zidovudine and Lamivudine. -
[1] NCAIDS, NCSTD, China CDC. Update on the AIDS/STD epidemic in China the third quarter of 2018. Chin J AIDS STD, 2018; 24, 1075. (In Chinese [2] The Chinese People's Political Consultative Conference. China has more than one million people infected with HIV. http://www.rmzxb.com.cn/c/2020-12-30/2750622.shtml. [2021-02-01]. (In Chinese) [3] The Antiretroviral Therapy (ART) Cohort Collaboration. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS, 2007; 21, 1185−97. doi: 10.1097/QAD.0b013e328133f285 [4] Zhang FJ, Dou ZH, Ma Y, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis, 2011; 11, 516−24. doi: 10.1016/S1473-3099(11)70097-4 [5] He L, Pan XH, Dou ZH, et al. The factors related to CD4+ T-cell recovery and viral suppression in patients who have low CD4+ T cell counts at the initiation of HAART: a retrospective study of the national HIV treatment sub-database of Zhejiang Province, China, 2014. PLoS One, 2016; 11, e0148915. doi: 10.1371/journal.pone.0148915 [6] Vidal F, Leal M, Alcamí J, et al. Current situation of the pharmacogenetics of immune recovery in treated HIV-infected patients. Pharmacogenomics, 2014; 15, 569−72. doi: 10.2217/pgs.14.1 [7] Olsen NJ, Kovacs WJ. Evidence that androgens modulate human thymic T cell output. J Investig Med, 2011; 59, 32−5. doi: 10.2310/JIM.0b013e318200dc98 [8] China CDC. The number of elderly people infected with HIV has increased fivefold in nine years. https://news.china.com/socialgd/10000169/20201202/39029425_all.html. [2021-02-01]. (In Chinese) [9] Gaardbo JC, Hartling HJ, Gerstoft J, et al. Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol, 2012; 2012, 670957. [10] Gazzola L, Tincati C, Bellistré GM, et al. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis, 2009; 48, 328−37. doi: 10.1086/695852 [11] Zhou C, Zhang W, Lu RR, et al. Benefits of early and immediate initiation of antiretroviral therapy among HIV patients in Chongqing, China. Biomed Environ Sci, 2020; 33, 282−5. -




 下载:
下载:



 Quick Links
Quick Links